Recent thymic emigrants (RTEs) are T cells that have just exited from the thymus, having completed an approximately 2-week journey that takes them from stem cell to committed T cell. Only 1% to 5% of thymocytes survive this complex maturation process that begins with T-cell receptor gene rearrangement and ends with a select population of lineage committed T cells that are both self-major histocompatibility complex (MHC)–restricted and self-tolerant.1 Throughout the lifetime of the individual, RTEs are essential for the maintenance of a diverse population of naive peripheral T cells, ready to further differentiate into appropriate effector T cells upon encounter with foreign antigen.
It has long been of interest to identify and analyze RTEs as a population distinct from the bulk of peripheral T cells, in order to quantify thymic output and to assess whether T-cell maturation continues after thymic egress. Understanding RTE biology is of particular importance for predicting recovery of the immune system following lymphoablative therapy or viral infection, and for the study of immunity in neonates (in which the bulk of the lymphoid periphery consists of RTEs) and in aged individuals (in which RTEs represent a small minority of peripheral T cells). Previous methods for tagging RTEs have included intrathymic injection of fluorochromes, transplantation of congenically marked thymus grafts, and analysis of T cell–receptor rearrangement excision circles. Each of these methods is problematic: they either result in cell death, thereby precluding functional analyses, or involve surgical manipulation that can alter the very parameters being measured.
Recent advances have highlighted new means of identifying RTEs in both unmanipulated mice and humans, allowing the isolation of live RTEs for functional and phenotypic analyses. In humans, CD4+ RTEs are specifically marked by expression of PTK7, a protein tyrosine kinase of unknown function in T cells.2 In the mouse, RTEs can be identified as fluorescent peripheral T cells in mice expressing a transgene encoding green fluorescent protein under the control of the RAG2 promoter.3 We have learned from these studies that thymic output (as a function of the size of the generative compartment) is relatively constant throughout life, that many more CD4+ RTEs emigrate than can ultimately be incorporated into the peripheral T-cell pool, and that in both mice3,–5 and humans,2 RTEs are phenotypically immature and functionally defective, compared with their mature counterparts.
Opiela et al have now contributed to this body of work by comparing the function and phenotype of RTEs in the neonatal and adult mouse.6 Such a comparison is clearly warranted, given the known differences in neonatal and adult T-cell biology. In the neonate, RTEs differentiate from fetal liver stem cells to enter a lymphopenic peripheral environment in which they constitute the majority of peripheral T cells while in healthy adults, RTEs comprise a minority of T cells in a lymphoreplete periphery. According to this latest study, stimulated neonatal RTEs secrete higher levels of effector cytokines (IL-2, IL-4 and IFNγ) than do adult RTEs.1 Furthermore, RTEs from neonates, but not from adults, proliferate in response to the key homeostatic cytokine IL-7 in the absence of T cell–receptor stimulation. CD4+ RTEs in humans also show increased IL-7–driven proliferation, although no comparative analysis of neonatal and adult RTEs was presented in this study.2 The heightened response of murine neonatal RTEs to IL-7 is accompanied by differences in the kinetics of IL-7Rα down-regulation and downstream transcription factor activation. Analysis of radiation chimeras has indicated that the key differences between neonatal and adult RTEs do not result solely from their distinct stem cell source. Instead, the availability of IL-7 and the density and identity of the cellular neighbors of RTEs may contribute to the distinct properties of adult and neonatal RTEs.
Now that the “hows” are beginning to be understood, attention is likely to turn to the “whys” of continued postthymic maturation of T cells and the functional and phenotypic differences between neonatal and adult RTEs. Does the 2- to 3-week transition period of postthymic T-cell maturation provide a means for further selecting the T-cell population for “best fit”? Do the functional defects allow the individual to purge self-reactive T-cells by permitting new emigrants to scan the periphery for tissue-specific antigens without the danger of eliciting autoimmunity? Neonates must uniquely cope with lymphopenia and the absence of mature peripheral T cells. Given that T cells undergoing homeostatic proliferation adopt a memory cell phenotype and heightened function,7 the IL-7–driven proliferation of neonatal RTEs may both help fill up empty space and provide a population of memorylike T cells. Clearly, much remains to be learned about how the youngest peripheral T cells cope with their adolescence and successfully transition into adulthood.
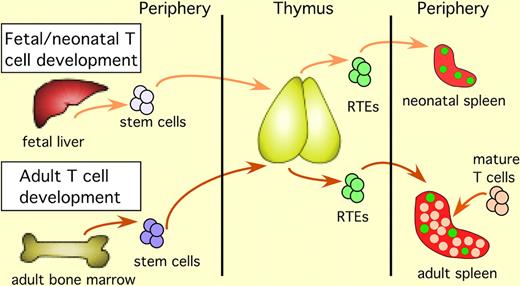
T-cell development in the fetus and the neonate begins with immigration of fetal liver–derived stem cells into the thymus. After intrathymic T-cell maturation is complete, RTEs exit the thymus and enter the lymphoid periphery. RTEs are marked by green fluorescence in mice carrying a transgene-encoding green fluorescent protein driven by the RAG2 promoter. RTEs in the neonate enter a lymphopenic periphery and constitute the majority of peripheral T cells. In the adult, stem cells arise from the bone marrow, and after completing intrathymic maturation, the resulting RTEs exit the thymus and enter a lymphoreplete periphery, where they are surrounded by a majority of mature T cells.
T-cell development in the fetus and the neonate begins with immigration of fetal liver–derived stem cells into the thymus. After intrathymic T-cell maturation is complete, RTEs exit the thymus and enter the lymphoid periphery. RTEs are marked by green fluorescence in mice carrying a transgene-encoding green fluorescent protein driven by the RAG2 promoter. RTEs in the neonate enter a lymphopenic periphery and constitute the majority of peripheral T cells. In the adult, stem cells arise from the bone marrow, and after completing intrathymic maturation, the resulting RTEs exit the thymus and enter a lymphoreplete periphery, where they are surrounded by a majority of mature T cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Acknowledgment:
This work was supported by National Institutes of Health grant R01 AI064318.
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal