Abstract
CD4+CD25+Foxp3+ T cells are regulatory/suppressor cells (Tregs) that include non-antigen (Ag)–specific as well as Ag-specific Tregs. How non–Ag-specific naive CD4+CD25+ Treg develop into specific Tregs is unknown. Here, we generated adaptive Tregs by culture of naive CD4+CD25+Foxp3+ T cells with allo-Ag and either interleukin-2 (IL-2) or IL-4. Within days, IL-2 enhanced interferon-γ receptor (Ifnγr) and Il-5 mRNA and IL-4 induced a reciprocal profile with de novo IL-5Rα and increased IFN-γ mRNA expression. Both IL-2– and IL-4–alloactivated CD4+CD25+ Tregs within 3 to 4 days of culture had enhanced capacity to induce tolerance to specific donor but not to third-party cardiac allografts. These hosts became tolerant as allografts functioned more than 250 days, with a physiologic ratio of less than 10% CD4+CD25+Foxp3+ T cells in the CD4+ population. CD4+CD25+ T cells from tolerant hosts given IL-2–cultured cells had increased Il-5 and Ifnγr mRNA. Those from hosts given IL-4–cultured cells had enhanced IL-5Rα mRNA expression and IL-5 enhanced their proliferation to donor but not third-party allo-Ag. Thus, IL-2 and IL-4 activated allo-Ag–specific Tregs with distinct phenotypes that were retained in vivo. These findings suggested that T-helper 1 (Th1) and Th2 responses activate 2 pathways of adaptive Ag-specific Tregs that mediate tolerance. We propose they be known as T-suppressor 1 (Ts1) and Ts2 cells.
Introduction
Bone marrow and organ transplantation are curative for an increasing number of diseases. The major barriers are graft-versus-host disease (GVHD) and rejection, which currently are inhibited by toxic nonspecific immunosuppression. Induction of donor-specific tolerance without the requirement for immunosuppressive drugs would be highly desirable. Adoptive therapy with ex vivo–induced antigen (Ag)–specific T regulatory cells (Tregs) has considerable potential.
CD4+CD25highFoxp3+ T cells are potent regulators of transplantation rejection1-5 and GVHD.6-8 Natural Tregs are produced as a separate lineage in the thymus that constitute 2% to 10% of peripheral CD4+ T cells,9 inhibit in a non–Ag-specific manner, and protect normal tissue from immune injury.2,10 The CD4+CD25+ T cells that mediate transplantation tolerance are Ag-specific,1-4 but how they develop and differ to natural non–Ag-specific CD4+CD25+ Tregs is poorly understood.9
In fully allogeneic models, naive CD4+CD25high T cells, if given at a ratio of 1:1 with naive CD4+ T cells, can totally prevent rejection5 and GVHD7 but only partially block GVHD at a ratio of 1:2.8 At a ratio of 1:10, naive CD4+CD25high T cells do not block rejection5 or GVHD.7 Given the very low number of CD4+CD25+ T cells in the T-cell population, it is impractical to prepare enough cells for therapeutic usage at a ratio of 1:1. Ex vivo polyclonal activation of CD4+CD25+ T cells with interleukin-2 (IL-2) and anti-CD3 monoclonal antibody (mAb)6 or IL-2, anti-CD3 and anti-CD2811 results in 200- to 250-fold cell number expansion but no enhanced Ag-specific regulatory capacity.12 Culture of CD4+CD25+ Tregs with allo-Ag and IL-2 for a week or more also increases cell numbers but does not induce high potency Ag-specific CD4+CD25+ Tregs, in that a ratio of 2:113 or 5:114 with naive T cells is required to suppress skin graft rejection. Similarly, in GVHD, a 7:10 ratio of IL-2– and allo-Ag–cultured Tregs to naive cells is less effective than fresh naive CD4+CD25+ T cells, in that they delay but do not fully prevent GVHD.8
In contrast, CD4+CD25+ T cells within CD4+ T cells from hosts with allo-Ag–specific tolerance to a graft can transfer allo-Ag–specific tolerance at an effective ratio of less than 1:20.2,3 These tolerant CD4+ T cells, when cultured in vitro, lose the capacity to transfer tolerance unless stimulated by specific donor Ag in media supplemented with T-cell cytokines.2,15,16 Which cytokines are most effective at maintaining specific Tregs is unknown, but IL-2 alone is insufficient.16 This suggests that Ag-specific CD4+CD25+ T cells may become dependent on cytokines other than IL-2. We have identified that interferon-γ (IFN-γ) and IL-5, but not other T-helper 1 (Th1) and Th2 cytokines, promoted proliferation and survival of Ag-specific tolerance mediating Tregs from rats tolerant to an allograft (B.M.H., M.N., K.M.P., N.D.V., G.T.T., S.J.H., unpublished data).
Th1 and Th2 responses promote different pathways of activation in other immune cells, including activated CD8+ T cells,17 B cells,18 and macrophages.19 As both Th1 and Th2 responses activate CD4+CD25+ T cells,20 we examined whether Th1 and Th2 cytokines promoted different pathways of activation of natural CD4+CD25+ T cells. We found that the only Th1 or Th2 cytokines that could markedly enhance proliferation of naive CD4+CD25+ T cells to allo-Ag were IL-2 or IL-4. Short-term culture of natural CD4+CD25+ T cells with allo-Ag and IL-2 or IL-4 produced allo-Ag–specific Tregs that at a ratio of 1:10 with naive CD4+ T cells inhibited rejection of specific donor but not third-party allografts. Within 3 days, IL-2– or IL-4–induced Ag-specific CD4+CD25+Foxp3+ Tregs had acquired different profiles of cytokine receptor and cytokine expression, consistent with 2 separate pathways of activation of Tregs by Th1 and Th2 responses.
Methods
Animals
DA (Rt-1a), PVG (Rt-1c), and Lewis (Rt-1l) rats were bred at the Liverpool Hospital Animal House or the Animal Resources Center (Perth, Australia). The University of New South Wales Animal Care and Ethics Committee approved all experiments.
Cytokines
IL-2, IFN-γ, IL-5, IL-10, IL-12p70, IL-13, IL-15, and IL-23 were produced from transfected CHO-K cell lines and characterized and quantified using bioassays, as described.21-24 The cell line for IL-4 was a kind gift from N. Barclay (Sir William Dunn School of Pathology, Oxford, United Kingdom). IL-2 was used at 100 units/mL and IL-4 at 125 units/mL, the dose that induced nearly maximal proliferation of CD4+CD25+ T cells in dilutional mixed lymphocyte culture (MLC) assays. This concentration inhibited proliferation of CD4+CD25− T cells, which peaked with lower concentrations of 5 to 25 units/mL (data not shown).
Lymphocyte preparation and culture
Cells were prepared from spleens and lymph nodes of DA rats and immunofluorescence staining performed as described.5,25 Antirat mAbs used were R7.2 (TCR-α,β), G4.18 (CD3), MRCOx35 (CD4), MRCOx8 (CD8), MRCOx39 (CD25), MRCOx33 (CD45RA), Cy5 annexin V (BD PharMingen, San Diego, CA), and fluorescein isothiocyanate (FITC) antimouse/rat Foxp3 (eBioscience, San Diego, CA).
T-cell subsets were enriched by indirect panning to deplete CD8+ T cells and B cells, followed by CD25 enrichment using phycoerythrin (PE)–conjugated MRCOx39 and anti-PE magnetic beads using the magnetic-activated cell separation system (Miltenyi Biotec, North Ryde, Australia) as described.5,26
MLCs were performed as described,5,26 and proliferation was measured by [3H]thymidine incorporation. Antigen-presenting cells (APCs) were irradiated (25 Gy) thymus cells, media was supplemented with 20% normal rat serum, and cytokines were in serum-free media at 50 to 200 units/mL. Syngeneic control counts were less than 200 cpm, equivalent to media alone.
Microcultures contained 1 to 2 × 105 responder and 2 × 104 stimulator cells/well with 4 to 6 replicates/group. Bulk cultures (25-cm2 flasks) were harvested at 2, 4, or 6 days; the CD4+CD25+ T cells were reseparated after culture by magnetic-activated cell separation before RNA extraction for cytokine and cytokine receptor mRNA analysis.
To test suppressive capacity of alloactivated CD4+CD25+ T cells, serial 2-fold dilutions of cells starting at 105 cells/well, were admixed with 105 naive CD4+CD25 T cells and in vitro proliferative response to priming, and third-party stimulator cells was assessed at day 4.
RT-PCR
RNA extraction, cDNA synthesis, and semiquantitative polymerase chain reaction (PCR) were performed as described.27 Primers and conditions for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Il-2, Ifnγ, Il-4, Il-5, Il-10, Il-13, and transforming growth factor-β (Tgf-β) were as described.27 Primers (forward; reverse) for rat cytokine receptors were: IFN-γ receptor (Ifnγr): (5′-AGAAGCACCAGAGCAGGAAGAAC-3′; 5′-CACGAGAACAAAGCAGGAAAAC-3′), Il-4rα:(5′-GGTGAGTGTGCTGTTGTTGCTGA-3′; 5′-AATGAGTCCCTGAATCCCTTGTG-3′), Il-5rα:(5′-CTTCTGCCACCTGTCAATTTTACC-3′; 5′-AACAAGCCAGGTGCAACGAAGAGA-3′); and for rat transcription factors were: Foxp3: (5′-GCTCCTGCTGCCTCGTAG-3′; 5′-TTGTGGAAGAACTCTGGAAAGG-3′), Gata3: (5′-ACTGTGGCGGCGAGATGGTA-3′; 5′-ATGAACGGGGAGATGTGGCT-3′), T-bet: (5′-AACCAGTATCCTGTTCCCAGC-3′; 5′-TGTCGCCACTGGAAGGATAG-3′).
Real-time reverse-transcribed PCR (RT-PCR) was performed using a Rotorgene (Corbett Research, Mortlake, Australia) with SYBR Green I and HotMaster Taq polymerase (Eppendorf, Hamburg, Germany) or the SensiMix DNA Kit (Quantace, London, United Kingdom). Gene copy numbers were derived from a standard curve run in parallel and were normalized against Gapdh expression.
Allograft adoptive transfer assay
DA recipient rats were given 7 Gy of whole body irradiation and grafted with fully allogeneic PVG or Lewis heterotopic heart allograft. Graft function was scored using a semiquantitative scale: 4 indicates robust contraction with normal auxiliary heart graft rate; 3, minor slowing of heart rate and/or minor reduced contraction; 2, obvious slowing and swelling of the graft with significant impairment of contraction on palpation; 1, marked slowing, very poor palpable contraction, or very reduced electrocardiogram (ECG) amplitude; and 0, no palpable contraction, equivalent to no ECG activity, as described.5
In this model, rejection is ablated unless hosts are restored with CD4+ T cells2,5,28,29 with 5 × 106 naive CD4+ T cells reconstituting severe rejection in 10 to 20 days.5 Some of these allografts recover days or weeks later and function long-term.5 As poor cardiac function would not support life if the graft were orthotopic, our aim was to assay the ability of activated Tregs to abolish severe rejection, defined as less than or equal to 2 using the semiquantitative score.
In preliminary studies, naive CD4+CD25+ T cells, coadministered at a ratio of 1:1 with naive CD4+ T cells, prevented severe rejection of all grafts; but at a ratio of 1:10, 8 of 9 rats had severe rejection episodes.5 In vitro alloactivation of naive CD4+CD25+ T cells with IL-2 did not activate effector T cells and suppressed rejection at a ratio of 1:1.5
Here we examined whether in vitro IL-2– or IL-4–alloactivated CD4+CD25+ Treg had enhanced capacity to suppress rejection at a ratio of 1:10 with naive CD4+ T cells. Thus, 5 × 105 CD4+CD25+ T cells activated with either IL-2 or IL-4 and PVG allo-Ag were adoptively transferred with 5 × 106 naive CD4+ T cells to irradiated DA hosts with either PVG or Lewis cardiac allografts. All groups had 6 to 10 animals.
Adoptive hosts that accepted their graft for more than 250 days were examined for graft histology, as described,5 and examined with an Olympus BH2 microscope with a 40× S plan Gpo objective (Olympus Australia, Mt Waverley, Australia), and photographed with a digital imaging camera, Spot RT Slider (Diagnostic Instruments, Sterling Heights, MI) using Spot RT Slider software, with no digital manipulation. Peripheral lymphocyte subset composition, and cytokine and cytokine receptor mRNA expression by peripheral CD4+CD25+ T cells. The reactivity of CD4+CD25+ and CD4+CD25− T cells from tolerant hosts to donor and third-party Ag was examined in MLC, as was the effect of IL-5 and IFN-γ on their proliferation. To obtain sufficient CD4+CD25+ T cells to undertake the studies, lymph nodes and spleens of all long-term surviving hosts were combined.
Statistics
Significance was determined using analysis of variance with a Bonferroni-Dunn post-hoc test on StatView 5.0. Graft survival used the Kaplan-Meier method, and differences were determined using the Mann-Whitney log-rank test on Statview (Abacus Concepts, Berkeley, CA). Significance was P less than .05.
Results
Effect of Th1 and Th2 cytokines on the naive CD4+CD25+ T cell response to allo-Ag in MLCs
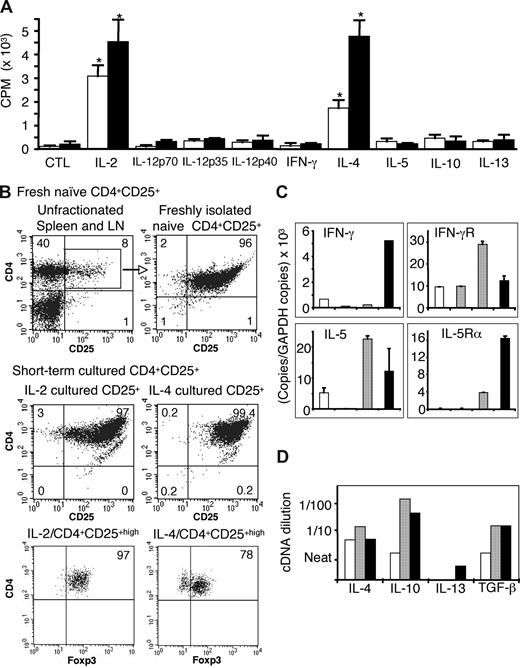
Either IL-2 or IL-4 enhanced proliferation of CD4+CD25+ T cells to both self- and allogeneic APCs (Figure 1A), with counts of 1500 to 5000 cpm and ongoing proliferation at 6 days. The proliferation to allo-Ag was always greater than to self in all 7 experiments. The original CD4+CD25+-enriched populations were more than or equal to 96% CD25+, more than 98% CD4+, less than 1% CD8+ (Figure 1B), with more than 99% TCR-α/β+ and CD3+, confirming they were T cells. After 3 days in culture with allo-Ag and IL-2 or IL-4, 99% of cells expressed CD4, CD25, TCR-α,β, and CD3. Foxp3 expression after culture with IL-2 (97%) and IL-4 (78%) (Figure 1B bottom panel) was similar to naive CD4+CD25+ T cells (88%), indicating retention of their suppressor phenotype. In contrast, CD4+CD25+ T cells cultured with allo-Ag alone had very low proliferation (< 500 cpm) peaking at 3 to 4 days (Figure 1A) and only approximately 30% of cells were CD4+CD25+highFoxp3+. At 24 hours and 96 hours, respectively, 45% and 50% of CD4+CD25+ T cells cultured with allo-Ag expressed annexin V, compared with 29% and 50% with IL-2, and 28% and 22% with IL-4, indicating both cytokines reduced early apoptosis.
Proliferation and phenotype of IL-2– and IL-4–cultured naive CD4+CD25+ T cells. (A) Representative MLC experiment showing proliferation of naive CD4+CD25+ T cells cultured for 4 days with self- (DA, □) or allogeneic (PVG, ■) APCs and different Th1 and Th2 cytokines. Proliferation induced by IL-2 and IL-4 was greater to allo-Ag than to self. CTL indicates cultured with no cytokine added. Data are mean plus or minus SD (n = 4). *P < .001 compared with CTL. (B) Representative fluorescence-activated cell sorter profiles showing CD4+CD25+ T cells retained CD25 and Foxp3 expression after culture. Expression of surface CD4 (Cy5), CD25 (PE), and intracellular Foxp3 (FITC) analyzed by 3-color staining. (Top panel) Naive unfractionated peripheral lymph node and spleen and freshly isolated CD4+CD25+ T cells preculture. (Middle and bottom panels) CD4+CD25+ T cells cultured with allo-Ag and either IL-2 (left) or IL-4 (right). (C) Expression of cytokines and cytokine receptors by CD4+CD25+ T cells: fresh (□) or cultured with allogeneic APCs (/PVG) with no cytokines (CTL, ▩), IL-2 (▨), or IL-4 (■) for 2 to 6 days. Representative quantitative real-time RT-PCR data expressed as ratio of specific gene copy number per 103 copies of Gapdh. Culture with IL-2 and allo-Ag increased expression of Ifnγ and Il-5 mRNA, whereas IL-4 and allo-Ag induced IL-5Rα and enhanced IFN-γ expression. (D) Composite semiquantitative RT-PCR of mRNA from CD4+CD25+ T cells cultured for 2 to 4 days with allogeneic APCs (/PVG) and no cytokine (□), IL-2 (▨), or IL-4 (■) (n = 4-5). Y-axis shows cDNA dilutions (near to 1 in 100) after normalization for Gapdh. PCRs were at 40 cycles, except for GAPDH (30 cycles).
Proliferation and phenotype of IL-2– and IL-4–cultured naive CD4+CD25+ T cells. (A) Representative MLC experiment showing proliferation of naive CD4+CD25+ T cells cultured for 4 days with self- (DA, □) or allogeneic (PVG, ■) APCs and different Th1 and Th2 cytokines. Proliferation induced by IL-2 and IL-4 was greater to allo-Ag than to self. CTL indicates cultured with no cytokine added. Data are mean plus or minus SD (n = 4). *P < .001 compared with CTL. (B) Representative fluorescence-activated cell sorter profiles showing CD4+CD25+ T cells retained CD25 and Foxp3 expression after culture. Expression of surface CD4 (Cy5), CD25 (PE), and intracellular Foxp3 (FITC) analyzed by 3-color staining. (Top panel) Naive unfractionated peripheral lymph node and spleen and freshly isolated CD4+CD25+ T cells preculture. (Middle and bottom panels) CD4+CD25+ T cells cultured with allo-Ag and either IL-2 (left) or IL-4 (right). (C) Expression of cytokines and cytokine receptors by CD4+CD25+ T cells: fresh (□) or cultured with allogeneic APCs (/PVG) with no cytokines (CTL, ▩), IL-2 (▨), or IL-4 (■) for 2 to 6 days. Representative quantitative real-time RT-PCR data expressed as ratio of specific gene copy number per 103 copies of Gapdh. Culture with IL-2 and allo-Ag increased expression of Ifnγ and Il-5 mRNA, whereas IL-4 and allo-Ag induced IL-5Rα and enhanced IFN-γ expression. (D) Composite semiquantitative RT-PCR of mRNA from CD4+CD25+ T cells cultured for 2 to 4 days with allogeneic APCs (/PVG) and no cytokine (□), IL-2 (▨), or IL-4 (■) (n = 4-5). Y-axis shows cDNA dilutions (near to 1 in 100) after normalization for Gapdh. PCRs were at 40 cycles, except for GAPDH (30 cycles).
Other Th1 cytokines, including IFN-γ and IL-12p70, as well as Th2 cytokines, including IL-5, IL-10, and IL-13, did not enhance proliferation (Figure 1A) in all 3 experiments. In one experiment, IL-15 and IL-23 did not induce proliferation of naive CD4+CD25+ T cells (data not shown).
Th1 cytokine and cytokine receptor mRNA expression in naive CD4+CD25+ T cells stimulated in MLCs with IL-2 or IL-4
There was no induction of Il-2 mRNA in any culture, consistent with Foxp3 inhibition of IL-2 transcription in CD4+CD25+ T cells and no induction of Th1 cells.30 Ifnγ mRNA expression was increased in CD4+CD25+ T cells cultured with allo-Ag and Il-4, but not Il-2 (Figure 1C), with the greatest differences (> 20 fold) observed at day 2 in culture. Ifnγr mRNA expression was enhanced on CD4+CD25+ T cells cultured with IL-2, but not with IL-4 or nil cytokine (Figure 1C), with the greatest differences observed at days 4 to 6. The data in Figure 1C were representative of 5 experiments. The Th1 transcription factor T-bet was not induced in either IL-2– or IL-4–cultured CD4+CD25+ T cells.
Th2 cytokine and cytokine receptor mRNA expression in naive CD4+CD25+ T cells stimulated in MLCs with IL-2 or IL-4
mRNA for Il-5rα, the IL-5 binding component of the receptor, was induced in CD4+CD25+ T cells cultured with IL-4 for 4 to 6 days (Figure 1C; representative of 4 experiments). Il-5rα mRNA was not detected on freshly isolated CD4+CD25+ T cells, nor was it induced by culture with IL-2 or nil cytokine and allo-Ag, or culture with self-stimulator cells and IL-2 or IL-4.
Il-5 mRNA expression tended to be higher after culture with IL-2 and allo-Ag at days 2 to 4 (Figure 1C) and in one experiment was 10-fold greater. In cultures with IL-4 and allo-Ag, Il-5 mRNA expression was similar to that observed in fresh CD4+CD25+ T cells.
Il-4 mRNA expression in cells cultured with either IL-2 or IL-4 (Figure 1D) was similar to fresh CD4+CD25+ T cells (data not shown). Il-4rα, the IL-4–binding component of the receptor, was enhanced after culture with either IL-2 or IL-4 in allogeneic MLCs but not in cultures with self-APC or nil cytokine (data not shown). Il-10 and Tgf-β mRNA expression was similar in IL-2– and IL-4–cultured cells (Figure 1D). The Th2 transcription factor, Gata3, was modestly increased with IL-4 but not with IL-2.
Comparison of the cytokine and cytokine receptor expression in CD4+CD25− T cells cultured in MLCs with either IL-2 or IL-4
In some experiments, CD4+CD25− T cells cultured, in parallel with CD4+CD25+ T cells, and had greater proliferation at 4 and 6 days.26 Foxp3+ cells did not increase in CD4+CD25− T cell cultures, with 3% before and 2% to 4% after culture with either IL-2 or IL-4. Annexin V expression was similar with no cytokine or IL-2 at 24 hours, 75% vs 73%, and at 96 hours, 21% vs 21%. There was reduced early apoptosis with IL-4 as only 24% expressed annexin V at 24 hours and 20% at 96 hours.
There was no induction of Il-5rα in any CD4+CD25− T-cell cultures (data not shown). In cultures with IL-2, there was also no enhanced expression of Il-5 mRNA in CD4+CD25− T cells, unlike the CD4+CD25+ T cells, which had enhanced Il-5 mRNA expression. There was increased Il-5 mRNA expression in CD4+CD25− T cells cultured with IL-4, consistent with induction of a Th2 profile. Ifnγr mRNA was detected in CD4+CD25− T cells cultured with either IL-2 or IL-4. CD4+CD25− T cells had no enhanced expression of Ifnγr mRNA in MLCs supplemented with either IL-2 or IL-4 compared with freshly isolated cells.
This confirmed that the distinct changes in cytokine and cytokine receptor mRNA expression in naive CD4+CD25+ T cells after alloactivation with IL-2 or IL-4 were not the result of induction of Th1 and Th2 phenotypes.
Capacity of IL-2– or IL-4–alloactivated CD4+CD25+ T cells to suppress in vitro
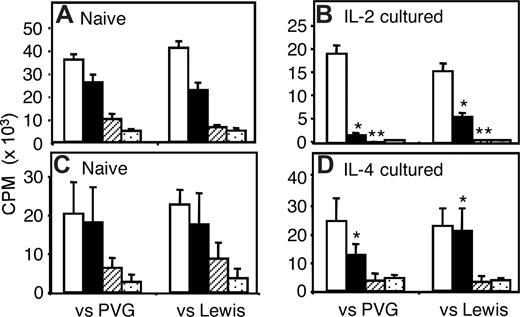
Limiting dilutions of CD4+CD25+ T cells with a constant number of CD4+CD25− T cells were assayed in MLCs. Both IL-2– and IL-4–alloactivated CD4+CD25+ T-cell populations had greater suppression of CD4+CD25− T-cell proliferation to specific priming Ag (PVG) than to third-party Lewis stimulator cells, manifest at a ratio of 1:16 and 1:32, respectively (Figure 2B,D). At higher ratios, there was non–Ag-specific suppression with similar effects against PVG or Lewis stimulators. Parallel experiments with naive CD4+CD25+ T cells showed similar suppression against PVG and Lewis at all ratios tested, with near-complete suppression only at a ratio of 1:1 (Figure 2A,C). The enhanced inhibition at lower ratios, by IL-2– and IL-4–alloactivated CD4+CD25+ T cells, suggested induction of allo-Ag–specific Tregs.
Ability of IL-2– or IL-4– and allo(PVG)–activated CD4+CD25+ Tregs to suppress in vitro proliferation of CD4+CD25− T cells in MLCs. Responses to specific PVG and third-party Lewis stimulators were compared. The effect of specific suppression was evident at ratios of activated CD4+CD25+ Tregs to CD4+CD25− T cells of 1:16 and 1:32, respectively. At ratios above this, there was non–Ag-specific suppression by both IL-2 and IL-4 allo-Ag–activated Tregs. (A,B) Proliferation of 1 × 105 naive CD4+CD25− T cells to PVG and Lewis APC cultured with naive (A) and IL-2/PVG–activated (B) CD4+CD25+ Tregs in different ratio; no Tregs (□), IL-2/PVG–activated CD4+CD25+ T regs, and CD4+CD25− T cells at ratios of 1:16 (squlf), 1:4 (▨), and 1:1 (▩). (C,D) Proliferation of 1 × 105 naive CD4+CD25− T cells to PVG and Lewis APCs cultured with naive (C) and IL-4/PVG–activated (D) CD4+CD25+ Tregs in different ratios; no CD4+CD25+ Tregs (□), IL-4/PVG–activated CD4+CD25+ Tregs, and CD4+CD25− T cells at ratios of 1:32 (■), 1:4 (▨), and 1:1 (▩). Significant differences at *P < .01; **P < .05.
Ability of IL-2– or IL-4– and allo(PVG)–activated CD4+CD25+ Tregs to suppress in vitro proliferation of CD4+CD25− T cells in MLCs. Responses to specific PVG and third-party Lewis stimulators were compared. The effect of specific suppression was evident at ratios of activated CD4+CD25+ Tregs to CD4+CD25− T cells of 1:16 and 1:32, respectively. At ratios above this, there was non–Ag-specific suppression by both IL-2 and IL-4 allo-Ag–activated Tregs. (A,B) Proliferation of 1 × 105 naive CD4+CD25− T cells to PVG and Lewis APC cultured with naive (A) and IL-2/PVG–activated (B) CD4+CD25+ Tregs in different ratio; no Tregs (□), IL-2/PVG–activated CD4+CD25+ T regs, and CD4+CD25− T cells at ratios of 1:16 (squlf), 1:4 (▨), and 1:1 (▩). (C,D) Proliferation of 1 × 105 naive CD4+CD25− T cells to PVG and Lewis APCs cultured with naive (C) and IL-4/PVG–activated (D) CD4+CD25+ Tregs in different ratios; no CD4+CD25+ Tregs (□), IL-4/PVG–activated CD4+CD25+ Tregs, and CD4+CD25− T cells at ratios of 1:32 (■), 1:4 (▨), and 1:1 (▩). Significant differences at *P < .01; **P < .05.
Examination of the capacity of IL-2– or IL-4–cultured CD4+CD25+ T cells to suppress allograft rejection and induce tolerance
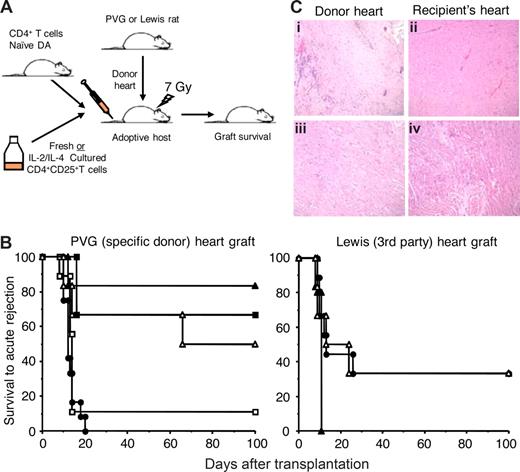
A total of 5 × 105 CD4+CD25+ T cells cultured with PVG allo-Ag and either IL-2 or IL-4 were transferred to irradiated hosts that had been grafted with either specific donor PVG or third-party Lewis cardiac grafts and restored with 5 × 106 naive CD4+ T cells (Figure 3A). At this ratio of 1:10, the transferred CD4+CD25+ T cells suppressed specific donor but not third-party allograft rejection (Figure 3B).
Graft survival without a severe rejection episode and histology of heart grafts from DA hosts that were restored with CD4+CD25+ T cells cultured with allogeneic APCs and IL-2 or IL-4. (A) Experimental plan to test the ability of IL-2 or IL-4, PVG-alloactivated CD4+CD25+ T cells from DA rats to suppress severe rejection of heterotopic adult heart grafts from PVG (specific) or Lewis (third-party) donors. All irradiated DA hosts were restored with 5 × 106 naive CD4+ T cells. CD4+CD25+ T cells, either fresh or cultured for 3 to 4 days with allogeneic APC (PVG) and IL-2 or IL-4, were cotransferred with naive CD4+ T cells at various ratios. (B) Heart graft survival results showing the ability of naive CD4+CD25+ T cells cultured with allogeneic APCs and IL-2 (Ts1 cells) or IL-4 (Ts2 cells) to suppress allograft rejection. Irradiated hosts restored with 5 × 106 naive CD4+ T cells (●) reject both PVG (n = 12) and Lewis (n = 9) grafts. CD4+CD25+ T cells cultured with IL-2 (▴) or IL-4 (▵) and PVG allo-Ag, mixed at a ratio of 1:10 with naive CD4+ T cells (5 × 105 CD4+CD25+ with 5 × 106 CD4+ cells), suppressed donor specific (PVG) (P = .007 for IL-2, n = 6 and P = .035 for IL-4 cultured, n = 6) but not third-party allograft rejection (NSD, n = 5 for IL-2 and n = 6 for IL-4 cultured). Naive CD4+CD25+ T cells suppressed severe rejection of PVG allografts at a ratio of 1:1 with naive CD4+ T cells (■) (P < .001, n = 9) but not at a ratio of 1:10 (□) (NSD, n = 9). Some control data have previously been published.5 Graft survival without a severe rejection episode is only shown to 100 days, but all grafts functioning at that time were monitored to at least 250 days and none had a rejection episode. (C) Photomicrographs (original magnification ×200) of hearts from DA hosts given CD4+CD25+ T cells cultured with PVG allo-Ag and IL-2 (i,ii) or IL-4 (iii,iv). Comparison of histology of PVG long surviving donor heart (left panels) to the recipient's own heart (right panels) showed similar morphology with preserved muscular structure except for scattered patches of infiltrating mononuclear cells in donor hearts.
Graft survival without a severe rejection episode and histology of heart grafts from DA hosts that were restored with CD4+CD25+ T cells cultured with allogeneic APCs and IL-2 or IL-4. (A) Experimental plan to test the ability of IL-2 or IL-4, PVG-alloactivated CD4+CD25+ T cells from DA rats to suppress severe rejection of heterotopic adult heart grafts from PVG (specific) or Lewis (third-party) donors. All irradiated DA hosts were restored with 5 × 106 naive CD4+ T cells. CD4+CD25+ T cells, either fresh or cultured for 3 to 4 days with allogeneic APC (PVG) and IL-2 or IL-4, were cotransferred with naive CD4+ T cells at various ratios. (B) Heart graft survival results showing the ability of naive CD4+CD25+ T cells cultured with allogeneic APCs and IL-2 (Ts1 cells) or IL-4 (Ts2 cells) to suppress allograft rejection. Irradiated hosts restored with 5 × 106 naive CD4+ T cells (●) reject both PVG (n = 12) and Lewis (n = 9) grafts. CD4+CD25+ T cells cultured with IL-2 (▴) or IL-4 (▵) and PVG allo-Ag, mixed at a ratio of 1:10 with naive CD4+ T cells (5 × 105 CD4+CD25+ with 5 × 106 CD4+ cells), suppressed donor specific (PVG) (P = .007 for IL-2, n = 6 and P = .035 for IL-4 cultured, n = 6) but not third-party allograft rejection (NSD, n = 5 for IL-2 and n = 6 for IL-4 cultured). Naive CD4+CD25+ T cells suppressed severe rejection of PVG allografts at a ratio of 1:1 with naive CD4+ T cells (■) (P < .001, n = 9) but not at a ratio of 1:10 (□) (NSD, n = 9). Some control data have previously been published.5 Graft survival without a severe rejection episode is only shown to 100 days, but all grafts functioning at that time were monitored to at least 250 days and none had a rejection episode. (C) Photomicrographs (original magnification ×200) of hearts from DA hosts given CD4+CD25+ T cells cultured with PVG allo-Ag and IL-2 (i,ii) or IL-4 (iii,iv). Comparison of histology of PVG long surviving donor heart (left panels) to the recipient's own heart (right panels) showed similar morphology with preserved muscular structure except for scattered patches of infiltrating mononuclear cells in donor hearts.
PVG grafts were accepted long term with IL-2–alloactivated Tregs (median survival time [MST] > 250 days, P = .007) and IL-4–alloactivated Tregs (MST 66- ≥ 250 days, P = .035), whereas hosts treated with 5 × 106 naive CD4+ T cells alone had severe rejection episode (rejection score < 2+) between 10 and 20 days (n = 12). All grafts that survived more than 100 days (Figure 3B) continued to function normally for more than 250 days without any immunosuppression. Fresh naive CD4+CD25+ T cells did not suppress early severe PVG graft rejection episode at a ratio of 1:10 with naive CD4+ T cells, but do so at a ratio of 1:1, as described5 (Figure 3B).
Rejection of third-party Lewis grafts by 5 × 106 naive CD4+ T cells was not prevented by cotransfer of either IL-2–alloactivated Tregs (MST, 11 days) or with IL-4–alloactivated Tregs (MST, 13-24 days). Time to severe rejection was not significantly different from rats restored with 5 × 106 naive CD4+ T cells alone (MST, 13 days). This demonstrated that IL-2- and IL-4–alloactivated CD4+CD25+ T cells suppressed rejection in an Ag-specific manner.
Examination of hosts with long surviving grafts after restoration with IL-2– or IL-4–alloactivated CD4+CD25+ T cells
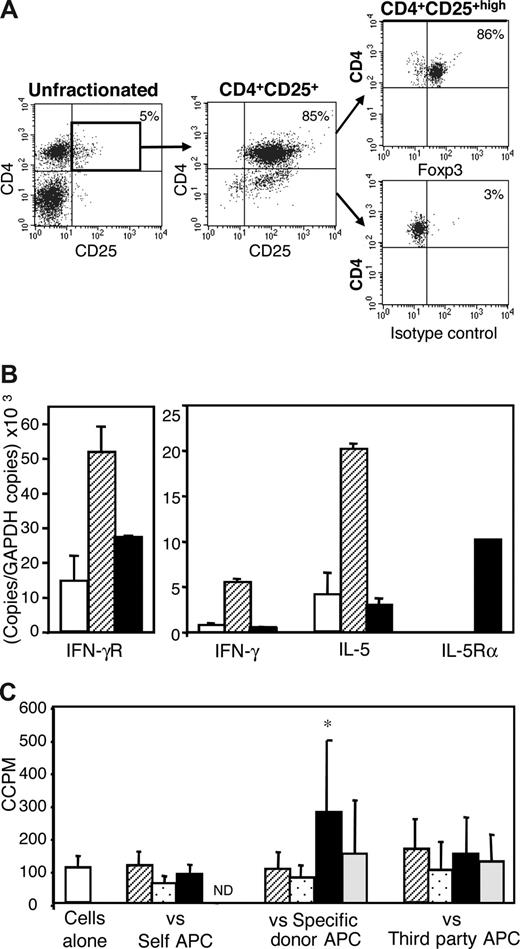
Specific donor heart grafts in animals restored with either IL-2– or IL-4–alloactivated CD4+CD25+ T cells and 5 × 106 naive CD4+ T cells that functioned more than 250 days had normal muscle morphology with only scattered areas of mononuclear cell infiltration (Figure 3C). These hosts had normal peripheral lymphocyte population, with only 5% CD4+CD25+ T cells. More than 80% of CD4+CD25+high T cells expressed Foxp3, indicating they were mainly Tregs (Figure 4A).
Phenotype and in vitro reactivity of CD4+CD25+ T cells from hosts with long surviving grafts given CD4+CD25+ T cells that had been cultured with allogeneic APCs and IL-2 or IL-4. (A) Fluorescence-activated cell sorter profiles showing normal proportions of CD4+CD25+ T cells in tolerant hosts and Foxp3 expression by the majority of CD4+CD25+high T cells. Expression of surface CD4 (Cy5), CD25 (PE), and intracellular Foxp3 (FITC) was analyzed by 3-color staining. Isotype control mAb staining showed specificity of Foxp3 Ab. (B) Cytokine and cytokine receptor expression profiles of fresh naive CD4+CD25+ T cells from normal DA rats (□) or CD4+CD25+ T cells from DA hosts with long surviving PVG allograft after being restored with IL-2–cultured Ts1 (▨) or IL-4-cultured Ts2 (■) cells. CD4+CD25+ T cells from Ts1 restored hosts had higher Ifnγr and Il-5 but no Il-5Rα mRNA expression. CD4+CD25+ T cells from hosts that had been restored with Ts2 cells had higher Il-5rα and low Il-5 mRNA. Thus, tolerant hosts' CD4+CD25+ T cells had a cytokine and cytokine receptor profile similar to the alloactivated CD4+CD25+ T-cell population given to induce tolerance. (C) In vitro reactivity of CD4+CD25+ T cells from DA hosts with long surviving PVG allografts after being restored with IL-4/PVG–activated (Ts2) CD4+CD25+ Tregs. CD4+CD25+ T cells from these tolerant hosts were cultured either without stimulator cells (□) or against self- (DA), specific donor (PVG), or third-party (Lewis) stimulator cells. Control cultures not supplemented with cytokine/mAb (▨) were compared with those supplemented with IFN-γ (▩), IL-5 (■), or a combination of IL-5 and anti-IL-5 mAb (▩). These cells had higher proliferation to specific donor allo-Ag in the presence of IL-5 that was blocked by anti-IL-5 mAb, consistent with the IL-5Rα expression on tolerant CD4+CD25+ T cells having a functional role.
Phenotype and in vitro reactivity of CD4+CD25+ T cells from hosts with long surviving grafts given CD4+CD25+ T cells that had been cultured with allogeneic APCs and IL-2 or IL-4. (A) Fluorescence-activated cell sorter profiles showing normal proportions of CD4+CD25+ T cells in tolerant hosts and Foxp3 expression by the majority of CD4+CD25+high T cells. Expression of surface CD4 (Cy5), CD25 (PE), and intracellular Foxp3 (FITC) was analyzed by 3-color staining. Isotype control mAb staining showed specificity of Foxp3 Ab. (B) Cytokine and cytokine receptor expression profiles of fresh naive CD4+CD25+ T cells from normal DA rats (□) or CD4+CD25+ T cells from DA hosts with long surviving PVG allograft after being restored with IL-2–cultured Ts1 (▨) or IL-4-cultured Ts2 (■) cells. CD4+CD25+ T cells from Ts1 restored hosts had higher Ifnγr and Il-5 but no Il-5Rα mRNA expression. CD4+CD25+ T cells from hosts that had been restored with Ts2 cells had higher Il-5rα and low Il-5 mRNA. Thus, tolerant hosts' CD4+CD25+ T cells had a cytokine and cytokine receptor profile similar to the alloactivated CD4+CD25+ T-cell population given to induce tolerance. (C) In vitro reactivity of CD4+CD25+ T cells from DA hosts with long surviving PVG allografts after being restored with IL-4/PVG–activated (Ts2) CD4+CD25+ Tregs. CD4+CD25+ T cells from these tolerant hosts were cultured either without stimulator cells (□) or against self- (DA), specific donor (PVG), or third-party (Lewis) stimulator cells. Control cultures not supplemented with cytokine/mAb (▨) were compared with those supplemented with IFN-γ (▩), IL-5 (■), or a combination of IL-5 and anti-IL-5 mAb (▩). These cells had higher proliferation to specific donor allo-Ag in the presence of IL-5 that was blocked by anti-IL-5 mAb, consistent with the IL-5Rα expression on tolerant CD4+CD25+ T cells having a functional role.
We also analyzed the phenotype and proliferation responses of CD4+ T-cell subsets from hosts made tolerant to an allograft by transfer of IL-2– or IL-4–alloactivated CD4+CD25+ T cells. CD4+CD25+ T cells from hosts restored with IL-2–activated Tregs had greater expression of Il-5 and Ifnγr mRNA than CD4+CD25+ T cells from naive rats or from tolerant hosts restored with IL-4–alloactivated Tregs (Figure 4B).
Il-5Rα mRNA was detected in CD4+CD25+ T cells from hosts restored with IL-4–activated Tregs but was not found in CD4+CD25+ T cells from naive rats or tolerant hosts restored with IL-2–activated Tregs. Ifnγ mRNA was increased in CD4+CD25+ T cells from hosts restored with IL-2–activated Tregs but not those given IL-4–activated Tregs. Ifnγ was the only marker not consistent with the original phenotype. It may be explained by Ifnγ mRNA induction in Th1 cells that express CD25. Thus, hosts had CD4+CD25+ T cells with a cytokine and cytokine receptor phenotypes similar to the alloactivated Tregs with which they were restored.
In vitro, CD4+CD25+ T cells from hosts made tolerant with IL-4–activated Tregs had significantly enhanced proliferation to specific donor APCs when cultured with IL-5 (P < .05), but not IFN-γ (Figure 4C). CD4+CD25+ T cells from hosts restored with IL-2–activated cells did not have enhanced proliferation to PVG allo-Ag in the presence of IL-5 (1447 ± 401 cpm) compared with cultures without cytokine (1511 ± 593 cpm). This demonstrated that the Il-5Rα expressed on the CD4+CD25+ T cells from tolerant hosts restored with IL-4–activated Tregs was functional and that IL-5 could promote proliferation of these cells.
IFN-γ suppressed proliferation of all cultured cells, including CD4+CD25− T cells as well as CD4+CD25+ T cells. In other studies, we reported that IFN-γ induces inducible nitric oxide synthase in the APC in MLC, which in turn produces nitric oxide that inhibits T-cell proliferation.5,26
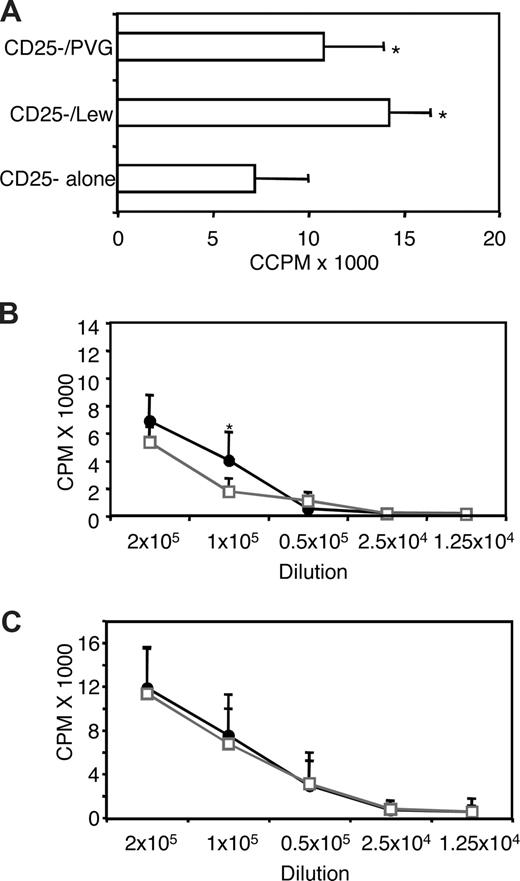
CD4+CD25− T cells from hosts given IL-2–activated Tregs had similar responses to PVG and Lewis (Figure 5A). The response to syngeneic cells was also high, but less than the response to allo-Ag, as observed with naive cells.5,26 The response of the CD4+ and CD4+CD25− T-cell subset from hosts given IL-4–alloactivated Tregs was tested against both specific donor PVG and third-party Lewis in a limiting dilution MLC. At all dilutions, the response to PVG was as great as that to Lewis for both CD4+ (Figure 5B) and CD4+CD25− T cells (Figure 5C). This demonstrated that there was no clonal deletion of alloreactive CD4+CD25− T cells in these hosts with long-surviving allografts.
Proliferation in MLCs of CD4+ and CD4+CD25− T cells from DA rats with long surviving PVG allografts after restoration with IL-2– or IL-4–alloactivated CD4+CD25+ Tregs. (A) The proliferative response of CD4+CD25− T cells from hosts restored with IL-2/PVG-alloactivated (Ts1) cells against PVG and Lewis stimulator cells was not significantly different, but both were greater than response without allogeneic stimulator cells (P < .03 for PVG and P < .001 for Lewis). (B,C) Proliferative response of CD4+ T cells (B) and CD4+CD25− T cells (C) from tolerant hosts restored with IL-4/PVG–alloactivated CD4+CD25+ T cells (Ts2) to PVG (●) and Lewis (□) stimulator cells in a limiting dilution assay. At all dilutions of responder cells, the proliferation to specific donor PVG was as great as to Lewis, indicating there was no clonal deletion. For CD4+ T cells, the response to PVG at 1 dilution was greater (*P < .05).
Proliferation in MLCs of CD4+ and CD4+CD25− T cells from DA rats with long surviving PVG allografts after restoration with IL-2– or IL-4–alloactivated CD4+CD25+ Tregs. (A) The proliferative response of CD4+CD25− T cells from hosts restored with IL-2/PVG-alloactivated (Ts1) cells against PVG and Lewis stimulator cells was not significantly different, but both were greater than response without allogeneic stimulator cells (P < .03 for PVG and P < .001 for Lewis). (B,C) Proliferative response of CD4+ T cells (B) and CD4+CD25− T cells (C) from tolerant hosts restored with IL-4/PVG–alloactivated CD4+CD25+ T cells (Ts2) to PVG (●) and Lewis (□) stimulator cells in a limiting dilution assay. At all dilutions of responder cells, the proliferation to specific donor PVG was as great as to Lewis, indicating there was no clonal deletion. For CD4+ T cells, the response to PVG at 1 dilution was greater (*P < .05).
Discussion
This study using rat CD4+CD25+ T cells found only IL-2 and IL-4, but not other Th1 or Th2 cytokines, activated these cells as described in humans.31 Our findings suggested Th1 and Th2 cytokines induced separate pathways of activation of CD4+CD25+ T cells, which we have termed T-suppressor 1 (Ts1) and Ts2 (Figure 6). This is not surprising as Th1 and Th2 cells, through the cytokines they produce, induce separate pathways of activation in other immune-associated cells. These include CD8+ T cells that differentiate to T cytotoxic (Tc) with IL-2 and IFN-γ or to Tc2 cells with IL-4,17 B cells that have different Ig isotype switching with IFN-γ or IL-4 and IL-5,18 and macrophages that differentiate to M1 with IFN-γ and to M2 with IL-4 and IL-13.19
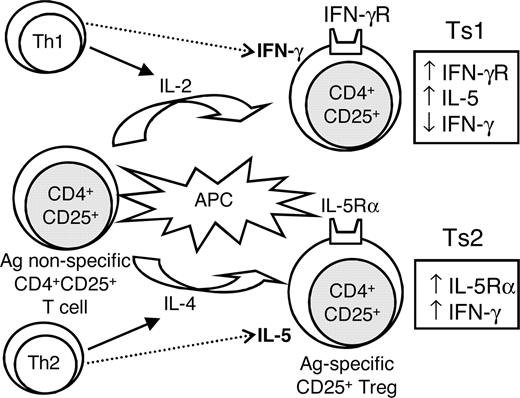
Proposed scheme of the initial activation of naive CD4+CD25+ T cells with allogeneic APCs and either IL-2 or IL-4, showing 2 separate activation pathways of CD4+CD25+ Tregs. We propose these be known as Ts1 and Ts2 cells, as they have distinct phenotypes, defined by the cytokines and cytokine receptors they express. Ts1 express IFN-γr, less IFN-γ, and more IL-5 than Ts2 cells, and Ts2 express IL-5rα and have greater IFN-γ mRNA induction. We hypothesize that expression of IFNγr or IL-5rα occurs as the Ts1 and Ts2 cells, respectively, become dependent on IFN-γ and IL-5 for survival and function as allo-Ag–specific Tregs.
Proposed scheme of the initial activation of naive CD4+CD25+ T cells with allogeneic APCs and either IL-2 or IL-4, showing 2 separate activation pathways of CD4+CD25+ Tregs. We propose these be known as Ts1 and Ts2 cells, as they have distinct phenotypes, defined by the cytokines and cytokine receptors they express. Ts1 express IFN-γr, less IFN-γ, and more IL-5 than Ts2 cells, and Ts2 express IL-5rα and have greater IFN-γ mRNA induction. We hypothesize that expression of IFNγr or IL-5rα occurs as the Ts1 and Ts2 cells, respectively, become dependent on IFN-γ and IL-5 for survival and function as allo-Ag–specific Tregs.
In vivo, Tregs develop in parallel with Th1 and Th2 responses.20 Th1 cells only transiently produce IL-2 and later produce IFN-γ and TNF-β.32 Th2 cells produce IL-4 early and later produce IL-5, IL-10, and IL-13.32 Our findings with Ts1 and Ts2 cells are consistent with IL-2 and IL-4 being critical cytokines for the early activation of Tregs. It is possible that they later become dependent on late Th1 and Th2 cytokines, such as IFN-γ and IL-5. Naive CD4+CD25+ T cells cultured with IL-2 and allo-Ag expressed Ifnγr and Il-4rα but not Il-5rα mRNA and maintain Il-5 but not Ifnγ mRNA expression. In contrast, naive CD4+CD25+ T cells activated with IL-4 and allo-Ag expressed IFN-γ, IL-5Rα, and Il-4rα but not Ifnγr mRNA. Neither IL-2– nor Il-4–activated Tregs expressed IL-2, but both expressed IL-4, IL-10, and Tgf-β mRNA.
These findings suggested that there are 2 separate pathways of activation of Tregs; Ts1 cells induced by Th1 cytokine (IL-2) and Ts2 cells activated by Th2 cytokine (IL-4). The phenotype of Ts1 and Ts2 cells was shown to be distinct from Th1 and Th2 cells; particularly, the enhanced expression of Il-5rα and Ifnγ mRNA by IL-4–alloactivated Ts2 cells and enhanced expression of Il-5 mRNA by IL-2–alloactivated Ts1 cells. Il-5rα has not been previously identified on T cells but is expressed by B cells, eosinophils and mast cells.33
Ts1 and Ts2 cells are different from T regulatory (Tr1) cells that are induced in culture with IL-10 or by IL-10–producing dendritic cells, and function via release of IL-10 and TGF-β.34 Tr1 cells do not express CD25 but do also produce IL-5.34 The cells we activated also differ in their cytokine profile from Th3 cells that mediate oral tolerance and produce TGF-β.35
CD4+CD25+ T cells are a heterogeneous population; and in our studies, more than 75% expressed Foxp3, the transcription factor that may define CD4+CD25+ Tregs.9,30 In our studies, culture with either IL-2 or IL-4 and allo-Ag increased the proportion of Foxp3+ cells, suggesting the initial Foxp3− cells are not the cells proliferating in MLCs. If IL-2 promoted activation of Foxp3− cells, it would have induced mRNA for Th1 transcription factor T-bet and for cytokines, such as Il-2 and Ifn-γ. Further, these cells would effect rejection. The opposite occurred in this study; IL-2–alloactivated CD4+CD25+ T cells had enhanced Foxp3 and no T-bet. They also had reduced Ifnγ and no Il-2 mRNA induction but enhanced expression of Il-5 mRNA, a Th2 cytokine. In addition, even 5 × 106 Il-2–alloactivated CD4+CD25+ T cells do not effect rejection.5 Similarly, if IL-4 promoted activation of CD4+CD25+Foxp3− T cells, it would have enhanced Th2 transcription factor Gata3 and cytokine expression, but there was no increased expression of Gata3, Il-4, Il-5, Il-10, or Il-13 mRNA in this study. Instead, there was enhanced expression of Foxp3 and Ifnγ mRNA, with de novo induction of Il-5rα mRNA, which is not expressed by Th2 cells.
In this study, more than 100 units/mL IL-2 or IL-4 was required to induce maximum proliferation CD4+CD25+ T cells in MLCs. This was 30-fold greater than required to induce maximal proliferation of CD4+CD25− T cells in MLCs. The doses of IL-2 and IL-4 used to promote Tregs partially suppressed the CD4+CD25− T-cell proliferation, however. Both IL-2- and IL-4–alloactivated Tregs suppressed rejection of specific donor allografts at the physiologic ratio of 1:10 to CD4+CD25− T cells. A 2- to 4-fold enhanced allo-Ag–specific suppression by Ts1 and Ts2 cells was observed in MLCs, albeit there was also enhanced non–Ag-specific suppression.
We think that this is the first study to show such rapid and enhanced allo-Ag–specific induction of activated CD4+CD25+ T cells that can regulate an allograft response. The Ts1 and Ts2 cells we studied had 10-fold greater suppressive capacity than fresh naive CD4+CD25+ T cells, which require a ratio of 1:1 to prevent severe rejection and induce tolerance.5 Naive CD4+CD25+ T cells given at a ratio of 1:10 with naive CD4+ T cells could not prevent severe rejection episodes, although grafts recovered later.5
Previous studies found that culture of naive CD4+CD25+ T cells with IL-2 and allo-Ag can produce CD4+CD25+ T cells that suppress GVHD8 and graft rejection,13,14,36 but these used longer culture times. CD4+CD25+ T cells cultured for 7 days suppressed rejection of skin allografts by naive CD25− cells only at a ratio of 5:1.14 A cell line of CD4+CD25+ T cells stimulated with IL-2 and autologous dendritic cells pulsed with an allopeptide given at a ratio of 2:1 with CD4+CD25− T cells suppressed skin graft rejection in thymectomized, T cell–depleted mice.13 Polyclonal activation of CD4+CD25+ T cells with IL-2 and anti-CD3/anti-CD28, before stimulating them with allo-Ag together with IL-2 and TGF-β, produced Tregs that protected specific donor but not third-party cardiac allografts from rejection, but the ratio of Tregs to CD25− T cells was not determined.36 In our studies, within 3 to 4 days in culture, CD4+CD25+ T cells acquired enhanced donor-specific suppressive capacity and inhibited rejection at a ratio of 1:10, which was a 20- to 50-fold lower ratio than reported for skin grafts.13,14
The ratio required with our alloactivated Tregs is similar to the physiologic ratio of less than 1:10 of CD4+CD25+/CD4+CD25− T cells in the CD4+ T cells that transfer tolerance from animals with long surviving cardiac grafts.2,29 These Ag-specific CD4+ Tregs lose their function in culture unless stimulated with both donor allo-Ag and T cell–derived cytokines,15,16 but IL-2 alone is not sufficient to maintain their full suppressor function.16 In our unpublished studies, IFN-γ or IL-5 alone can maintain Ag-specific CD4+ Tregs, but IL-4 alone cannot. Therefore, we hypothesized that activation of Ag-specific CD4+CD25+ Tregs may initially be dependent on IL-2, but they may later depend on other T-cell cytokines. This led us to examine whether culture with allo-Ag and IL-2 or IL-4 can induce expression of the receptors for IFN-γ and IL-5 on CD4+CD25+ T cells. Taken together, these findings suggest that the lack of enhanced suppressive potency with longer cultures of CD4+CD25+ T cells reported in other studies13,14 may be the result of lack of other cytokines.
The notion that Il-5rα and Ifnγr expression is important in the function of allo-Ag–specific CD4+CD25+ Treg was supported by the continued expression of Ifnγr and Il-5 mRNA by CD4+CD25+ T cells from tolerant hosts restored with Ts1 cells more than 250 days earlier. In addition, by the expression of Il-5Rα, mRNA in CD4+CD25+ T cells in tolerant hosts restored with Ts2 cells and the ability of Il-5 to enhance proliferation of these cells in MLCs to specific donor allo-Ag but not to third-party or self-Ag. These findings suggest that the expression of Il-5rα is a marker of an Ag-specific CD4+CD25+ Treg.
There is now clear evidence that Th2 cells reactive to allo-Ag effect rejection, rather than mediate tolerance.37,38 We are not aware of any previous reports showing that IL-4 can promote CD4+CD25+ Tregs that prevent allograft rejection. The ability of IL-4 to activate Ag-specific CD4+CD25+ Treg may explain why IL-421 or a Th2 response may under certain conditions promote transplantation tolerance induction.39,40 Thus, tolerance induced by Th2 responses may be the result of IL-4 and other Th2 cytokines promoting Tregs, not the Th2 cells themselves.
Tolerant hosts restored with either IL-2– or IL-4–alloactivated CD4+CD25+ T cells had a normal proportion of CD4+CD25+Foxp3+ T cells, which was less than 10% of CD4+T cells. Thus, tolerance was not the result of an abnormally high ratio of CD4+CD25+ T cells to CD4+CD25− T cells. The restoration of a physiologic ratio probably occurred because of normal homeostatic mechanism that have been described in irradiated41 and normal hosts.42
The regeneration of CD4+CD25− T cells in animals with long surviving grafts was not associated with clonal deletion, as CD4+ T cells had similar alloreactivity to donor allo-Ag and third-party in MLCs. Thus, transferred alloactivated CD4+CD25+ T cells did not deplete donor-reactive cells with the potential to effect rejection. In this respect, these animals were similar to transplantation-tolerant adult hosts, which have CD4+ T cells with the capacity to respond to donor allo-Ag in vitro43,44 and have Ag-specific adaptive CD4+CD25+ T cells that maintain tolerance.1-4 These adult-tolerant hosts also have a ratio of CD4+CD25+ to CD4+CD25− T cells of less than 1:10.2,3,44
We have shown that IL-5 can promote allograft survival and may be important in tolerance induction, associated with reduction in IL-2 and IFN-γ mRNA expression.22 Other Tregs express IL-5, but its function in these cells has not been examined.34 We have found that IL-5 therapy promoted recovery from active experimental autoimmune neuritis, and this was associated with induction of more Il-5Rα–expressing cells, and CD4+CD25+ T cells whose proliferation to specific Ag was enhanced by IL-5 (G.T.T., S.J.H., Carter, N.D.V., K.M.P., M. Killingsworth, B.M.H., manuscript in preparation). The findings in this study suggest that IL-5 therapy may promote Ag-specific Ts2 cells.
The expression of Ifnγr on Ts1 cells and the effect of IFN-γ produced by Ts2 cells is harder to isolate, given the multiple and paradoxical effects of IFN-γ. Sawitzki et al45 found that IFN-γ is an effector of Tregs in transplantation tolerance, and our study suggested that this could be by Ts2 cells. Tr1 cells also produce IFN-γ,34 however. IFN-γ plays a key role in transplantation tolerance induction by costimulation blockade,46 in spontaneous acceptance of liver allografts47 and transplantation tolerance maintenance45 but is not always essential for transplantation tolerance induction.48 IFN-γ inhibits Th2 cell development49 and induces apoptosis of activated T cells.50 Release of IFN-γ by activated Th1 cells can induce inducible nitric oxide synthase in macrophages, which in turn produce nitric oxide that limits alloreactive T-cell proliferation.5,26 Our findings suggested that IFN-γ may promote Ts1 cells.
As production of IL-2 and IL-4 is early and transient in the immune response,32 we propose that activated Tregs must acquire receptors for cytokines that are produced by Th1 and Th2 cells late in the immune response. For Th1 responses, this could be IFN-γ, and in Th2 responses, IL-5, consistent as with Ts1 and Ts2, respectively, expressing Ifnγr and Il-5rα mRNA (Figure 6). This requirement for other cytokines could explain why longer cultures of CD4+CD25+ T cells with IL-2 and allo-Ag do not increase allo-Ag–specific Tregs.13,14
This hypothesis is consistent with our original description of Ag-specific CD4+CD25+ Treg in transplantation tolerance, which die without T cell–derived cytokines.16 IL-216 or IL-4 does not fully maintain tolerance transferring cells, but either IL-5 or IFN-γ can (B.M.H., M.N., K.M.P., N.D.V., G.T.T., S.J.H., manuscript in preparation). These findings led us to examine Ifnγr and Il-5rα expression in this study and demonstrate that expression of these cytokine receptors is functionally important for Ts1 and Ts2 cells in the maintenance of allo-Ag–specific tolerance. The maintenance of the Tregs in adoptive hosts may depend on the production of cytokines produced by the coadministered CD4+ T cells, which are activated by allo-Ag and induced to express cytokines even in the presence of tolerance transferring T cells.44
The rapid development of alloantigen-specific Tregs in culture may allow ex vivo induction of these cells for use to prevent GVHD and allograft rejection. The identification of 2 pathways of Treg activation with different cytokine and cytokine receptor expression suggests that these cells may have different suppressor mechanisms.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms N. Carter, Dr J. Chen, Associate Professor M. Killingworth, and Mr M. Botros for technical assistance and Radiation Oncology Unit and Blood Bank at Liverpool Hospital for use of irradiators. Some cytokine clones were kindly provided by Neil Barclay, MRC Cellular Immunology Unit (Oxford, United Kingdom) and X. Y. He, Liverpool Hospital (Liverpool, Australia).
This work was supported by a generous donation from Bob and Jack Ingham of Ingham Enterprises (Liverpool, Australia), Liverpool Hospital, and University of New South Wales, especially Vice Chancellor Professor Rory Hume as well as unencumbered grants from Bayer Pharmaceuticals/Schering, Australia. Grants were from the South Western Sydney Area Health Foundation, Juvenile Diabetes Foundation of Australia, and Multiple Sclerosis Australia.
Authorship
Contribution: N.D.V. designed and performed experiments, grew IL-2– and IL-4–activated T cells, and drafted and wrote the manuscript; K.M.P. planned and performed experiments, grew IL-2– and IL-4–activated T cells, and drafted and wrote the manuscript; M.N. planned and performed heart graft experiments; G.T.T. handled cytokine production and developed RT-PCR analysis; C.R. developed in vitro assays; R.B. planned and conducted experiments with heart grafts; and S.J.H. and B.M.H. planned and interpreted all aspects of project and drafted and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce M. Hall, Department of Medicine, University of New South Wales, Level 3, Biomedical Building, Australian Technology Park, Eveleigh, NSW 2015, Australia; e-mail: b.hall@unsw.edu.au.
References
Author notes
*N.D.V. and K.M.P. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal