Abstract
Ras-associated protein 1 (Rap1), a small GTPase, attracted attention because of its involvement in several aspects of cell adhesion, including integrin- and cadherin-mediated adhesion. Yet, the role of Rap1 genes and of Rap1 effectors for angiogenesis has not been investigated. Human umbilical vein endothelial cells (HUVECs) express Rap1a and Rap1b mRNA. To determine the contribution of Rap1 activity for angiogenesis, we overexpressed Rap1GAP1, a GTPase-activating protein that inhibits Rap1 activity. Overexpression of Rap1GAP1 significantly blocked angiogenic sprouting and tube-forming activity of HUVECs as well as migration and integrin-dependent adhesion. Silencing of Rap1a, Rap1b, or both significantly blocked HUVECs sprouting under basal and basic fibroblast growth factor-stimulated conditions and reduced HUVEC migration and integrin-dependent adhesion. We found that Rap1a and Rap1b are essential for the conformational activation of β1-integrins in endothelial cells. Furthermore, silencing of Rap1a and Rap1b prevented phosphorylation of tyrosine 397 in focal adhesion kinase (FAK) and vascular endothelial growth factor-induced Akt1-activation. Rap1a−/−-deficient and Rap1a+/− heterozygote mice displayed reduced neovascularization after hind limb ischemia compared with wild-type mice. Silencing of RAPL significantly blocked the Rap1-induced sprouting of HUVECs, suggesting that the angiogenic activity of Rap1 is partly mediated by RAPL. Our data demonstrate a critical role of Rap1 in the regulation of β1-integrin affinity, adhesion, and migration in endothelial cells and in postnatal neovascularization.
Introduction
The term angiogenesis refers to the formation of new blood capillaries from preexisting ones.1,2 Angiogenesis is implicated in many physiologic and pathologic conditions, including embryonic development, wound healing, tumor growth, rheumatoid arthritis, and proliferative retinopathy.1,2 Vascular endothelial growth factor (VEGF) is an essential cytokine for vasculogenesis and angiogenesis.3 Through its receptors, which include 2 distinct tyrosine kinases, VEGF exerts multiple effects on endothelial cells, including proliferation, rapid induction of endothelial permeability, promotion of endothelial cell survival, stimulation of migration, and induction of gene expression.3 Besides VEGF, the fibroblast growth factors (FGFs) are also implicated in angiogenesis.4 However, the downstream signaling pathways mediating the angiogenic effects of angiogenic growth factors, such as VEGF and FGFs, are poorly understood.
Integrins are heterodimeric transmembrane proteins consisting of noncovalent bound α- and β-subunits mediating cell adhesion to extracellular matrix proteins and bidirectional signaling.5 Beyond angiogenic growth factors, there is evidence that integrins are implicated in angiogenesis.6 Specifically, β1-integrins were shown to play an important role in angiogenesis.6 Moreover, angiogenic factors, such as VEGF, affect integrin activity and function in endothelial cells.7
Ras-associated proteins (Rap) define a family of highly homologous small guanosine triphosphate (GTP)–binding proteins belonging to the Ras superfamily, which includes 5 members, Rap1a, Rap1b, Rap2a, Rap2b, and the recently discovered Rap2c, which are grouped into 2 subfamilies, Rap1 and Rap2, based on their sequence homology.8-12 Small GTPases cycle between an inactive guanosine diphosphate (GDP)–bound conformation and an active GTP-bound conformation. In their active conformation, small GTPases interact with effector proteins, which induce downstream signaling. The GDP/GTP cycle is highly regulated by guanine nucleotide exchange factors (GEFs) that induce the release of the bound GDP to be replaced by the more abundant GTP and by GTPase-activating proteins (GAP) that promotes GTP hydrolysis.13
Rap1a and Rap1b were shown to be essential for inside-out integrin activation and for integrin-dependent cell-matrix and cell-cell adhesion of various cell types, such as leukocytes, platelets, ovarial carcinoma cells, fibroblasts, and progenitor cells.10,11,14-24 In addition, while this study was under preparation for submission, Chrzanowska-Wodnicka et al have demonstrated that Rap1b is involved in angiogenesis.25 Moreover, inactivation of Rap1 by overexpression of Rap1GAPII inhibited wound healing in endothelial cells.26 However, the role of Rap1 for integrin activity regulation, integrin-dependent adhesion, and angiogenic sprouting of endothelial cells has not been investigated so far. Furthermore, the role of Rap1a for the ischemia-induced neovascularization is unclear.
Active (GTP-bound) Rap1a was shown to associate with effector proteins, such as RAPL and RIAM, thus mediating the activation of integrins and integrin-dependent adhesion in leukocytes.27,28 Beyond integrin-dependent adhesion, Rap1 regulates junctional adhesion, thereby modulating permeability of endothelial cell monolayers.29-33
Although Rap1 was shown to be important for the regulation of several physiologic processes, little information is available about the role of Rap1 in integrin signaling in endothelial cells and angiogenesis. In the present study, we demonstrated that both Rap1a and Rap1b play a key role in integrin-dependent angiogenic functions, such as sprouting, tube formation, migration, and adhesion of endothelial cells in vitro. In line with these results, Rap1a heterozygote and Rap1a-deficient mice displayed a decreased angiogenic response in the matrigel assay and in the hind limb ischemia model compared with wild-type mice. Moreover, we provide insights into the Rap1 downstream signaling pathways and demonstrate that RAPL partly mediates the angiogenic effects of Rap1.
Methods
Cells
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Basel, Switzerland) and cultured in endothelial basal medium (EBM) supplemented with 1 μg/mL hydrocortisone, 12 μg/mL bovine brain extract, 50 μg/mL gentamicin, 50 ng/mL amphotericin-B, 10 ng/mL epidermal growth factor, and 10% fetal calf serum until the third passage.
Plasmid constructs and transfection
HUVECs (3.5 × 105 cells/6-cm well) were grown to 60% to 70% confluence and then transfected with 3 μg plasmids and 20 μL Superfect (QIAGEN, Hilden, Germany), resulting in a transfection efficiency of approximately 50% as previously described.34,35 The Rap1GAP1 (pcDNA-Flag-Rap1GAP1) was kindly provided by Dr P. J. Stork (Vollum Insitute, Oregon Health and Science University, Portland, OR); the plasmids EGFP-Rap1a, the constitutive active mutant EGFP-Rap1aV12, and GFP-RBDRalGDS were kindly provided by Dr M. R. Philips (New York University, New York, NY).36 We subcloned Rap1a and Rap1aV12 from the EGFP-Rap1a and EGFP-Rap1aV12 vectors in the pcDNA3.1-His vector.
RNA interference
To silence Rap1 and RAPL expression, we performed transfection of small-interfering RNA (siRNA) duplex using GeneTrans II (MoBiTec, Göttingen, Germany). Rap1a, Rap1b, and RAPL siRNAs were synthesized by Eurogentec (Cologne, Germany). The Rap1a target sequence was 5′-GCAAGACAGTGGTGTAACT-3′ (Rap1a siRNA I), and the Rap1b target sequence was 5′-GTCTGCTTTGACTGTACAA-3′ (Rap1b siRNA I). The human RAPL siRNA target sequence was 5′-CTGGAAGACTGCTTCTTCA-3′ (RAPL siRNA I). A nonrelated, scrambled siRNA was used as a control. Additional sequences are available on request.
Western blot analysis
For Western blot analysis, HUVECs were lysed with 100 μL lysis buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycoltetraacetic acid, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mmol Na3VO4, 1 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 10 mmol NaF) for 15 minutes on ice. After centrifugation for 15 minutes at 14 000g (4°C), the protein content of the samples was determined according to the Bradford method. Proteins were loaded onto sodium dodecyl sulfate–polyacrylamide gels and blotted onto polyvinylidene difluoride membranes (Millipore, Schwalbach, Germany). Western blots were performed by using antibodies directed against Rap1 (1/1000; Upstate Biotechnology, Hamburg, Germany), phosphotyrosine 397 focal adhesion kinase (FAK; 1/1000; Biosource, Hamburg, Germany), FAK (1/1000; BD Biosciences, Heidelberg, Germany), phospho-extracellular signal-regulated kinase 1/2 (Erk1/2; 1/1000; Cell Signaling Technology, Freiburg, Germany), Erk1/2 (1/1000; Cell Signaling Technology), FLAG (1/1000; Sigma-Aldrich, Munich, Germany), and α-tubulin (1/1000; Dianova, Hamburg, Germany). Enhanced chemiluminescence was performed according to the manufacturer's instructions (Amersham Biosciences, Freiburg, Germany). Densitometry was performed where indicated for the quantification of the Western blots.
Reverse-transcription PCR
Total RNA was isolated using the RNAeasy Mini Kit (QIAGEN). Afterward, 1 μg RNA from each sample was reverse-transcribed into cDNA and subjected to conventional polymerase chain reaction (PCR). Primer sequences for PCR were: Rap1a 5′-CGATTGCCAACAGTGTATGCTCG-3′ and 5′-ACACCACTGTCTTGCTAAATTCTG-3′; Rap1b 5′-TTTATTCCATCACAGCACAGTCC-3′ and 5′-TTTCTGTTAATTTGCCGCACTAGG-3′ and RAPL 5′-CCTGGACGAGGAACTGGAAGAC-3′ and 5′-CAACCATGAACTTCTTGAGCAGCC-3′.
Tube formation assay
A total of 200 μL Matrigel Basement Membrane Matrix (BD Biosciences) was coated for 2 hours at 37°C within a 12-well plate (Greiner Bio-One, Frickenhausen, Germany). Transfected HUVECs were detached by trypsinization, and after neutralization of trypsin, cells were resuspended in EBM containing 10% fetal calf serum (FCS). Then, HUVECs (105) were seeded in 1 mL EBM (10% FCS) on the Matrigel Basement Membrane Matrix. Tube length was quantified after 24 hours by measuring the cumulative tube length in 5 random microscopic fields with a computer-assisted microscope (Axiovert 100; Carl Zeiss, Jena, Germany) using the software Axiovision 4.5 (Carl Zeiss).
Spheroid-based angiogenic assay
Endothelial-cell spheroids of defined cell numbers were generated as described previously.37 In brief, HUVECs were suspended in culture medium containing 0.2% (wt/vol) carboxymethylcellulose (Sigma-Aldrich) and seeded in round-bottom 96-well plates, which do not support cell adhesion (Greiner Bio-One). Under these conditions, all suspended cells contribute to the formation of a single spheroid per well of defined size and cell number (400 cells/spheroid). Spheroids were generated overnight and then embedded into rat collagen I (BD Biosciences). The spheroid-containing collagen was rapidly transferred into prewarmed 24-well plates and allowed to polymerize (for 30 minutes). Then, 100 μL EBM with or without human basic fibroblast growth factor (bFGF, 50 ng/mL; PeproTech, Hamburg, Germany) was added into the wells. In the experiments using siRNA silencing, HUVECs were transfected 48 hours before the formation of the spheroids with the indicated siRNAs. For the experiments with the neutralizing β1 antibodies, murine IgG1 (30 μg/mL; Ancell, Loerrach, Germany) or anti–β1-integrin (clone 6S6, 30 μg/mL; Millipore) was added to the collagen gel. After 24 hours, pictures were taken using an Axiovert 100 microscope and a Plan-NEOFLUAR 10× objective. In vitro capillary sprouting was quantified by measuring the cumulative length of sprouts per each spheroid using AxioVision Rel 4.5 digital imaging software (Carl Zeiss). The mean cumulative sprout length per spheroid was calculated after evaluation of 10 to 15 spheroids/condition.
Cell-matrix adhesion
Ninety-six–well plates were coated overnight at 4°C with 1 μg/mL soluble recombinant human collagen I (Millipore) or 2.5 μg/mL human fibronectin (Roche, Mannheim, Germany) or 2.5 μg/mL human vitronectin (Millipore) in phosphate-buffered saline and then blocked for 1 hour at room temperature with 3% (wt/vol) heat-inactivated (2 hours, 56°C) bovine serum albumin (BSA; Sigma-Aldrich). HUVECs were stained with 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester and after detachment with trypsin were resuspended in EBM containing 0.05% BSA. Then, cells were seeded at 50 000 cells/well in 100 μL in the wells for 60 minutes at 37°C. After removal of nonadhering cells by washing with warm EBM, adherent cells were quantified in triplicates with a fluorescence plate reader (Synergy HT; Bio-Tek Instruments, Bad Friedrichshall, Germany).
HUVEC migration
Transwell membranes (8 μm; Costar, Bodenheim, Germany) were coated on both sides with fibronectin (2.5 μg/mL; Roche) or human collagen type I (1 μg/mL; Millipore) overnight at 4°C. HUVECs were detached by trypsinization, and after neutralization of trypsin, cells were resuspended in serum-free EBM containing 0.05% BSA. Then, HUVECs (50 000 cells/well) were incubated in the upper chamber at 37°C in 5% CO2 and allowed to migrate for 5 hours toward the lower chamber in the presence or absence of 50 ng/mL VEGF (PeproTech, London, United Kingdom). Cells remaining on the upper surface of the transwell membranes were mechanically removed, and cells that had migrated to the lower surface were fixed with 4% formaldehyde. For quantification, cell nuclei were stained with 4′-6-diamidino-2-phenylindole dihydrochloride. Cells migrated into the lower chamber were counted in 5 random microscopic fields using a fluorescence microscope (Axiovert 100; Carl Zeiss).
Activation of Rap1 in migrating HUVECs
HUVECs were plated on glass slides coated with 2.5 μg/mL fibronectin (Roche Diagnostics). After 36 hours, HUVECs were transfected with GFP-RBDRalGDS. Six hours after transfection, a scratch wound was created with a cell scraper. After 3 hours, cells were fixed in 3% formaldehyde in phosphate-buffered saline for 10 minutes at room temperature, and 6-diamidino-2-phenylindole dihydrochloride nuclear staining was performed. Cells were analyzed with a confocal microscope for localization of the active Rap1 (LSM510; Carl Zeiss).
Total and phospho-Akt1–ELISA
The experiments were performed 48 hours after transfection with the respective siRNAs. Transfected HUVECs were serum-starved for 2 hours in EBM (0% FCS) and then stimulated where indicated with VEGF (50 ng/mL) for 5 minutes. After lysis with the Akt–enzyme-linked immunosorbent assay (ELISA) lysis buffer, Akt-ELISAs were performed (total Akt1-ELISA and phospho-Akt1 [Ser473]–ELISA) using a commercial Akt1-ELISA Kit (Cell Signaling Technology). Absorbances were measured with a plate reader (Synergy HT; Bio-Tek Instruments).
Immunofluorescence staining
The method for the immunofluorescence staining is described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Animal experiments
All the animal experiments were approved from the Regional Board of Land Hessen (Darmstadt, Germany). The animal experiments are described in Document S1.
Statistical analysis
Continuous variables are expressed as mean plus or minus SEM. Comparisons between groups were analyzed by t test (2-sided) or analysis of variance (post-hoc test: least significant difference) for experiments with more than 2 subgroups (SPSS software; SPSS, München, Germany). P values less than .05 were considered statistically significant.
Results
Expression of Rap1a and Rap1b in human endothelial cells
To characterize the expression of Rap1a and Rap1b in human endothelial cells, we performed reverse transcription (RT)–PCR. HUVECs expressed both Rap1a and Rap1b mRNA as assessed by RT-PCR (Figure S1A). Moreover, analysis of a mRNA expression microarray revealed that Rap1a and Rap1b are similarly expressed in HUVECs and in human microvascular endothelial cells (Figure S1A,B). We confirmed the expression of Rap1 in HUVECs by Western blot (data not shown). Because VEGF and bFGF are relevant angiogenic growth factors, we investigated their effects on Rap1 activation by using Rap1 activity assays. Both VEGF and bFGF rapidly increased the active GTP-bound Rap1 reaching a maximum between 2 and 5 minutes after stimulation (Figure S1C,D). This activation was reversible within 15 minutes (Figure S1D). All microarray data have been deposited with Gene Expression Omnibus under accession number GSE2040.38
Role of Rap1 activity for angiogenic sprouting and tube formation
Because angiogenic growth factors rapidly increase Rap1 activity, we next investigated whether Rap1 activity is required for angiogenic sprouting and tube formation of endothelial cells in vitro. Rap1GAP1 is a GTPase-activating protein, which specifically inhibits Rap1a and Rap1b activity.39 To investigate the role of Rap1 activity in angiogenesis, we used a 3-dimensional spheroidal system of endothelial differentiation and in vitro angiogenic sprouting.37 Endothelial cell spheroids were embedded in collagen gels, and outgrowth of capillary-like structures was assessed. Overexpression of Rap1GAP1 significantly blocked the basal and bFGF-induced angiogenic sprouting of HUVECs in this spheroidal culture system (Figure 1A,B). Consistent with these data, overexpression of Rap1GAP1 led to a significant impairment of tube/network-forming activity of HUVECs in another angiogenic assay, the matrigel assay (Figure 1C). Overexpression of Rap1a or a constitutively active Rap1aV12 mutant significantly increased the angiogenic sprouting of HUVECs compared with mock-transfected cells (Figure 1D). Moreover, the Rap1aV12-induced sprouting of HUVECs was inhibited by a neutralizing β1-integrin antibody (Figure 1E). In addition, the neutralizing β1-integrin antibody also inhibited bFGF-induced angiogenic sprouting of HUVECs (Figure S4B). In conclusion, these data demonstrate that Rap1 activity is required and sufficient to promote in vitro angiogenesis in a β1-integrin–dependent manner.
Effect of Rap1GAP1 overexpression on in vitro angiogenesis. (A) HUVECs were transfected with Rap1GAP1-FLAG or empty vector. After 24 hours, cells were lysed and subjected to Western blot analysis using a Flag-tag specific antibody. An antibody directed against tubulin was used as loading control. (B) Three-dimensional in vitro angiogenic sprouting in a spheroidal culture system with collagen-embedded spheroids of Rap1GAP1-FLAG- versus mock-transfected endothelial cells in the presence or absence (control) of 50 ng/mL bFGF. The mean cumulative length of sprouts per spheroid was assessed after 24 hours (*P < .05 vs empty vector, **P < .05 vs empty vector + bFGF, n = 9). (C) Statistical analysis and representative micrographs of the tube-forming activity. HUVECs were seeded on Matrigel Basement Membrane Matrix 24 hours after transfection with the indicated plasmids (n = 3). The length of capillary-like structures/networks was measured in 5 different high-power fields by light microscopy after 24 hours (*P < .05 vs empty vector). Bar represents 200 μm. The wells were viewed with an Axiovert 100M inverted microscope using as objective a Plan-NEOFLUAR (at 10×/0.30). Images were acquired using an Axiocam MR digital camera and were processed using AxioVision Rel 4.5 digital imaging software (all from Carl Zeiss, Jena, Germany). (D) Three-dimensional in vitro angiogenesis with collagen-embedded spheroids of Rap1a-, Rap1aV12-, or mock-transfected HUVECs (n = 7; *P < .05 vs empty vector). (E) Three-dimensional in vitro angiogenic sprouting in a spheroidal culture system with collagen-embedded endothelial spheroids of Rap1aV12- or mock-transfected HUVECs. The sprouting assay was performed in the presence of blocking monoclonal β1-integrin antibodies or murine isotype control antibodies. Data are presented as mean plus or minus SEM (n = 5, *P < .05 vs Rap1aV12 + IgG).
Effect of Rap1GAP1 overexpression on in vitro angiogenesis. (A) HUVECs were transfected with Rap1GAP1-FLAG or empty vector. After 24 hours, cells were lysed and subjected to Western blot analysis using a Flag-tag specific antibody. An antibody directed against tubulin was used as loading control. (B) Three-dimensional in vitro angiogenic sprouting in a spheroidal culture system with collagen-embedded spheroids of Rap1GAP1-FLAG- versus mock-transfected endothelial cells in the presence or absence (control) of 50 ng/mL bFGF. The mean cumulative length of sprouts per spheroid was assessed after 24 hours (*P < .05 vs empty vector, **P < .05 vs empty vector + bFGF, n = 9). (C) Statistical analysis and representative micrographs of the tube-forming activity. HUVECs were seeded on Matrigel Basement Membrane Matrix 24 hours after transfection with the indicated plasmids (n = 3). The length of capillary-like structures/networks was measured in 5 different high-power fields by light microscopy after 24 hours (*P < .05 vs empty vector). Bar represents 200 μm. The wells were viewed with an Axiovert 100M inverted microscope using as objective a Plan-NEOFLUAR (at 10×/0.30). Images were acquired using an Axiocam MR digital camera and were processed using AxioVision Rel 4.5 digital imaging software (all from Carl Zeiss, Jena, Germany). (D) Three-dimensional in vitro angiogenesis with collagen-embedded spheroids of Rap1a-, Rap1aV12-, or mock-transfected HUVECs (n = 7; *P < .05 vs empty vector). (E) Three-dimensional in vitro angiogenic sprouting in a spheroidal culture system with collagen-embedded endothelial spheroids of Rap1aV12- or mock-transfected HUVECs. The sprouting assay was performed in the presence of blocking monoclonal β1-integrin antibodies or murine isotype control antibodies. Data are presented as mean plus or minus SEM (n = 5, *P < .05 vs Rap1aV12 + IgG).
Role of Rap1a and Rap1b for angiogenic sprouting
Having demonstrated that Rap1 activity is essential for the angiogenic sprouting of endothelial cells in vitro, we next sought to separately study the role of endogenous Rap1a and Rap1b for angiogenesis. For this purpose, we designed siRNA sequences specifically targeting Rap1a and Rap1b. RT-PCR and Western blot confirmed the efficient and specific suppression of Rap1a and Rap1b by the respective siRNA oligonucleotides (Figures 2A, S2). Interestingly, silencing of Rap1a or Rap1b by siRNA transfection significantly blocked the angiogenic sprouting of HUVECs under basal conditions and on stimulation with bFGF (Figure 2B). Simultaneous silencing of Rap1a and Rap1b induced a minor further decrease in sprout formation, which did not achieve statistical significance compared with silencing of the single genes (Figure 2B). As a control for the specificity of this approach, a second Rap1a- and Rap1b-specific siRNA was generated and gave identical results in terms of angiogenic sprouting (data not shown). Remarkably, silencing of both Rap1a and Rap1b did not significantly influence the proliferation rate of HUVECs (data not shown). Taken together, Rap1a and Rap1b are essential for in vitro angiogenesis without affecting proliferation.
Silencing of Rap1 inhibits in vitro angiogenesis. Endothelial cells transfected with siRNAs targeted against Rap1a, Rap1b, or scrambled controls. Two different sequences were used as indicated by I and II. (A) Expression of Rap1a and Rap1b mRNA was assessed by RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serves as loading control. (B) Statistical analysis and representative images of spheroidal sprouting assay performed with endothelial cells transfected as indicated with Rap1a-, Rap1b-, Rap1a-, and Rap1b-siRNA (sequence I) or scrambled siRNA in the absence or presence of 50 ng/mL bFGF (n = 13). The mean cumulative length of sprouts per spheroid was assessed after 24 hours (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + bFGF). Bar represents 100 μm. The wells were viewed with an Axiovert 100M inverted microscope using as objective a Plan-NEOFLUAR (at 10×/0.30). Images were acquired using a digital camera Axiocam MR and were processed using AxioVision Rel 4.5 digital imaging software (all from Carl Zeiss).
Silencing of Rap1 inhibits in vitro angiogenesis. Endothelial cells transfected with siRNAs targeted against Rap1a, Rap1b, or scrambled controls. Two different sequences were used as indicated by I and II. (A) Expression of Rap1a and Rap1b mRNA was assessed by RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serves as loading control. (B) Statistical analysis and representative images of spheroidal sprouting assay performed with endothelial cells transfected as indicated with Rap1a-, Rap1b-, Rap1a-, and Rap1b-siRNA (sequence I) or scrambled siRNA in the absence or presence of 50 ng/mL bFGF (n = 13). The mean cumulative length of sprouts per spheroid was assessed after 24 hours (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + bFGF). Bar represents 100 μm. The wells were viewed with an Axiovert 100M inverted microscope using as objective a Plan-NEOFLUAR (at 10×/0.30). Images were acquired using a digital camera Axiocam MR and were processed using AxioVision Rel 4.5 digital imaging software (all from Carl Zeiss).
Role of Rap1a and Rap1b for endothelial cell migration
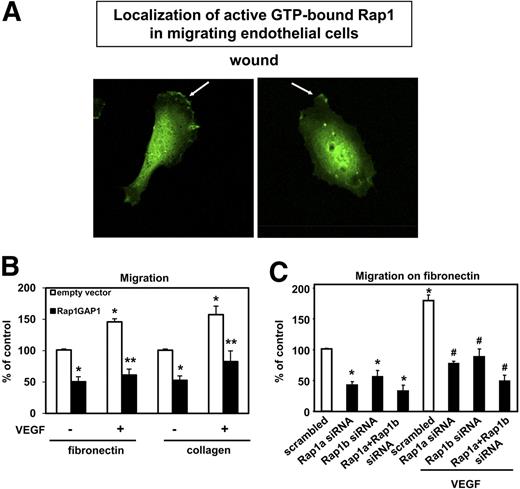
Endothelial cell migration is an essential step during angiogenesis. Therefore, we investigated the localization of active (GTP-bound) Rap1 in migrating endothelial cells. For this purpose, we overexpressed a construct containing the Ras-binding domain (RBD) of RalGDS fused with GFP as an indicator of Rap1 activity in HUVECs.36,40 Interestingly, active (GTP-bound) Rap1 partly accumulated at the plasma membrane preferentially in the leading front of migrating HUVECs in a scratch wound assay compared with nonmigrating endothelial cells, which displayed a more random localization of active Rap1 on their cell membrane or localization of active Rap1 to cell-cell contacts (Figure 3A and data not shown). These data suggested that Rap1 activity may be involved in the regulation of endothelial cell migration. To test this hypothesis, we overexpressed Rap1GAP1 in HUVECs and performed migration assays on the matrix proteins collagen and fibronectin. Remarkably, inhibition of Rap1 activity significantly blocked basal and VEGF-stimulated migration of HUVECs on both matrix proteins (Figure 3B). Moreover, silencing of Rap1a and/or Rap1b inhibited the basal and the VEGF-induced migration of HUVECs on fibronectin (Figure 3C) and on collagen (Figure S3A).
Silencing of Rap1 inhibits endothelial migration. (A) HUVECs were transfected with GFP-RBDRalGDS. Six hours after transfection, a wound was created. Representative images of 3 different experiments are depicted, showing recruitment of the GFP-RBDRalGDS (as an indicator of Rap1 activity) at the leading edge of migrating endothelial cells. Micrographs were acquired with an LSM 510 confocal microscope fitted with a Plan-Neofluar 40×/1.3 numeric aperture (NA) oil objective and LSM5 image acquisition software (all from Carl Zeiss). (B) HUVECs were transfected with Rap1GAP1-FLAG or empty vector. After 24 hours, cells were seeded in the upper chamber of modified Boyden chambers coated with fibronectin (n = 5) or collagen (n = 5). Endothelial cell migration was stimulated using VEGF (50 ng/mL) as chemoattractant where indicated. Data are presented as mean of migrated cell % of control plus or minus SEM (*P < .05 vs empty vector, **P < .05 vs empty vector + VEGF). (C) Migration assay on fibronectin (n = 5) with endothelial cells transfected with siRNAs targeted against Rap1a, Rap1b Rap1a/Rap1b, or scrambled siRNA. After 48 hours, cells were seeded in the upper chamber of modified Boyden chambers. Endothelial cell migration was assessed using VEGF (50 ng/mL) as chemoattractant. Data are presented as mean of migrated cell % of control plus or minus SEM (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + VEGF).
Silencing of Rap1 inhibits endothelial migration. (A) HUVECs were transfected with GFP-RBDRalGDS. Six hours after transfection, a wound was created. Representative images of 3 different experiments are depicted, showing recruitment of the GFP-RBDRalGDS (as an indicator of Rap1 activity) at the leading edge of migrating endothelial cells. Micrographs were acquired with an LSM 510 confocal microscope fitted with a Plan-Neofluar 40×/1.3 numeric aperture (NA) oil objective and LSM5 image acquisition software (all from Carl Zeiss). (B) HUVECs were transfected with Rap1GAP1-FLAG or empty vector. After 24 hours, cells were seeded in the upper chamber of modified Boyden chambers coated with fibronectin (n = 5) or collagen (n = 5). Endothelial cell migration was stimulated using VEGF (50 ng/mL) as chemoattractant where indicated. Data are presented as mean of migrated cell % of control plus or minus SEM (*P < .05 vs empty vector, **P < .05 vs empty vector + VEGF). (C) Migration assay on fibronectin (n = 5) with endothelial cells transfected with siRNAs targeted against Rap1a, Rap1b Rap1a/Rap1b, or scrambled siRNA. After 48 hours, cells were seeded in the upper chamber of modified Boyden chambers. Endothelial cell migration was assessed using VEGF (50 ng/mL) as chemoattractant. Data are presented as mean of migrated cell % of control plus or minus SEM (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + VEGF).
Role of Rap1a and Rap1b for endothelial cell adhesion and for β1-integrin affinity regulation
The regulation of cell adhesion to matrix proteins is essential for cell migration41 and angiogenesis. Therefore, we studied the role of Rap1 activity for the integrin-dependent endothelial cell adhesion on fibronectin and collagen. Overexpression of Rap1GAP1 significantly reduced the adhesion of HUVECs on extracellular matrix proteins fibronectin and collagen (Figure 4A). In addition, silencing of Rap1a, Rap1b, or of Rap1a and Rap1b significantly blocked the adhesion of HUVEC on the matrix proteins fibronectin (Figure 4B), collagen, and vitronectin, which is the major ligand for αVβ3- and αVβ5-integrins (Figure S3B,C). Because β1-integrins are mediating cell adhesion to fibronectin and to collagen and are essential for the Rap1aV12-induced in vitro sprout formation in endothelial cells (Figure 1E), we investigated the role of Rap1 for β1-integrin expression. Interestingly, inhibition of Rap1 activity by overexpression of Rap1GAP1 did not affect the surface expression of β1-integrins in HUVECs (Figure S3D). These data prompted us to investigate the role of Rap1a and Rap1b on integrin affinity, which is promoted by conformational changes of the integrin subunits.42 For this purpose, we performed immunofluorescent staining using an antibody (clone HUTS21) that recognizes the active conformation of β1-integrins. Stimulation of HUVECs with 8-pCPT-cAMP, a specific activator of the guanine nucleotide exchange factor Epac, which specifically increases Rap1 activity, enhanced the staining with HUTS21 compared with nonstimulated endothelial cells (Figure 4C). Silencing of Rap1a and Rap1b reduced the active conformation of β1-integrins under basal conditions and on stimulation with 8-pCPT-cAMP compared with scrambled siRNA-transfected HUVECs (Figure 4C), suggesting that active Rap1 is essential for the regulation of β1-integrin affinity in endothelial cells. Taken together, these data indicate that Rap1a and Rap1b promote angiogenesis by mediating β1-integrin affinity regulation, thereby mediating endothelial cell adhesion and migration.
Inhibition of Rap1 reduced endothelial adhesion. (A) HUVECs were transfected with Rap1GAP1-FLAG or empty vector, and adhesion assays were performed with transfected endothelial cells. After 24 hours, cells were allowed to adhere for one hour on fibronectin (n = 6) or collagen (n = 9; *P < .05 vs empty vector). (B) Adhesion assay with endothelial cells transfected with siRNAs targeted against Rap1a, Rap1b, Rap1a/Rap1b, or scrambled siRNA. After 48 hours, cells were allowed to adhere for 1 hour on fibronectin-coated wells (n = 5). Data are presented as percentage of adhering cells plus or minus SEM (*P < .05 vs scrambled). (C) Twelve hours after transfection, HUVEC cells were grown on 4-well chamber slides. At 48 hours after transfection, serum-starved cells were left untreated or stimulated with 100 μM 8-pCPT-2′-O-Me-cAMP for 10 minutes. Immunofluorescence was performed using HUTS21 antibodies. Representative pictures from 3 different experiments are depicted. Bar represents 10 μm. Micrographs were acquired with an LSM 510 confocal microscope fitted with a Plan-Neofluar 40×/1.3 NA oil objective and LSM5 image acquisition software (all from Carl Zeiss).
Inhibition of Rap1 reduced endothelial adhesion. (A) HUVECs were transfected with Rap1GAP1-FLAG or empty vector, and adhesion assays were performed with transfected endothelial cells. After 24 hours, cells were allowed to adhere for one hour on fibronectin (n = 6) or collagen (n = 9; *P < .05 vs empty vector). (B) Adhesion assay with endothelial cells transfected with siRNAs targeted against Rap1a, Rap1b, Rap1a/Rap1b, or scrambled siRNA. After 48 hours, cells were allowed to adhere for 1 hour on fibronectin-coated wells (n = 5). Data are presented as percentage of adhering cells plus or minus SEM (*P < .05 vs scrambled). (C) Twelve hours after transfection, HUVEC cells were grown on 4-well chamber slides. At 48 hours after transfection, serum-starved cells were left untreated or stimulated with 100 μM 8-pCPT-2′-O-Me-cAMP for 10 minutes. Immunofluorescence was performed using HUTS21 antibodies. Representative pictures from 3 different experiments are depicted. Bar represents 10 μm. Micrographs were acquired with an LSM 510 confocal microscope fitted with a Plan-Neofluar 40×/1.3 NA oil objective and LSM5 image acquisition software (all from Carl Zeiss).
Role of Rap1a and Rap1b for angiogenic signaling
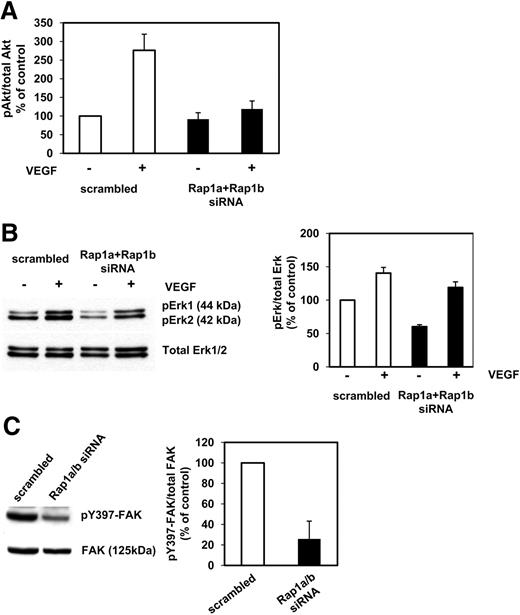
Silencing of Rap1a and Rap1b blocked angiogenic sprouting, migration, and adhesion of endothelial cells. Therefore, we investigated the role of Rap1a and Rap1b for angiogenic signaling in endothelial cells. Silencing of Rap1a and Rap1b blocked the VEGF-induced phosphorylation of Akt1 on serine 473 in adherent HUVECs as assessed by an Akt-phospho-ELISA (Figure 5A). In addition, knockdown of Rap1a and Rap1b reduced the phosphorylation/activation of ERK under basal conditions but had only a slight inhibitory effect on VEGF-induced ERK phosphorylation (Figure 5B). Moreover, silencing of Rap1a and Rap1b reduced the phosphorylation of FAK on tyrosine 397 (Figure 5C). In conclusion, these data demonstrate that Rap1 is required for appropriate angiogenic signaling in endothelial cells.
Altered angiogenic cell signaling in Rap1-silenced endothelial cells. Endothelial cells transfected with siRNAs targeted against Rap1a/Rap1b or scrambled siRNA. (A) After 48 hours, HUVECs were serum-starved for 3 hours and then left untreated or stimulated with VEGF (50 ng/mL) for 5 minutes. Cell lysates were subjected to phospho-Akt1–Ser473- or total Akt1-ELISA analysis. (B) After 48 hours, HUVECs were serum-starved for 3 hours and then left untreated or stimulated with VEGF (50 ng/mL) for 10 minutes. Cell lysates were subjected to Western blot analysis using phospho Erk1/2 or total Erk1/2. (C) Transfected HUVECs were lysed 48 hours after transfection. Cell lysates were subjected to Western blot analysis using antibodies against phospho-Y397-FAK, FAK, Rap1, and tubulin.
Altered angiogenic cell signaling in Rap1-silenced endothelial cells. Endothelial cells transfected with siRNAs targeted against Rap1a/Rap1b or scrambled siRNA. (A) After 48 hours, HUVECs were serum-starved for 3 hours and then left untreated or stimulated with VEGF (50 ng/mL) for 5 minutes. Cell lysates were subjected to phospho-Akt1–Ser473- or total Akt1-ELISA analysis. (B) After 48 hours, HUVECs were serum-starved for 3 hours and then left untreated or stimulated with VEGF (50 ng/mL) for 10 minutes. Cell lysates were subjected to Western blot analysis using phospho Erk1/2 or total Erk1/2. (C) Transfected HUVECs were lysed 48 hours after transfection. Cell lysates were subjected to Western blot analysis using antibodies against phospho-Y397-FAK, FAK, Rap1, and tubulin.
Role of Rap1a for in vivo angiogenesis
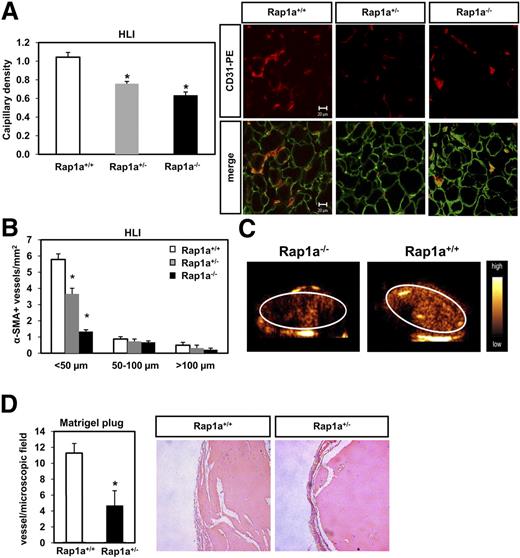
Next, we studied the role of Rap1a for in vivo angiogenesis. Rap1a−/− mice (in C57Bl/J background) are born with a substantially reduced mendelian ratio: 32.3%:59.1%:8.6% ratio of Rap1a+/+, Rap1a+/− and Rap1a−/− mice, respectively (313 mice born from heterozygote mating). We studied the role of the Rap1a in postnatal angiogenesis by using the Rap1a+/− heterozygote mice and the Rap1a-deficient mice (Rap1a−/−). Cells isolated from Rap1a+/− heterozygote mice displayed approximately 50% reduction of Rap1a mRNA (data not shown). Moreover, we investigated the role of Rap1a for the ischemia-induced neovascularization using the murine hind limb ischemia model. Strikingly, Rap1a−/− and Rap1a+/− mice displayed a reduced capillary (Figure 6A) and arteriole density (Figure 6B) 14 days after the induction of hind limb ischemia compared with wild-type mice. In line with these results, the perfusion of ischemic muscles detected by ultrasound was reduced in Rap1a−/− mice compared with wild-type mice (Figure 6C). In an additional angiogenic model, Rap1a+/− heterozygote mice displayed a significant reduction in capillary formation in a matrigel assay compared with wild-type (Rap1a+/+) mice (Figure 6D). In conclusion, Rap1a is essential for postnatal angiogenesis in vivo.
Role of Rap1a for neovascularization capacity in vivo. (A,B) Rap1a+/+ (n = 11), Rap1a+/− (n = 11), and Rap1a−/− (n = 4) mice were subjected to hind limb ischemia as described in “Methods.” (A) Capillary density (ratio of the number of capillaries to the number of myocytes) was determined in 8-μm frozen sections of ischemic muscles. Representative images of ischemic muscles are shown on the left panel (CD31, red fluorescence; laminin, green fluorescence). A quantitative analysis of capillary density is shown. Data are presented as mean plus or minus SEM (*P < .05 vs Rap1a+/+). Bar represents 20 μm. Micrographs were acquired with an LSM 510 confocal microscope fitted with a Plan-Neofluar 40×/1.3 NA oil objective and LSM5 image acquisition software (all from Carl Zeiss). (B) Conductance vessels in the adductor muscles were identified by size and smooth muscle actin (SMA) staining using a Cy3-labeled mouse monoclonal antibody for SMA. The number of small (< 50 μm), medium (50-100 μm), and large vessels was determined separately. Data are presented as mean plus or minus SEM (*P < .05 vs Rap1a+/+, < 50 μm). Evaluation was performed in a blinded fashion. (C) The perfusion of ischemic limbs was assessed by high frequency ultrasound in Rap1a−/− and Rap1a+/+ (wild-type) mice. (D) Statistical summary of blood vessel infiltration in Matrigel sections stained with an anti-SMA antibody in wild-type and Rap1a+/− mice. Quantitative results are presented as mean plus or minus SEM; n = 4 (Rap1a+/+), n = 4 (Rap1a+/−). Evaluation was performed in a blinded fashion. Sections of Matrigel plugs were stained with hematoxylin and eosin. Bar represents 20 μm (right panel).
Role of Rap1a for neovascularization capacity in vivo. (A,B) Rap1a+/+ (n = 11), Rap1a+/− (n = 11), and Rap1a−/− (n = 4) mice were subjected to hind limb ischemia as described in “Methods.” (A) Capillary density (ratio of the number of capillaries to the number of myocytes) was determined in 8-μm frozen sections of ischemic muscles. Representative images of ischemic muscles are shown on the left panel (CD31, red fluorescence; laminin, green fluorescence). A quantitative analysis of capillary density is shown. Data are presented as mean plus or minus SEM (*P < .05 vs Rap1a+/+). Bar represents 20 μm. Micrographs were acquired with an LSM 510 confocal microscope fitted with a Plan-Neofluar 40×/1.3 NA oil objective and LSM5 image acquisition software (all from Carl Zeiss). (B) Conductance vessels in the adductor muscles were identified by size and smooth muscle actin (SMA) staining using a Cy3-labeled mouse monoclonal antibody for SMA. The number of small (< 50 μm), medium (50-100 μm), and large vessels was determined separately. Data are presented as mean plus or minus SEM (*P < .05 vs Rap1a+/+, < 50 μm). Evaluation was performed in a blinded fashion. (C) The perfusion of ischemic limbs was assessed by high frequency ultrasound in Rap1a−/− and Rap1a+/+ (wild-type) mice. (D) Statistical summary of blood vessel infiltration in Matrigel sections stained with an anti-SMA antibody in wild-type and Rap1a+/− mice. Quantitative results are presented as mean plus or minus SEM; n = 4 (Rap1a+/+), n = 4 (Rap1a+/−). Evaluation was performed in a blinded fashion. Sections of Matrigel plugs were stained with hematoxylin and eosin. Bar represents 20 μm (right panel).
RAPL is a downstream effector of Rap1-mediating angiogenesis
Having demonstrated the importance of Rap1a and Rap1b for angiogenesis, we finally aimed to identify components of the downstream signaling pathway mediating these effects. RAPL is an effector of Rap1, which associates with activated (GTP-bound) Rap1 and mediates integrin activation in lymphocytes.27,43 Therefore, we studied the role of RAPL in angiogenesis. HUVECs expressed mRNA for RAPL (Figure 7A). Silencing of RAPL significantly blocked the angiogenic sprouting and migration of HUVECs (Figure 7B,C). As a control for the specificity of this approach, a second RAPL-specific siRNA was generated and gave identical results in terms of angiogenic sprouting (Figure S4). To investigate the role of RAPL as a possible downstream effector of Rap1 in angiogenesis, we overexpressed the constitutively active mutant Rap1aV12 in combination with scrambled or RAPL siRNA. Whereas the Rap1aV12 mutant increased angiogenic sprouting in scrambled siRNA-transfected cells, the silencing of RAPL inhibited the Rap1aV12-induced sprouting (Figure 7D,E). However, Rap1aV12 still slightly but significantly increased sprouting formation in HUVECs when cotransfected with RAPL siRNA compared with HUVECs transfected with RAPL siRNA and empty vector, indicating the existence of additional downstream effectors beyond RAPL to mediate angiogenesis (Figure 7E). Taken together, these data demonstrate that RAPL is a downstream mediator of Rap1-induced angiogenesis but also suggest that additional downstream angiogenic effectors of Rap1 may exist.
RAPL contributes to the angiogenic effect of Rap1a. Endothelial cells transfected with siRNAs targeted against RAPL or scrambled control. (A) Twenty-four hours later, expression of RAPL mRNA was assessed by RT-PCR; GAPDH serves as loading control. (B) Statistical summary of spheroid assays with RAPL siRNA or scrambled transfected endothelial cells. Spheroids were stimulated with or without 50 ng/mL bFGF (n = 3). Cumulative length of all sprouts originating from each spheroid was quantified after 24 hours. Statistical summary represents the mean plus or minus SEM (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + bFGF). (C) Forty-eight hours after transfection, migration assays on fibronectin were performed (n = 5). Data are presented as mean of migrated cell % of control plus or minus SEM (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + VEGF). (D) RT-PCR analysis of RAPL and Rap1aV12 expression by RT-PCR. GAPDH serves as loading control. (E) Statistical summary and representative micrographs of spheroid assay performed to analyze the effect of simultaneous RAPL silencing and Rap1aV12 overexpression on sprouting capacity of HUVEC (n = 5). Data are given as mean plus or minus SEM (*P < .05 vs scrambled siRNA + Rap1aV12; **P < .05 vs scrambled siRNA + pcDNA3.1; #P < .05 RAPL siRNA + pcDNA3.1). Bar represents 100 μm. The wells were viewed with an Axiovert 100M inverted microscope using as objective a Plan-NEOFLUAR (at 10×/0.30). Images were acquired using a digital camera Axiocam MR and were processed using AxioVision Rel 4.5 digital imaging software (all from Carl Zeiss).
RAPL contributes to the angiogenic effect of Rap1a. Endothelial cells transfected with siRNAs targeted against RAPL or scrambled control. (A) Twenty-four hours later, expression of RAPL mRNA was assessed by RT-PCR; GAPDH serves as loading control. (B) Statistical summary of spheroid assays with RAPL siRNA or scrambled transfected endothelial cells. Spheroids were stimulated with or without 50 ng/mL bFGF (n = 3). Cumulative length of all sprouts originating from each spheroid was quantified after 24 hours. Statistical summary represents the mean plus or minus SEM (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + bFGF). (C) Forty-eight hours after transfection, migration assays on fibronectin were performed (n = 5). Data are presented as mean of migrated cell % of control plus or minus SEM (*P < .05 vs scrambled siRNA, #P < .05 vs scrambled siRNA + VEGF). (D) RT-PCR analysis of RAPL and Rap1aV12 expression by RT-PCR. GAPDH serves as loading control. (E) Statistical summary and representative micrographs of spheroid assay performed to analyze the effect of simultaneous RAPL silencing and Rap1aV12 overexpression on sprouting capacity of HUVEC (n = 5). Data are given as mean plus or minus SEM (*P < .05 vs scrambled siRNA + Rap1aV12; **P < .05 vs scrambled siRNA + pcDNA3.1; #P < .05 RAPL siRNA + pcDNA3.1). Bar represents 100 μm. The wells were viewed with an Axiovert 100M inverted microscope using as objective a Plan-NEOFLUAR (at 10×/0.30). Images were acquired using a digital camera Axiocam MR and were processed using AxioVision Rel 4.5 digital imaging software (all from Carl Zeiss).
Discussion
The present study underscores the relevance of Rap1a and Rap1b for integrin function in endothelial cells and in vitro and in vivo angiogenesis. Specifically, the data of the present study revealed that (1) human endothelial cells express the Rap1a and Rap1b genes; (2) angiogenic growth factors can rapidly increase Rap1 activity in endothelial cells; (3) Rap1a and Rap1b are essential for in vitro angiogenic sprouting, migration, adhesion, and integrin affinity regulation in endothelial cells; (4) Rap1a and Rap1b are essential for the VEGF-induced phosphorylation of Akt in adherent endothelial cells; (5) using the Rap1a heterozygote mice, we demonstrate that Rap1a is essential for the in vivo neovascularization; and finally (6) we identified RAPL as an important effector of Rap1 mediating angiogenic sprouting. Thus, the present study provides insights into the role of integrin regulation for angiogenesis in endothelial cells and unravels a new function of Rap1 as key mediator of neovascularization.
In this work, we demonstrated that angiogenic growth factors, such as VEGF and bFGF, rapidly increase Rap1 activity. However, the underlying mechanism is unclear. It is conceivable that a GEF, which potentially acts downstream of the VEGF and bFGF receptors, could mediate this effect. A possible candidate GEF is C3G, a GEF of Rap1, which mediates signals from receptor tyrosine kinases.44 Indeed, it was shown that C3G can mediate VEGF- and bFGF-induced signaling in endothelial cells.45,46 However, the role of C3G for the bFGF- and VEGF-induced Rap1-activation in endothelial cells is not established. Further studies are required to clarify the mechanism of VEGF- and bFGF-induced Rap1 activation in endothelial cells.
Remarkably, inhibition of Rap1 activity, or knockdown of Rap1a or Rap1b, blocked the angiogenic sprouting and tube-forming activity of endothelial cells. In line with these results, the Rap1a heterozygote mice displayed a reduced angiogenic response in an in vivo matrigel plug assay and in the hind-limb ischemia model. Interestingly, a recent study showed that the Rap1b-deficient mice also displayed angiogenic defects.47 In addition, generation of knockout mice for C3G and PDZ-GEF, 2 GEFs of Rap1, led to defective vascular morphogenesis.48,49 Taken together, our data clearly demonstrate that Rap1a and Rap1b are involved in the sprouting of endothelial cells and in vivo angiogenesis. However, some of the single Rap1a- and Rap1b-deficient mice are born displaying no vascular defects under normal conditions. The modest phenotype might be explained by a redundant function of Rap1a and Rap1b, and it is conceivable that mice lacking the endothelial Rap1a and Rap1b may display a more severe vascular phenotype during embryonic vascular development than the single-deficient mice. Another possibility is that, during vascular development, additional signaling pathways may compensate for Rap1a and Rap1b. In line with these results, silencing of Rap1a and Rap1b did not completely abolish the angiogenic sprouting, migration, and adhesion of endothelial cells, suggesting that additional molecular pathways may mediate these angiogenic effects.
Cell migration is an essential angiogenic function of endothelial cells. The role of Rap1 in cell migration is controversially discussed. It was demonstrated that the migration of C3G-deficient fibroblasts was increased compared with wild-type cells and that expression of active Rap1 blocked this effect.50 Similarly, overexpression of Rap1V12 blocked cell motility in carcinoma cells.51 However, in other studies, inhibition of Rap1 blocked cell motility and activation of Rap1 correlated with increased migratory capacity.21,24,26,52 The data of the present study revealed that overexpression of Rap1GAP1 or silencing of Rap1a and/or Rap1b inhibited the integrin-dependent endothelial cell migration on fibronectin and collagen. Furthermore, active Rap1 was localized at the front of migrating endothelial cells. These data clearly demonstrate that Rap1a and Rap1b are essential for endothelial cell migration.
Rap1 was shown to be an essential activator of adhesion in a variety of cells (eg, platelets, leukocytes, fibroblasts).11,53 However, the role of Rap1 for the interaction of endothelial integrins to extracellular matrix proteins has not been investigated so far. Because endothelial cell migration on matrix proteins was reduced by inhibition of Rap1, we additionally studied the role of Rap1a and Rap1b on integrin affinity and integrin-dependent interaction of endothelial cells with extracellular matrix proteins. Strikingly, inhibition of Rap1 activity or knockdown of Rap1a and/or Rap1b significantly blocked the β1-integrin–dependent adhesion of endothelial cells to fibronectin and collagen and the αVβ3/αVβ5-integrin–dependent adhesion to vitronectin. This effect was mediated, at least for the β1-integrins, by an inhibitory effect on integrin affinity. Activated integrins were shown to localize in the front of migrating endothelial cells in a Rac1-dependent manner.54 Because active Rap1 and activated integrins localize at the leading front of migrating endothelial cells and Rap1 can increase and affect the localization of Rac1 activity,55 it is conceivable that inhibition of Rap1 may affect migration through an inhibitory effect on integrin activity and on the localization of activated integrins.
Interestingly, silencing of Rap1a and Rap1b inhibited the VEGF-induced Akt phosphorylation in adherent endothelial cells. A possible explanation for this effect could be that silencing of Rap1a and Rap1b affects the adhesion-dependent Akt phosphorylation and activity mediated by outside-in integrin signaling. Indeed, it has been demonstrated that integrin-dependent outside-in signaling acts synergistically to receptor tyrosine kinases affecting their downstream signaling.56,57 Another possibility is that Rap1 or a Rap1 effector acts downstream of the VEGFR2 as essential cofactor for Akt phosphorylation. Independent on the mechanism by which Rap1 interferes with Akt signaling, VEGF-induced Akt phosphorylation regulates endothelial nitric oxide synthase (eNOS),34 Girdin,58 as well as ACAP1 phosphorylation,59 all involved in migration, thereby contributing to the migratory defect in Rap1a- and Rap1b-silenced endothelial cells. Akt may also affect integrin affinity and adhesion.60 Besides Akt phosphorylation, silencing of Rap1a and Rap1b impaired phosphorylation of FAK on tyrosine residue 397 in endothelial cells. This effect is also indicative of an impaired integrin-dependent outside-in signaling, which may affect endothelial cell motility.61 Furthermore, silencing of Rap1 and Rap1b affected basal ERK phosphorylation. However, VEGF-induced phosphorylation of ERK was not significantly affected. In line with these results, silencing of Rap1a and Rap1b did not affect endothelial cell proliferation, which is controlled by the ERK pathway.
Finally, in the present work, we provide new evidence that RAPL is an important mediator of Rap1-induced angiogenesis. Silencing of RAPL as well as silencing of Rap1a and/or Rap1b blocked the migration and angiogenic sprouting of endothelial cells. Consistent with the present findings, another group reported that overexpression of a RAPL mutant perturbed wound healing26 and that RAPL is involved in the lymphocyte migration.62 Overexpression of constitutive active Rap1aV12 partly rescued the angiogenic sprouting defect induced by silencing of RAPL in endothelial cells, suggesting that other effector proteins may also mediate the Rap1aV12-induced angiogenic sprouting independent on RAPL.
In leukocytes, RAPL associates with the β2-integrin, lymphocyte function-associated antigen 1 (LFA-1).63 However, LFA-1 is not expressed in endothelial cells but only in hematopoietic cells; therefore, another mechanism accounts for the effects of RAPL in endothelial cells. Moreover, we could not detect any direct association of RAPL with the α5- or β1-integrin subunit in coimmunoprecipitation experiments (data not shown). In immunofluorescent stainings for β1-integrins, we only observed, to a limited extent, colocalization of GFP-RAPL with the β1-integrin subunit or localization of GFP-RAPL in the vicinity of β1-integrin subunits near the membrane of migrating cells (data not shown), indicating that the majority of RAPL does not bind directly to the β1-integrin, at least in the absence of stimulation with growth factors. It is also conceivable that RAPL may only transiently associate with the β1-integrins or that RAPL affects only indirectly integrin function in endothelial cells through signaling via other intermediate effector molecules. Another possibility is that RAPL mediates migration and 3-dimensional angiogenic sprouting of endothelial cells downstream of Rap1 independent of integrins. In this regard, it was shown that Rap1/RAPL may affect microtubule extension in endothelial cells.26 However, silencing of Rap1a and Rap1b in HUVECs did not affect the extension of microtubule during migration in a scratch wound assay (data not shown), suggesting that the Rap1/RAPL axis, at least in our experimental setting, affects migration and angiogenic sprouting through a mechanism distinct of microtubule regulation. Another possibility is that RAPL may affect downstream of Rap1 the migratory capacity and angiogenic sprouting of endothelial cells through an effect on cell polarization or actin polymerization/protrusion, processes necessary for directional cell migration. Indeed, it was shown that RAPL induces downstream of Rap1 the localization of the CXC chemokine receptor 4 (CXCR4) to the leading front of leukocytes (polarization),27 raising the possibility that RAPL may also, in endothelial cells, affect the localization of growth factor receptors during migration and sprouting. Additional studies are mandatory to elucidate the molecular mechanism by which RAPL affects migration and angiogenic sprouting of endothelial cells downstream of Rap1.
Interestingly, RIAM is another effector of Rap1, which can affect integrin function in leukocytes.28 However, we could not detect RIAM protein expression in endothelial cells (data not shown). Additional studies are required to identify new Rap1 effectors, which are involved in mediating Rap1-induced angiogenesis.
Beyond the regulation of integrin affinity and integrin-dependent adhesion to extracellular matrix proteins, which affect the migratory capacity of endothelial cells and their sprouting activity, Rap1 was also shown to regulate the assembly of VE-cadherin and to regulate vascular permeability.29,31-33 This is an additional pathway, which could affect angiogenesis.
Taken together, Rap1a and Rap1b are key mediators of angiogenesis by affecting β1-integrin affinity and integrin-dependent adhesion and migration in endothelial cells. Moreover, the angiogenic effects of Rap1 are mediated, at least in part, through the effector protein RAPL. It is conceivable that regulation of Rap1 could be a therapeutic target for antiangiogenic and proangiogenic approaches to treat patients with pathologic angiogenesis or ischemic disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peggy Schuster for excellent technical assistance, Ariane Fischer for the animal experiments, and Dr P. J. Stork (Vollum Institute, Oregon Health and Science University, Portland, OR) and Dr M. R. Philips (New York University, New York, NY) for the provided plasmids.
This work was supported by Deutsche Forschungsgemeinschaft, Transregional Collaborative Research Center SFB/TR23 Project A2 (E.C., S.D.). The generation of the Rap1a−/− was supported by the Deutsche Krebshilfe (grant 10-728 sche2; J.S.).
Authorship
Contribution: G.C. performed and designed experiments and performed manuscript correction; S.G. performed manuscript correction and contributed to the genotyping; A.O. performed proliferation experiments and manuscript correction; J.S. provided the Rap1a+/− and Rap1a −/− mice; T.B., M.J., and F.K. performed imaging with contrast-enhanced sonography; R.H. performed manuscript correction, provided the Rap1a+/− and Rap1a−/− mice, and contributed to the genotyping; A.M.Z. performed manuscript correction; S.D. designed experiments and performed manuscript correction; and E.C. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanouil Chavakis, Molecular Cardiology, Department of Internal Medicine III, J. W. Goethe University of Frankfurt, Theodor Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: Chavakis@em.uni-frankfurt.de.
References
Author notes
*S.D. and E.C. contributed equally to this manuscript.