Abstract
Therapeutic options for advanced B-cell acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) are limited. Available treatments can also deplete T lymphocytes, leaving patients at risk of life-threatening infections. In the National Cancer Institute cell line screen, the structurally unique natural product silvestrol produces an unusual pattern of cytotoxicity that suggests activity in leukemia and selectivity for B cells. We investigated silvestrol efficacy using primary human B-leukemia cells, established B-leukemia cell lines, and animal models. In CLL cells, silvestrol LC50 (concentration lethal to 50%) is 6.9 nM at 72 hours. At this concentration, there is no difference in sensitivity of cells from patients with or without the del(17p13.1) abnormality. In isolated cells and whole blood, silvestrol is more cytotoxic toward B cells than T cells. Silvestrol causes early reduction in Mcl-1 expression due to translational inhibition with subsequent mitochondrial damage, as evidenced by reactive oxygen species generation and membrane depolarization. In vivo, silvestrol causes significant B-cell reduction in Eμ-Tcl-1 transgenic mice and significantly extends survival of 697 xenograft severe combined immunodeficient (SCID) mice without discernible toxicity. These data indicate silvestrol has efficacy against B cells in vitro and in vivo and identify translational inhibition as a potential therapeutic target in B-cell leukemias.
Introduction
Chronic lymphocytic leukemia (CLL) is an incurable disease defined by an elevation of CD5+/CD19+ malignant monoclonal B lymphocytes that interfere with normal immune function, leading to infections and other complications. Available therapies are often ineffective for patients with advanced disease, or can have severe side effects such as T-cell depletion that increase infection risk.1 In addition, even after successful treatment, drug resistance eventually develops through mechanisms including dysfunction of the p53 tumor suppressor pathway. Although deletion of 17p13.1 (the chromosomal site of the p53 gene) is uncommon in CLL at diagnosis, this abnormality increases in frequency with disease progression and is strongly associated with drug resistance and reduced progression-free survival.2,3 Adult B-cell acute lymphoblastic leukemia (ALL) presents challenges very similar to refractory CLL, and regardless of initial treatment success, disease-free survival 5 years after diagnosis is less than 30%.4 Furthermore, there is a lack of therapeutic options now and in the immediate future, as drugs currently under development for refractory ALL are similar in mechanism to those already available, suggesting they will produce only modest improvements in responses.5 Agents with novel and selective mechanisms of action are desperately needed to improve survival in both patient populations
Cyclopenta[b]benzofuran constituents of plants from the genus Aglaia of the family Meliaceae have been of considerable interest since rocaglamide was found to exhibit antileukemic activity.6 Subsequently, several groups showed antiproliferative activity of related molecules using cancer cell lines7-9 (reviewed in Kim et al10 ), as well as human tumor cells.11 Kinghorn and colleagues isolated silvestrol, a structurally unique cyclopenta[b]benzofuran from the Indonesian plant Aglaia foveolata Pannell, and fully characterized its structure and absolute configuration.12 The total synthesis of silvestrol has subsequently been reported by 2 groups.13,14 Silvestrol has potency against lung, breast, and prostate cancer cell lines in vitro, with LC50 in the nanomolar range.12,15 This compound was also tested in vivo in the P388 leukemia model16 and hollow fiber assay,17 in which it demonstrated promising antitumor activity without significant weight loss. Silvestrol isolated from Aglaia leptantha Miq, collected in Malaysia, has also been found to inhibit the growth of PC-3 human prostate cancer cells in a murine xenograft study.18
The pattern of cytotoxicity produced by silvestrol in the NCI 60-cell line screen19 suggests an unusual mechanism of action as well as efficacy against B-cell leukemia. Results from this screen showed that in 5 of the 6 leukemia lines used, silvestrol produces a total growth inhibition (TGI)20 approximately 10-fold greater than the average of all cell lines tested, demonstrating an increased sensitivity of these cells to silvestrol. The single leukemia line with below-average TGI, indicating relative resistance, was the T-lymphoblastic line CCRF-CEM. We therefore investigated silvestrol efficacy in B-cell leukemias using cell lines, primary leukemia cells, and murine models of ALL and CLL.
Methods
Reagents
Isolation and characterization of silvestrol has been described.12 Z-VAD-FMK and Boc-D-FMK (Enzyme Systems Products, Aurora, OH) were used at 100 micromolar (μM). JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) and dihydroethidium were obtained from Molecular Probes (Eugene, OR). Fludarabine (9-β-d-arabinofuranosyl-2-fluoroadenine; F-ara A) and cycloheximide (CHX) were obtained from Sigma-Aldrich (St Louis, MO). Flavopiridol was provided by the NCI Cancer Therapy Evaluation Program.
Cells and cell lines
Peripheral blood was collected from patients with CLL, B- or T-cell ALL, or T-cell prolymphocytic leukemia (T-PLL) after written consent on an OSU Institutional Review Board–approved protocol was obtained in accordance with the Declaration of Helsinki. CLL patients were newly diagnosed or without treatment for a minimum of 30 days at time of collection. Samples used for this work (N = 70, 40 male) had a confirmed diagnosis of their disease and significantly elevated peripheral leukocyte counts (> 20 × 109/L) but were otherwise unselected based on disease stage or prognostic subgroup. Occurrence of del(17p13.1) was determined in CLL patient samples by fluorescence in situ hybridization as described,21 and in each positive case at least 70% of cells showed this deletion. Leukemic B cells or T cells were negatively selected using RosetteSep cocktails (StemCell Technologies, Vancouver, BC). ALL samples were derived from peripheral blood from newly diagnosed patients. Normal cells were obtained from Red Cross leukocyte reduction filters22 or partial leukocyte preparations, and B or T lymphocytes or natural killer (NK) cells were negatively selected using RosetteSep reagents. Normal samples were from anonymous donors, and therefore age, race, and sex were unknown. Cells were isolated by Ficoll density gradient centrifugation and dual-stained for flow cytometry with antibodies to CD19, CD3, or CD56 (BD Biosciences, San Jose, CA) to verify selection efficiency. The 697 B-cell ALL line was obtained from DSMZ (Braunschweig, Germany), and the JeKo-1 and Mino mantle cell lymphoma cell lines and Ramos Burkitt lymphoma line were obtained from ATCC (Manassas, VA). Cells were incubated at 37°C and 5% CO2 in RPMI 1640 with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (Sigma-Aldrich).
Viability and flow cytometric assays
MTT viability assays were performed as described,23 and LC50 was calculated using Prism (GraphPad Software, San Diego, CA). Cell viability was also measured using propidium iodide (PI) flow cytometry following the manufacturer's instructions (BD Pharmingen, San Diego, CA). Reactive oxygen species (ROS) and mitochondrial membrane depolarization were detected by flow cytometry on live (PI-negative) cells as previously described using dihydroethidium24 or JC-1,25 respectively. NK cells were incubated with or without 80 nanomolar (nM) silvestrol for 20 hours in the presence of plastic-bound herceptin or anti-CD19 IgG. NK-cell functional activation was determined by increased CD107a expression in CD3−/CD56+/PI− cells relative to the no-antibody control, as described by Alter et al.26
Additional analyses
Immunoblots were performed as described previously.23 Antibody to polyADP-ribose polymerase (PARP) was from EMD Biosciences (La Jolla, CA). Antibodies to caspase 7 (AF823), caspase 3 (AF605), and caspase 9 (AF8301) were from R&D Systems (Minneapolis, MN). Antibodies to caspase 8 (1C12), eIF2α (9721), 4EBP1 (9455), Bmf (4692), Bak (3814), and Smac/DIABLO (2954) were from Cell Signaling (Danvers, MA). Remaining antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Bands were quantified on a ChemiDoc system with Quantity One software (BioRad Laboratories, Hercules, CA). Caspase activity assays were performed as previously described.27 Mitochondrial fractionations were performed using reagents from Pierce (Rockford, IL) as reported.25 In vitro translational inhibition experiments were conducted using the Transcend kit with rabbit reticulocyte lysate according to the manufacturer's instructions (Promega, Madison, WI). The Mcl-1 expression vector was the kind gift of Dr Ruth Craig, Dartmouth Medical School (Hanover, NH). Mitochondria were fractionated using a kit according to the manufacturer's instructions (BioVision, Mountain View, CA). Fluorescence polarization assays were conducted as described28 by Drs Dayong Zhai and Paul W. Diaz in the laboratory of John C. Reed (The Burnham Institute for Medical Research, La Jolla, CA).
Real-time reverse transcription–polymerase chain reaction
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). Real-time reverse transcription–polymerase chain reaction (RT-PCR) for Mcl-1 was performed on an ABI 7900 in the OSU Comprehensive Cancer Center Nucleic Acids Shared Resource. TaqMan Universal Master Mix, primers, and labeled probes were used according to the manufacturer's procedure (Applied Biosystems, Foster City CA), using 18S RNA as an endogenous control. Mean threshold cycle (Ct) values were calculated by PRISM software (Applied Biosystems) to determine fold differences according to the manufacturer's instructions.
Whole-blood experiments
Peripheral blood from CLL patients and healthy volunteers was obtained in Vacutainer acid citrate dextrose tubes (BD, Franklin Lakes NJ). For CLL patients, previous diagnostic flow cytometric analysis confirmed the diagnosis of CLL; normal B cells were undetectable in these samples. Blood was untreated or treated with 80 nM silvestrol or 1.0 μM F-ara A. Tubes were incubated at 37°C with gentle mixing for 0, 24, and 48 hours. All samples were run in duplicate. At each time point, percentages and absolute cell numbers were immediately assessed by flow cytometry. After white blood cell (WBC) count using a Coulter AcTdiff Analyzer (Beckman-Coulter, Miami, FL), WBC counts were adjusted to 10 × 109 and whole-blood staining was performed with predetermined volumes of directly conjugated monoclonal antibodies. After staining, red blood cells were lysed using ImmunoPrep Whole Blood Lysing Reagent (Beckman-Coulter). Two tubes were used for analysis: tube 1: CD13-PE, HLA-DR-PC5, CD14-FITC, CD45-ECD; tube 2: CD19-PC5, CD3-FITC, CD16/CD56-PE, CD45-ECD. All reagents were from Beckman-Coulter except CD16/56 (BD Pharmingen). Four-color flow cytometric analysis was performed on an FC500 cytometer equipped with CXP software (Beckman-Coulter). Erythrocytes, platelets, dead cells, and debris were excluded based on CD45 staining and forward/side scatter characteristics, with gating strategy established in preliminary experiments using 7AAD. Percentages of B and T lymphocytes for each sample were calculated and expressed as percentages of the total lymphocytes analyzed. All data are shown relative to time-matched untreated samples. In untreated samples, only minor reductions were observed at 24 or 48 hours in both the T- and B-lymphocyte subsets. In contrast, numbers of monocytes, NK cells, and granulocytes dropped substantially over the incubation period (data not shown), and effects on these subsets were therefore not investigated.
In vivo experiments
The Eμ-Tcl-1 CLL29,30 and the 697 SCID ALL31,32 models have been described. Eμ-Tcl-1 breeding pairs were obtained from Dr Carlo Croce, OSU. Mice were housed in a clean environment and supplied with sterile food and water ad libitum. Silvestrol in sterile saline (< 1% DMSO) was injected intraperitoneally at 1.5 mg/kg per injection as noted in the figure legends. Mice were humanely killed upon development of hind-limb paralysis or other disease criteria causing discomfort. All experiments were carried out under protocols approved by the OSU Institutional Animal Care and Use Committee.
Statistics
As several of the experiments used cells from the same donor for different treatments, linear mixed models were used to take account of the dependency of observations from the same sample. Main effects and differences were estimated from these models. For survival analysis, the log-rank test was used and a Kaplan-Meier survival curve was generated. The Holm procedure was used to correct type I error for multiple comparisons or multiple endpoints.33 P value less than .05 was considered significant for single comparisons or after adjustment for multiple comparisons or multiple endpoints.
Results
Cytotoxicity of silvestrol
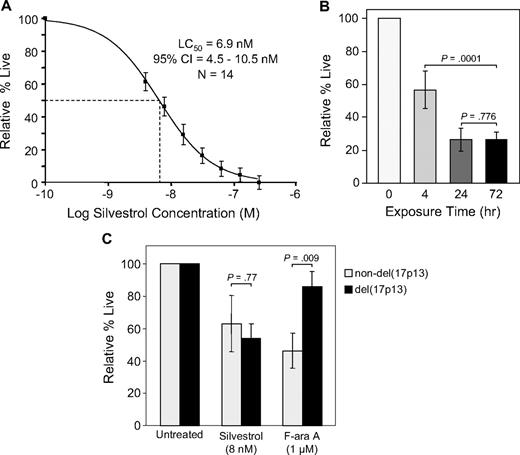
In CLL patient tumor cells, silvestrol-induced cell death was minimal at 24 hours but increased in a dose- and time-dependent manner. With 72 hours of silvestrol treatment, the average LC50 was 6.9 nM (95% CI = 4.5-10.5; n = 14; Figure 1A). In the same sample set, incubation with a pharmacologically relevant concentration of fludarabine (F-ara A, 1.0 μM) produced 66% viability relative to time-matched untreated cells. Using normal peripheral blood mononuclear cells (PBMCs) under identical conditions, 50% cell death relative to untreated cells was not observed until a silvestrol concentration at least 10-fold higher than the CLL cell LC50 was reached. Above this level there was little additional cell death, and thus the LC50 could not be properly calculated for normal PBMCs. In B-cell ALL cell line 697 and the B-lymphoma lines Ramos, JeKo-1, and Mino, the 72-hour IC50 was less than 5.0 nM (n = 3 each; data not shown). Cells were also tested from 2 patients with B-cell ALL, in which silvestrol produced an estimated LC50 below 7 nM at 72 hours, and 2 T-cell PLL samples, in which 50% cell death was not reached at 72 hours even at silvestrol concentrations up to 250 nM (data not shown).
Silvestrol cytotoxicity. (A) Silvestrol cytotoxicity in chronic lymphocytic leukemia (CLL) patient cells: CD19+ cells from CLL patients (n = 14) were incubated with or without silvestrol at various concentrations for 72 hours, and viability was determined by MTT assay. Viability was calculated relative to time-matched untreated controls. (B) Time dependency of silvestrol cytotoxicity: CLL patient samples (n = 4) were incubated with 0 or 80 nM silvestrol for 4, 24, or 72 hours. At each time point, cells were centrifuged and resuspended in fresh media without drug. Incubations were each continued to a total of 72 hours, at which point viability was determined by MTT assay. Results for each exposure time are shown relative to time-matched untreated cells, set at 100%. Bars show plus or minus SD. (C) Silvestrol cytotoxicity in del(17p13) cells: CLL cells from patients with or without the del(17p13) chromosomal abnormality (n = 7 for each subset) were incubated with silvestrol at 8 nM (approximate LC50) or 1 μM F-ara A, and viability was determined by MTT assay at 72 hours. Results are shown relative to time-matched untreated cells. Bars show plus or minus SD.
Silvestrol cytotoxicity. (A) Silvestrol cytotoxicity in chronic lymphocytic leukemia (CLL) patient cells: CD19+ cells from CLL patients (n = 14) were incubated with or without silvestrol at various concentrations for 72 hours, and viability was determined by MTT assay. Viability was calculated relative to time-matched untreated controls. (B) Time dependency of silvestrol cytotoxicity: CLL patient samples (n = 4) were incubated with 0 or 80 nM silvestrol for 4, 24, or 72 hours. At each time point, cells were centrifuged and resuspended in fresh media without drug. Incubations were each continued to a total of 72 hours, at which point viability was determined by MTT assay. Results for each exposure time are shown relative to time-matched untreated cells, set at 100%. Bars show plus or minus SD. (C) Silvestrol cytotoxicity in del(17p13) cells: CLL cells from patients with or without the del(17p13) chromosomal abnormality (n = 7 for each subset) were incubated with silvestrol at 8 nM (approximate LC50) or 1 μM F-ara A, and viability was determined by MTT assay at 72 hours. Results are shown relative to time-matched untreated cells. Bars show plus or minus SD.
Although maximal silvestrol cytotoxicity was observed at 72 hours, mechanistic investigations in CLL leukemia cells ex vivo are complicated by normal spontaneous apoptosis over time. Therefore, to more clearly determine the biologic effects of silvestrol, we performed experiments at earlier time points, using a higher concentration (80 nM) of silvestrol. This concentration is still well below the micromolar doses (estimated assuming equal distribution) that were administered in preliminary in vivo experiments.12 Experiments were also conducted to determine the required exposure time for silvestrol-mediated cytotoxicity in CLL cells. CLL samples (n = 4) were incubated in 0 or 80 nM silvestrol. After 4 or 24 hours, cells were washed and incubated in media without drug. Viability was then examined after a total of 72 hours in each case. Four hours of silvestrol exposure resulted in an average of 56% cytotoxicity at 72 hours relative to time-matched untreated cells. Within 24 hours of exposure, there was no difference in cytotoxicity between cells removed from silvestrol and cells with continuous exposure (Figure 1B). These results show that even though the cell death induced by silvestrol is maximal at approximately 72 hours, briefer exposures still produce substantial effects that are not reversed with removal of the agent.
Chromosomal deletions such as del(17p13.1) are one of the strongest laboratory predictors in CLL of poor response to chemotherapy.3 We therefore used silvestrol at the 72-hour LC50 to investigate the sensitivity of CLL leukemia cells with versus without the del(17p13.1) abnormality (n = 7 for each subset). Under these conditions, there was no significant difference in silvestrol sensitivity between these 2 patient subsets (P = .77). In contrast, a significant difference was observed using 1.0 μM fludarabine (P = .009; Figure 1C). This result suggests that, unlike fludarabine, silvestrol induces cell death through a p53-independent mechanism in CLL leukemia cells. To date, we have detected no difference in silvestrol cytotoxicity between CLL samples from IgVH gene–mutated versus unmutated patients. However, there are not yet sufficient sample numbers to state this conclusion with statistical significance.
Selectivity of silvestrol
Due to the relative silvestrol insensitivity of the T-cell line CCRF-CEM and normal PBMCs, of which approximately 70% are T cells, we compared the efficacy of silvestrol on normal T and B cells derived from healthy volunteers. T cells (81%-86% CD3+, n = 4) and B cells (74%-88% CD19+, n = 4) were isolated from peripheral blood of healthy donors, incubated with or without silvestrol for 48 hours, and assessed for viability. Results were compared with the untreated cells at the same time point. Silvestrol was significantly more cytotoxic to B cells (41.2% ± 3.3%) relative to T cells (84.9% ± 3.3%; P < .001) averaged across all concentrations tested (Figure 2A). To confirm B-cell selectivity in an assay with greater physiologic relevance, we incubated whole blood from CLL patients (n = 5) or healthy volunteers (n = 4) with or without 80 nM silvestrol or 1 μM fludarabine and assessed cell viability over time by flow cytometry. In blood from CLL patients, silvestrol was significantly more cytotoxic toward B cells compared with T cells (P < .001), causing a 75% reduction in CD19+ cells as early as 24 hours and a greater than 90% reduction by 48 hours relative to the untreated time-matched samples (Figure 2B). Importantly, this B cell–selective cytotoxicity was less prominent in samples from healthy volunteers compared with CLL patients. In spleen cells from leukemic Eμ-Tcl-1 mice29 tested ex vivo, silvestrol also produced a greater decrease in B cells than in T cells compared with fludarabine, flavopiridol, or cycloheximide (n = 3, data not shown). We also investigated the impact of silvestrol on NK cells, using surface expression of CD107a as a surrogate for NK-cell function.26 NK cells from healthy volunteers (n = 4) were incubated for 20 hours with or without 80 nM silvestrol in the presence or absence of an irrelevant antibody bound to plastic plates, and then CD107a expression on CD3−/CD56+ cells was assessed by flow cytometry. Silvestrol blocked antibody-mediated induction of CD107a expression (5% of antibody-incubated cells were CD107a+ with silvestrol vs 17% without; P = .001). Throughout the experiment, percentage of PI-negative cells remained unchanged at 95% to 98%. This experiment suggests that NK-cell function is inhibited, at least temporarily, in the presence of silvestrol.
Silvestrol produces B-cell selective cytotoxicity. (A) Silvestrol effects on isolated normal B and T lymphocytes: B cells (n = 4, 74%-88% CD19+) and T cells (n = 4, 81%-86% CD3+) were selected from peripheral blood of healthy volunteers and incubated in media with or without silvestrol. Viability was assessed at 48 hours by MTT assay and results were calculated relative to the time-matched untreated samples in each case. Bars show plus or minus SD. (B) Silvestrol effects on B and T cells in whole blood: Peripheral blood from either CLL patients (left, n = 5) or healthy volunteers (right, n = 4) was incubated at 37°C with mixing, with or without 80 nM silvestrol ( ) or 1 μM F-ara A (
) or 1 μM F-ara A ( ). At the indicated times, an aliquot was analyzed by flow cytometry for CD19+ cells (striped bars) and CD3+ cells (solid bars). Data are shown relative to time-matched untreated samples. Bars represent plus or minus SD.
). At the indicated times, an aliquot was analyzed by flow cytometry for CD19+ cells (striped bars) and CD3+ cells (solid bars). Data are shown relative to time-matched untreated samples. Bars represent plus or minus SD.
Silvestrol produces B-cell selective cytotoxicity. (A) Silvestrol effects on isolated normal B and T lymphocytes: B cells (n = 4, 74%-88% CD19+) and T cells (n = 4, 81%-86% CD3+) were selected from peripheral blood of healthy volunteers and incubated in media with or without silvestrol. Viability was assessed at 48 hours by MTT assay and results were calculated relative to the time-matched untreated samples in each case. Bars show plus or minus SD. (B) Silvestrol effects on B and T cells in whole blood: Peripheral blood from either CLL patients (left, n = 5) or healthy volunteers (right, n = 4) was incubated at 37°C with mixing, with or without 80 nM silvestrol ( ) or 1 μM F-ara A (
) or 1 μM F-ara A ( ). At the indicated times, an aliquot was analyzed by flow cytometry for CD19+ cells (striped bars) and CD3+ cells (solid bars). Data are shown relative to time-matched untreated samples. Bars represent plus or minus SD.
). At the indicated times, an aliquot was analyzed by flow cytometry for CD19+ cells (striped bars) and CD3+ cells (solid bars). Data are shown relative to time-matched untreated samples. Bars represent plus or minus SD.
Mechanism of cell death
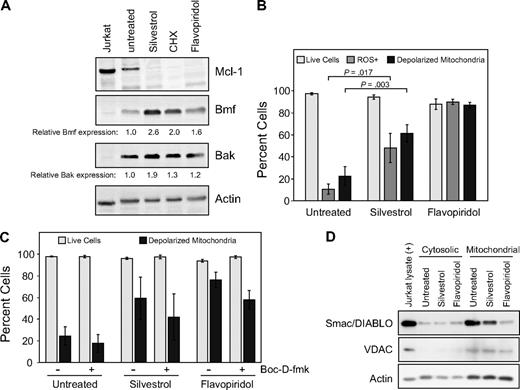
As the Bcl-2 family protein Mcl-1 is an important inhibitor of apoptosis in CLL, we investigated the effect of silvestrol on Mcl-1 expression in CLL patient cells before cell death. By immunoblot, we observed a substantial reduction in Mcl-1 protein with silvestrol in most cases as early as 4 hours, and in all samples before 16 hours. Mcl-1 reduction preceded cell death as measured by either MTT assay or PI uptake, and also could be observed before substantial processing of the caspase substrate polyADP-ribose polymerase (PARP), suggesting the caspase independence of this particular effect. Furthermore, pan-caspase inhibitors Z-VAD-fmk and Boc-D-fmk were unable to effectively block silvestrol-mediated Mcl-1 reduction, although they did prevent PARP cleavage (Figure 3A). These inhibitors also moderately reduced silvestrol-mediated cytotoxicity at later time points (24-48 hours), as examined by both PI uptake and MTT assays. However this effect did not reach statistical significance (data not shown), suggesting the possibility that silvestrol also induces non–caspase-mediated cell death. Because CLL cells in culture typically exhibit variable levels of spontaneous apoptosis together with caspase activation, we confirmed this Mcl-1 observation using the 697 ALL cell line. As shown in Figure 3B, Mcl-1 protein is reduced after silvestrol treatment before evidence of caspase activation as determined by cleavage of PARP. Because Mcl-1 is a known substrate of the proteasome, we then tested whether the proteasome inhibitor bortezomib could inhibit silvestrol-mediated Mcl-1 loss. Bortezomib alone increased the level of Mcl-1 protein with as little as 4 hours of exposure, indicating that proteasomal degradation of Mcl-1 is ongoing in untreated CLL patient cells ex vivo (Figure 3C). However, bortezomib was unable to substantially inhibit silvestrol-induced Mcl-1 protein reduction. These results suggest that Mcl-1 reduction is an early consequence of silvestrol exposure, and is not due only to apoptosis-related caspase or proteasome activity.
Silvestrol effects on Mcl-1 expression. (A) Silvestrol mediates caspase-independent Mcl-1 reduction: CLL cells treated with 80 nM silvestrol, 100 μM cycloheximide (CHX), or 25 μM F-ara A for 16 hours with or without caspase inhibitors Z-VAD-fmk or Boc-D-fmk (100 μM) were analyzed for Mcl-1 protein expression by immunoblot. Results are shown from 1 of 4 identical experiments. All samples tested to date showed substantial Mcl-1 protein reduction by this time point after silvestrol treatment. (B) Silvestrol-mediated Mcl-1 reduction in 697 acute lymphoblastic leukemia (ALL) cells is distinct from caspase activity: 697 ALL cell line was incubated for 8 hours with or without 80 nM silvestrol or 200 nM flavopiridol, and lysates were examined by immunoblot. (C) Silvestrol-mediated Mcl-1 reduction is independent of proteasome activity: CLL patient cells were treated with 80 nM silvestrol, 100 μM cycloheximide (CHX), or 200 nM flavopiridol for 4 or 8 hours, with or without 50 nM bortezomib. Lysates were analyzed for PARP and Mcl-1 expression by immunoblot.
Silvestrol effects on Mcl-1 expression. (A) Silvestrol mediates caspase-independent Mcl-1 reduction: CLL cells treated with 80 nM silvestrol, 100 μM cycloheximide (CHX), or 25 μM F-ara A for 16 hours with or without caspase inhibitors Z-VAD-fmk or Boc-D-fmk (100 μM) were analyzed for Mcl-1 protein expression by immunoblot. Results are shown from 1 of 4 identical experiments. All samples tested to date showed substantial Mcl-1 protein reduction by this time point after silvestrol treatment. (B) Silvestrol-mediated Mcl-1 reduction in 697 acute lymphoblastic leukemia (ALL) cells is distinct from caspase activity: 697 ALL cell line was incubated for 8 hours with or without 80 nM silvestrol or 200 nM flavopiridol, and lysates were examined by immunoblot. (C) Silvestrol-mediated Mcl-1 reduction is independent of proteasome activity: CLL patient cells were treated with 80 nM silvestrol, 100 μM cycloheximide (CHX), or 200 nM flavopiridol for 4 or 8 hours, with or without 50 nM bortezomib. Lysates were analyzed for PARP and Mcl-1 expression by immunoblot.
To determine whether silvestrol-mediated Mcl-1 reduction occurs at the transcriptional level, we assessed Mcl-1 mRNA levels in CLL cells treated with silvestrol for 4 or 12 hours using real-time RT-PCR. Mcl-1 mRNA levels in silvestrol-treated cells were not significantly different from those in untreated cells at the same time points (Figure 4A). Flavopiridol was included as a control in this experiment as it strongly decreases Mcl-1 in CLL cells at the mRNA level.34,35 We next tested whether silvestrol instead blocks translation, using an in vitro translation system with either Mcl-1 or luciferase mRNA produced in a separate reaction. Translation of both Mcl-1 (Figure 4B) and luciferase (not shown) was effectively reduced by silvestrol and the positive control cycloheximide, but not by flavopiridol. To investigate this mechanism further, we examined key control points of the endoplasmic reticulum (ER) stress pathway (eIF2a) and the mTOR pathway (4EBP), both of which can be affected by cytotoxic agents and can produce translational inhibition when activated or inhibited, respectively. Phosphorylation of eIF2α (Figure 4C) and 4EBP (Figure 4D) in silvestrol-treated 697 ALL cells was not affected by silvestrol treatment at 2.5 hours, a time point by which Mcl-1 protein is notably diminished. These results suggest that the mechanism of silvestrol-mediated Mcl-1 translational inhibition is not through the common mTOR and ER stress pathways. Other components of these pathways remain to be fully investigated.
Mechanism of silvestrol-mediated Mcl-1reduction. (A) Effect of silvestrol on Mcl-1 transcription: RNA was extracted from CLL patient cells treated either with 80 nM silvestrol, 200 nM flavopiridol, or 100 μM cycloheximide (CHX) for 4 or 12 hours. n = 6 for untreated and silvestrol treated; n = 3 for flavopiridol and CHX treated. Mcl-1 message level was analyzed by real-time RT-PCR and was normalized relative to 18S RNA. Data are expressed as fold change in Mcl-1 message level over the time-matched untreated sample. Increases in Mcl-1 mRNA with silvestrol treatment were not significant. Bars show plus or minus SD. (B) Translation inhibition by silvestrol: In vitro translation reactions using in vitro–transcribed Mcl-1 mRNA were prepared in the presence or absence of silvestrol, cycloheximide, or flavopiridol as indicated. Reactions were separated by SDS-PAGE and detected using anti–Mcl-1 antibody. Translation assay control reactions contained luciferase mRNA or no mRNA. Lysate from the 697 cell line was included as an Mcl-1 protein control. This figure is from a single immunoblot; a vertical line was inserted to indicate the deletion of an irrelevant lane. (C) Silvestrol does not affect eIF2α phosphorylation: 697 ALL cells were treated with or without silvestrol (80 nM) or arsenic (200 μM) for 2.5 hours and lysates analyzed by immunoblot. HeLa cells were included as control. (D) Silvestrol does not affect 4EBP phosphorylation: 697 ALL cells were serum starved for 3 hours, then were treated with or without silvestrol (80 nM) or rapamycin (20 nM) for 2.5 hours in the presence of 20% fetal bovine serum. Lysates were analyzed by immunoblot.
Mechanism of silvestrol-mediated Mcl-1reduction. (A) Effect of silvestrol on Mcl-1 transcription: RNA was extracted from CLL patient cells treated either with 80 nM silvestrol, 200 nM flavopiridol, or 100 μM cycloheximide (CHX) for 4 or 12 hours. n = 6 for untreated and silvestrol treated; n = 3 for flavopiridol and CHX treated. Mcl-1 message level was analyzed by real-time RT-PCR and was normalized relative to 18S RNA. Data are expressed as fold change in Mcl-1 message level over the time-matched untreated sample. Increases in Mcl-1 mRNA with silvestrol treatment were not significant. Bars show plus or minus SD. (B) Translation inhibition by silvestrol: In vitro translation reactions using in vitro–transcribed Mcl-1 mRNA were prepared in the presence or absence of silvestrol, cycloheximide, or flavopiridol as indicated. Reactions were separated by SDS-PAGE and detected using anti–Mcl-1 antibody. Translation assay control reactions contained luciferase mRNA or no mRNA. Lysate from the 697 cell line was included as an Mcl-1 protein control. This figure is from a single immunoblot; a vertical line was inserted to indicate the deletion of an irrelevant lane. (C) Silvestrol does not affect eIF2α phosphorylation: 697 ALL cells were treated with or without silvestrol (80 nM) or arsenic (200 μM) for 2.5 hours and lysates analyzed by immunoblot. HeLa cells were included as control. (D) Silvestrol does not affect 4EBP phosphorylation: 697 ALL cells were serum starved for 3 hours, then were treated with or without silvestrol (80 nM) or rapamycin (20 nM) for 2.5 hours in the presence of 20% fetal bovine serum. Lysates were analyzed by immunoblot.
Because of the observed impact of silvestrol on Mcl-1 protein, we examined the expression of additional Bcl-2 family members in silvestrol-treated CLL patient cells (n = 3 or 4 for each protein tested) at 16 hours, before increase in cell death is detected by PI uptake. Interestingly, at this time we noted an increase in the proapoptotic BH3-domain–only proteins Bmf and Bak (Figure 5A), suggesting that the strong reduction of Mcl-1 protein is not a general effect of silvestrol. No changes were observed in levels of Bcl-2, Bax, Bik, Bok, or PUMA at this time point. In addition, no changes in Noxa were detected and Bid appeared to be diminished, although the levels of these 2 proteins in untreated CLL cells were too low to reach firm conclusions. To rule out direct interaction of silvestrol with Mcl-1 or other antiapoptotic Bcl-2 family members, fluorescence polarization competition assays with labeled BH3 peptides were performed as described.28 No interactions were detected between silvestrol and Mcl-1, Bcl-2, Bcl-XL, Bcl-W, Bcl-B, or Bfl-1 in these experiments (data not shown).
Apoptotic mechanism of silvestrol. (A) Silvestrol causes increase in proapoptotic Bcl-2 family members in CLL cells: CLL patient cells were treated with silvestrol (80 nm), cycloheximide (CHX, 100 μM), or flavopiridol (200 nM) for 16 hours. Cells were analyzed by immunoblot for Bmf, Bak, and other Bcl-2 family members. Blot is representative of 4 CLL patient samples tested. Numbers below the lanes show densitometry data for Bmf and Bak levels in treated samples relative to untreated, normalized to the actin control. (B) Silvestrol induces reactive oxygen species (ROS) generation and mitochondrial depolarization in CLL cells: CLL patient cells (n = 3) were treated with silvestrol (80 nm) or flavopiridol (200 nM) for 16 hours. Cells were analyzed by flow cytometry using dihydroethidium (DHE) to detect ROS, JC-1 to detect mitochondrial depolarization, and propidium iodide (PI) to detect cell death. Percentages of live cells (PI negative) are shown as  . Percentages of cells showing ROS generation (DHE positive) are shown as
. Percentages of cells showing ROS generation (DHE positive) are shown as  and percentages of cells with depolarized mitochondria (monomeric JC-1) are shown as ■. Bars show plus or minus SD. (C) Silvestrol-induced mitochondrial depolarization: CLL patient cells (n = 4) were treated as in panel A, with or without the caspase inhibitor Boc-D-fmk (100 μM). Cells were analyzed at 16 hours by flow cytometry using PI and JC-1. The percentages of cells with intact mitochondria are shown as
and percentages of cells with depolarized mitochondria (monomeric JC-1) are shown as ■. Bars show plus or minus SD. (C) Silvestrol-induced mitochondrial depolarization: CLL patient cells (n = 4) were treated as in panel A, with or without the caspase inhibitor Boc-D-fmk (100 μM). Cells were analyzed at 16 hours by flow cytometry using PI and JC-1. The percentages of cells with intact mitochondria are shown as  , and PI-negative cells are shown as ■. Bars show plus or minus SD. Boc-D-fmk efficacy was confirmed by reversal of annexin-FITC positivity in treated cells (not shown). Although Boc-D-fmk reduced mitochondrial depolarization in silvestrol-treated CLL cells, this effect did not reach statistical significance relative to the untreated sample. (D) Silvestrol causes reduction in mitochondrial Smac/DIABLO: CLL patient cells treated with 80 nM silvestrol or 200 nM flavopiridol for 16 hours were subfractionated and analyzed by immunoblot. Voltage-dependent anion channel (VDAC) was used as a control for mitochondrial fractionation.
, and PI-negative cells are shown as ■. Bars show plus or minus SD. Boc-D-fmk efficacy was confirmed by reversal of annexin-FITC positivity in treated cells (not shown). Although Boc-D-fmk reduced mitochondrial depolarization in silvestrol-treated CLL cells, this effect did not reach statistical significance relative to the untreated sample. (D) Silvestrol causes reduction in mitochondrial Smac/DIABLO: CLL patient cells treated with 80 nM silvestrol or 200 nM flavopiridol for 16 hours were subfractionated and analyzed by immunoblot. Voltage-dependent anion channel (VDAC) was used as a control for mitochondrial fractionation.
Apoptotic mechanism of silvestrol. (A) Silvestrol causes increase in proapoptotic Bcl-2 family members in CLL cells: CLL patient cells were treated with silvestrol (80 nm), cycloheximide (CHX, 100 μM), or flavopiridol (200 nM) for 16 hours. Cells were analyzed by immunoblot for Bmf, Bak, and other Bcl-2 family members. Blot is representative of 4 CLL patient samples tested. Numbers below the lanes show densitometry data for Bmf and Bak levels in treated samples relative to untreated, normalized to the actin control. (B) Silvestrol induces reactive oxygen species (ROS) generation and mitochondrial depolarization in CLL cells: CLL patient cells (n = 3) were treated with silvestrol (80 nm) or flavopiridol (200 nM) for 16 hours. Cells were analyzed by flow cytometry using dihydroethidium (DHE) to detect ROS, JC-1 to detect mitochondrial depolarization, and propidium iodide (PI) to detect cell death. Percentages of live cells (PI negative) are shown as  . Percentages of cells showing ROS generation (DHE positive) are shown as
. Percentages of cells showing ROS generation (DHE positive) are shown as  and percentages of cells with depolarized mitochondria (monomeric JC-1) are shown as ■. Bars show plus or minus SD. (C) Silvestrol-induced mitochondrial depolarization: CLL patient cells (n = 4) were treated as in panel A, with or without the caspase inhibitor Boc-D-fmk (100 μM). Cells were analyzed at 16 hours by flow cytometry using PI and JC-1. The percentages of cells with intact mitochondria are shown as
and percentages of cells with depolarized mitochondria (monomeric JC-1) are shown as ■. Bars show plus or minus SD. (C) Silvestrol-induced mitochondrial depolarization: CLL patient cells (n = 4) were treated as in panel A, with or without the caspase inhibitor Boc-D-fmk (100 μM). Cells were analyzed at 16 hours by flow cytometry using PI and JC-1. The percentages of cells with intact mitochondria are shown as  , and PI-negative cells are shown as ■. Bars show plus or minus SD. Boc-D-fmk efficacy was confirmed by reversal of annexin-FITC positivity in treated cells (not shown). Although Boc-D-fmk reduced mitochondrial depolarization in silvestrol-treated CLL cells, this effect did not reach statistical significance relative to the untreated sample. (D) Silvestrol causes reduction in mitochondrial Smac/DIABLO: CLL patient cells treated with 80 nM silvestrol or 200 nM flavopiridol for 16 hours were subfractionated and analyzed by immunoblot. Voltage-dependent anion channel (VDAC) was used as a control for mitochondrial fractionation.
, and PI-negative cells are shown as ■. Bars show plus or minus SD. Boc-D-fmk efficacy was confirmed by reversal of annexin-FITC positivity in treated cells (not shown). Although Boc-D-fmk reduced mitochondrial depolarization in silvestrol-treated CLL cells, this effect did not reach statistical significance relative to the untreated sample. (D) Silvestrol causes reduction in mitochondrial Smac/DIABLO: CLL patient cells treated with 80 nM silvestrol or 200 nM flavopiridol for 16 hours were subfractionated and analyzed by immunoblot. Voltage-dependent anion channel (VDAC) was used as a control for mitochondrial fractionation.
Because alteration of Bcl-2 family proteins is expected to impact mitochondria, we next examined mitochondrial effects of silvestrol in CLL cells at 16 hours, by which time Mcl-1 protein levels are reproducibly diminished. As shown in Figure 5B, silvestrol induces both increased ROS generation and mitochondrial membrane depolarization in CLL cells. These effects are detectable well before cell death as measured by either MTT assay or PI uptake, and gradually increase concurrent with caspase activation (as detected by PARP cleavage) and the onset of apoptosis (as detected by the binding of annexin-V; data not shown). Although the broad caspase inhibitor Boc-D-fmk appeared to reduce silvestrol-mediated mitochondrial membrane depolarization (Figure 5C), this effect overall did not reach statistical significance. We observed reduction in Smac/DIABLO in the mitochondria of silvestrol-treated CLL cells in 2 of 3 patient samples tested (Figure 5D), indicating at least in some cases that silvestrol treatment may result in permeabilization of the outer mitochondrial membrane. However, release of cytochrome c was not reliably detected at this time point (data not shown). Together, these results indicate that silvestrol promotes multiple effects on Bcl-2 family members and concomitant mitochondrial damage. Caspase activity appears to contribute to silvestrol-mediated cell death, but the inability of caspase inhibitors to effectively block this suggests that additional or alternative pathways may be involved. For each of these parameters, qualitatively similar results were observed at 24 hours, although at this point increased cell death was evident.
Kim et al15 reported that in the LNCaP human prostate carcinoma cell line, silvestrol activated the mitochondrial pathway of apoptosis but did not activate effector caspases 3 and 7. We therefore investigated the pattern of caspase activation induced by silvestrol in CLL patient cells. At 16 hours, minor activation of caspases 8, 9, 3, and 7 was detected in CLL samples by immunoblot as assessed by reduction of the full-length proenzymes and/or increased levels of the processed forms (Figure 6). In each case, these changes were accompanied by cleavage of PARP, indicating functional caspase activation. We confirmed these results using fluorometric caspase activation assays in CLL patient cells, performed before detectable cell death by PI flow cytometry or MTT assay. At 16 hours, silvestrol caused 1.5-fold, 2.7-fold, and 4.1-fold increases in activity of caspase 3, 8, and 9, respectively, relative to time-matched untreated cells (n = 4, data not shown).
Silvestrol effects on caspase activation. CLL patient cells were treated either with 80 nM silvestrol or 200 nM flavopiridol for 16 hours. Caspases were assessed by immunoblot. Controls are Jurkat cells with or without ultraviolet irradiation. Representative results from 1 of 4 patient samples are shown.
Silvestrol effects on caspase activation. CLL patient cells were treated either with 80 nM silvestrol or 200 nM flavopiridol for 16 hours. Caspases were assessed by immunoblot. Controls are Jurkat cells with or without ultraviolet irradiation. Representative results from 1 of 4 patient samples are shown.
In vivo efficacy and selectivity
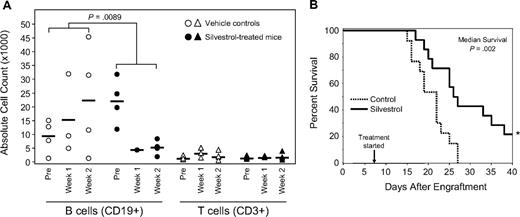
Eμ-Tcl-1 transgenic mice were selected to evaluate the in vivo efficacy of silvestrol. These animals develop a monoclonal CD5+/CD19+ B-cell lymphocytosis very similar to human IgVH-unmutated CLL in biologic and clinical features as well as response to fludarabine.29,30,36 Based on previous work12 and preliminary experiments using nontransgenic mice of the same background (C3H/B6), a silvestrol dose of 1.5 mg/kg per day for 5 days, administered intraperitoneally for 2 weeks, was chosen. Nonleukemic animals survived treatment without weight loss or other evidence of toxicity. Mice with leukemia as defined by elevated WBC counts were then treated either with silvestrol or vehicle control (n = 4 each). WBC counts and percentage of T (CD3+) and B (CD19+) cells were monitored before and after treatment by blood smear and flow cytometry, respectively (Figure 7A). The reduction of B cells in silvestrol-treated animals versus controls at week 2 was significant (P = .009). No significant changes were observed in T-cell numbers. To better understand effects on survival, we engrafted SCID mice with the 697 B-ALL cell line as reported.31,32 Animals were monitored for 1 week before beginning treatment with either vehicle (n = 13) or silvestrol (n = 14, 1.0 mg/kg intraperitoneally on Monday, Wednesday, and Friday). After 3 weeks, mice began to develop signs of disease as manifested by ruffled fur, weight loss, and hind-limb paralysis requiring killing as soon as 23 days after engraftment. In killed mice, tumors were detected in kidney, ovary, and liver as reported, although circulating tumor cells were not detected. By day 27, all control animals had died, indicating the aggressiveness of this model. Mice treated with silvestrol showed a significant increase in median survival relative to the saline controls (P = .002), with 3 of 14 silvestrol-treated animals alive without disease or weight loss more than 6 weeks after engraftment (Figure 7B). Treatment was halted and these mice were followed for an additional 6 weeks. No evidence of either disease or toxicity was noted on pathologic examination.
In vivo efficacy of silvestrol. (A) B-cell selectivity of silvestrol in vivo: Eμ-TCL1 mice with leukemia were injected intraperitoneally either with vehicle or 1.5 mg/kg silvestrol (n = 4 each) daily for 5 days for 2 weeks. Percentages of B and T cells were assessed by CD3/CD19 flow cytometry and total lymphocytes by blood smear. Each shape represents 1 animal; circles represent B cells, and triangles represent T cells. Horizontal bars are the average for the group. B-cell reduction in silvestrol-treated mice relative to that in vehicle-treated control mice at week 2 was significant (P = .009). No significant changes in T cells were detected. (B) In vivo efficacy of silvestrol in a xenograft model of ALL: SCID mice that received a transplant of 697 ALL cells were injected intraperitoneally with vehicle only (n = 13) or silvestrol (n = 14) at 1.5 mg/kg every other day, starting 1 week after engraftment. Survival difference between the treated and untreated groups was significant (P = .002). * indicates 3 silvestrol-treated mice survived to 6 weeks after engraftment without symptoms of disease or toxicity. These mice were then followed without treatment for an additional 6 weeks and killed for examination. No evidence of disease or toxicity was found.
In vivo efficacy of silvestrol. (A) B-cell selectivity of silvestrol in vivo: Eμ-TCL1 mice with leukemia were injected intraperitoneally either with vehicle or 1.5 mg/kg silvestrol (n = 4 each) daily for 5 days for 2 weeks. Percentages of B and T cells were assessed by CD3/CD19 flow cytometry and total lymphocytes by blood smear. Each shape represents 1 animal; circles represent B cells, and triangles represent T cells. Horizontal bars are the average for the group. B-cell reduction in silvestrol-treated mice relative to that in vehicle-treated control mice at week 2 was significant (P = .009). No significant changes in T cells were detected. (B) In vivo efficacy of silvestrol in a xenograft model of ALL: SCID mice that received a transplant of 697 ALL cells were injected intraperitoneally with vehicle only (n = 13) or silvestrol (n = 14) at 1.5 mg/kg every other day, starting 1 week after engraftment. Survival difference between the treated and untreated groups was significant (P = .002). * indicates 3 silvestrol-treated mice survived to 6 weeks after engraftment without symptoms of disease or toxicity. These mice were then followed without treatment for an additional 6 weeks and killed for examination. No evidence of disease or toxicity was found.
Discussion
The novel agent silvestrol is described here for the first time to possess significant preclinical activity against B-cell malignancies. In particular, we demonstrate that silvestrol has potent and selective activity against B cells relative to T cells, both tumor and normal, and maintains this B-cell selectivity in vivo in the Eμ-Tcl-1 murine CLL model. Furthermore, we show that silvestrol produces a significant survival improvement in an aggressive ALL xenograft model. Silvestrol, isolated from the plant Aglaia foveolata by activity-guided fractionation,12 is a unique member of the cyclopenta[b]benzofuran class of natural compounds and bears a bulky dioxanyl group unprecedented in nature.12 Thus, silvestrol truly represents a new chemotype. Because relatively small amounts of silvestrol have been isolated to date, knowledge of how the unique dioxanyl ring system influences biologic activity is limited. However, its presence results in substantially greater potency of silvestrol in several biologic test systems compared with cyclopenta[b]benzofuran derivatives lacking this feature. In addition, the structural uniqueness of silvestrol may indicate qualitatively different activity than related compounds. Identifying and testing structurally unusual agents is a key strategy in drug discovery to improve chances of finding agents with increased potency, target specificity, and novel mechanisms of action.
The cellular target(s) for the cyclopenta[b]benzofuran class of agents is not completely understood, and may vary by cell type. These agents have previously been hypothesized to work by inhibition of nucleotide and/or protein synthesis9 or by inhibition of NF-kB37 or NF-AT.38 Zhu et al recently described a potential mechanism of rocaglamide action involving p38 MAPK activation and ERK suppression.11 In a prostate cancer cell line, silvestrol was shown to produce a p53-independent blockade at the G2/M checkpoint39 and induce apoptosis via the mitochondrial pathway, but with an unusual lack of caspase 3 and caspase 7 activation.15 Bordeleau et al40 recently reported that silvestrol interferes with translation by affecting the interaction of eukaryotic initiation factor 4A (eIF4A) with mRNA. Here we identify a specific effect, the early and dramatic reduction of the antiapoptotic Mcl-1 protein by translational inhibition, by which silvestrol likely induces cell death in B-leukemia cells. The strong impact of silvestrol on Mcl-1 is important, as Mcl-1 is commonly up-regulated in CLL cells after B-cell receptor signaling and in the presence of stromal cells, and is associated both with resistance to therapy and the presence of other poor prognosis markers.41 Mcl-1 expression is highly regulated, and can be affected by a variety of mechanisms at the mRNA level through transcriptional control42,43 and at the protein level via stress-induced translational inhibition44 as well as degradation by caspases or the proteasome pathway.45-48 We and others reported that reduction in Mcl-1 is, by itself, sufficient to cause apoptosis in CLL cells.49 The potential impact of Mcl-1 as a therapeutic target is significant, as it is down-regulated by multiple effective antileukemic therapies including flavopiridol,34,35,50 and is elevated in CLL patients who have a poor response to rituximab.51
Like Bcl-2, Mcl-1 acts to stabilize the mitochondrial membrane by binding and sequestering proapoptotic BH3-only Bcl-2 family members such as Bax and Bak.52-54 Such interactions can be displaced by initiator BH3-only proteins including Bim and Bmf. The silvestrol-mediated rapid loss of Mcl-1 and concurrent increases in Bmf and Bak may work together to force the release of effector BH3 proteins Bax and Bak and their assembly on the outer mitochondrial membrane. This would result in membrane permeabilization, release of Smac/DIABLO, and apoptosome-mediated caspase 9 activation. In support of this, we demonstrate an early loss in mitochondrial membrane potential. Interestingly, we observe a concurrent increase in ROS generation. High levels of ROS overwhelm the antioxidant protection of cells, potently inducing apoptosis via oxidative damage to membranes, peptides, and DNA. Although increased ROS generation is not unusual during chemically induced apoptosis, its occurrence well before cell death suggests that it may play either an initiating or accelerating role in the cytotoxic process after silvestrol treatment. The mechanism of ROS increase and its role in silvestrol-mediated cell death is currently under investigation. Consistent with mitochondrial damage, we also detected reduction in mitochondrial Smac/DIABLO 16 hours after silvestrol treatment in some, but not all, CLL patient samples. Unlike flavopiridol,25 silvestrol did not appear to affect mitochondrial cytochrome c levels at this time point. The release of cytochrome c and Smac/DIABLO is believed to be via different mechanisms and with different relations to caspase activation.55 Establishing the role of each of these events in silvestrol-mediated apoptosis is important, and will require additional detailed investigation.
Multiple groups have shown that Mcl-1 expression correlates with drug resistance in leukemic cells, and we and others have reported that Mcl-1 reduction by itself is sufficient to induce death in CLL patient cells and ALL cell lines.49 We further demonstrated that Mcl-1 reduction sensitizes CLL tumor cells to the effects of rituximab.49 These observations suggest that silvestrol will have even more pronounced effects in CLL when combined with currently available therapies, particularly those rendered less effective by strong Mcl-1 expression. This idea is also supported by Bordeleau et al's report, in which silvestrol potentiated the effects of doxorubicin in PTEN-deficient cells.40 The mechanism of Mcl-1 protein reduction in silvestrol-treated CLL patient cells appears to be due to translational inhibition as proposed both for silvestrol40 and for other rocaglamides,8,9 as Mcl-1 mRNA levels are not diminished, inhibitors of caspases and the proteasome pathway fail to block Mcl-1 protein reduction, and protein synthesis inhibition is noted in vitro. Because of its dynamic regulation and short half-life, Mcl-1 is likely to be particularly sensitive to translational inhibition.
Therapeutic agents that inhibit translation include mTOR inhibitors such as rapamycin, which block translation initiation by preventing phosphorylation of 4EBP and subsequent release of cap-binding protein eIF4E to the eIF4F multisubunit translation initiation complex. Alternatively, translation inhibition is observed as a result of endoplasmic reticulum (ER) stress after changes in oxygen, glucose, or calcium homeostasis.56 ER stress results in blockage of translation initiation through PERK-mediated phosphorylation of eIF2α, again preventing assembly of the essential eIF4F complex. Our data indicate that silvestrol's mechanism of action differs from these 2 pathways in that neither eIF4B nor eIF2α is affected in phosphorylation by silvestrol at concentrations and times that cause Mcl-1 depletion. Bordeleau et al40 describe a potential mechanism by which silvestrol blocks translation, showing that silvestrol increases ATP-dependent binding of the eukaryotic initiation factor 4A (eIF4A) to capped mRNA. This in turn might prevent translation initiation by sequestering free eIF4A, which is rate-limiting in the assembly of the eIF4F complex. Although this must be validated in B cells, currently there are no agents available for CLL or ALL therapy that directly impact translation. Our data reported here suggest that translational inhibition may be an effective new therapeutic target in CLL and ALL, and possibly in other B-cell diseases as well.
We also demonstrate that the efficacy and the B-cell selectivity of silvestrol is evident in vivo using 2 different mouse B-cell tumor models. Many drugs currently used to treat CLL and ALL result in prolonged reduction of T cells, predisposing patients to life-threatening infections. For this reason, silvestrol may have a clear advantage over agents now in use for B-cell malignancies. Significant prolongation of survival in an aggressive engraftment model is also encouraging, especially as dose, formulation, and schedule of silvestrol have not yet been optimized and its pharmacokinetic properties have not yet been fully investigated. Our in vivo results are in contrast to the report by Bordeleau et al, in which silvestrol as a single agent showed no survival advantage in mice with PTEN-deficient tumors, but provided advantage only in combination with doxorubicin.40 This difference may be due to the notable B-cell selectivity we identified; other cell types we have tested, including T cells and spleen cells from nonleukemic mice, also showed reduced sensitivity. This relative lack of silvestrol cytotoxicity on non-B cells suggests an exciting potential for a positive therapeutic index and moderate side effects of this novel agent.
Based in part on the data presented here, silvestrol is now undergoing preclinical development by the NCI Developmental Therapeutics Program's Drug Development Group at the stage IIA level in anticipation of future clinical testing. The unique aspects of silvestrol described here indicate that it could have a valuable role, either alone or in combination, for the treatment of patients with B-cell malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof:
An additional paper describing total synthesis of silvestrol was recently published.57
Acknowledgments
Special thanks to Dr John C. Reed and laboratory members Dayong Zhai and Paul W. Diaz (The Burnham Institute for Medical Research) for conducting the silvestrol fluorescence polarization assays, and Dr Ruth W. Craig (Dartmouth Medical School) for providing the Mcl-1 expression vector. The authors also thank members of the Byrd and Grever laboratories for experimental assistance and helpful discussions, and the many CLL patients who donate blood for our studies.
This work was supported in part by the NCI (P01 CA081534), the CLL Global Research Foundation (Houston, TX), the American Cancer Society (Atlanta, GA; Institutional Research Grant), the Samuel Waxman Cancer Research Foundation (New York, NY), the Leukemia & Lymphoma Society (White Plains, NY), and the D. Warren Brown Foundation (Columbus, OH). Silvestrol was provided through a National Cooperative Drug Discovery Group (CA52956) and NCI P01 (CA125066; A.D.K.).
National Institutes of Health
Authorship
Contribution: D.M.L. directed the research and authored the paper; R.B.E. conducted the in vitro experiments and assisted in writing the paper; D.A.W. assisted with experiments and provided critical reading of the paper; J.D.S. performed the in vivo experiments, M.A.V., M.E.D., and D.M.R. assisted with in vitro experiments; G.L. assisted in experimental design and directed the whole-blood studies, which were performed in his laboratory; A.J.J. assisted with experimental design and critical reading of the paper; B.-N.S. purified and provided silvestrol; V.M.G. assisted with the in vivo studies; N.A.H. oversaw the interphase cytogenetic characterization of CLL samples; T.S.L. provided CLL patient samples; A.L. and X.Z., under the guidance of D.J., performed biostatistical analysis of all the experimental data presented; D.J.N. provided key advice and expertise in the preclinical evaluation strategy of silvestrol; J.C.B. assisted in experimental design and interpretation as well as critical reading of the paper, provided CLL patient samples, and is the co–principal investigator of the laboratory in which work was conducted; A.D.K. led the isolation and characterization of silvestrol, provided silvestrol for these studies, and assisted in writing the paper; and M.R.G. oversaw the research design and paper writing and is the principal investigator of the laboratory in which this work was conducted; and all authors approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David M. Lucas, OSUCCC Bldg, Rm 455, 410 West 12th Ave, Columbus, OH 43210; e-mail: david.lucas@osumc.edu.