Abstract
The diagnosis of chronic lymphocytic leukemia (CLL) in asymptomatic patients has historically been based on documenting a characteristic lymphocyte clone and the presence of lymphocytosis. There are minimal data regarding which lymphocyte parameter (absolute lymphocyte count [ALC] or B-cell count) and what threshold should be used for diagnosis. We analyzed the relationship of ALC and B-cell count with clinical outcome in 459 patients with a clonal population of CLL phenotype to determine (1) whether the CLL diagnosis should be based on ALC or B-cell count, (2) what lymphocyte threshold should be used for diagnosis, and (3) whether any lymphocyte count has independent prognostic value after accounting for biologic/molecular prognostic markers. B-cell count and ALC had similar value for predicting treatment-free survival (TFS) and overall survival as continuous variables, but as binary factors, a B-cell threshold of 11 × 109/L best predicted survival. B-cell count remained an independent predictor of TFS after controlling for ZAP-70, IGHV, CD38, or fluorescence in situ hybridization (FISH) results (all P < .001). These analyses support basing the diagnosis of CLL on B-cell count and retaining the size of the B-cell count in the diagnostic criteria. Using clinically relevant criteria to distinguish between monoclonal B-cell lymphocytosis (MBL) and CLL could minimize patient distress caused by labeling asymptomatic people at low risk for adverse clinical consequences as having CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common lymphoid malignancies1 and is defined by the presence of a clonal population of CD5+ B lymphocytes having a characteristic immunophenotype in conjunction with an elevation in the peripheral blood lymphocyte count.2-4 After multiple early efforts at classification,5-7 consensus criteria for the diagnosis of CLL were developed in 1988 as part of international efforts to standardize the diagnosis.2 Although these criteria were guided by extensive clinical experience and knowledge of the immunophenotypic characteristic of CLL B cells, no data were available to determine what lymphocyte threshold should be used as the basis for diagnosis because automated blood counters and flow cytometric immunophenotyping were not routinely available clinical tests. In the absence of definitive data, these diagnostic criteria used absolute lymphocyte counts (ALC) above 5 to 15 × 109 as the threshold for diagnosis.2,5,7-9 At the time, these criteria were adequate for management of most patients because CLL was usually diagnosed when patients developed symptoms (marrow failure, lymphadenopathy, or B-symptoms), and only a minority of patients were diagnosed based on the incidental finding of an ALC above a defined threshold.10-13
The evolution of flow cytometry in routine clinical practice in the 1990s facilitated the discrimination between clonal and reactive lymphocytosis and the differentiation of CLL from other lymphoproliferative disorders. In addition, flow cytometry allowed for the measurement of the size of the clonal lymphocyte population and detection of small clonal lymphocyte populations of CLL phenotype in asymptomatic people with normal lymphocyte counts. This technologic progress had 2 major effects on the diagnostic criteria for CLL. First, the improved ability to distinguish clonal from reactive processes and other lymphoproliferative disorders led to a lowering of the ALC required for diagnosis (≥ 5 × 109/L).2,3 Second, the sensitivity of this method led to the discovery that 3% of adults over the age of 40 have circulating clonal lymphocyte populations with CLL phenotype.14 This observation led to creation of a new diagnostic entity, monoclonal B-cell lymphocytosis (MBL),15 whose natural history continues to be defined.16
The introduction of automated blood counters and the lowering of the ALC threshold for diagnosis led to a dramatic increase in the number of patients incidentally diagnosed with CLL after discovery of an elevated ALC on a differential blood count obtained for other reasons.11,12,17 Such early stage patients now comprise 55% to 70% of CLL diagnoses.11,12,18 This information along with recognition of MBL created a need to determine which asymptomatic patients with circulating lymphocyte clones were likely to progress to a clinical disease. Formal criteria for the diagnosis of MBL were developed in 200515 to help address this need; however, the use of the B-cell count rather than ALC in these criteria created uncertainty of where the line between MBL and CLL should be drawn.19 To create consistency between the MBL and CLL diagnostic criteria, the 2008 revisions to the CLL diagnostic criteria also propose using B-cell count rather than ALC as the basis for diagnosis and adopted the B-cell threshold used in the MBL criteria to distinguish CLL (≥ 5 × 109) from MBL (< 5 × 109).4 These proposed changes from ALC to B-cell count ultimately led to a reclassification of large numbers of patients previously considered to have CLL.19
Because the diagnosis of “leukemia” may lead to profound psychological distress,20 classifying patients as having CLL rather than MBL should be based, in part, on a patient's likelihood of developing clinical symptoms, requiring chemotherapeutic treatment, and dying of the disease.19,21 As outlined, the evolution of the lymphocyte parameter (ALC vs B-cell count) and threshold used for diagnosis of CLL have been driven largely by technologic advances and availability rather than objective clinical data determining how these features relate to a patient's clinical outcome.2-4 Here, we analyze the relationship of these lymphocyte counts with clinical outcome in a cohort of patients to inform the discussion of (1) whether the CLL diagnosis should be based on ALC or B-cell count, (2) what lymphocyte threshold (if any) predicts the risk of requiring chemotherapeutic treatment and dying of the disease, and (3) whether any lymphocyte count has independent prognostic value after accounting for biologic/molecular prognostic markers.
Methods
Patients
The Mayo Clinic CLL Database includes all patients with a diagnosis of CLL seen in the Division of Hematology at Mayo Clinic Rochester (MCR) who permit their records be used for research purposes.18,22-26 Clinical information regarding date of diagnosis, physical examination, clinical stage (Rai), prognostic parameters, treatment history, and disease-related complications are abstracted from clinical records on all patients at the time of inclusion and maintained on an ongoing, prospective basis. Results of prognostic testing performed as part of clinical or research studies are also included in the database. This includes evaluation of IGHV gene mutation analysis, ZAP-70 status, CD38 status, and cytogenetics abnormalities by interphase fluorescence in situ hybridization (FISH) testing using methods previously described by our group.27-29
With the approval of the Mayo Clinic Institutional Review Board and in accord with federal regulations and informed consent obtained in accordance with the Declaration of Helsinki, we used this database to identify all Rai stage 0 patients diagnosed with CLL between January 1, 2000, and December 31, 2007, who were evaluated and had flow cytometry at Mayo Clinic within 12 months of diagnosis. All these patients had an ALC of at least 5.0 × 109/L within 6 months of flow cytometric analysis and fulfilled the 1996 criteria for CLL.3 All peripheral blood samples were evaluated by multicolor flow cytometric immunophenotyping analysis on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) according to previously described methods using fluorochrome-conjugated antibodies.30 The proportion of B cells, T cells, and natural killer (NK) cells were determined in each case, normalized to 100% lymphocytes and then an absolute count of each lymphocyte subset (B cells, T cells, NK cells) was calculated using a simultaneously run complete blood count (CBC) with differential counts. For the 35 of 459 patients (7.6%) who did not have a CBC within 1 week of flow cytometric analysis, the ALC was determined from the flow specimen.
Statistical methods
Overall survival (OS) was defined as the time between the date of diagnosis to the date of death or last follow-up. Treatment-free survival (TFS) was defined as the time between date of diagnosis and the date of initiation of first treatment or date of last follow-up at which patient was known to be untreated. The accepted indications to initiate treatment were based on the NCI-WG 1996 criteria during the study interval.3 Patients receiving early treatment as part of experimental protocols prior to meeting National Cancer Institute-Working Group (NCI-WG) 1996 criteria to initiate therapy were censored as untreated on the date experimental therapy was administered. Estimates of survival were calculated using the Kaplan-Meier method. Cox proportional hazard models were used to model the relationship of cell counts (eg, ALC, B-cell count) or other prognostic parameters with overall and TFS survival. Likelihood ratio tests were used to test effects of individual factors either individually or jointly. Determination of optimal thresholds for ALC and B-cell count relating to OS and TFS was performed using a method based on log-rank statistics described by Contal and O'Quigley.31 Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated from the Cox models. Because the HR measures magnitude of risk rather than a model's ability to accurately classify subjects, Harrell's C statistics32 were used to further evaluate the discriminatory value of the prognostic factors in terms of survival and TFS. The C statistic estimates the proportion of correct predictions of the model, (c = 1 indicates perfect discrimination between poor survivors and good survivors; c = 0.5 equivalent to chance). The standard error of the c-statistic was calculated based on 1000 bootstrap samples. P values less than .05 were considered significant.
Results
We identified 459 consecutive patients diagnosed with Rai stage 0 CLL between January 1, 2000, and December 31, 2007, who were evaluated in the MCR Division of Hematology and had flow cytometry at Mayo Clinic within 12 months of diagnosis (median time from CLL diagnosis to Mayo flow cytometry < 1 day). None of these patients had palpable lymphadenopathy or hepatosplenomegaly. The clinical characteristics of these patients are shown in Table 1. Because CD38, ZAP-70, IGHV gene mutation status, and FISH analysis were not routinely performed during the entirety of the study period, results were not available for all patients. Median follow-up for vital status and treatment status were 34 months and 17 months, respectively. As of last follow-up, 51 patients had been treated and 42 patients had died.
Patient characteristics
| Characteristic . | n = 459 . |
|---|---|
| Age at diagnosis, y | |
| Median (Q1, Q3) | 65 (58, 73) |
| < 50 | 41 (9) |
| 50-60 | 121 (26) |
| 61-70 | 141 (31) |
| > 70 | 156 (34) |
| Sex | |
| Male | 278 (61) |
| Female | 181 (39) |
| Median ALC at diagnosis, ×109/L (Q1, Q3) | 8.8 (6.5, 14.2) |
| Median B-cell count at diagnosis, ×109/L (Q1, Q3) | 5.9 (3.7, 11.2) |
| ALC at diagnosis, ×109/L | |
| < 10.0 | 272 (59) |
| 10.0-25.0 | 145 (32) |
| > 25.0-50.0 | 30 (7) |
| > 50.0 | 13 (3) |
| CD38 | |
| Positive, ≥ 30% | 83 (19) |
| Negative, < 30% | 355 (81) |
| Missing | 21 |
| ZAP-70 | |
| Positive, ≥ 20% | 63 (23) |
| Negative, < 20% | 215 (77) |
| Missing | 181 |
| IGHV gene mutation status | |
| Unmutated | 87 (31) |
| Mutated | 190 (69) |
| Missing | 182 |
| FISH result | |
| 13q− | 149 (50) |
| Normal | 81 (27) |
| +12 | 42 (14) |
| 11q− | 16 (5) |
| 17p− | 11 (4) |
| Other (6q−) | 2 (1) |
| Missing | 158 |
| Characteristic . | n = 459 . |
|---|---|
| Age at diagnosis, y | |
| Median (Q1, Q3) | 65 (58, 73) |
| < 50 | 41 (9) |
| 50-60 | 121 (26) |
| 61-70 | 141 (31) |
| > 70 | 156 (34) |
| Sex | |
| Male | 278 (61) |
| Female | 181 (39) |
| Median ALC at diagnosis, ×109/L (Q1, Q3) | 8.8 (6.5, 14.2) |
| Median B-cell count at diagnosis, ×109/L (Q1, Q3) | 5.9 (3.7, 11.2) |
| ALC at diagnosis, ×109/L | |
| < 10.0 | 272 (59) |
| 10.0-25.0 | 145 (32) |
| > 25.0-50.0 | 30 (7) |
| > 50.0 | 13 (3) |
| CD38 | |
| Positive, ≥ 30% | 83 (19) |
| Negative, < 30% | 355 (81) |
| Missing | 21 |
| ZAP-70 | |
| Positive, ≥ 20% | 63 (23) |
| Negative, < 20% | 215 (77) |
| Missing | 181 |
| IGHV gene mutation status | |
| Unmutated | 87 (31) |
| Mutated | 190 (69) |
| Missing | 182 |
| FISH result | |
| 13q− | 149 (50) |
| Normal | 81 (27) |
| +12 | 42 (14) |
| 11q− | 16 (5) |
| 17p− | 11 (4) |
| Other (6q−) | 2 (1) |
| Missing | 158 |
All values are no. (%) unless otherwise indicated.
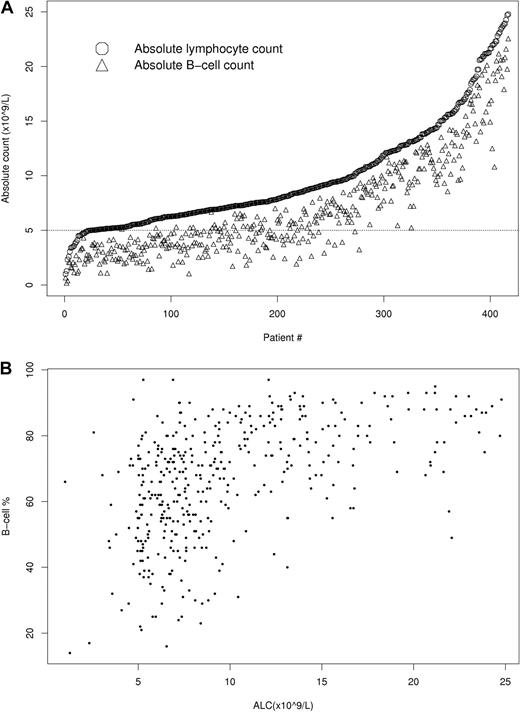
Although all patients had an ALC of at least 5 × 109/L and met the 1996 CLL diagnostic criteria,3 B-cell counts ranged from 0.2 to 378.7 × 109/L and 190 (41%) patients would be reclassified as MBL using the 2008 CLL diagnostic criteria.4 The relationship between ALC and B-lymphocyte count at diagnosis is shown in Figure 1. All patients with a B-cell count less than 5.0 × 109/L had an ALC less than 10.4 × 109/L.
Relationship of ALC and B-cell count. (A) Relationship of ALC and B-cell count in people with ALC ≤ 25 × 109/L (n = 417). (B) Percentage of ALC composed of B cells shows significant variability among people with an ALC of 25 × 109/L (n = 417) or below.
Relationship of ALC and B-cell count. (A) Relationship of ALC and B-cell count in people with ALC ≤ 25 × 109/L (n = 417). (B) Percentage of ALC composed of B cells shows significant variability among people with an ALC of 25 × 109/L (n = 417) or below.
To evaluate what proportion of B cells were monoclonal B cells, we explored the relationship between absolute B-cell count and percent monoclonal B cells in a subset of 110 patients in our cohort who were seen between January 2000 and December 2002. In all cases, more than 86% of B cells were monoclonal B cells and only 2 patients had less than 90% monoclonal B cells. In 90 of 110 cases, only rare (< 0.1 × 109/L) polyclonal B cells were observed and in the remaining 20 patients, the range was 0.1 to 0.8 × 109/L. These results indicate that polyclonal B cells represent a very small fraction of total B cells among patients with an ALC of at least 5 × 109/L and suggest the absolute CD19 positive B-cell count is a reliably measure of clonal B cells in such patients.
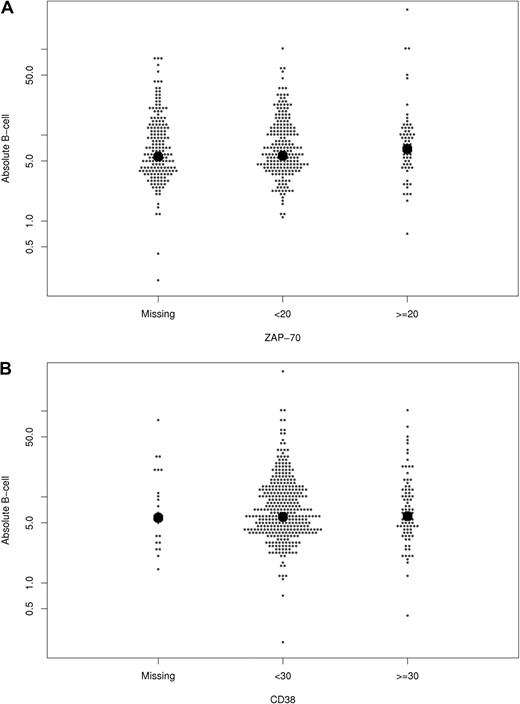
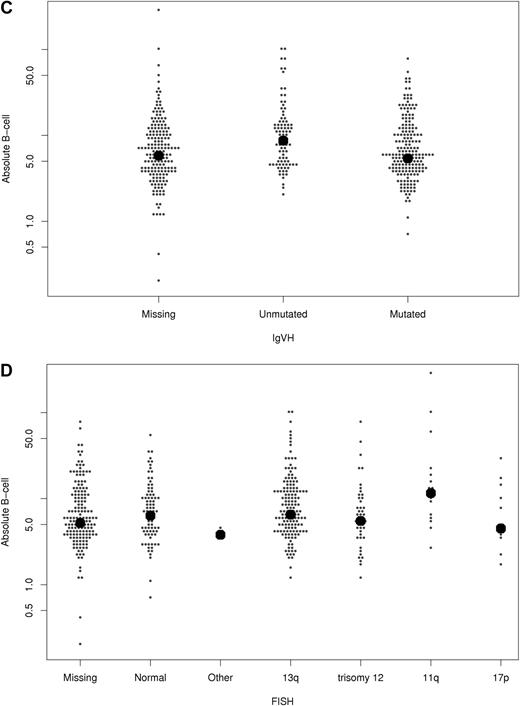
Next, the relationship between B-cell count at diagnosis and CD38, ZAP-70, IGHV gene mutation status, and FISH results were explored (Figure 2 and Table 2). The median B-cell count at diagnosis was slightly higher among patients with unmutated IGHV genes and those with 11q− on FISH. No statistically significant differences in median B-cell count were observed based on sex, CD38 expression, or ZAP-70 expression.
Relationship between absolute B-cell count at diagnosis and other prognostic parameters. The relationship of B-cell count (y-axis; ×109/L) with ZAP-70 status (A), CD38 (B), IGHV mutation status (C), and FISH category (D) is shown. Each dot represents one patient. The bold dots represent median values.
Relationship between absolute B-cell count at diagnosis and other prognostic parameters. The relationship of B-cell count (y-axis; ×109/L) with ZAP-70 status (A), CD38 (B), IGHV mutation status (C), and FISH category (D) is shown. Each dot represents one patient. The bold dots represent median values.
Median absolute B-cell count at diagnosis
| Prognostic factors/variable . | No. of patients . | Median B-cell count, ×109/L (1st, 3rd quartile) . | Difference in median B-cell count, P . |
|---|---|---|---|
| All patients | 459 | 5.9 (3.7, 11.2) | |
| Sex | .25 | ||
| Male | 278 | 5.8 (3.8, 11.8) | |
| Female | 181 | 5.9 (3.5, 10.4) | |
| CD38 | .76 | ||
| Positive, ≥ 30% | 83 | 6.0 (3.7, 11.2) | |
| Negative, < 30% | 355 | 5.9 (3.7, 11.2) | |
| ZAP-70 | .60 | ||
| Positive, ≥ 20% | 63 | 6.9 (4.2, 10.9) | |
| Negative, < 20% | 215 | 5.8 (3.8, 10.9) | |
| IGHV gene mutation status | .003 | ||
| Unmutated | 87 | 8.7 (4.4, 13.8) | |
| Mutated | 190 | 5.4 (3.7, 10.4) | |
| FISH result | .05 | ||
| 13q− | 149 | 6.5 (4.1, 11.9) | |
| Normal | 81 | 6.3 (3.7, 10.3) | |
| +12 | 42 | 5.5 (3.3, 9.7) | |
| 11q− | 16 | 11.6 (5.7, 19.2) | |
| 17p− | 11 | 4.5 (3.2, 12.7) |
| Prognostic factors/variable . | No. of patients . | Median B-cell count, ×109/L (1st, 3rd quartile) . | Difference in median B-cell count, P . |
|---|---|---|---|
| All patients | 459 | 5.9 (3.7, 11.2) | |
| Sex | .25 | ||
| Male | 278 | 5.8 (3.8, 11.8) | |
| Female | 181 | 5.9 (3.5, 10.4) | |
| CD38 | .76 | ||
| Positive, ≥ 30% | 83 | 6.0 (3.7, 11.2) | |
| Negative, < 30% | 355 | 5.9 (3.7, 11.2) | |
| ZAP-70 | .60 | ||
| Positive, ≥ 20% | 63 | 6.9 (4.2, 10.9) | |
| Negative, < 20% | 215 | 5.8 (3.8, 10.9) | |
| IGHV gene mutation status | .003 | ||
| Unmutated | 87 | 8.7 (4.4, 13.8) | |
| Mutated | 190 | 5.4 (3.7, 10.4) | |
| FISH result | .05 | ||
| 13q− | 149 | 6.5 (4.1, 11.9) | |
| Normal | 81 | 6.3 (3.7, 10.3) | |
| +12 | 42 | 5.5 (3.3, 9.7) | |
| 11q− | 16 | 11.6 (5.7, 19.2) | |
| 17p− | 11 | 4.5 (3.2, 12.7) |
The ALC is a composite value representing the sum of the absolute B-cell, T-cell, and NK-cell counts that has been used historically as a surrogate measure of clonal lymphocytes. To assess whether B-cell count or ALC more strongly related to clinical outcome, we evaluated the relationship of these variables with TFS and OS. When treated as a continuous variables (ie, measuring the risk of each 1.0 × 109/L increase in the cell count), both ALC and B-cell count were related to TFS (HR ALC = 1.02, P < .001, 95% CI = 1.01-1.03; HR B-cell count = 1.02, P < .001, 95% CI = 1.02-1.03) and OS (HR ALC = 1.02, P = .04, 95% CI = 1.00-1.04; HR B-cell count = 1.02, P = .02, 95% CI = 1.01-1.04).
We next evaluated whether measurement of B-cell counts allowed a more accurate predictor of patient outcome than ALC using the c-statistic, a measure of concordance between observed and predicted survival.32 The c-statistic evaluates a model's ability to accurately classify the outcome of a subject as opposed to the HR which measures the magnitude of risk of experiencing an outcome. A c value of 1 would indicate that a higher count was a perfect discriminator of poor survival.32 Both B-cell count (c = 0.71; 95% CI = 0.62-0.80; P < .001) and ALC (c = 0.68; 95% CI = 0.57-0.78; P < .001) predicted TFS. Both B-cell count (c = 0.59; 95% CI = 0.48-0.72; P = .02) and ALC (c = 0.58; 95% CI = 0.47-0.7; P = .04) also predicted OS. Notably, the combination of the B-cell count and non-B lymphocyte count (T-cell and NK cells) provided a better predictor of both TFS (c = 0.74; P < .001) and OS (c = 0.65; P = .01) than either B-cell count or ALC. This observation was due to the fact that the effect of the T- and NK-cell count were the opposite of the effect of B-cell count (ie, as B-cell count increased, survival decreased; as T and NK counts increased, survival increased) making the ALC summary measure less accurate than the prognostic value of its components.
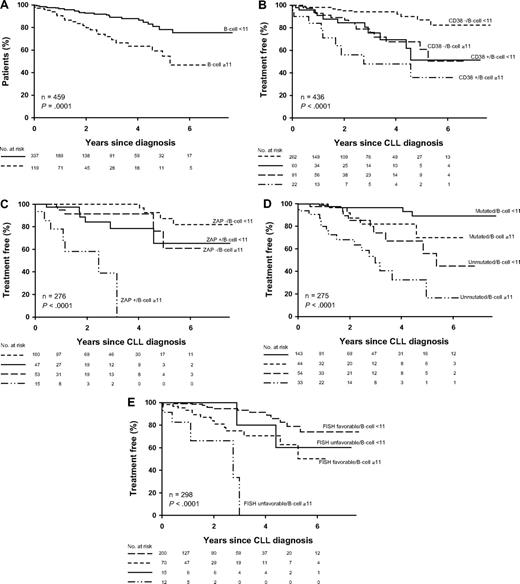
Because B-cell count and ALC were related to TFS and OS as continuous variables, we next evaluated what threshold (nearest 1.0 × 109/L) at diagnosis best related to an patient's risk of death. The B-cell threshold that best predicted OS was 11 × 109/L (HR = 2.36, P = .01; c = 0.60). This threshold also predicted TFS (HR = 3.02, P = .001; c = 0.64; Figure 3A). Although the B-cell threshold used in the current diagnostic criteria (5 × 109/L)4,15 was also able to predict TFS (HR = 3.24, P = .004; c = 0.63), it did not predict OS (HR = 1.64, P = .13; c = 0.55), and its c-statistic for TFS was slightly lower than that observed using the 11 × 109/L threshold. With respect to ALC, a threshold of 12 × 109/L was able to predict TFS (HR = 2.28, P = .003; c = 0.62) but not OS (HR = 1.62, P = .12; c = 0.56). The ALC threshold used in the previous diagnostic criteria (5 × 109/L)2 did not predict either TFS (HR = 1.72, P = .56; c = 0.51) or OS (HR = 0.87, P = .86; c = 0.49).
TFS stratified by B-cell count greater than or less than 11 × 109/L (panel A; n = 459; P = .001) and in conjunction with CD38 status (panel B; n = 436; P < .001), ZAP-70 status (panel C; n = 276; P < .001), IGHV gene mutation status (panel D; n = 275; P < .001), and by FISH risk category (E; n = 298; P < .001). For FISH risk category, those with either a 17p− or 11q− on FISH were considered to have an “unfavorable” FISH result while those with other FISH defects (eg, 13q−, trisomy 12, 6q−) or normal results on FISH were considered to have a “favorable” FISH result.
TFS stratified by B-cell count greater than or less than 11 × 109/L (panel A; n = 459; P = .001) and in conjunction with CD38 status (panel B; n = 436; P < .001), ZAP-70 status (panel C; n = 276; P < .001), IGHV gene mutation status (panel D; n = 275; P < .001), and by FISH risk category (E; n = 298; P < .001). For FISH risk category, those with either a 17p− or 11q− on FISH were considered to have an “unfavorable” FISH result while those with other FISH defects (eg, 13q−, trisomy 12, 6q−) or normal results on FISH were considered to have a “favorable” FISH result.
To compare the predictive value of these B-cell and ALC thresholds for TFS and OS with other prognostic parameters, the c-statistic and hazard ratios for TFS and OS were calculated for IGHV mutation status, ZAP-70 status, CD38 status, and FISH (Table 3). According to the c-statistic, B-cell count, IGHV, and FISH appeared to be the best predictors of OS and were of similar value with respect to predicting survival. In contrast, ZAP-70 and IGHV mutation analysis were the strongest predictors of TFS while the predictive value of the B-cell count was similar to or slightly better than FISH and CD38.
Univariate hazard ratios and C-statistic values for lymphocyte count and other prognostic variables
| . | Time to treatment . | Overall survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| n (events) . | C-statistic (SE) . | HR (95% CI) . | Difference in time to treatment, P . | n (events) . | C-statistic (SE) . | HR (95% CI) . | Difference in survival, P . | |
| B-cell count ≥ 11 vs < 11 × 109/L | 456 (51) | 0.64 (0.04) | 3.02 (1.74-5.23) | <.001 | 459 (42) | 0.60 (0.05) | 2.36 (1.28-4.36) | .01 |
| ALC ≥ 12 vs < 12 × 109/L | 456 (51) | 0.62 (0.04) | 2.28 (1.31-3.97) | .003 | 459 (42) | 0.56 (0.04) | 1.62 (0.89-2.99) | .12 |
| CD38 | 435 (48) | 0.64 (0.04) | 3.21 (1.82-5.70) | <.001 | 438 (37) | 0.56 (0.04) | 2.17 (1.10-4.25) | .03 |
| ZAP-70 | 275 (22) | 0.76 (0.05) | 5.82 (2.48-13.66) | <.001 | 278 (9) | 0.51 (0.09) | 2.77 (0.74-10.37) | .14 |
| IGHV (unmutated) | 274 (35) | 0.72 (0.04) | 6.25 (3.03-12.5) | <.001 | 277 (17) | 0.62 (0.07) | 2.86 (1.10-7.69) | .03 |
| FISH | 297 (34) | 0.58 (0.04) | 3.48 (1.51-8.01) | .01 | 299 (15) | 0.59 (0.08) | 4.47 (1.52-13.12) | .01 |
| Favorable (normal, 13q−, +12) | ||||||||
| Unfavorable (17p−, 11q−) | ||||||||
| . | Time to treatment . | Overall survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| n (events) . | C-statistic (SE) . | HR (95% CI) . | Difference in time to treatment, P . | n (events) . | C-statistic (SE) . | HR (95% CI) . | Difference in survival, P . | |
| B-cell count ≥ 11 vs < 11 × 109/L | 456 (51) | 0.64 (0.04) | 3.02 (1.74-5.23) | <.001 | 459 (42) | 0.60 (0.05) | 2.36 (1.28-4.36) | .01 |
| ALC ≥ 12 vs < 12 × 109/L | 456 (51) | 0.62 (0.04) | 2.28 (1.31-3.97) | .003 | 459 (42) | 0.56 (0.04) | 1.62 (0.89-2.99) | .12 |
| CD38 | 435 (48) | 0.64 (0.04) | 3.21 (1.82-5.70) | <.001 | 438 (37) | 0.56 (0.04) | 2.17 (1.10-4.25) | .03 |
| ZAP-70 | 275 (22) | 0.76 (0.05) | 5.82 (2.48-13.66) | <.001 | 278 (9) | 0.51 (0.09) | 2.77 (0.74-10.37) | .14 |
| IGHV (unmutated) | 274 (35) | 0.72 (0.04) | 6.25 (3.03-12.5) | <.001 | 277 (17) | 0.62 (0.07) | 2.86 (1.10-7.69) | .03 |
| FISH | 297 (34) | 0.58 (0.04) | 3.48 (1.51-8.01) | .01 | 299 (15) | 0.59 (0.08) | 4.47 (1.52-13.12) | .01 |
| Favorable (normal, 13q−, +12) | ||||||||
| Unfavorable (17p−, 11q−) | ||||||||
Finally, we evaluated the ability of the B-cell count to predict TFS and OS independent of IGHV mutation status, ZAP-70 status, CD38 status, and FISH. Because not all patients had all prognostic tests performed, the predictive value of the B-cell count (< or ≥ 11 × 109) independent of the other prognostic variable was assessed separately for each variable. With respect to TFS, B-cell count retained prognostic value independent of IGHV mutation status, ZAP-70 status, CD38 status, and FISH (Figure 3B-E). With respect to OS, B-cell count retained prognostic value independent of CD38 status and FISH; however, after controlling for IGHV mutation status or ZAP-70 status, both B-cell count and the second variable (ZAP-70; IGVH) were no longer statistically significant (Table 4). Based on the C-statistic, the combination of B-cell count and ZAP-70 was the best predictor of TFS, while the combination of B-cell count and FISH was the best predictor of OS.
Adjusted hazard ratios for lymphocyte count and other prognostic variables
| . | Treatment-free survival . | Overall survival . | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | C-statistic . | HR (95% CI) . | P . | C-statistic . | |
| 0.72 | 0.65 | |||||
| CD38+ | 3.20 (1.80-5.66) | .001 | 2.14 (1.09-4.20) | .04 | ||
| B cell ≥ 11 | 3.10 (1.76-5.47) | .001 | 2.63 (1.37-5.03) | .001 | ||
| 0.85 | 0.67 | |||||
| ZAP+ | 8.33 (3.37-20.61) | <.001 | 2.88 (0.77-10.79) | .13 | ||
| B cell ≥11 | 6.86 (2.77-17.01) | <.001 | 3.41 (0.85-13.71) | .09 | ||
| 0.78 | 0.63 | |||||
| Unmutated | 5.26 (2.56-11.11) | <.001 | 2.38 (0.88-6.25) | .08 | ||
| B cell ≥ 11 | 3.06 (1.54-6.07) | .001 | 2.54 (0.94-6.81) | .07 | ||
| 0.73 | 0.74 | |||||
| FISH (17p− or 11q−) | 3.97 (2.00-7.88) | .01 | 3.63 (1.22-10.76) | .03 | ||
| B cell ≥ 11 | 3.67 (1.58-8.50) | <.001 | 3.61 (1.25-10.48) | .02 | ||
| . | Treatment-free survival . | Overall survival . | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | C-statistic . | HR (95% CI) . | P . | C-statistic . | |
| 0.72 | 0.65 | |||||
| CD38+ | 3.20 (1.80-5.66) | .001 | 2.14 (1.09-4.20) | .04 | ||
| B cell ≥ 11 | 3.10 (1.76-5.47) | .001 | 2.63 (1.37-5.03) | .001 | ||
| 0.85 | 0.67 | |||||
| ZAP+ | 8.33 (3.37-20.61) | <.001 | 2.88 (0.77-10.79) | .13 | ||
| B cell ≥11 | 6.86 (2.77-17.01) | <.001 | 3.41 (0.85-13.71) | .09 | ||
| 0.78 | 0.63 | |||||
| Unmutated | 5.26 (2.56-11.11) | <.001 | 2.38 (0.88-6.25) | .08 | ||
| B cell ≥ 11 | 3.06 (1.54-6.07) | .001 | 2.54 (0.94-6.81) | .07 | ||
| 0.73 | 0.74 | |||||
| FISH (17p− or 11q−) | 3.97 (2.00-7.88) | .01 | 3.63 (1.22-10.76) | .03 | ||
| B cell ≥ 11 | 3.67 (1.58-8.50) | <.001 | 3.61 (1.25-10.48) | .02 | ||
Top row in each pair indicates the HR of the listed prognostic factor after controlling for B-cell count. The bottom row in each pair indicates the HR for B-cell count after controlling for the prognostic variable listed in row above.
Discussion
The diagnosis of CLL among asymptomatic people has historically been based on the presence of a characteristic lymphocyte clone and the ALC. While establishing the presence of a clonal population in the era of flow cytometry is easily accomplished, there are no clear data regarding what lymphocyte threshold should be used to establish the diagnosis of CLL or whether the criteria should be based on ALC or B-cell count. Our findings suggest that although B-cell count and ALC have similar ability to predict TFS and OS as continuous variables, B-cell count appears to be a better predictor of TFS and OS when a defined lymphocyte threshold is used. Indeed a higher non–B-cell component in the ALC (T and NK cells) is associated with a longer TFS and OS, which could explain why the B-cell count appears to be a better measure of clinical outcome.26 These findings provide supportive evidence to justify the recent proposal to change to a B-cell count based diagnostic system.4
As emphasized by the current MBL criteria,15 having a laboratory abnormality (such as elevated ALC or B-cell count) does not define a disease. Indeed, even the presence of a clonal cell population is not equivalent to the diagnosis of malignancy as recognized in the classification of other premalignant conditions such as monoclonal gammopathy of undetermined significance (MGUS). As this example illustrates, the distinction between a malignant and premalignant condition is best made based on disease-related symptoms or a person's risk of adverse clinical outcome. In this regard, the size of the B-cell count among patients with a clonal population of cells of CLL phenotype in the peripheral blood does appear to relate to a patient's risk of requiring therapy and death. In the current cohort, a B-cell count of 11 × 109 was the threshold that best stratified patient's risk of death and also predicted TFS. Accordingly, using this threshold to distinguish between MBL and CLL would tie the classification to clinically meaningful patient outcomes.
Several studies suggest that the molecular/biologic characteristics (eg, ZAP-70 expression, IGHV mutation status) of the leukemic clone are powerful predictors of an individual patient's clinical outcome29,33-38 even among subjects with very small clonal populations.19,39 Once the presence of a clonal population is established, this observation raises the question of whether biologic features of the clonal cells rather than lymphocyte count may be the best way to determine a patient's risk of developing clinical symptoms, requiring treatment, and dying of disease. If this hypothesis is true, such features could be incorporated into the diagnostic criteria used to distinguish CLL from MBL. In this respect, our analysis demonstrates that the hazard ratios for TFS and death using a B-cell count of at least 11 × 109 are similar to those for other established molecular/biologic prognostic factors. Furthermore, the B-cell count remained an independent predictor of TFS after controlling for ZAP-70, IGHV, CD38, or FISH test results where the combination of B-cell count with ZAP-70 expression status appeared to be the best predictor of TFS (c = 0.85). These findings indicate that the B-cell count remains a predictor of patient outcome independent of the currently known molecular/biologic prognostic factors and provides evidence for retaining the B-cell count in the diagnostic criteria.
In a recent study,16 Rawstron and colleagues reported the clinical outcome of a cohort of 185 patients with a clonal population of CLL phenotype and a B-cell count of less than < 5 × 109/L. The purpose of this study was to describe the outcome of patients meeting the current definition of MBL. B-cell count (< or ≥ 1.9 × 109/L) at the time of MBL diagnosis was the only factor independently associated with progressive lymphocytosis (P = .01) although no association was observed with either OS or TFS.16 Our findings are consistent with and complementary to this report. Rather than describe the outcome of subjects with B-cell counts below the traditional but arbitrarily derived 5 × 109/L threshold, our study included all patients with isolated lymphocytosis (no evidence of lymphadenopathy, organomegaly, or cytopenias) and a circulating clone of CLL phenotype to determine what lymphocyte threshold best predicts clinic outcome (TFS and survival). The inability of a B-cell threshold below 5 × 109/L to predict either TFS or OS16 in Rawstron et al's analysis is consistent with our results that suggest that higher B-cell thresholds best relate to clinical outcome.
Switching from an ALC to a B-cell count based diagnostic criteria4,15 does create a number of challenges.40 First, the optimal way to measure B-cell count for such analysis has not been defined.40 Second, such a shift could necessitate repeated flow cytometric analysis (and attendant cost) to monitor the B-cell count among people with a documented clonal lymphocyte population but whose initial count is below the diagnostic threshold. The relationship between B-cell count and ALC illustrated in Figure 1 suggests that a practical and fiscally responsible approach would be to simply monitor the ALC among people with a B-cell count below the diagnostic threshold and to reclassify them as having CLL when the ALC indicates their B-cell count has likely crossed the threshold. For example, if the threshold to classify CLL were considered a B-cell count of at least 11.0 × 109/L, it could be inferred that nearly all patients with a documented clone who later develop an ALC consistently above 20 × 109/L will have a B-cell count of at least 11.0 × 109/L and fulfill the diagnostic criteria (Figure 1A). Similarly, if the threshold to diagnose CLL remains a B-cell count of 5.0 × 109/L or less, it could be inferred that nearly all patients with a documented clone who develop an ALC consistently above 11 × 109/L will fulfill the diagnostic criteria. A complementary alternative approach would be to simply monitor the ALC and only repeat flow cytometry to quantify the B-cell count when the ALC implies that the B-cell count is likely to be above the diagnostic threshold.
Our study is subject to a number of limitations. First, not all patients had molecular/biologic prognostic parameters assessed and hence we were unable to perform a comprehensive multivariate analysis that included all factors. Second, by design, the study was limited to patients without lymphadenopathy, organomegaly, and cytopenia and hence does not pertain to the clinical outcome among patients with more-advanced-stage disease. Finally, although the analysis convincingly demonstrates a relationship between the B-cell count and clinical outcome, the optimal B-cell threshold to predict TFS and OS needs to be validated in independent series of patients monitored prospectively.
The criteria to diagnose CLL have undergone substantial revisions over the past 30 years. The recent recognition that 3% of adults over the age of 40 have a circulating clonal population of lymphocytes with the CLL phenotype heightens the importance of developing evidence-based and clinically relevant criteria to differentiate between MBL and CLL. Our findings lend evidence to support the recent proposal by Hallek and colleagues4 to base the diagnosis of CLL on B-cell count rather than ALC. Our results also provide justification to retaining the B-cell count as part of the diagnostic criteria in the era of molecular/biologic prognostic markers but imply that a threshold of 11 × 109/L is the B-cell count that best predicts patients' TFS and risk of death. Continued efforts to differentiate between CLL and MBL based on a patient's likelihood of developing clinical symptoms, requiring chemotherapeutic treatment, and dying of the disease are necessary to minimize unnecessary psychological distress caused by labeling asymptomatic people with a laboratory abnormality and low risk for adverse clinical consequences as having leukemia.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge support through grants from the NCI (Rockville, MD; CA 113408 to T.D.S.) and Gabrielle's Angel Foundation for Cancer Research (New York, NY; T.D.S.).
National Institutes of Health
Authorship
Contribution: T.D.S., N.E.K., T.G.C., C.S.Z., and C.A.H. were responsible for the concept of the paper; T.D.S., N.E.K., G.J., T.G.C., C.S.Z., D.J., W.G.M., and C.A.H. designed the research; T.D.S., D.J., J.B., L.Z., S.L.S., and C.A.H. performed the research; G.J., S.S., T.D.S., W.G.M., C.H. analyzed and interpreted data; and T.D.S., N.E.K., G.J., T.G.C., C.S.Z., D.J., W.G.M., S.L.S., and C.H. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tait D. Shanafelt, MD, 200 First St SW, Rochester, MN 55905; e-mail: shanafelt.tait@mayo.edu.