Abstract
Mesenchymal stem cells (MSCs), which potentially transdifferentiate into multiple cell types, are increasingly reported to be beneficial in models of organ system injury. However, the molecular mechanisms underlying interactions between MSCs and host cells, in particular endothelial cells (ECs), remain unclear. We show here in a matrigel angiogenesis assay that MSCs are capable of inhibiting capillary growth. After addition of MSCs to EC-derived capillaries in matrigel at EC:MSC ratio of 1:1, MSCs migrated toward the capillaries, intercalated between ECs, established Cx43-based intercellular gap junctional communication (GJC) with ECs, and increased production of reactive oxygen species (ROS). These events led to EC apoptosis and capillary degeneration. In an in vivo tumor model, direct MSC inoculation into subcutaneous melanomas induced apoptosis and abrogated tumor growth. Thus, our findings show for the first time that at high numbers, MSCs are potentially cytotoxic and that when injected locally in tumor tissue they might be effective antiangiogenesis agents suitable for cancer therapy.
Introduction
Intense interest in the therapeutic application of bone marrow–derived mesenchymal stem cells (MSCs) arises from the possibility that MSCs promote vascular repair. In animal models, intravenous injections of MSCs protected against heart failure by enhancing cardiac myocyte survival1 and blocked lipopolysaccharide-induced acute lung injury by reducing total cell and proinflammatory cytokines in the lung.2 In a collagen gel model, MSCs promoted survival of capillaries grown from human umbilical vein endothelial cells (HUVECs).3 Despite these findings, the lack of conclusive evidence supporting a beneficial effect of MSCs in the clinical setting4 indicates that mechanisms underlying MSC–endothelial cell (EC) interactions require better understanding.
Several reports indicate that these interactions result from direct contact between MSCs and host cells. The MSC-induced responses include induction of gene transcription in ECs,3 mitochondrial transfer in A549 cells,5 and interleukin-10 (IL-10) secretion in alveolar macrophages.6 In the context of tumor growth, MSCs recruit ECs to induce angiogenesis in stable tissue7 as well as in tumors,8 raising the possibility that MSCs might promote tumor growth. By contrast, intravenously injected MSCs are capable of abrogating growth of the Kaposi sarcoma,9 suggesting that MSCs potentially possess cytotoxic properties. However, the mechanisms by which MSCs engage ECs are not understood and might involve gap junctional communication (GJC), as proposed for MSC-cardiomycyte interactions.10
Here, we addressed MSC-EC interactions in a capillary culture with the expectation that MSCs would enhance angiogenesis. However, surprisingly, addition of MSCs caused dose-dependent EC cytotoxicity that was attributable to the formation of MSC-EC GJC and the production of MSC-derived reactive oxygen species (ROS). The combined effect of these responses was capillary destruction. Further, in an in vivo melanoma model, MSCs inhibited tumor growth by abrogating growth of the tumor vasculature. These results indicate a novel property of MSCs, namely as cytotoxic agents that inhibit the formation of neocapillary networks.
Methods
Reagents
Cell culture.
M199 medium, F-12 nutrient mixture medium, lipofectin, Opti-MEM, and antibiotics (50 U/mL penicillin G and 50 μg/mL streptomycin) were obtained from Invitrogen (Carlsbad, CA). G418 were obtained from Calbiochem (San Diego, CA). Dulbecco modified Eagle medium (DMEM) was obtained from Mediatech (Herndon, VA). Fetal bovine serum (FBS) and bovine calf serum were obtained from Hyclone (Logan, UT).
Fluorophores.
Mito Tracker Deep Red (MTDR) (500 nM), calcein red-orange AM (13 μM), Hoechst 33342 (5 μg/mL), and dichlorodihydrofluorescein diacetate (DCFH-DA) (10 μM) were obtained from Molecular Probes (Eugene, OR).
Inhibitors.
Glycerrhetinic acid (GA) (5 μM), N-acetyl-L-cysteine (NAC) (10 mM), and polyethylene glycol polyethylene glycol-conjugated (PEG)–catalase (100 U/mL) were obtained from Sigma-Aldrich (St Louis, MO). Cx 43 peptides gap 26 (VCYDKSFPISHVR; 160 μM), and gap 27 (SRPTEKTIFII; 190 μM) were purchased from Alpha Diagnostic International (San Antonio, TX).
Antibodies.
Antibodies against CD11b/c (integrin αM and αX chains; macrophage common antigen), CD29 (integrin β1 chain), CD45 (leukocyte common antigen), CD54 (intracellular cell adhesion molecule-1; intercellular adhesion molecule 1 [ICAM-1]), CD59 (membrane attack complex-inhibitory protein), and CD90 (Thy-1) were obtained from BD Biosciences (San Jose, CA). Antibodies against CD31 (platelet/endothelial cell adhesion molecule 1; PECAM1), CD34 (hematopoietic stem cells antigen), CD44 (homing cell adhesion molecule; H-CAM), p-Tyr, and vascular endothelial (VE)–cadherin polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–Tie-2 monoclonal antibody was purchased from Santa Cruz. Anti-connexin 43 monoclonal antibody was obtained from Invitrogen. Cleaved caspase-3 monoclonal antibody was obtained from Cell Signaling Technology (Danvers, MA). Alexa Fluor 488–conjugated donkey anti–goat Ab and Alexa Fluor 546–conjugated goat anti–mouse Ab were purchased from Invitrogen.
MSC harvesting and culture
Sprague-Dawley rats (Taconic, Hudson, NY) and C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were MSC donors. Animal procedures were approved by the Institutional Animal Care and Use Committee of St Luke's-Roosevelt Hospital Center (New York, NY). Under sterile conditions, animals were anesthetized (pentobarbital 35 mg/kg intraperitoneally), the femur and tibia excised, the epiphyses removed, and, through needles inserted in the bone lumen, bone marrow was flushed out in a solution consisting of DMEM containing 10% FBS and 1% of an antibiotic mixture. The marrow was plated in tissue culture flasks, and nonadherent hematopoietic cell populations were removed at day 3, followed by new media replenishment every 2 days. Adherent MSCs were harvested and passed at low density (100-200 cells/cm2) and maintained in a humidified incubator (37°C; 5% CO2) under subconfluent conditions to prevent cell differentiation. For flow cytometry (FACscan; BD Biosciences), trypsinized MSCs were incubated with fluorescein isothiocyanate (FITC)–labeled monoclonal antibodies (30 minutes, 4°C).
Cell culture
Rat lung microvascular endothelial cells (RLMECs) were cultured and characterized as we previously described.11,12 Rat neonatal lung fibroblasts (RNLFBs) were provided by Dr Kevin Costa (Columbia University, New York, NY). Mouse lung fibroblasts (MLFBs) were harvested from C57BL/6J mouse lung.13 Chinese hamster ovary (CHO) cells and mouse B16F10 melanoma cells were obtained from ATCC (Manassas, VA). Media were DMEM for fibroblasts and melanoma cells and F-12 for CHO cells. Culture conditions were in 5% CO2 at 37°C.
RLMECs were transfected with one of the following: pEGFP-C1 vector (Clontech, Palo Alto, CA),14 mutant Cx43 (Cx43 T154A) obtained as a gift from Dr G. Sosinsky (University of California, San Diego), and empty vector. To make the empty vector, the Cx43T154A region was removed by digestion of pcDNA3.1 GFP-Cx43mt at the HindIII restriction site. The observed band representing the Cx43T154A region was visualized on 1% agarose gel (data not shown). The vectors were transfected using lipofectin (Invitrogen) following the manufacturer's instructions. Green fluorescence protein (GFP)–positive cells were sorted (FACSAria Cell Sorter; BD Biosciences) and maintained with G418 (500 μg/mL).
Matrigel assays
Capillary growth.
Aliquots (300 μL) of growth factor–containing matrigel matrix (phenol red free, BD Biosciences) were plated into individual wells of 24-well tissue culture plates (BD Biosciences) and allowed to polymerize at 37°C for at least 30 minutes. GFP-expressing rat lung microvascular endothelial cells RLMECs (excitation 488 nm) were seeded (1.25 × 105 cells/cm2) with RLMEC culture media on the polymerized matrigel. After 1 day, once capillary networks had formed, MTDR-stained MSCs (excitation, 644 nm), CHO cells, or RNLFBs were seeded with RLMECs culture media on the capillaries. Inhibitors (GA, gap peptides, NAC, and PEG-catalase) were added simultaneously with MSCs, CHO cells, or RNLFBs. The same view fields were observed periodically over time under an inverted fluorescence microscope (IX 81; Olympus America, Melville, NY) or a laser scanning microscope (LSM 510 Meta; Zeiss, Thornwood, NY). For quantification of capillary number, the total circumscribed area surrounded by GFP-EC capillaries from 5 randomly chosen areas per well were counted and analyzed using Metamorph software (Universal Imaging, Downingtown, PA).
Insert experiments.
To prevent physical contact between RLMECs and MSCs, RLMECs were cultured in matrigel at the bottom of a well, while MSCs were cultured in the same well inside a cell culture insert (3.0 μm, BD Biosciences). We visually confirmed the absence of physical contact between the insert and the matrigel. At the end of the experiment, we removed the insert to confirm that no fluorescence from the MTDR-loaded MSCs had leached into the underlying matrigel.
Time-lapse microscopy.
To detect MSC migration toward EC-derived capillaries, we microscopically imaged matrigel cultures in a temperature-controlled culture dish (Delta T4; Bioptechs, Butler, PA) held at 37°C. Humidified air containing 5% CO2 flowed under the lid of culture dish during image acquisition at 1 image per hour for 24 hours.
Apoptosis assay.
Three days after seeding MSCs or RNLFBs on day-old capillaries, TdT-mediated dUTP nick end labeling (TUNEL) staining and cleaved caspase-3 expression assays were performed. For TUNEL staining, we used a commercially available kit (In situ Cell Death Detection; Roche, Mannheim, Germany). For cleaved caspase-3 expression assay, we recovered the capillaries from matrigel (Cell Recovery Solution; BD Biosciences) and carried out immunoblotting with mAb against cleaved caspase-3.
Fluorescence recovery after photobleaching
Quantification of GJC in matrigel capillaries was carried out by fluorescence recovery after photobleaching (FRAP) in targeted MSCs viewed by confocal microscopy (Zeiss LSM 510 Meta, 40× water objective). Capillary cultures were derived from GFP-expressing ECs. MSCs loaded with MTDRs were added to the capillary network. For FRAP, capillaries with embedded MSCs were loaded with calcein red-orange acetoxymethyl, then the capillaries were washed 3 times with buffer. We confirmed the absence of fluorescence interference among the 3 fluorophores. A targeted MSC was subjected to high power laser excitation to photobleach calcein fluorescence throughout the cell as confirmed by Z stack images. Subsequent fluorescence recovery was imaged at 1 image per minute for 20 minutes. Images were analyzed by Metamorph software.
Immunocytochemistry
We followed our previously described methods.14 Briefly, MSCs or RLMEC monolayers grown on glass coverslips were fixed (3.7% formaldehyde in phosphate-buffered saline [PBS]), pH 7.4, 20 minutes, 22°C), rinsed (3× PBS), and permeabilized (0.1% Triton X-100). After adding blocking serum (5% normal horse serum in PBS, 1 hour at 22°C), cells were incubated with diluted primary antibodies (1:50; 1 hour at 22°C), and washed (3 × PBS). Fluorescence-conjugated secondary antibodies were then added (1:200, 1 hour at 22°C) and washed 3× with PBS. The glass coverslips were mounted upside down on object slides using fluorescent-mounting medium (Dako, Carpinteria, CA). Images were taken by fluorescent microscopy.
Reactive oxygen species quantification
Reactive oxygen species (ROS) were detected by the DCF method.15 To matrigel capillaries we added DCFH-DA, which de-esterifies intracellularly to DCFH, the substrate that ROS oxidize to fluorescent DCF. Matrigel capillaries were incubated with DCFH-DA in PBS at a final concentration of 10 μM for 1 hour and washed 3× with PBS. Background subtracted capillary fluorescence was acquired and analyzed using Metamorph software.
Mouse tumor growth
Mouse melanoma cells were mixed in matrigel (500 μL) containing heparin (60 U/mL). The mixture was subcutaneously injected in the lower right flank of age-matched male C57BL/6J mice (2-3 months old). Tumor size was quantified by calipers and volume calculated in cubic millimeters as reported.16 Tumors were recovered, photographed, and weighed. For histopathologic analyses, tumor tissues were fixed with 4% paraformaldehyde and hematoxylin and eosin staining was performed (AML Laboratories, Rosedale, MD). Blood vessels were identified by the presence of lumens containing red cells. In minced tumor tissue, we assayed hemoglobin by the Drabkin method (Sigma-Aldrich), and we detected endothelial markers and cleaved caspase-3 by immunoblotting and immunoprecipitation.
Immunoblotting and immunoprecipitation
Tumor tissue was homogenized (Tissue Tearor; Biospec Products, Bartlesville, OK) and lysed with radioimmunoprecipitation assay (RIPA) buffer (1 mM sodium orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride [PMSF]). Cultured cells were lysed with RIPA buffer at 0°C. After clearing (16 000g, 20 minutes), lysate protein concentration was determined (protein assay kit; Pierce, Rockford, IL). For immunoprecipitation, lysates containing 2 mg total protein were precleared with appropriate control IgG for 30 minutes at 4°C with 20 μL of prewashed protein A/G and agarose reagent (Santa Cruz Biotechnology) followed by incubation with primary antibodies (4 μg, overnight). Antibody-antigen complexes were precipitated with 50 μL of prewashed protein A/G and agarose reagent for 3 hours at 4°C. Unbound proteins were washed 3 times with RIPA buffer and once with PBS. Bound proteins were eluted by boiling in 50 μL of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for 5 minutes. The proteins from immunoprecipitation or for direct immunoblot were separated in SDS-polyacrylamide gel electrophoresis, transferred overnight at 4°C onto nitrocellulose membrane, and visualized by immunoblotting against appropriate antibodies.
Statistical analyses
All data are reported as mean plus or minus SD. Statistical significance was assessed using 2-tailed Student t test for 2 groups and by the analysis of variance (ANOVA)-Tukey test for more than 2 groups. Significance was accepted at P values less than .05.
Results
MSCs intercalate in neocapillary networks
Analyses by fluorescence-activated cell sorting (FACS) and optical immunofluorescence indicated that MSCs were positive for CD29, CD44, CD54, CD59, and CD90, but negative for macrophage (CD11b/c) and leukocyte (CD45) markers (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), as well as for the hematopoietic stem cell marker (CD34), Tie-2, and VE-cadherin (data not shown). These findings are consistent with previous characterizations of MSCs.17-19
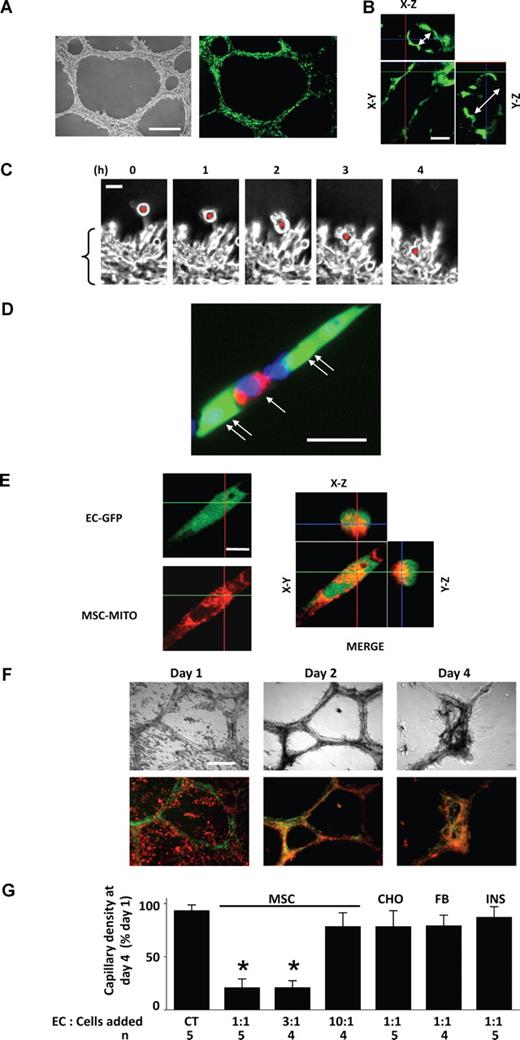
Within 1 day of seeding in matrigel, RLMECs formed stable neocapillary networks with well formed lumens (Figures 1A,B). Upon addition to day-old capillaries, MSCs immediately migrated (24 ± 6 μm/hour) toward the capillaries (Figure 1C) and subsequently, within 4 hours they intercalated among capillary ECs (Figure 1C,D). Consistent with previous reports,5,20 confocal microscopy of the matrigel cultures revealed mitochondrial transfer from MSCs to ECs (Figure 1E).
Incorporation of mitochondria-stained MSCs in GFP-expressing EC capillaries in matrigel. (A) Phase contrast (left) and fluorescence (right) images show day-old, GFP-expressing, RLMEC-derived capillaries. Bar = 500 μm. (B) Confocal microscopy of a capillary shows the lumen (double-headed arrows) in z-axis images. Bar = 50 μm. (C) Phase contrast images taken at 1-hour intervals show migration of a single MSC (red dot) toward capillaries (bracket). Bar = 20 μm. (D) High magnification image shows Mito Tracker Deep Red (MTDR)–loaded MSC (single arrow) intercalated between ECs (double arrows) in a single, day-old capillary. The nucleus is Hoechst 33342-stained (blue). Bar = 25 μm. (E) Confocal microscopy of a single EC from a capillary network shows GFP fluorescence (EC-GFP) and MTDR fluorescence of MSC mitochondria (MSC-MITO). The merged image shows in different planes the incorporation of MSC mitochondria (red) in EC (green). Images were taken 1 day after adding MTDR-stained MSC to day-old capillaries. Bar = 10 μm. All imaging data replicated at least 6 times. (F) Images are by phase contrast (top) and fluorescence (bottom) microscopy on days indicated after addition of mitochondria-stained MSC (red) to day-old capillaries (green) at EC:MSC ratio of 1:1. Bar = 500 μm. (G) Group data show responses to addition of the indicated ratio of ECs to MSC, CHO cells, or RNLFB (FB) to day-old capillaries. Capillary density in capillaries to which no cells were added (CT) was 7 (± 2) capillaries per low-power (4×) field. In one group (INS), MSCs were grown in an insert, which was placed adjacent to the matrigel culture. Data are mean plus or minus SD. *P < .05, significantly different from CT.
Incorporation of mitochondria-stained MSCs in GFP-expressing EC capillaries in matrigel. (A) Phase contrast (left) and fluorescence (right) images show day-old, GFP-expressing, RLMEC-derived capillaries. Bar = 500 μm. (B) Confocal microscopy of a capillary shows the lumen (double-headed arrows) in z-axis images. Bar = 50 μm. (C) Phase contrast images taken at 1-hour intervals show migration of a single MSC (red dot) toward capillaries (bracket). Bar = 20 μm. (D) High magnification image shows Mito Tracker Deep Red (MTDR)–loaded MSC (single arrow) intercalated between ECs (double arrows) in a single, day-old capillary. The nucleus is Hoechst 33342-stained (blue). Bar = 25 μm. (E) Confocal microscopy of a single EC from a capillary network shows GFP fluorescence (EC-GFP) and MTDR fluorescence of MSC mitochondria (MSC-MITO). The merged image shows in different planes the incorporation of MSC mitochondria (red) in EC (green). Images were taken 1 day after adding MTDR-stained MSC to day-old capillaries. Bar = 10 μm. All imaging data replicated at least 6 times. (F) Images are by phase contrast (top) and fluorescence (bottom) microscopy on days indicated after addition of mitochondria-stained MSC (red) to day-old capillaries (green) at EC:MSC ratio of 1:1. Bar = 500 μm. (G) Group data show responses to addition of the indicated ratio of ECs to MSC, CHO cells, or RNLFB (FB) to day-old capillaries. Capillary density in capillaries to which no cells were added (CT) was 7 (± 2) capillaries per low-power (4×) field. In one group (INS), MSCs were grown in an insert, which was placed adjacent to the matrigel culture. Data are mean plus or minus SD. *P < .05, significantly different from CT.
MSCs induce apoptosis of neocapillary networks
In the report by Spees et al,5 mitochondrial transfer from MSCs rescued aerobic respiration in mammalian cells with nonfunctional mitochondria. However, here, addition of MSCs to day-old EC-derived capillaries at EC:MSC ratio of 1:1 or 3:1 induced capillary degeneration. On day 1, MSC fluorescence was distinct from the capillary fluorescence (Figure 1F), indicating that MSCs lay separate from the capillaries. However, by day 2, discreet MSCs were no longer visible and the fluorescence staining was diffuse (Figure 1F), indicating that the MSCs had merged in the capillary network. By day 4, complete capillary collapse was evident (Figures 1F,G). In contrast, nontreated capillaries (Figure S2) or day-old capillaries treated with MSCs at EC:MSC ratio of 10:1 (Figure 1G) remained intact for more than 7 days. Addition of CHO cells or RNLFBs to ECs at 1:1 ratio did not cause capillary degeneration (Figure 1G), indicating that the effect was MSC specific. For MSCs grown on inserts that were placed approximately 1 mM above the matrigel well, no capillary degeneration was evident (Figure 1G), indicating that direct physical contact between MSCs and ECs was necessary for the degenerative effect.
By TUNEL staining, capillaries undergoing MSC-induced degeneration were more fluorescent than nontreated or RNLFB-treated capillaries (Figure 2A). Expression of the apoptosis indicator, cleaved (activated) caspase-321 was detectable in MSC-treated capillaries, but not in nontreated or RNLFB-treated capillaries (Figure 2B). These findings indicated that EC apoptosis caused the capillary degeneration.
MSC-induced apoptosis of EC-derived capillaries in matrigel. (A) Images are phase contrast (Ph) and TUNEL fluorescence in 4-day-old capillary cultures to which no cells were added (CT), or MSCs were added after 1 day of culture at 1:1 ratio to EC (MSC). Bar = 500 μm. Bar diagram shows corresponding group data. FB, at EC:RNLFB ratio of 1:1. Data are mean plus or minus SD; n = 3 for each group. *P < .05, significantly different from CT. (B) Immunoblot for cleaved caspase-3 in 4-day-old capillaries exposed to no cells (CT), MSC, or FB as above. Actin expression is shown in the bottom panel. Replicated 3 times.
MSC-induced apoptosis of EC-derived capillaries in matrigel. (A) Images are phase contrast (Ph) and TUNEL fluorescence in 4-day-old capillary cultures to which no cells were added (CT), or MSCs were added after 1 day of culture at 1:1 ratio to EC (MSC). Bar = 500 μm. Bar diagram shows corresponding group data. FB, at EC:RNLFB ratio of 1:1. Data are mean plus or minus SD; n = 3 for each group. *P < .05, significantly different from CT. (B) Immunoblot for cleaved caspase-3 in 4-day-old capillaries exposed to no cells (CT), MSC, or FB as above. Actin expression is shown in the bottom panel. Replicated 3 times.
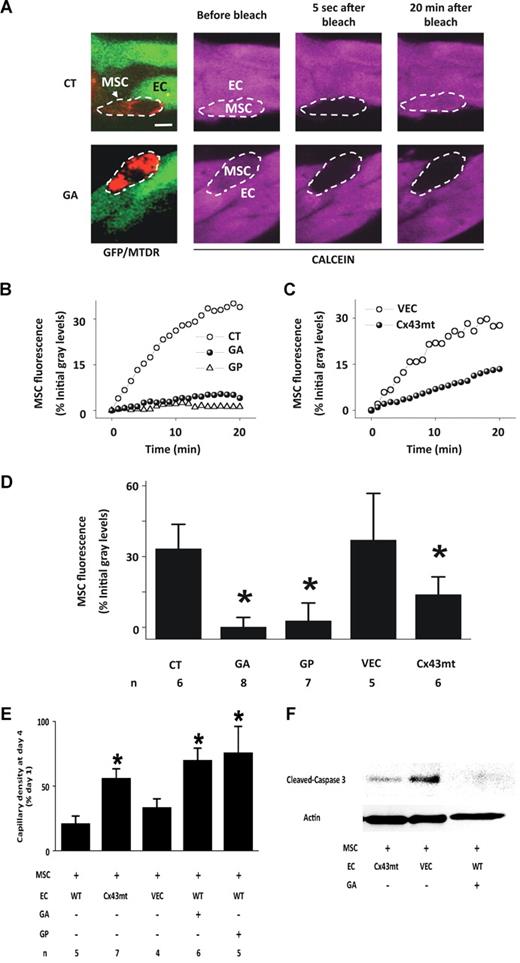
Connexin 43 (Cx43)–dependent GJC determines cell-cell signaling in capillaries22 and cocultured MSCs and ECs establish Cx43-containing gap junctional channels23 that are potentially capable of communicating apoptosis-inducing signals.24-26 Immunofluorescence confirmed the presence of Cx43 in both MSCs and ECs (Figure S3). To determine GJC between capillary-embedded MSCs and adjoining ECs, we quantified fluorescence recovery after FRAP. For cell identification, we loaded MSCs with the red-emitting fluorophore, MTDR, before adding the cells to GFP-expressing capillaries (Figure 3A). We loaded capillaries with the cytosolic fluorophore, calcein orange AM. Photobleaching calcein fluorescence in a single MSC was followed by fluorescence recovery (Figure 3A,B,D), indicating that the cytosolic dye diffused from adjoining ECs to the photobleached MSCs. In contrast, in the presence of the gap junctional blockers, GA, and the Cx43-specific gap peptides, gap26/27,27 FRAP was completely blocked (Figure 3A,B,D). These findings suggested the presence of Cx43-dependent GJC between MSCs and ECs.
Gap junctional communication between MSCs and ECs in matrigel capillaries. (A) Images show red fluorescence of mitochondria-stained MSCs (arrow) juxtaposed with GFP-expressing capillaries (green). MTDR, Mitotracker deep red. Capillaries were loaded with calcein (purple). By switching fluorescence filters, single MSC (dashed ovals) were identified by red mitochondrial fluorescence under calcein loaded conditions. Note that 20 minutes after calcein fluorescence in the MSC was photobleached, fluorescence was recovered in control (CT) but not in GA-treated capillaries. Bar = 10 μm. (B,C) Time course of FRAP in single EC-associated MSCs in untreated (CT), GA-treated (GA), and gap peptide–treated (GP) cultures, and for single EC-associated MSCs in GFP-Cx43T154A- (Cx43mt) or empty vector–expressing capillaries (VECs). (D) Group data show FRAP in MSCs 20 minutes after bleaching. Mean plus or minus SD. *P < .05 relative to CT. (E) Group data show capillary density in 4-day-old capillaries to which MSCs were added at 1:1 ratio to EC on day 1. WT, wild-type; other abbreviations are as above. Mean plus or minus SD; n indicates number of experiments. *P < .05 relative to WT. (F) Immunoblot for cleaved caspase-3 in 4-day-old capillaries exposed to MSCs as above. Actin expression is shown in the bottom panel. Replicated 3 times.
Gap junctional communication between MSCs and ECs in matrigel capillaries. (A) Images show red fluorescence of mitochondria-stained MSCs (arrow) juxtaposed with GFP-expressing capillaries (green). MTDR, Mitotracker deep red. Capillaries were loaded with calcein (purple). By switching fluorescence filters, single MSC (dashed ovals) were identified by red mitochondrial fluorescence under calcein loaded conditions. Note that 20 minutes after calcein fluorescence in the MSC was photobleached, fluorescence was recovered in control (CT) but not in GA-treated capillaries. Bar = 10 μm. (B,C) Time course of FRAP in single EC-associated MSCs in untreated (CT), GA-treated (GA), and gap peptide–treated (GP) cultures, and for single EC-associated MSCs in GFP-Cx43T154A- (Cx43mt) or empty vector–expressing capillaries (VECs). (D) Group data show FRAP in MSCs 20 minutes after bleaching. Mean plus or minus SD. *P < .05 relative to CT. (E) Group data show capillary density in 4-day-old capillaries to which MSCs were added at 1:1 ratio to EC on day 1. WT, wild-type; other abbreviations are as above. Mean plus or minus SD; n indicates number of experiments. *P < .05 relative to WT. (F) Immunoblot for cleaved caspase-3 in 4-day-old capillaries exposed to MSCs as above. Actin expression is shown in the bottom panel. Replicated 3 times.
To further delineate the Cx43 role in RLMECs, we expressed full-length GFP-Cx43 cDNA containing a mutation on threonine 154 (Cx43T154A), which blocks Cx43-dependent GJC,28 or the GFP-linked empty vector. FRAP was absent in monolayers of mutant RLMECs (Figure S4), indicating that GJC was completely blocked in these cells. Although the mutant RLMECs formed capillaries (Figure S5), FRAP was markedly inhibited in MSCs embedded in the mutant capillaries (Figure 3C,D), but not in those embedded in capillaries expressing the empty vector. These findings indicate that MSCs and ECs formed functional, Cx43-based GJC.
Importantly, when we added MSCs to day-old mutant capillaries, capillary degeneration was markedly, but not completely abrogated (Figure 3E), and cleaved caspase-3 expression was diminished (Figure 3F). The degenerative response was also inhibited in the presence of the gap junctional blockers (Figure 3E,F). Taking these findings together with the FRAP data, we conclude that the MSC-induced capillary degeneration resulted from formation of Cx43-based GJC between MSCs and ECs.
MSC-derived ROS cause capillary degeneration
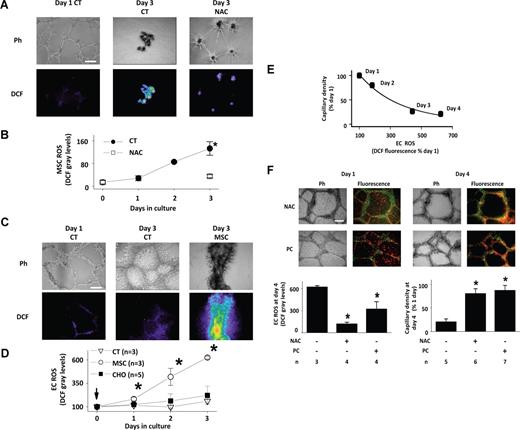
Because ROS production induces apoptosis,29 we considered the extent to which MSCs produce ROS. As different from EC-derived capillaries, in matrigel seeded with MSC alone, capillaries formed in one day, but degenerated in 3 days (Figure 4A). In MSC capillaries loaded with 2′,7′-DCFH-DA, ROS-sensitive DCF fluorescence steadily increased during the 3-day growth period, indicating increasing ROS production (Figure 4A,B). Addition of the antioxidant NAC abrogated both the ROS increase as well as the capillary degeneration (Figure 4A,B), indicating that the effect was ROS-dependent.
MSC-induced ROS production in matrigel. (A) Images show phase contrast (Ph) and DCF fluorescence (DCF) of MSC capillaries after indicated days of culture under nontreated (CT) and NAC-treated (NAC) conditions. NAC was added simultaneous with MSC seeding. Fluorescence was background corrected. Bar = 500 μm. (B) Plot shows time course of MSC ROS production in nontreated (CT) or NAC-treated (NAC) MSC capillaries. Mean plus or minus SD n = 3 (each group). *P < .05 versus NAC. (C) Images show phase contrast (Ph) and DCF fluorescence (DCF) of EC capillaries after indicated days of culture under untreated conditions (CT), or after addition of MSCs to day-old capillaries at 1:1 ratio (MSC). Bar = 500 μm. (D) Plots show time dependent effects of adding MSCs or CHO cells to day-old EC capillaries at 1:1 ratio. Mean plus or minus SD. *P < .05 relative to control (CT) at same time point. (E) Exponential regression of mean capillary density against mean EC fluorescence (R2 = 0.9887; P < .05). (F) Images show mitochondria-stained MSCs (red) juxtaposed with day-old EC-GFP capillaries (green) that were treated NAC or PEG-catalase (PC). Days of capillary culture are indicated. Bar = 500 μm. Group data are mean plus or minus SD. *P < .05 relative to untreated group.
MSC-induced ROS production in matrigel. (A) Images show phase contrast (Ph) and DCF fluorescence (DCF) of MSC capillaries after indicated days of culture under nontreated (CT) and NAC-treated (NAC) conditions. NAC was added simultaneous with MSC seeding. Fluorescence was background corrected. Bar = 500 μm. (B) Plot shows time course of MSC ROS production in nontreated (CT) or NAC-treated (NAC) MSC capillaries. Mean plus or minus SD n = 3 (each group). *P < .05 versus NAC. (C) Images show phase contrast (Ph) and DCF fluorescence (DCF) of EC capillaries after indicated days of culture under untreated conditions (CT), or after addition of MSCs to day-old capillaries at 1:1 ratio (MSC). Bar = 500 μm. (D) Plots show time dependent effects of adding MSCs or CHO cells to day-old EC capillaries at 1:1 ratio. Mean plus or minus SD. *P < .05 relative to control (CT) at same time point. (E) Exponential regression of mean capillary density against mean EC fluorescence (R2 = 0.9887; P < .05). (F) Images show mitochondria-stained MSCs (red) juxtaposed with day-old EC-GFP capillaries (green) that were treated NAC or PEG-catalase (PC). Days of capillary culture are indicated. Bar = 500 μm. Group data are mean plus or minus SD. *P < .05 relative to untreated group.
As different from MSCs, in day-old EC capillaries ROS production was low for up to 4 days of culture (Figure 4C,D) and no structural changes were evident by phase contrast. However, addition of MSCs progressively increased ROS (Figure 4C,D). Addition of CHO cells (Figure 4D) and RNLFB (data not shown) did not cause capillary ROS production, indicating that the effect was MSC specific. The time course of the ROS increase correlated with the extent of capillary degeneration (Figure 4E).
Fluorescence images in Figure 4F show MSCs added to day-old, NAC, or PEG-catalase–treated EC capillaries. The mitochondria-stained MSCs and the GFP-expressing ECs were easily distinguished by their respective red and green fluorescence emissions. However, on day 4 the fluorescence changed to yellow-orange, indicating that MSCs had intercalated in the capillary network. Despite the intercalation, the phase and fluorescence images as well as the group data for DCF fluorescence and capillary density indicated that both NAC and PEG-catalase blocked both the MSC-induced ROS production and the capillary degeneration. These findings affirm that the degeneration was ROS induced. Because cells internalize PEG-catalase,15 we interpret that the inhibition by PEG-catalase indicates intracellular H2O2 as the critical factor underlying these responses.
MSCs inhibit tumor angiogenesis
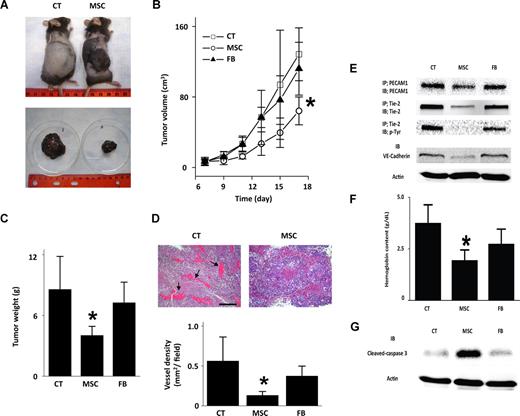
MSCs are reported to both promote30 and abrogate9 tumor growth. To determine the effects of MSCs on angiogenesis, we established the mouse melanoma model in subcutaneously implanted matrigel plugs seeded with B16-F10 melanoma cells.31,32 Implant melanomas grew steadily, reaching substantial dimensions in 18 days (Figure 5A-C). However, in 7-day-old melanomas, at which point tumor size was 700 mm3, direct inoculation of 106 MSCs abrogated further tumor growth (Figure 5A-C). Inoculations of MLFBs were without effect (Figure 5B,C), ruling out nonspecificity of the MSC-induced response. Analyses of tumor histologic sections indicated lower vascular density in MSC-inoculated than noninoculated tumors (Figure 5D). Immunoblot analyses of the MSC-inoculated tumors revealed decreases in the EC markers, PECAM1 and VE-cadherin. Tie-2 expression and phosphorylation that reflect ongoing tumor angiogenesis33 (Figure 5E) and tissue hemoglobin levels that indicate blood content (Figure 5F) were also each lower in MSC-treated than nontreated tumors. Further, cleaved caspase-3 expression was higher in MSC-inoculated tumors than in both noninoculated and MLFB-inoculated tumors (Figure 5G), suggesting that MSCs induced apoptosis. Together, these results indicate that MSCs inhibited tumor growth by inhibiting tumor angiogenesis.
Abrogation of mouse tumor growth by MSCs. Data are for tumors grown for 18 days after subcutaneous injections of B16F10 melanoma cells. The tumors were untreated (CT) or inoculated with 106 MSCs or mouse lung fibroblasts (FB) on day 7 of tumor growth. (A) Photographs show tumors in situ (top) and the excised tumor masses (bottom). Replicated 4 times. (B,C) Time-dependent changes in tumors. Mean plus or minus SD; n = 4 for each group. *P < .05 relative to CT. (D) Histologic sections show blood vessels in an untreated (left image,  ) and MSC-inoculated (right image) tumors. Bar = 300 μm. Bar graphs show vessel density quantified in histologic sections. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (E) Expression of indicated EC markers in tumor tissue. Lysates were subjected to immunoprecipitation (IP) and immunoblot (IB) analyses as indicated. Replicated 3 times. (F) Group data show hemoglobin content of tumor tissue. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (G) Immunoblots for indicated proteins in tumor lysates. Replicated 3 times.
) and MSC-inoculated (right image) tumors. Bar = 300 μm. Bar graphs show vessel density quantified in histologic sections. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (E) Expression of indicated EC markers in tumor tissue. Lysates were subjected to immunoprecipitation (IP) and immunoblot (IB) analyses as indicated. Replicated 3 times. (F) Group data show hemoglobin content of tumor tissue. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (G) Immunoblots for indicated proteins in tumor lysates. Replicated 3 times.
Abrogation of mouse tumor growth by MSCs. Data are for tumors grown for 18 days after subcutaneous injections of B16F10 melanoma cells. The tumors were untreated (CT) or inoculated with 106 MSCs or mouse lung fibroblasts (FB) on day 7 of tumor growth. (A) Photographs show tumors in situ (top) and the excised tumor masses (bottom). Replicated 4 times. (B,C) Time-dependent changes in tumors. Mean plus or minus SD; n = 4 for each group. *P < .05 relative to CT. (D) Histologic sections show blood vessels in an untreated (left image,  ) and MSC-inoculated (right image) tumors. Bar = 300 μm. Bar graphs show vessel density quantified in histologic sections. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (E) Expression of indicated EC markers in tumor tissue. Lysates were subjected to immunoprecipitation (IP) and immunoblot (IB) analyses as indicated. Replicated 3 times. (F) Group data show hemoglobin content of tumor tissue. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (G) Immunoblots for indicated proteins in tumor lysates. Replicated 3 times.
) and MSC-inoculated (right image) tumors. Bar = 300 μm. Bar graphs show vessel density quantified in histologic sections. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (E) Expression of indicated EC markers in tumor tissue. Lysates were subjected to immunoprecipitation (IP) and immunoblot (IB) analyses as indicated. Replicated 3 times. (F) Group data show hemoglobin content of tumor tissue. Mean plus or minus SD; n = 4 each group. *P < .05 relative to CT. (G) Immunoblots for indicated proteins in tumor lysates. Replicated 3 times.
Discussion
Our data are the first evidence that MSCs cause dose-dependent apoptosis of capillaries in both matrigel cultures as well as in a tumor model. The matrigel experiments indicated that the MSC-induced capillary cytotoxicity required direct contact between MSCs and ECs. Because MSCs secrete several growth factors and cytokines,34,35 we placed an insert containing MSCs adjacent to the matrigel capillaries to block direct contact but not the diffusion of soluble factors between the cell types. Under these conditions no cytotoxicity occurred, ruling out a role for MSC-secreted factors. Direct contact between these cell types was evident in that after addition to capillary cultures, MSCs rapidly intercalated between ECs.
Also critical was the formation of GJC between MSCs and ECs. This was evident in our FRAP studies that showed the existence of dye communication between embedded MSCs and their adjoining ECs. We confirmed previous findings that wild-type MSCs express Cx43 and are therefore, capable of forming GJC channels with other cell types.23 Importantly, pharmacologic inhibition of GJC by glycerrhetinic acid blocked the MSC-induced cytotoxic response. Because pharmacologic inhibition might induce nonspecific effects, in separate experiments we treated the capillaries with a mixture of the peptides gap26 and gap27 that ligate extracellular loops of Cx43, thereby specifically inhibiting Cx43-dependent GJC.27 In capillaries from both these treatment groups, the MSC-induced network degeneration was markedly inhibited, indicating that the cytotoxic effect was primarily Cx43-dependent.
The Cx43 role was further defined in experiments in which we grew capillaries from ECs expressing the communication-blocking Cx43 mutant, Cx43T154A.28 In these mutant capillaries, GJC between embedded MSCs and ECs was markedly, but not completely blocked. The partial blockade might be attributable to heterotypic GJC channels between Cx43 in MSCs and Cx37 or Cx40 in ECs.36 As similar to the glycerrhetinic acid and the gap peptide experiments, the MSC-induced network degeneration was markedly inhibited in mutant capillaries. However, the inhibition was partial, since as we discuss above, GJC was not completely blocked. Nevertheless taken together, our findings indicate that the MSC-induced cytotoxic effect was primarily Cx43 dependent. We conclude that cell communication between MSCs and ECs24-26,37 transmitted a cytotoxic signal throughout the network.
Our DCF experiments identified ROS as the transmitted signal. Although ROS production has been previously reported in bone marrow–derived hematopoietic cells,38 we show here for the first time that MSCs are capable of generating ROS. Because intracellular DCF remains cell bound after being formed by the oxidation of its precursor, DCFH,39 constant levels of DCF fluorescence indicate low ROS production. When grown as monolayers, ROS production was low in both MSCs and ECs. ROS production was also low in EC-derived capillaries grown in matrigel. By contrast, ROS production increased progressively and at a markedly higher rate in MSC capillaries, or when we added MSCs to EC-derived capillaries. The augmented ROS response was not a nonspecific matrigel effect, because ROS production was low in EC capillaries. Thus, we show that MSCs trigger ROS production when either themselves forming capillaries, or when migrating toward EC-derived capillaries.
The origins of the MSC ROS are unclear. Cellular processes that are generally considered a source for ROS include the mitochondrial, the xanthine/xanthine oxidase and the NADPH oxidase systems.40 Because MSCs transfer mitochondria to host cells,5 it is possible that the transferred mitochondria are activated and thereby increase ROS production. Here, this possibility was supported by fluorescence data indicating that MSCs indeed transferred mitochondria to ECs. As reported for trophoblast fusion,41 the underlying processes might entail MSC-EC mitochondrial transfer as a result of cell fusion subsequent to formation of Cx43-dependent GJC channels. The ROS were clearly responsible for the capillary degeneration, because ROS production correlated with the extent of the degenerative response and the ROS inhibitor, NAC blocked both the ROS production as well as the MSC-induced cytotoxicity. These findings affirm that high levels of ROS induced apoptosis.42 Taking these results together with the GJC data, we conclude that MSCs established GJC with ECs to communicate ROS generating signals in the capillaries,22 leading to capillary degeneration.
We point out that the cytotoxic effects of MSCs were evident only when the cells were added to matrigel in high numbers, namely at EC:MSC ratio of 1:1 or 1:3. The cytotoxicity dropped off when the number of cells added decreased by an order of magnitude. This dose dependence might explain the difference between our findings and those of reported studies in which MSCs caused no cytotoxicity, but instead promoted angiogenesis by secreting growth factors,8,43 stabilized engineered blood vessel due to a differentiation into perivascular cells,3 or inhibited lymphocyte and neutrophil apoptosis.44,45 In these reported studies the effective numbers of MSCs interacting with host cells might have been lower than the numbers required to effect cytotoxicity.
Nevertheless, our studies carry the caution that interaction of a large number of MSCs with host cells might prove cytotoxic. The potential for such interactions needs to be considered when MSCs are given for therapeutic applications by either intravenous injection,2 or by intrapulmonary instillation.6 The large numbers of MSCs that are required for cytotoxic effects, as shown here, are unlikely to be achieved in capillary beds with normal blood flow, because the cells are likely to be flushed away. However, cytotoxic effects might become evident to the extent that administered MSCs aggregate in say, the reticulo-endothelial system such as in liver and spleen.
This prediction was realized to some extent when we directly injected 106 MSCs in subcutaneous melanomas that had already grown for a week. The MSC injections markedly retarded further increase in tumor size. To the extent of this antitumorigenic effect, our findings agree with those of Khakoo et al, who reported inhibition of Kaposi sarcoma by vascular administration of MSCs.9 Our findings indicate, furthermore, that the antimelanoma effect was associated with loss of tumor hemoglobin, loss of tumor vasculature, and depletion of several EC markers in tumor tissue. Further, increased expression of cleaved caspase-3 in tumor tissue indicated that as similar to matrigel-grown capillaries, MSCs induced apoptosis in tumor tissue. Taking these findings together, we suggest that the intratumoral MSC injections induced apoptosis of the tumor vasculature, thereby decreasing tumor viability.
In conclusion, our findings indicate that MSCs, when administered in large numbers, are cytotoxic to newly forming vessels. The extent to which the cytotoxicity might extend to other cell types and indeed to stable vasculature remains uncertain. Nevertheless, we suggest that our findings are therapeutically relevant from at least 2 standpoints. First, direct MSC injection in solid tumors, as we show here might promote tumor regression to the extent that the tumor vasculature undergoes apoptosis. Second, the potential cytotoxicity to host cells must be taken into consideration when designing MSC-based cell therapy. We believe that these cytotoxic effects need to be better understood to develop effective MSC-based therapeutic modalities.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by National Institutes of Health grants HL36024, HL57556, and HL69514 (Bethesda, MD; J.B.).
National Institutes of Health
Authorship
Contribution: K.O. designed, performed, and analyzed the research and wrote the paper; S.D., S.H., and S.Q. performed and analyzed the research; S.B. analyzed the research; and J.B. supervised, designed, and analyzed the research and wrote the paper.
Conflict-of-interest disclosure: J.B. is a consultant for Daiichi-Sankyo (Tokyo, Japan). The remaining authors declare no competing financial interests.
Correspondence: Jahar Bhattacharya, Columbia University Medical Center, 630 West 168th St, BB 8-812, New York, NY 10032; e-mail: jb39@columbia.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal