Abstract
Imatinib mesylate has become the treatment of choice for chronic myeloid leukemia (CML): the standard dose for chronic- phase (CP) CML is 400 mg daily. Response rates are different according to Sokal score, being significantly lower in intermediate and high Sokal risk patients. Phase 1 and 2 trials have shown a dose-response effect and high-dose imatinib trials in early CP CML showed better results compared with standard dose. Our study is the first prospective trial planned to evaluate the efficacy and tolerability of high-dose imatinib in previously untreated intermediate Sokal risk CML patients. Seventy-eight patients were treated with 400 mg imatinib twice daily: complete cytogenetic response (CCgR) rates at 12 and 24 months were 88% and 91%; moreover, at 12 and 24 months 56% and 73% of CCgR patients achieved a major molecular response. The incidence of adverse events was slightly higher than reported by the most important standard-dose trials. With a median follow-up of 24 months, 3 patients progressed to advanced phase. In intermediate Sokal risk newly diagnosed CML patients, high-dose imatinib induced rapid and high response rates, apparently faster than those documented in the International Randomized Study of IFN and Imatinib for the same risk category. These clinical trials are registered at www.clinicaltrials.gov as no. NCT00510926.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder arising from a multipotent stem cell marked by a specific chromosome abnormality, the Philadelphia chromosome (Ph), which results from a reciprocal t(9;22)(q34;q11) chromosomal translocation1-3 ; the molecular consequence of this translocation is a fusion gene, BCR-ABL, encoding a constitutively active tyrosine kinase.4,5 Imatinib mesylate (Glivec; Novartis, Basel, Switzerland) is a 2-phenylaminopyrimidine derivative active in Ph-positive CML in all phases of the disease through a selective inhibition of the BCR-ABL protein.6-13 For chronic-phase (CP) CML, the recommended dose is 400 mg daily, which produces high response rates. Particularly, the International Randomized Study of IFN and Imatinib (IRIS) study reported a complete hematologic response (CHR) rate, a complete cytogenetic response (CCgR) rate, and a major molecular response (MMR) rate at 12 months of 95%, 68%, and 39%, respectively. In patients who obtained a CCgR, the 1-year rate of MMR was 57%.14,15 These early responses produced a long-term (60 months) 93% survival without progression to the accelerated or blastic phase (ABP) and an 83% event-free survival.16 Two scoring systems, including prognostic variables identified by multivariate analysis, are available for disease risk evaluation: the Sokal score and the Hasford score. The Sokal risk formulation (variables included: age, spleen size, platelet count, and percentage of blasts in the peripheral blood) was based on conventional chemotherapy-treated patients,17 whereas the Hasford formulation (same variables, plus percentage of eosinophils and basophils in the peripheral blood) was based on alpha-interferon (α-IFN)–treated patients.18 A strong relationship between risk and outcome has been evident in all phases of CML history (conventional chemotherapy,19 recombinant α-IFN alone,20-24 recombinant α-IFN associated with low-dose cytarabine,25,26 imatinib alone,14-16 and imatinib associated with pegylated α-IFN27,28 ), and both scores have confirmed their predictive value. The Sokal scoring system has been applied to stratify the patients by risk in all the largest clinical imatinib trials. At 12 months, according to the Sokal score, the IRIS study reported significantly different CCgR rates and MMR rates in CCgR patients: 76% to 66%, 67% to 45%, and 49% to 38% for low, intermediate, and high Sokal risk patients, respectively.14 At 60 months, the estimated progression rates for patients in the 3 groups were 3%, 8%, and 17%, respectively; all the differences were statistically significant.16 Based on these data, in our trial we referred to the Sokal score.
Several observations suggest that higher doses of imatinib may be more effective than standard doses: (1) a clear dose-response effect has been observed in the phase 1 study, and no maximum tolerated dose was identified up to 1000 mg/day8,9 ; (2) 600 mg daily is more effective in ABP disease11-13 ; (3) responses have been reported after dose escalation to 800 mg in patients resistant to 400 mg daily29,30 ; (4) high-dose imatinib in late and early CP produces faster results than standard doses31-35 ; (5) several mutations within the BCR-ABL structure resulting in resistance to standard doses may be overridden or prevented through a high-dose treatment policy36-40 ; and (6) plasma and intracellular imatinib levels may influence responses in CP.41-44
The rationale of our study is based on the relationships between risk and response, and on the therapeutic profile of high-dose imatinib. Our study is the first prospective, controlled, and multi-institutional trial designed to evaluate the efficacy and tolerability of imatinib at high dose in a selected (Sokal intermediate) risk category of previously untreated early CP CML patients.
Methods
Study group
Patients were required to have a Ph-positive and BCR-ABL–positive CML in the first chronic phase (from diagnosis to imatinib start, ≤ 6 months), an intermediate Sokal risk score (defined as Sokal score between 0.8 and 1.2), no previous treatment (hydroxyurea only allowed), 18 years or older, and to have an adequate performance status (Eastern Cooperative Oncology Group: 0-2), serum creatinine less than 2 mg/dL, total bilirubin, and aspartate aminotransferase and alanine aminotransferase less than 3 times upper limit of normal. Women of childbearing potential were required to have a negative pregnancy test before starting imatinib, and all fertile patients were asked to use an acceptable method to avoid pregnancy. All the patients provided written informed consent before the enrollment. The study was reviewed and approved by the Internal Review Board of all the participating institutions and performed in accordance with the Declaration of Helsinki. The trial (serial number ICSG on CML/021) was submitted to 71 hematologic centers belonging to the GIMEMA (Italian Group for Hematologic Malignancies of the Adult) CML Working Party (formerly, ICSG on CML, Italian Cooperative Study Group on CML): 25 centers enrolled at least one patient in this study. Seventy-eight patients were eligible.
Treatment and dose modifications
Imatinib was administered orally 400 mg daily for the first 2 weeks and 400 mg twice a day from the third week onward. Drug safety parameters were evaluated at each visit and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 2.0. Dose reductions of imatinib for nonhematologic or hematologic toxic effects were performed as follows: for persistent grade 2 or 3 nonhematologic toxic effects, therapy was interrupted until recovery to grade 1 or less. For grade 2 nonhematologic toxic effects, imatinib was resumed at a dose of 400 mg daily; for grade 3 nonhematologic adverse events, it was resumed at a reduced dose of 300 mg daily for 2 weeks and then reescalated to 400 mg daily, provided that a nonhematologic toxicity of grade 3 did not recur. If a grade 2 or 3 nonhematologic toxicity recurred for more than 3 times, the treatment was adapted to the maximum tolerated dose. For a grade 4 nonhematologic toxicity, the patient was declared off-protocol: subsequent treatment was unrestricted. For severe hematologic toxicity (grade 3 or grade 4), treatment was interrupted until the white blood cell recovered to more than 2.0 × 109/L and/or platelets to more than 50 × 109/L, then resumed at 400 mg daily for 2 weeks (grade 3 toxicity) or 4 weeks (grade 4 toxicity), and thereafter reescalated to 800 mg daily, provided that a new grade 3 or 4 hematologic toxicity did not recur. In case of recurrence of more than 3 episodes of grade 3 or 4 hematologic toxicity, the dose was adapted to the MTD.
Dose escalation was not allowed. In case of contemporary hematologic and nonhematologic toxicities, dose adaptation was regulated based on the most severe adverse event.
Treatment monitoring and response evaluation
Blood count was performed at enrollment, weekly during the first 3 months, every 2 weeks during the next 3 months, then monthly until the completion of the 12 months of treatment, and every 3 months thereafter. A CHR was defined as a white blood cell count of less than 10 × 109/L, a platelet count of less than 450 × 109/L, no immature cells (blasts, promyelocytes, myelocytes) in the peripheral blood, and disappearance of all signs and symptoms related to leukemia (including palpable splenomegaly). Serum chemistry was performed at enrollment and every week for the first 12 months, and then every 3 months. Bone marrow examinations, including cytologic and cytogenetic (both conventional and fluorescent in situ hybridization [FISH]) analyses, were done at baseline, after 6 and 12 months on treatment, and every 6 months thereafter or in case of failure or disease progression. Conventional cytogenetic analysis was performed using the G-banding technique. Marrow specimens were examined on direct or short-term (24-hour) cultures; at least 20 metaphases were analyzed. FISH was performed using BCR/ABL extra-signal, D-FISH, or dual-color dual-fusion probes; at least 300 cells were analyzed. Cytogenetic response, based on results of conventional analysis, was categorized as: complete (CCgR), 0% Ph-positive metaphases; partial (PCgR), 1% to 35% Ph-positive metaphases; major (MCgR), 0% to 35% Ph-positive metaphases; minor, 36% to 65% Ph-positive metaphases; minimal (mCgR), 66% to 95% Ph-positive metaphases; no response, more than 95% Ph-positive metaphases. Qualitative reverse transcription–polymerase chain reaction (RT-PCR) for BCR-ABL transcript was routinely performed at enrollment for determining the type of transcript. Peripheral blood (PB) samples for real-time quantitative PCR (RQ-PCR) were collected before therapy, after 3, 6, and 12 months, and every 6 months thereafter. All molecular analyses were centralized in Bologna, Italy. Mononuclear cells were isolated from 20 mL PB after separation on a Ficoll-Hypaque gradient. RNA extraction, RT-PCR, and RQ-PCR were performed as already described.45-47 ABL was used as housekeeping gene to correct for differences in RNA quality and/or RT efficacy.45-47 BCR-ABL and ABL plasmid dilutions (Ipsogen, Marseille, France) were used as standards, and the final results were calculated as the ratios BCR-ABL/ABL and expressed as a percentage. All experiments were performed in duplicate, and the results were expressed as percentage ratio to ABL. The BCR-ABL/ABL ratios were further multiplied by a conversion factor to set the results on an international scale as defined in the IRIS study.15,48,49 Samples yielding an ABL threshold cycle (Ct) more than 30, corresponding to less than 1000 ABL transcript copies, were considered as having degraded RNA and discarded. We defined an MMR as a BCR-ABL/ABL ratio less than 0.1% and a complete molecular response (CMR) as a ratio less than 0.005. To confirm a CMR value for a single assay, our definition requires that the analysis gave an ABL copy number superior to 30 000 corresponding to a 4.5-log sensitivity of the assay45 and that the samples negative by RQ-PCR were analyzed by nested PCR46,47 : a CMR was defined as nested PCR negativity.
Statistical methods
Sample size estimation has been performed according to the Fleming single-stage procedure.50 If, at the end of the trial, at least 56 successes are observed, the treatment will be accepted for a phase 3 trial. An analysis of the primary endpoint (CCgR at 12 months) has been made on all the patients enrolled, according to the intention to treat. Patients were categorized by their best cytogenetic and molecular response. Time to event was calculated from the date of start of treatment until the appearance of the event. Survival was calculated from the date of treatment start until the date of death. Event-free curve was estimated by the Kaplan-Meier method.
Results
Study group
From January 2004 until May 2005, 78 intermediate Sokal risk CML patients in early CP have been enrolled; all the patients are fully evaluable for responses and survival. Detailed demographic and hematologic data of the study group are presented in Table 1. The median age was 56 years (range, 26-79 years), and 32 patients (41%) were 60 years of age or older at enrollment. Hasford risk stratification was as follows: 38% low and 62% intermediate; no patient had high Hasford score. At enrollment, there were 9 patients (12%) with der(9) deletion, 4 (5%) with variant translocation, and 2 (3%) with additional chromosomal abnormalities: loss of chromosome Y (1) trisomy of chromosome 8 (1). There were 48 (62%) patients who had received treatment with hydroxyurea before imatinib, whereas the remaining 30 received imatinib as first-line treatment.
Characteristics of the study group at diagnosis
| Parameter . | Value . |
|---|---|
| Age, y, median (range) | 56 (26-79) |
| 60 years or older, n (%) | 32 (41) |
| Sex, male/female, n (%) | 46/32 (59/41) |
| Performance status = 2, n (%) | 2 (3) |
| Splenomegaly, cm, median (range) | 3 (0-15) |
| Hemoglobin level, g/L, median (range) | 120 (64-161) |
| WBC count,109/L, median (range) | 56 (2-405) |
| Platelet count, 109/L, median (range) | 392 (129-1140) |
| Peripheral blasts, %, median (range) | 2 (0-8) |
| Eosinophils, %, median (range) | 2 (0-8) |
| Basophils, %, median (range) | 2 (0-16) |
| Clonal evolution present, n (%) | 2 (3) |
| Variant translocation present, n (%) | 4 (5) |
| Prior hydroxyurea, N (%) | 48 (62) |
| Hasford score, n (%) | |
| Low | 30 (38) |
| Intermediate | 48 (62) |
| High | 0 (0) |
| Parameter . | Value . |
|---|---|
| Age, y, median (range) | 56 (26-79) |
| 60 years or older, n (%) | 32 (41) |
| Sex, male/female, n (%) | 46/32 (59/41) |
| Performance status = 2, n (%) | 2 (3) |
| Splenomegaly, cm, median (range) | 3 (0-15) |
| Hemoglobin level, g/L, median (range) | 120 (64-161) |
| WBC count,109/L, median (range) | 56 (2-405) |
| Platelet count, 109/L, median (range) | 392 (129-1140) |
| Peripheral blasts, %, median (range) | 2 (0-8) |
| Eosinophils, %, median (range) | 2 (0-8) |
| Basophils, %, median (range) | 2 (0-16) |
| Clonal evolution present, n (%) | 2 (3) |
| Variant translocation present, n (%) | 4 (5) |
| Prior hydroxyurea, N (%) | 48 (62) |
| Hasford score, n (%) | |
| Low | 30 (38) |
| Intermediate | 48 (62) |
| High | 0 (0) |
WBC indicates white blood cell.
Hematologic response
Of the 78 patients, 76 (97%) achieved a CHR as best hematologic response (Table 2). After 3 months, 64 patients (82%) reached a CHR, with 14 patients (18%) still showing minor signs of active disease (palpable spleen 1 cm below the costal margin: 10 patients; palpable spleen and < 5% precursors in the PB: 4 patients). All these patients except 2 achieved a CHR at month 6.
Response to high-dose imatinib therapy
| Response . | n (%) . |
|---|---|
| Complete hematologic | 76 (97) |
| Cytogenetic | |
| Complete | 71 (91) |
| Major | 73 (94) |
| Molecular | |
| Complete | 21 (27) |
| Major | 54 (69) |
| Response . | n (%) . |
|---|---|
| Complete hematologic | 76 (97) |
| Cytogenetic | |
| Complete | 71 (91) |
| Major | 73 (94) |
| Molecular | |
| Complete | 21 (27) |
| Major | 54 (69) |
Study group N = 78. Patients were categorized by their best response.
Cytogenetic response
CCgR.
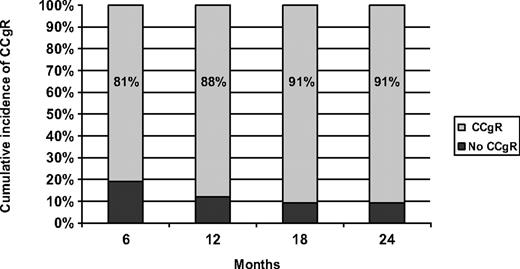
The time to cytogenetic response was short (Figure 1): 63 patients (81%) achieved a CCgR within 6 months. The primary endpoint was the CCgR rate at 12 months: 69 patients (88%) reached a CCgR as best cytogenetic response within 12 months, whereas the 12-month cumulative incidence of MCgR was 94%. Two additional cases obtained the first CCgR beyond 12 months, for a cumulative incidence of CCgR of 91% (Table 3). The median observation time between the first occurrence of the CCgR and the most recent evaluation was 12 months (range, 1-30 months): only 1 patient lost the CCgR, whereas 2 CCgR cases died. The 2 deaths in CCgR patients were probably unrelated to CML, 1 resulting from a high-grade non-Hodgkin lymphoma and the second from pulmonary thromboembolism after a lung infection with normal blood counts.
Cumulative incidence of complete cytogenetic response in the study group.
Patient disposition and cytogenetic response
| Cytogenetic response . | n . | Notes . | |
|---|---|---|---|
| Overall CCgR | 71 | ||
| Time to CCgR ≤ 12 months | 69 | ||
| Time to CCgR > 12 months | 2 | ||
| CML-unrelated death | 2 | Pulmonary embolism (1); NHL (1) | |
| Stable CCgR | 68 | ||
| Loss of CCgR | 1 | CP | |
| Not CCgR | 7 | ||
| AE grade IV | 1 | CP (detailed in text) | |
| Loss of CHR | 1 | CP | |
| ABP | 1 | Allogeneic BMT | |
| No CgR < 6 months (100% Ph+) | 3 | ABP (2); CP (1) | |
| PCgR at 12 months | 1 | Allogeneic BMT | |
| Total | 78 | ||
| Cytogenetic response . | n . | Notes . | |
|---|---|---|---|
| Overall CCgR | 71 | ||
| Time to CCgR ≤ 12 months | 69 | ||
| Time to CCgR > 12 months | 2 | ||
| CML-unrelated death | 2 | Pulmonary embolism (1); NHL (1) | |
| Stable CCgR | 68 | ||
| Loss of CCgR | 1 | CP | |
| Not CCgR | 7 | ||
| AE grade IV | 1 | CP (detailed in text) | |
| Loss of CHR | 1 | CP | |
| ABP | 1 | Allogeneic BMT | |
| No CgR < 6 months (100% Ph+) | 3 | ABP (2); CP (1) | |
| PCgR at 12 months | 1 | Allogeneic BMT | |
| Total | 78 | ||
CCgR indicates complete cytogenetic response; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; CP, chronic phase; AE, adverse event; CHR, complete hematologic response; ABP, accelerated or blastic phase; BMT, bone marrow transplantation; and PCgR, partial cytogenetic response. Bold values indicate totals.
No CCgR.
Seven patients never achieved a CCgR. One patient reached a PCgR at 12 months as best cytogentic response (suboptimal response; submitted to allogeneic stem cell transplantation from HLA-matched donor and alive in complete remission 7 months after transplantation). Three patients were cytogenetic refractory (100% Ph-positive after 6 months): 2 of them progressed to ABP (1 died after disease transformation) and 1 of them is still alive in CP. One patient reached a PCgR at 6 months but progressed to ABP at month 9 (alive in complete remission 11 months after allogeneic stem cell transplantation from HLA identical sibling donor). One patient (mCgR as best response) lost the CHR and is alive in CP. The remaining patient experienced a long-lasting liver toxicity (ranging between grade 2 and grade 4) and was declared off protocol according to the study criteria: after 4 months of suspension he resumed imatinib by degrees (final dose of 400 mg daily) without any further liver toxicity and achieved a stable CCgR.
Molecular response
Of the 78 patients, 54 (69%) reached an MMR as best response, and 20 (26%) had undetectable BCR-ABL transcript, confirmed by nested PCR (CMR). The molecular response was also evaluated in patients who obtained a CCgR as best cytogenetic response (71 patients): there was a consistent and continuous decline of BCR-ABL/ABL ratio over time, with an MMR rate of 38%, 56%, 64%, and 73% at 6, 12, 18, and 24 months, respectively. Overall, the cumulative incidence of MMR among CCgR patients was 76%, with 28% patients achieving a CMR.
Survival and events
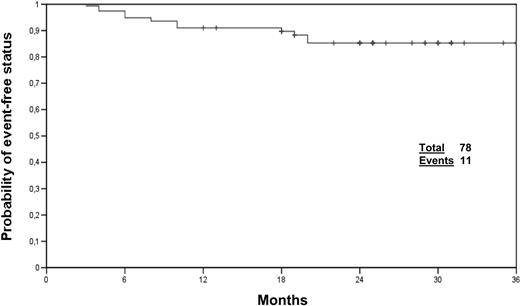
The median follow-up was 24 months, and all the patients had at least 12 months of observation (range, 12-37 months; 97% of patients ≥ 18 months, 87% of patients ≥ 24 months). At the time of writing, 75 patients (96%) are alive and 3 patients (4%) progressed to advanced phase. Of the 78 patients, 67 (85%) are alive without any event and still on imatinib therapy, whereas 11 patients experimented one event, as follows: 1 lost to follow-up, 1 loss of CCgR, 2 deaths in CCgR (probably unrelated to CML), 1 allogeneic transplantation for suboptimal response, 1 progression to ABP, 3 cytogenetic refractoriness, 1 loss of CHR, and 1 grade 4 nonhematologic adverse event (Figure 2).
Compliance and toxicity
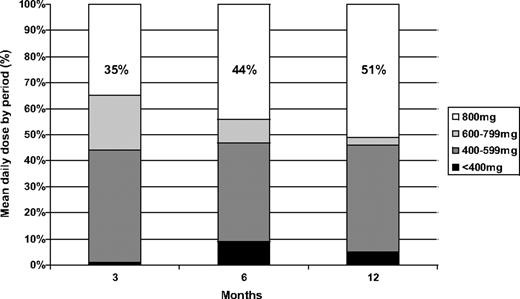
We calculated the mean daily dose received by the patients in the first 12 months (Figure 3). During the first quarter (months 1-3), 56% of the patients received a mean daily dose more than or equal to 600 mg daily (62% of them, or 35% of the whole patient population, received the full scheduled dose of 400 mg daily for 14 days and 800 mg daily thereafter). During the second quarter (months 4-6), 53% of the patients assumed a mean dose of 600 mg or more daily (83% of them, or 44% of the whole patient population, received the full scheduled dose). Finally, during the last 6 months (months 7-12), 54% of the patients received a mean dose of 600 mg or more daily (94% of them, or 51% of the whole patient population, received the full scheduled dose). A mean daily dose of 600 mg or more daily always corresponds to a median dose of 800 mg during the period. At the end of the first year, 56% of the patients still on treatment were receiving 800 mg daily, 40% 400 mg daily, and 4% 300 mg daily.
Skin rash, myalgia and bone pain, gastrointestinal intolerance, fluid retention, and asthenia were common, slightly more frequent than reported by the most important standard-dose imatinib published trial.14 Severe (grade 3 and 4) nonhematologic adverse events were uncommon: skin rash (9%), muscle/bone pain (9%), nausea/vomiting (6%), and diarrhea (5%). Grade 3 and 4 hematologic adverse events were as follows: leukopenia (18%), thrombocytopenia (17%), and anemia (9%) (Table 4). Toxicities were more frequent during the first 3 months of treatment, and they were generally reversible with temporary treatment interruption or dose reduction: 65% of the patients required a transient withdrawal of imatinib for nonhematologic toxicity grade 2 or more or hematologic toxicity grade 3 or more, but only one patient was declared off-study for a grade 4 long-lasting liver toxicity (the patient gradually started taking imatinib again until a dose of 400 mg daily, without further liver toxicity, achieving a CCgR). The most common reason for treatment discontinuation was hematologic toxicity (leukopenia, 18%; and thrombocytopenia, 17%). In some instances, the dose reduction was required for more than one of the toxicities listed.
Adverse events
| Adverse event . | All grades, n (%) . | Grade 3 or 4, n (%) . |
|---|---|---|
| Skin rash | 20 (26) | 7 (9) |
| Myalgia/cramps/bone pain | 39 (50) | 7 (9) |
| Nausea/vomiting | 35 (45) | 5 (6) |
| Diarrhea | 29 (37) | 4 (5) |
| Superficial edema | 49 (63) | 2 (3) |
| Fatigue | 38 (49) | 2 (3) |
| Leukopenia | 56 (72) | 14 (18) |
| Thrombocytopenia | 53 (68) | 13 (17) |
| Anemia | 39 (50) | 7 (9) |
| Liver toxicity | 31 (40) | 2 (3) |
| Adverse event . | All grades, n (%) . | Grade 3 or 4, n (%) . |
|---|---|---|
| Skin rash | 20 (26) | 7 (9) |
| Myalgia/cramps/bone pain | 39 (50) | 7 (9) |
| Nausea/vomiting | 35 (45) | 5 (6) |
| Diarrhea | 29 (37) | 4 (5) |
| Superficial edema | 49 (63) | 2 (3) |
| Fatigue | 38 (49) | 2 (3) |
| Leukopenia | 56 (72) | 14 (18) |
| Thrombocytopenia | 53 (68) | 13 (17) |
| Anemia | 39 (50) | 7 (9) |
| Liver toxicity | 31 (40) | 2 (3) |
Discussion
High-dose imatinib (600-800 mg daily) has a strong rationale: the phase 1 trial showed a direct relationship between the dose and the response,8,9 and high-dose imatinib clinical trials produced better results than standard dose in advanced phase,11-13 in late chronic phase after α-IFN failure,31 and in early chronic phase for all risk categories.32-35 Moreover, dose escalation to 600 or 800 mg daily allows for the recovery of a proportion of patients who failed to achieve a hematologic and cytogenetic response with lower doses,29,30 and data from clinical trials of Philadelphia-positive acute lymphoblastic leukemias51-55 support the superior efficacy of high-dose imatinib. Finally, the pharmacokinetic analysis suggests a relationship between the area under the plasma concentration time curve of imatinib and the response,41-44 and a high-dose treatment should be an efficacious strategy to overcome resistance induced by some BCR-ABL point mutations.36-40
Our study is the first one planned to investigate the efficacy of and the compliance to high-dose imatinib in a particular Sokal risk category: we treated 78 newly diagnosed Ph-positive CML patients in early chronic phase, at intermediate Sokal risk, with imatinib 400 mg orally twice a day. At 12 months, the cumulative incidence of CCgR was 88% (confidence interval [CI] 84%-92%) and the cumulative incidence of MMR of CCgR patients was 56% (CI, 50%-62%). Both these results seems to be superior compared with those obtained in the IRIS trial for the same Sokal risk category with the same observation time: after 1 year of treatment, 67% CCgR rate and 45% MMR rate in CCgR patients.15 Moreover, our results are similar to the ones of the low Sokal score category of the IRIS study at 12 months (CCgR rate of 76% and MMR rate of 66%), suggesting that a “risk related” high-dose treatment policy may flatten the differences of early response among the risk categories. It should not be overlooked that imatinib standard-dose induced a cumulative incidence of CCgR in intermediate-risk patients comparable with that obtained in our study (91%; CI, 88%-94%), but with a much longer follow-up: 87% estimated rate of CCgR at 60 months for all Sokal risk categories and 82% for intermediate Sokal risk patients only.16 Given that the CCgR has been a confirmed surrogate marker of long-term survival both for the α-IFN and for the imatinib therapy,56,57 our results should translate in a good long-term outcome. Another strong marker of long-term survival for the imatinib therapy is the molecular response: an MMR in CCgR patients correlates with a 100% long-term progression-free survival.58-62 The molecular response rate observed in our study confirms the efficacy of high-dose imatinib: overall, 76% of CCgR patients obtained an MMR, whereas the IRIS study reported 80% of MMR in CCgR patients, but considering all risk categories and after 4 years of observation.16 The median observation period of our study (24 months) is still short, and the overall survival and progression-free survival, as expected, are very high (both 96%): 2 patients died of causes unrelated to CML and another one after disease transformation; 3 patients progressed to ABP (one death, one survival with active disease, and one recovery with allogeneic stem cell transplantation). Two considerations are important: (1) it is not clear whether an earlier achievement of CCgR and MMR will translate to a better long-term outcome; and (2) the IRIS trial offered the possibility of a dose escalation of imatinib to 400 mg twice daily in case of suboptimal response at 12 months (as currently recommended in the clinical practice63 ), but our trial was not planned to evaluate whether high-dose imatinib as first-line treatment in this Sokal risk category would obtain a better overall therapeutic effect with respect to a standard dose policy with dose escalation in case of resistance. The potential long-term benefits of a high-dose treatment front-line in intermediate Sokal risk patients need to be determined through a phase 3 trial. Kantarjian et al32 treated 114 CML patients in first chronic phase with imatinib 800 mg daily, obtaining an overall CCgR rate of 90% and an overall survival probability of 98% (median follow-up, 15 months; range, 3-27 months), in line with our study. The overall incidence of MMR of the 112 evaluable patients was 63%, comparable with our 76% for CCgR patients only and 69% for all the patients. The CMR rate was 28%; in our study, the CMR was 26% (28% of CCgR patients). Hughes et al35 conducted a trial in 103 newly diagnosed CML patients using imatinib 600 mg/day, with dose escalation to 800 mg/day for suboptimal response and combination of intermittent standard-dose cytosine arabinoside if the response criteria were still not met after a further 3 months. The estimated cumulative incidences of CCgR and MMR were 88% and 47% at 12 months, 90% and 73% at 24 months, respectively. In both studies, Sokal risk was not available for all the cases, but at least 36% and 41% of the reported patients, respectively, were low Sokal risk at diagnosis.32,35 On the other hand, Baccarani et al64 presented at the 2008 American Society of Hematology (ASH) Meeting the results of a phase 3 trial comparing 400 mg and 800 mg daily of imatinib in Sokal high-risk CML patients in early CP: based on an intention-to-treat analysis, this study did not show a significant benefit of 800 mg over 400 mg, but the patients who could comply with the high dose had a better cytogenetic outcome.
As shown in Figure 3, the compliance to high-dose imatinib in our study was satisfactory. The rules for dose adaptation (see “Treatment and dose modifications” in “Methods”) in case of hematologic and nonhematologic adverse events were strict, and dose reductions were performed more often than in other clinical trials31,32 because of the multicentric nature of our study, which included small clinical centers. Particularly, in case of any adverse event requiring a dose reduction, the subsequent dose was set at 400 mg daily, and not at 600 mg as in the M. D. Anderson Hospital's trial. Despite these considerations, approximately 55% of all the patients received a mean daily dose more than 600 mg daily (Figure 3), high-dose therapy, during the study period, and more than 50% of the patients are still taking the full dose after 1 year of therapy. The reported adverse events were the same as for the conventional doses of imatinib14,16 (Table 4): the most common severe (grade 3 or 4) toxicity was hematologic (18% leukopenia, 17% thrombocytopenia), whereas the most frequent nonhematologic grade 3 or 4 adverse events were skin rash (9%), myalgias (9%), nausea/vomiting (6%), diarrhea (5%), and fluid retention (3%). Most of these events were resolved by temporary suspension of imatinib or dose reduction. Only one patient was removed from the study, resulting from grade 4 liver toxicity: this patient later resumed taking imatinib 400 mg daily, without any further complications. No unexpected adverse events were recorded. The incidence of adverse events is slightly higher with respect to other studies with imatinib 400 mg14,16 and 600 mg35 ; however, it was possible to treat a large proportion of patients with high-dose imatinib.
In conclusion, our study indicates that 800 mg imatinib is effective in rapidly inducing a very high rate of cytogenetic and molecular response in intermediate Sokal risk patients; the results obtained within 12 months seem to be better than those documented in the IRIS study for intermediate-risk patients treated with 400 mg daily with a similar observation time, and comparable with and even better than those reported for the low Sokal score category. Furthermore, the overall compliance to high-dose imatinib within the frame of a nationwide multicenter trial (25 centers contributed at least one patient each) was acceptable. The running phase 3 trials, in addition to the present one and to the results of already published studies, should contribute to determining the cost-effectiveness of high-dose imatinib and, if so, whether it is to be implemented in all the naive CML patients or, conversely, in a risk-adapted way.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Katia Vecchi for her valuable assistance.
This study was sponsored by the GIMEMA CML Working Party (formerly Italian Cooperative Study Group on Chronic Myeloid Leukemia).
This work was supported by COFIN 2003, FIRB 2001, Associazione Italiana per la Ricerca sul Cancro, Consiglio Nazionale delle Ricerche, Fondazione del Monte di Bologna e Ravenna, European LeukemiaNet, Bologna AIL.
Authorship
Contribution: F.C. and G.R. drafted the manuscript and analyzed the data; M.A., S.S., and I.I. performed molecular analysis; N.T. and S.L. performed cytogenetic analysis; F.C., F. Palandri, M. Breccia, G.R.C., F.S., G. Specchia, P.G., F.I., F. Pane, G. Saglio, G.A., and G.M. enrolled the study patients and collected clinical data; and M. Baccarani and G.R. supervised the study and gave final approval for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete membership list of the GIMEMA CML Working Party appears in the Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Fausto Castagnetti, Institute of Hematology and Medical Oncology “L. e A. Seràgnoli,” Sant'Orsola-Malpighi Hospital, Via Massarenti 9, 40138 Bologna, Italy; e-mail: faucast@inwind.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal