Abstract
Notch signaling is absolutely required for β-selection during mouse T-cell development, both for differentiation and proliferation. In this report, we investigated whether Notch has an equally important role during human T-cell development. We show that human CD34+ thymocytes can differentiate into CD4+CD8β+ double positive (DP) thymocytes in the absence of Notch signaling. While these DP cells phenotypically resemble human β-selected cells, they lack a T-cell receptor (TCR)–β chain. Therefore, we characterized the β-selection checkpoint in human T-cell development, using CD28 as a differential marker at the immature single positive CD4+CD3−CD8α− stage. Through intracellular TCR-β staining and gene expression analysis, we show that CD4+CD3−CD8α−CD28+ thymocytes have passed the β-selection checkpoint, in contrast to CD4+CD3−CD8α−CD28− cells. These CD4+CD3−CD8α−CD28+ thymocytes can efficiently differentiate into CD3+TCRαβ+ human T cells in the absence of Notch signaling. Importantly, preselection CD4+CD3−CD8α−CD28− thymocytes can also differentiate into CD3+TCRαβ+ human T cells without Notch activation when provided with a rearranged TCR-β chain. Proliferation of human thymocytes, however, is clearly Notch-dependent. Thus, we have characterized the β-selection checkpoint during human T-cell development and show that human thymocytes require Notch signaling for proliferation but not for differentiation at this stage of development.
Introduction
T-cell development is a well ordered process that is characterized by several discrete developmental stages.1 In mice, early thymocyte progenitors differentiate into DN2 cells and subsequently into DN3a thymocytes, during which Notch activation continues to increase,2,3 thereby maintaining differentiation along the T-lineage pathway4,5 and supporting T-cell receptor (TCR) rearrangements.6 Successful TCR-β chain production results in β-selection, characterized by CD27 up-regulation (DN3b),2 extensive proliferation, and rapid differentiation into DN4 and immature single positive (ISP) CD8+ cells and next into CD4+CD8+ double positive (DP) thymocytes. Pre-TCR and Notch signaling are simultaneously required for differentiation and proliferation at the β-selection checkpoint.7-10 After β-selection, a sharp decline in Notch activity occurs in DN3b thymocytes4,5 that is maintained in DN4/ISP, DP, and single positive thymocytes, suggesting a less stringent role for Notch signaling at these stages, despite still contributing to T-cell development in an in vitro culture system.10 This transition is also characterized by a temporary down-regulation of the RAG genes, the permanent repression of pTa expression, and the induction of TCR-α rearrangements as indicated by TCR-Cα expression.2 TCR-γδ T cells, which diverge from TCR-αβ lineage cells at the DN2/DN3 stages of development, are less stringently dependent on Notch signaling compared with αβ-lineage cells.2,10-12
In human, certain aspects of T-cell development and the role that Notch plays in them are less clear, although it is obvious that Notch is essential to induce and support human T-cell development.13 Human CD34+CD1−CD7− cells comprise the most immature postnatal thymocyte subset,14,15 and CD7 up-regulation specifies NK/T-cell fate.14,16 Notch is important for proliferation of these uncommitted CD34+CD1− thymocytes and to maintain T-lineage fidelity.17 Up-regulation of CD1 marks T-lineage commitment and subsequent ISP CD4+ cells first express CD8α and then CD8β as they become DP thymocytes.18 The β-selection checkpoint has not been well characterized during this process. TCR-β+ cells can be detected in ISP4+ cells, and their frequency increases in the CD4+CD8α+ stage.19 Recently some markers have been suggested that could clarify this process.18 As a result, the role of Notch signaling has not been established during human β-selection. This is crucial because human CD34+ thymocytes have been shown to differentiate into DP cells in the absence of both a TCR-β chain and Notch signaling,20 a clear difference compared with the mouse.5,8,10
To further investigate the Notch requirement during early human T-cell development, we now used the dominant-negative mutant of Mastermind-like-1 (DNMAML1) as well as the OP9 coculture system and confirm that T-lineage–committed precursors can efficiently generate DP cells in the absence of Notch signaling. We also pinpointed the β-selection checkpoint in human and show, by introducing a TCR-β chain in unselected thymocytes, that differentiation through this checkpoint can occur in the absence of Notch signaling. Proliferation, however, is clearly Notch-dependent.
Methods
Monoclonal antibodies
The following monoclonal antibodies were used from BD Biosciences (Franklin Lakes, NJ): FITC (fluorescein isothiocyanate)–conjugated CD3, CD7, CD8α, TCR-γδ; phycoerythrin (PE)-conjugated CD4, CD19, CD45, CD56, TCR-γδ; allophycocyanin (APC)-conjugated CD3, CD7, CD34, human leukocyte antigen (HLA)–DR. The following monoclonal antibodies were used from Miltenyi Biotec (Bergisch Gladbach, Germany): PE-conjugated TCR-αβ and APC-conjugated CD4, CD28, CD45, CD71. CD8β-PE was from Beckman Coulter (Fullerton, CA); CD4 PE-Cy7, CD3 Alexa-750, and streptavidin-PE-Cy5.5 from eBiosciences (San Diego, CA); and TCR-β PE from Ancell (Bayport, MN). CD1a-FITC was obtained from ATCC (Manassas, VA) and CD8β-biotin was a generous gift from E. Reinherz (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA). Rat anti–mouse monoclonal antibody (mAb) CD45-cychrome (30F1 1.1; BD Biosciences) was used to gate out mouse cells during flow cytometry, and anti-FcgRII/III mAb (clone 2.4.G2; Dr J. Unkeless, Mount Sinai School of Medicine, New York, NY) was used to block murine Fc receptors.
Isolation of cord blood and thymocyte progenitors
Pediatric thymuses and cord blood (CB) samples were obtained and used according to the guidelines of the Medical Ethical Commission of Ghent University Hospital (Ghent, Belgium), and informed consent was obtained in accordance with the Declaration of Helsinki. The 6-color flow cytometric analysis of human postnatal thymocytes was performed on a LSRII flow cytometer using FACSDiva software (BD Biosciences). All other flow cytometric data were generated of a FACSCalibur using Cellquest software (BD Biosciences). Cell sorting of populations was performed on a FACSVantage using Cellquest software. CD34+Lin− CB and CD34+ thymocytes were enriched using MACS with CD34 microbeads (Miltenyi Biotec) as described.20 CB cells were sorted as CD34+CD19−CD56− (CD34+Lin−) cells. Enriched CD34+ thymocytes were, where necessary, sorted after CD34-APC, CD1-FITC, and CD4-PE labeling into CD34+CD1−CD4− and CD34+CD1+CD4− fractions. To obtain ISP4+ cells, thymocytes were depleted for CD3/CD8 using Dynal beads sheep anti–mouse IgG (Invitrogen, Carlsbad, CA) and afterward labeled with CD34-FITC, CD3-FITC, CD8α-FITC, CD4-PE, and CD28-APC to sort CD34−CD3−CD8α−CD4+CD28− and CD34−CD3−CD8α−CD4+CD28+ cells.
Plasmid construction and viral production
The MigR1 control vector and the DNMAML1-EGFP (enhanced green fluorescent protein) fusion construct were generously provided by Warren Pear (Department of Immunology, University of Pennsylvania, Philadelphia, PA).21 For virus production, Phoenix-A cells were transiently transfected,22 and viral supernatant collected after 48 and 72 hours. TCR-β was amplified from cDNA from Jurkat cells using Platinum Pfx (Invitrogen) with the following primers: 5′-ATCTCAGAATTCTCTGCTCTCACTCTGCCATGGAC-3′ (forward) and 5′-ATTGTACTCGAGTCAGAAATCCTTTCTCTTGACCATG-3′ (reverse) containing EcoRI and XhoI restriction sites (italic sequences) respectively that were used for cloning in the retroviral LZRS control vector.22 Two versions were generated, one containing EGFP, the other containing nerve growth factor receptor (NGFR) as a marker gene. Infectious retrovirus was produced as described.22 Viral supernatants were stored in aliquots at −70°C until use. Retroviral transduction of CB cells22 and thymocytes23 has been described.
Fetal thymus organ cultures and OP9 cocultures
Fetal thymus organ cultures (FTOCs), with or without γ-secretase inhibition, were performed as described.20 For DNMAML1 experiments, 104 EGFP+ cells were added per lobe for each retroviral construct and for both CB and thymocyte experiments. For TCRβ experiments in FTOC, 4 × 103 EGFP+ cells were added per lobe. OP9 cocultures were initiated with 5 × 103 CD34+CD1+ thymocytes or with 104 CD4+CD3−CD8α−CD28− and CD4+CD3−CD8α−CD28+ thymocytes. For OP9 cocultures with transduced CD34+CD1− thymocytes, 4 to 5 × 103 EGFP+ transduced cells were plated. OP9 cocultures were performed as described24 with the exception that all cultures contained 2.5 ng/mL stem cell factor (SCF; a kind gift of Amgen, Thousand Oaks, CA) in addition to 5 ng/mL of both IL-7 and Flt3L (R&D Systems, Minneapolis, MN). Cocultures were harvested by forceful pipetting at the indicated time points.
CFSE labeling and intracellular staining
Intracellular staining of TCR-β was performed after initial cell surface staining using Fix & Perm reagents (Imtec) as described.20
Carboxyfluorescein succinimidyl ester (CFSE) labeling was performed by incubating the cells for 4 minutes at 37°C in phosphate buffered saline (PBS) containing 5 μM CFSE (Invitrogen). Cells were washed 3 times in cold PBS containing 30% fetal calf serum and once in PBS without serum before initiation of OP9 stromal cocultures.
Gene expression analysis
Cells were resuspended in Trizol (Invitrogen) or RLT buffer (QIAGEN, Valencia, CA) and stored at −70°C before RNA extraction. RNA was isolated using RNeasy (QIAGEN) and converted into cDNA using Superscript RT II (Invitrogen). Real-time PCR reactions were performed using qPCR Core kit for SYBR Green I (Eurogentec) on a 7300 Real-time PCR system (Applied Biosystems, Foster City, CA). Primer sequences are available upon request. In the cases of DNMAML1 and MigR1 transduced cells, thymocytes were recultured for 24 hours on OP9-DL1 after transduction to reinduce Notch activation after the 2-day transduction period in which Notch signals were absent. For these experiments, relative expression levels were calculated for each gene using the ΔΔCt method using β-actin for normalization. Gene expression data for freshly isolated thymocytes subset are presented as relative expression levels using the ΔCt method using β-actin for normalization.
Results
DNMAML1 allows the further differentiation of CD34+ thymocytes into CD4+CD8β+ DP cells
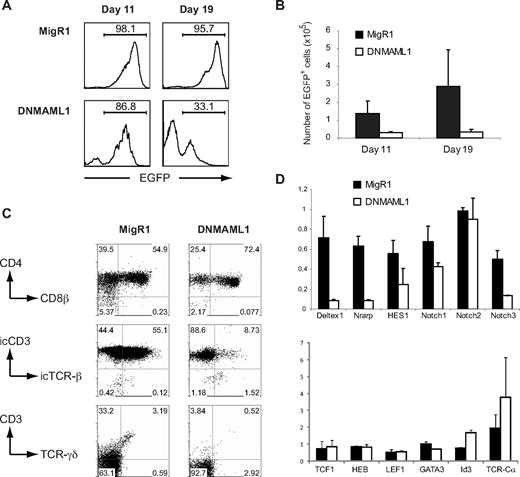
To investigate the requirement for Notch signaling during the early intrathymic stages of human T-cell development, we chose to inhibit Notch signaling using overexpression of DNMAML1.21 The efficacy of DNMAML1 to inhibit Notch signaling in human precursor cells was shown by the inhibition of T-cell development and the induction of B- and natural killer (NK)–cell development in CD34+ CB progenitors that were cultured in FTOC (Figure S1; available on the Blood website; see the Supplemental Materials link at the top of the online article), consistent with previous experiments that used γ-secretase inhibition to block Notch signaling.20 In contrast to CB cells, thymocytes have already received in vivo Notch signaling and therefore, to bypass this initial need for Notch signaling at the induction phase of T-cell development, we retrovirally transduced CD34+ human postnatal thymocytes with the control or DNMAML1 and cultured the cells in FTOC to study further T-cell differentiation. The frequency of EGFP+ DNMAML1 transduced thymocytes decreased in FTOC (Figure 1A) and this resulted in a reduction of EGFP+ cells compared with the MigR1 control (Figure 1B). The EGFP− cells in these DNMAML1 cultures are human CD7+ thymocytes that may have silenced the retroviral vector (Figure S2A). Strikingly, DNMAML1 transduced thymocytes differentiated further along the T-cell pathway into CD4+CD8β+ DP thymocytes (Figure 1C). However, unlike control transduced cells or in vivo CD4+CD8β+ DP thymocytes,17 the majority of DNMAML1 transduced CD4+CD8β+ DP cells failed to express the TCR-β chain intracellularly (Figure 1C). In addition, the development of TCR-γδ T cells was impaired.
DNMAML allows differentiation of CD34+ thymocytes into CD4+CD8β+ DP thymocytes in FTOC. (A-C) Human CD34+ thymocytes, sorted for EGFP after infection with MigR1 and DNMAML1 retroviral constructs, were subjected to FTOC. Cultures were analyzed after 11 and 19 days as indicated, and at each time point, 3 lobes of each viral construct were pooled. Results shown are derived from 3 independent experiments. (A) Reduction in the frequency of DNMAML1 transduced EGFP+ cells in FTOC compared with the frequency of control MigR1 transduced EGFP+ thymic progenitors. Numbers in histograms indicate the percentage of EGFP+ cells. (B) Reduction in the absolute number of DNMAML1 (□) transduced EGFP+ cells that are generated in FTOC after 11 and 19 days of culture compared with the MigR1 (■) control. Numbers indicate the average number of EGFP+ cells (± SD) that were generated per lob, initiated with 10 000 EGFP+ cells. (C) Flow cytometric analysis of FTOCs after 11 days of culture, gated on EGFP+ cells as shown in histograms on the left in panel A. Numbers in dot plots indicate the percentage of cells in the indicated areas. (D) Gene expression analysis of genes involved in Notch signaling (top) and T-cell development (bottom) in CD34+ thymocytes, transduced with MigR1 (■) control and DNMAML1 (□) and sorted for human CD45 and EGFP after 24 hours of OP9-DL1 coculture as shown in Figure S2B. Expression levels for all genes are presented in units relative to those from EGFP− cells from the same cultures and normalized to β-actin, calculated by the ΔΔCT method. Data shown are the average of 2 independent experiments that consisted of triplicate wells each, and error bars indicate data range.
DNMAML allows differentiation of CD34+ thymocytes into CD4+CD8β+ DP thymocytes in FTOC. (A-C) Human CD34+ thymocytes, sorted for EGFP after infection with MigR1 and DNMAML1 retroviral constructs, were subjected to FTOC. Cultures were analyzed after 11 and 19 days as indicated, and at each time point, 3 lobes of each viral construct were pooled. Results shown are derived from 3 independent experiments. (A) Reduction in the frequency of DNMAML1 transduced EGFP+ cells in FTOC compared with the frequency of control MigR1 transduced EGFP+ thymic progenitors. Numbers in histograms indicate the percentage of EGFP+ cells. (B) Reduction in the absolute number of DNMAML1 (□) transduced EGFP+ cells that are generated in FTOC after 11 and 19 days of culture compared with the MigR1 (■) control. Numbers indicate the average number of EGFP+ cells (± SD) that were generated per lob, initiated with 10 000 EGFP+ cells. (C) Flow cytometric analysis of FTOCs after 11 days of culture, gated on EGFP+ cells as shown in histograms on the left in panel A. Numbers in dot plots indicate the percentage of cells in the indicated areas. (D) Gene expression analysis of genes involved in Notch signaling (top) and T-cell development (bottom) in CD34+ thymocytes, transduced with MigR1 (■) control and DNMAML1 (□) and sorted for human CD45 and EGFP after 24 hours of OP9-DL1 coculture as shown in Figure S2B. Expression levels for all genes are presented in units relative to those from EGFP− cells from the same cultures and normalized to β-actin, calculated by the ΔΔCT method. Data shown are the average of 2 independent experiments that consisted of triplicate wells each, and error bars indicate data range.
Gene expression analysis further confirmed that the Notch pathway was severely inhibited as a result of DNMAML1 overexpression. Expression of the Notch1 target genes Deltex1, Nrarp, HES1, and Notch3 was strongly reduced in DNMAML1 transduced CD34+ human thymocytes in comparison to control transduced cells, and also a slight reduction in Notch1 expression was observed (Figure 1D). The expression of TCF1, HEB, LEF, and GATA-3, transcription factors that are also essential during T-cell development, was unaffected by DNMAML1 (Figure 1D). In contrast, Id3 and TCR-Cα expression was up-regulated in DNMAML1 transduced cells compared with the controls.
Thus, despite the inhibition in Notch signaling, other T-lineage specific molecular pathways remain active and DNMAML1 transduced thymocytes can further differentiate along the T-cell pathway.
Committed T-cell precursors efficiently differentiate into DP thymocytes in short-term OP9 cocultures in the absence of Notch/DL1 signaling
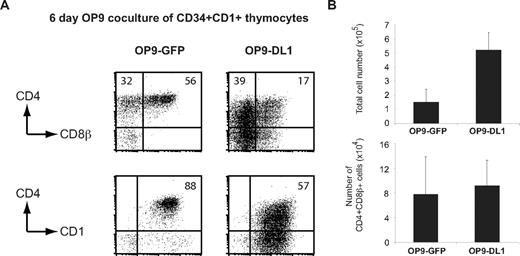
To further investigate the Notch-independent generation of human CD4+CD8β+ DP thymocytes, we initiated OP9-control and OP9-DL1 coculture experiments. Since CD34+ thymocytes contain both uncommitted as well as committed T-cell precursors, and because it has been shown that Notch signaling is important for the expansion of the earliest intrathymic human CD34+CD1− population, as well as for commitment to the T-cell lineage,17 we initiated these cultures with T-cell committed CD34+CD1+ human thymocytes. This approach allows a more direct interpretation of the Notch-dependent effects on T-cell precursors because these cells no longer have the option to divert into other hematopoietic lineages. After 6 days of coculture, a higher frequency of CD4+CD8β+ DP thymocytes, coexpressing the immature T-cell marker CD1, was observed on OP9-control stromal cells in comparison to cells cocultured on OP9-DL1 (Figure 2A). In OP9-DL1 cocultures, thymocytes only efficiently differentiated into CD4+CD8β+ DP cells after 14 days of culture (Figure S3A) and were stuck at an immature T-cell stage at day 6 but did not differentiate into a non-T–cell lineage (Figure S3B). Although the total cell yield was reduced in the absence of Notch/DL1 signaling, the total number of CD4+CD8β+ DP thymocytes was similar in both culture conditions after 6 days of coculture (Figure 2B).
Committed T-cell precursors efficiently differentiate in the absence Notch/DL signaling. (A) Freshly isolated CD34+CD1+ thymocytes were cocultured on OP9-control and OP9-DL1 stromal cells. Dot plots show CD4, CD8β, and CD1 profile in flow cytometry after 6 days of coculture. Numbers in dot plots indicate the percentage of cells in the indicated areas. (B) Average total number of cells (± SD) and CD4+CD8β+ cells generated from OP9 cocultures as shown in panel A. Data are derived from 3 independent experiments.
Committed T-cell precursors efficiently differentiate in the absence Notch/DL signaling. (A) Freshly isolated CD34+CD1+ thymocytes were cocultured on OP9-control and OP9-DL1 stromal cells. Dot plots show CD4, CD8β, and CD1 profile in flow cytometry after 6 days of coculture. Numbers in dot plots indicate the percentage of cells in the indicated areas. (B) Average total number of cells (± SD) and CD4+CD8β+ cells generated from OP9 cocultures as shown in panel A. Data are derived from 3 independent experiments.
Thus, human committed T-cell precursors can efficiently differentiate into CD4+CD8β+ DP thymocytes, independent of Notch signaling.
Human β-selection checkpoint
The CD4+CD8β+ phenotype is normally associated with β-selected cells. Thus, our results seem to indicate that immature human thymocytes can differentiate through the β-selection checkpoint in the absence of Notch signaling, in contrast to during mouse T-cell differentiation. Therefore, we investigated this further by defining the β-selection checkpoint in human using surface markers that were recently proposed.18
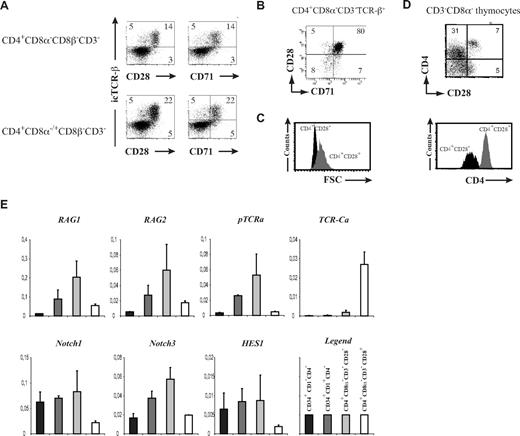
We performed 6-color flow cytometry (Figure 3A) on human postnatal thymocytes, analyzing CD3, CD4, CD8α, CD8β, intracellular TCR-β in combination with either CD28 or CD71 (Figure 3A). When gating on the CD4+CD8α−CD8β−CD3− ISP or CD4+CD8β−CD3− population (including both CD4+CD8α−CD8β−CD3− ISP and CD4+CD8α+CD8β−CD3− early DP cells), we indeed detected a population of intracellular TCR-β+ cells, of which the frequency increased according to the developmental stage and whose expression coincided with CD28 and CD71 (Figure 3A). Most CD4+CD8α−CD3−TCR-β+ thymocytes coexpress both CD28 and CD71 (Figure 3B). Relative forward scatter (FSC) analysis shows that CD4+CD8α−CD8β−CD3−CD28+ cells are larger compared with their CD28− counterpart, in agreement with pre and post β-selection stages in the mouse (Figure 3C). In addition, CD4 levels were increased in β-selected cells (Figure 3D).
Characterization of human β-selection. (A) Flow cytometric analysis of human postnatal thymocytes, showing intracellular TCR-β staining versus surface CD28 and CD71, gated on CD4+CD8α−CD8β−CD3− (top) or CD4+CD8α−/+CD8β−CD3− (bottom) cells. Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 3 independent stainings. (B) Flow cytometric analysis of TCR-β+ gated CD4+CD8α−CD3− thymocytes, showing coexpression of CD28 and CD71. Numbers in quadrants indicate the percentage of the corresponding population. (C) Relative cell size difference in CD4+CD3−CD8α−CD28− (CD4+CD28−, black histogram) versus CD4+CD3−CD8α−CD28+ (CD4+CD28+, gray histogram) thymocytes as shown by FSC analysis. (D) Dot plot shows flow cytometric analysis of CD3−CD8α− human postnatal thymocytes, showing CD4 versus CD28 expression. The histogram underneath highlights the CD4 expression level difference in CD4+CD3−CD8α−CD28− (CD4+CD28−, black histogram) versus CD4+CD3−CD8α−CD28+ (CD4+CD28+, gray histogram) thymocytes. Data shown are representative of at least 3 independent analyses. (E) Quantitative real-time RT-PCR gene expression analysis of Notch and T cell–related genes in early human postnatal thymocyte subsets, ordered according to developmental progression along the T-cell pathway. Data shown are the average (± SEM) from 2 sets of independent samples, relative to β-actin levels.
Characterization of human β-selection. (A) Flow cytometric analysis of human postnatal thymocytes, showing intracellular TCR-β staining versus surface CD28 and CD71, gated on CD4+CD8α−CD8β−CD3− (top) or CD4+CD8α−/+CD8β−CD3− (bottom) cells. Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 3 independent stainings. (B) Flow cytometric analysis of TCR-β+ gated CD4+CD8α−CD3− thymocytes, showing coexpression of CD28 and CD71. Numbers in quadrants indicate the percentage of the corresponding population. (C) Relative cell size difference in CD4+CD3−CD8α−CD28− (CD4+CD28−, black histogram) versus CD4+CD3−CD8α−CD28+ (CD4+CD28+, gray histogram) thymocytes as shown by FSC analysis. (D) Dot plot shows flow cytometric analysis of CD3−CD8α− human postnatal thymocytes, showing CD4 versus CD28 expression. The histogram underneath highlights the CD4 expression level difference in CD4+CD3−CD8α−CD28− (CD4+CD28−, black histogram) versus CD4+CD3−CD8α−CD28+ (CD4+CD28+, gray histogram) thymocytes. Data shown are representative of at least 3 independent analyses. (E) Quantitative real-time RT-PCR gene expression analysis of Notch and T cell–related genes in early human postnatal thymocyte subsets, ordered according to developmental progression along the T-cell pathway. Data shown are the average (± SEM) from 2 sets of independent samples, relative to β-actin levels.
Quantitative RT-PCR analysis revealed significant changes in gene expression as a result of the β-selection process. While RAG1, RAG2, and pTα expression is gradually up-regulated from the most immature CD34+CD1− stage into the ISP CD4+CD3−CD8α−CD28− (Figure 3E) stage, the expression of these genes is sharply down-regulated in CD4+CD3−CD8α−CD28+ thymocytes (Figure 3E). In contrast, expression of TCR-Cα, which marks opening of the TCR-α locus before rearrangement at the DP stage, is strongly and specifically induced in CD4+CD3−CD8α−CD28+ thymocytes while virtually undetectable in earlier stages of T-cell development. Expression of the Notch receptors Notch1 and Notch3 was also down-regulated in CD4+CD3−CD8α−CD28+ cells, as well as the target gene HES1 (Figure 3E).
Thus, differential CD28 expression distinguished TCR-β+ from TCR-β− human thymocytes at the ISP4+ stage and characterizes the human β-selection checkpoint. This developmental transition is associated with significant changes in the expression of genes that are critically involved in T-cell development.
Notch signaling effects on the development of thymocytes before and after β-selection
To determine the Notch signaling requirements for the further development of human thymocytes before and after β-selection, CD4+CD3−CD8α−CD28− and CD4+CD3−CD8α−CD28+ cells (CD4+CD28− and CD4+CD28+ respectively in Figure 4) were sorted and cultured on OP9-GFP and OP9-DL1. After 6 days of culture, most CD28− ISP cells had lost CD4 expression on OP9-DL1 and had not differentiated into CD4+CD8β+ DP cells, despite up-regulating CD28 and CD71. A significant portion differentiated into TCR-γδ cells (Figure 4A) and the TCR-αβ potential of CD4+CD28− thymocytes on OP9-DL1 was only detectable after 13 days of coculture (Figure S4). In contrast, in OP9-GFP cocultures, the cells differentiated into CD4high and DP cells, and less efficiently into TCR-γδ T cells, but no up-regulation of CD28 and CD71 was observed (Figure 4A). β-selected CD28+ ISP cells maintained CD4, CD28, and CD71 expression on OP9-DL1 but few had differentiated into CD4+CD8β+ DP cells. We also observed a significant population of TCR-γδ T cells but only a minor fraction of TCR-αβ T cells. In contrast, on OP9-GFP, β-selected cells rapidly differentiated into CD4+CD8β+ DP and CD3+TCR-αβ+ cells but not TCR-γδ T cells and failed to maintain CD28 and CD71 expression (Figure 4A). In the presence of Notch signaling on OP9-DL1 stromal cells, β-selected CD28+ISP cells displayed slower kinetics with respect to their differentiation into CD4+CD8β+ DP and CD3+TCR-αβ+ cells (Figure S4).
Human β-selection and Notch signaling requirements. (A) Flow cytometric analysis of human CD4+CD3−CD8α−CD28− (CD4+CD28−) and CD4+CD3−CD8α−CD28+ (CD4+CD28+) postnatal thymocytes after 6 days of coculture on OP9-GFP or OP9-DL1 stromal cells. Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 5 independent experiments. (B) Flow cytometric analysis of CFSE labeled CD4+CD28− and CD4+CD28+ thymocytes after OP9-DL1 (solid line) and OP9-GFP (dotted line) stromal coculture shows Notch-DL1 dependent optimal proliferation of both populations. (C) Corresponding total, CD3+TCR-αβ+, and CD3+TCR-γδ+ cellularity for cultures depicted in panel A. Data represent mean (± SEM; n = 5). * indicates significant differences (P < .05).
Human β-selection and Notch signaling requirements. (A) Flow cytometric analysis of human CD4+CD3−CD8α−CD28− (CD4+CD28−) and CD4+CD3−CD8α−CD28+ (CD4+CD28+) postnatal thymocytes after 6 days of coculture on OP9-GFP or OP9-DL1 stromal cells. Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 5 independent experiments. (B) Flow cytometric analysis of CFSE labeled CD4+CD28− and CD4+CD28+ thymocytes after OP9-DL1 (solid line) and OP9-GFP (dotted line) stromal coculture shows Notch-DL1 dependent optimal proliferation of both populations. (C) Corresponding total, CD3+TCR-αβ+, and CD3+TCR-γδ+ cellularity for cultures depicted in panel A. Data represent mean (± SEM; n = 5). * indicates significant differences (P < .05).
Proliferation of the CD28− and CD28+ populations was directly assessed by CFSE labeling and monitoring of CFSE dilution. A significant reduction in proliferation for both the CD28− and CD28+ populations in OP9-GFP cultures was observed compared with when cultured on OP9-DL1 (Figure 4B), resulting in higher total cell numbers on OP9-DL1 (Figure 4C). CD3+TCR-γδ+ cells were most efficiently generated on OP9-DL1 cells compared with on OP9-GFP cultures. However, after 6 days of culture, CD3+TCR-αβ+ cells were consistently more efficiently generated on OP9-GFP cells compared with on OP9-DL1 (Figure 4C). Analysis after 2 weeks indicated the loss of the OP9-GFP cultures (data not shown), presumably due to lack of proliferation.
These results show that developmental progression of β-selected thymocytes into CD3+TCR-αβ+ T cells can occur in the absence of Notch/DL signaling, consistent with the observed shutdown of the Notch pathway by gene expression analysis (Figure 3C).
Notch signaling is required for proliferation, but not for differentiation during β-selection
Since CD4+CD3−CD8α−CD28− cells fail to generate a TCR-β chain in the absence of Notch signaling and CD4+CD3−CD8α−CD28+ thymocytes have already passed β-selection (in the presence of Notch signaling), it was still unclear whether Notch signaling is required in conjunction with pre-TCR signaling7,8 to allow differentiation into human CD3+TCR-αβ+ cells. To investigate the direct requirement for Notch signaling during β-selection, we retrovirally transduced TCR-β into CD4+CD3−CD8α−CD28− thymocytes and cocultured the cells on OP9-GFP and OP9-DL1 stromal cells (Figure 5A). After 6 days, cocultures with control transduced cells on OP9-DL1 and OP9-GFP displayed low frequencies of CD3+TCR-αβ+ cells (Figure 5B). TCR-β transduced cells generated higher frequencies of DP and CD3+TCR-αβ+ cells in the absence of DL1 (Figure 5B). However, despite expressing high levels of CD4 and CD1, consistent with cells that passed the β-selection checkpoint, TCR-β transduced cells failed to up-regulate CD28 in the absence of Notch signaling. Analysis of proliferation with CFSE labeling showed that introduction of TCR-β chain enhanced cellular proliferation on OP9-DL1 cells compared with control transduced cells (Figure 5C), but that Notch signaling was required for optimal proliferation (Figure 5D). The capacity of early human thymocytes to differentiate into CD3+TCR-αβ+ cells in the absence of Notch signaling was also confirmed with TCR-β transduction of the most immature CD34+CD1− precursors in the OP9 system (Figure S5) and in the more physiologic FTOC model (Figure S6). This shows that human T-cell precursors do not require Notch signaling at the β-selection checkpoint to differentiate into CD3+TCR-αβ+ thymocytes. Proliferation, however, is clearly reduced in the absence of Notch signaling.
Notch signaling is required for proliferation, but not for differentiation during β-selection. (A) Experimental outline. (B) Flow cytometric analysis of NGFR+ gated control or TCR-β transduced human CD4+CD3−CD8α−CD28− thymocytes after 6 days of OP9-DL1 and OP9-GFP coculture. Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 3 independent experiments. (C) Flow cytometric analysis of CFSE-labeled control or TCR-β–transduced CD4+CD3−CD8α−CD28− thymocytes after 6 days of OP9-DL1 coculture. Dot plots show NGFR (marker gene for control and TCR-β transduction) expression versus CFSE label for control (top) and TCR-β (bottom) transduced cells. Numbers to the right of dot plots show ratio of CFSElow versus CFSEhigh labeled cells for transduced and untransduced cells for both sets of transduction, indicating enhanced proliferation after TCR-β transduction. (D) Flow cytometric analysis of CFSE labeled TCR-β transduced CD4+CD28− thymocytes after OP9-DL1 (solid line) and OP9-GFP (dotted line) stromal coculture shows Notch-DL1 dependent optimal proliferation in conjunction with pre-TCR signaling.
Notch signaling is required for proliferation, but not for differentiation during β-selection. (A) Experimental outline. (B) Flow cytometric analysis of NGFR+ gated control or TCR-β transduced human CD4+CD3−CD8α−CD28− thymocytes after 6 days of OP9-DL1 and OP9-GFP coculture. Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 3 independent experiments. (C) Flow cytometric analysis of CFSE-labeled control or TCR-β–transduced CD4+CD3−CD8α−CD28− thymocytes after 6 days of OP9-DL1 coculture. Dot plots show NGFR (marker gene for control and TCR-β transduction) expression versus CFSE label for control (top) and TCR-β (bottom) transduced cells. Numbers to the right of dot plots show ratio of CFSElow versus CFSEhigh labeled cells for transduced and untransduced cells for both sets of transduction, indicating enhanced proliferation after TCR-β transduction. (D) Flow cytometric analysis of CFSE labeled TCR-β transduced CD4+CD28− thymocytes after OP9-DL1 (solid line) and OP9-GFP (dotted line) stromal coculture shows Notch-DL1 dependent optimal proliferation in conjunction with pre-TCR signaling.
Discussion
During mouse T-cell development, Notch signaling is essential to support developmental progression along the T-cell lineage5 and for differentiation and proliferation at the β-selection checkpoint.7,8 Here, we show that human postnatal thymocytes can differentiate into CD4+CD8β+ DP thymocytes in the absence of Notch signaling. This also occurs independently of pre-TCR signaling as the cells fail to generate a TCR-β chain. Through characterization of the human β-selection checkpoint, using CD28 as a marker that is up-regulated at this development stage, we show that preselection ISP CD4+CD3−CD8α−CD28− thymocytes can differentiate into CD3+TCR-αβ+ T cells when provided with at functional TCR-β chain. Although differentiation can occur in the absence of Notch signaling, proliferation of human thymocytes is clearly Notch dependent as shown by CFSE staining.
Previously, we have shown that inhibition of Notch signaling through addition of the γ-secretase inhibitor DAPT accelerates differentiation of early human thymocytes into CD4+CD8β+ DP thymocytes.20 Since γ-secretase inhibitors may also affect the expression of other surface markers that could be of importance for normal T-cell differentiation, we excluded this as a possible cause for our observations as in this report both DNMAML1 overexpression and absence of DL1 induced Notch signaling in the OP9 coculture system provide similar results. The inhibition of Notch signaling by DNMAML1, which in human has only been documented in Notch transformed T-ALL cells25 but not in primary cells, is clearly demonstrated through gene expression analysis and the observation that DNMAML1 transduced CB precursors adapt B and NK cell fates when cultured in a thymic microenvironment. Thus, human early thymocytes can differentiate in the absence of Notch signaling into CD4+CD8β+ DP thymocytes that resemble cells that passed through the β-selection checkpoint.26
Notch signaling does remain important, however, for the generation of a functional TCR-β chain. At these early stages, Notch1 signaling is critical for mediating V to DJ rearrangements at the TCR-β locus as demonstrated in both mouse6 and human.20 Thus, our data suggest that the generation of DP thymocytes in human can occur in the absence of pre-TCR signaling. This, in fact, has also been observed in conditional Notch1-deficient animals.6 But while these mouse DP cells were generated in the presence of potentially active Notch2, -3, and -4,6 the differentiation of the most immature human thymocytes into CD4+CD8β+ DP cells, occurred in the complete absence of Notch signaling because both DAPT27 and DNMAML121 interfere with the Notch activation through all 4 Notch receptors.
To further investigate this Notch independent differentiation and to dissect the role of Notch signaling during human β-selection more clearly, we first tried to enhance the characterization of this developmental checkpoint in human T-cell development. While CD8β expression clearly identifies thymocytes that are virtually 100% positive for intracellular TCR-β,26 certain earlier stages of human T-cell development also contain TCR-β+ thymocytes.18,19 This prompted us to further characterize this stage, using markers that were recently proposed.18 In the mouse, differential CD27 expression separates DN3 thymocytes before and after β-selection, allowing us to dissect the molecular changes at this critical developmental stage.2 In addition, CD28 correlates with TCR-β expression in mouse DN3 cells.28 Here, we show that intracellular TCR-β expression in human ISP4+ thymocytes, a stage comparable with the mouse DN3 stage, correlates with the expression of CD28. Also CD71, marking cycling thymocytes,29 is up-regulated, in agreement with the increased cell size of CD28+ISP4+ cells and the fact that β-selection induces proliferation. This stage is also characterized by distinct changes in gene expression. Notch receptors and target genes are down-regulated after β-selection, indicating that this pathway becomes less important during later differentiation, similarly as during mouse T-cell development.2 In addition, the expression of the RAG and pTα genes is turned off because the pre-TCR complex has been fully generated, and TCR-α transcripts, which are characteristic at the start of TCR-α rearrangements, are initiated, also in correlation with the β-selection checkpoint in the mouse.2 Thus, we have identified this critical stage during human T-cell development.
At the β-selection checkpoint, 2 separate phenomena occur. One aspect is the differentiation into cells that express the CD4 and CD8 surface markers, being DP thymocytes.2 Our data show that, in human, this can occur in the absence of Notch signaling as discussed above, albeit at the expense of the generation of a functional TCR-β chain. This process occurs quite efficiently, as shown by the similar amount of DP cells that are generated in short-term culture with committed T-cell precursors. Inhibition of Notch signaling in immature TCR-β− thymocytes seems to accelerate differentiation toward the DP stage,20 perhaps because Notch signaling induces such an amount of proliferation in early thymocytes that differentiation is prevented.17 The CD4+CD8β+ DP cells that are generated in the absence of Notch signaling show increased levels of CD4 and CD1, and gene expression analysis of DNMAML1 transduced cells indicated increased Id3 and TCR-Cα expression, events that also occur during normal β-selection.2,18,30 When provided with a functional TCR-β chain, thymocytes in a developmental stage prior β-selection are capable of differentiating into cells containing a full CD3+TCR-αβ+ TCR complex. In addition, this process is very efficient and occurs much faster compared with that when Notch signaling is present. Thus, Notch-starvation of human thymocytes seems to remove a barrier that prevents further differentiation of the cells. These observations are very different from mouse experiments, as no DP thymocytes or surface TCR-β+ thymocytes can be generated from DN3 thymocytes in the absence of Notch signaling.7,10
A second major consequence of β-selection is extensive proliferation. This is clearly Notch-dependent as observed through CFSE staining and cell number analysis. Accordingly, CD28 and CD71, both important for thymocyte proliferation31,32 require Notch signaling to be expressed after pre-TCR signaling. Thus, there is a clear demarcation between the requirements for Notch signaling at the level of differentiation versus proliferation during human T-cell development.
One consistent observation is that human T-cell differentiation is delayed on OP9-DL1 compared with in the absence of DL1. This is apparent for both CD34+ thymocytes, as well as for the later CD4+CD8−CD3−CD28− and CD4+CD8−CD3−CD28+ subsets. We believe that this is the result of continuous proliferation that inhibits cellular differentiation. Toribio and colleagues17 have initially reported the Notch-dependent proliferation of CD34+ human thymocytes and concluded that Notch signaling, in conjunction with IL-7, is important for expansion of the early T-cell precursor pool. This is in agreement with our observation that differentiation from CD34+ thymocytes is slower in the presence of Notch signaling compared with in its absence. In contrast, total cell numbers are increased on OP9-DL1, presumably due to the increased proliferation. We also observe this delay in differentiation in the pre β-selection CD4+CD8−CD3−CD28− cells and show, through CFSE labeling, that these cells proliferate more compared with Notch-deprived cells that show faster differentiation. Since TCR rearrangements mainly occur at this stage, we believe that the Notch-induced proliferation, and consequent DNA synthesis, inhibits efficient TCR rearrangements that normally occur in noncycling resting cells, thereby reducing efficient differentiation as progression toward the next developmental stage depends on the success of these recombination events. A similar rationale can be used to explain the delay in differentiation for β-selected CD4+CD8−CD3−CD28+ human thymocytes as they still need to generate a TCR-α chain through TCR rearrangements before a full CD3-TCR-αβ complex can be expressed. Thus, modulation of Notch signaling is critical to balance between proliferation and differentiation. In vivo, this precise regulation at specific developmental stages is presumably regulated through migration toward different niches that have appropriate densities of Notch ligands.
Since our results show that human β-selection can occur in the absence of Notch signaling, this indicates that, for TCR-αβ T-cell development, the Notch-dependent to Notch-independent transition occurs earlier during human development compared with during mouse T-cell development. In this respect, human TCR-αβ T-cell development closely resembles mouse TCR-γδ T-cell development, as this developmental process is also less Notch-dependent.2,10-12 Combined with the observation that high concentrations of Notch signaling through overexpression of intracellular Notch increase human TCR-γδ T-cell development at the expense of αβ T-cell development,13,33 which also contrasts mouse data,34 these observations suggest a reverse Notch dependency for the TCR-αβ/γδ lineage decision point between mouse and human. The reduced dependence of human αβ-lineage differentiation on Notch signaling suggests that other signaling pathways could be responsible for regulating survival at this developmental stage.9 Alternatively, human thymocytes might be more robust compared with mouse cells so that they allow the detection of these differentiated thymocytes. In this respect, it is interesting to note that one report has also described the generation of DP cells in the absence of Notch signaling after a short 2-day culture period.35 In each case, our observations clearly indicate differences in the role of Notch signaling during human and mouse T-cell development. Given the role of Notch signaling in the malignant transformation of T-lineage cells,36 these differences highlight the importance of further studies on human cells because they are critical for understanding the molecular mechanisms behind transformation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Warren S. Pear (University of Pennsylvania, Philadelphia, PA) for the generous gift of the MigR1 and DNMAML1 retroviral constructs, Juan Carlos Zúñiga-Pflücker (University of Toronto, Toronto, ON) for the OP9-DL1 cells, and C. Collier for animal care.
This work was supported by the Odysseus program of the Fund for Scientific Research Flanders (FWO) and grants from the FWO, the Flemish Institute for the Advancement of Scientific-Technological Research in the Industry (IWT), and the Concerted Research Action of Ghent University (GOA). T.T. is a postdoctoral researcher of the FWO. I.V.d.W. is a PhD student supported by a grant from the IWT.
Authorship
Contribution: T.T. designed and performed research, analyzed and interpreted data, and wrote the paper; I.V.d.W. and G.D.S. performed research; M.D.S. performed research and analyzed and interpreted data; B.V. and G.L. analyzed and interpreted data; and J.P. designed research and analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tom Taghon, Department of Clinical Chemistry, Microbiology, and Immunology, Ghent University, University Hospital Ghent, 4BlokA, De Pintelaan 185, B-9000 Ghent, Belgium; e-mail: Tom.Taghon@ugent.be.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal