Abstract
Gram-negative bacterial infections, unlike viral infections, do not typically protect against subsequent viral infections. This is puzzling given that lipopolysaccharide (LPS) and double-stranded (ds) RNA both activate the TIR domain–containing adaptor-inducing interferon β (TRIF) pathway and, thus, are both capable of eliciting an antiviral response by stimulating type I interferon (IFN) production. We demonstrate herein that SH2-containing inositol-5′-phosphatase (SHIP) protein levels are dramatically increased in murine macrophages via the MyD88-dependent pathway, by up-regulating autocrine-acting transforming growth factor-β (TGFβ). The increased SHIP then mediates, via inhibition of the phosphatidylinositol-3-kinase (PI3K) pathway, cytosine-phosphate-guanosine (CPG)– and LPS-induced tolerance and cross-tolerance and restrains IFN-β production induced by a subsequent exposure to LPS or dsRNA. Intriguingly, we found, using isoform-specific PI3K inhibitors, that LPS- or cytosine-phosphate-guanosine-induced interleukin-6 (IL-6) is positively regulated by p110α, -γ, and -δ but negatively regulated by p110β. This may explain some of the controversy concerning the role of PI3K in Toll-like receptor–induced cytokine production. Consistent with our in vitro findings, SHIP−/− mice overproduce IFN-β in response to LPS, and this leads to antiviral hypothermia. Thus, up-regulation of SHIP in response to Gram-negative bacterial infections probably explains the inability of such infections to protect against subsequent viral infections.
Introduction
We reported in 2004 that the hematopoietic-restricted, SH2-containing inositol-5′-phosphatase (SHIP, also known as SHIP1) was markedly up-regulated in bone marrow–derived macrophages (BMmφs) and mast cells (BMMCs) on exposure to lipopolysaccharide (LPS, also known as endotoxin).1 This increase in SHIP was mediated by the production of autocrine-acting transforming growth factor-β (TGF-β) and blocked the production of pro-inflammatory cytokines and nitric oxide in response to a subsequent exposure to LPS.1 Consistent with these results, we found that SHIP−/− BMmφs, BMMCs, and SHIP−/− mice did not display endotoxin tolerance.1 Several questions arose as a result of this study, including whether SHIP plays a role in the tolerance induced by Toll-like receptors (TLRs) other than TLR4, whether TGF-β and SHIP are up-regulated via the MyD88-dependent or -independent pathway, and whether SHIP negatively regulates both of these pathways. To address these questions, we took advantage of differences in signaling initiated by TLR3, TLR4, and TLR9.
TLR3, in response to binding double-stranded RNA (dsRNA) in endosomes, activates cells exclusively through MyD88-independent pathways by recruiting TRIF, which activates the IκB kinase (IKK)–related kinases, TBK1 and IKK-ϵ. These, together with the adaptors TANK and NAP1, phosphorylate the transcription factor IRF3, allowing it to dimerize and translocate to the nucleus where it collaborates with nuclear factor-κB (NF-κB) and AP-1 to drive the transcription of many genes, including interferon-β (IFN-β), a key effector of antiviral immunity.2,3 dsRHA is also bound within the cytoplasm by the RNA helicases, RIG-1 and Mda5, and triggers MyD88-independent signaling. Specifically, these 2 RIG-1-like receptors, once bound to dsRNA, then associate with the mitochondrial-anchored adaptor, MAVS via CARD-CARD interactions. This association links RIG-1-like receptors to the TRAF3 complex, which subsequently drives type I IFN transcription by stimulating TBK1 and IKKϵ to phosphorylate IRF3.3,4
TLR4, in response to LPS binding at the cell surface, activates both the MyD88-dependent and -independent pathways. Specifically, it uses a MyD88/TIRAP heterodimer5 to stimulate a cascade involving IRAK4, TRAF6, and TAK1 to activate both MAP kinase family members and the transcription factor NF-κB, and these collaborate to activate the transcription of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6).6 TLR4 also can activate type I IFN production via the MyD88-independent pathway using a heterodimer of TRIF and TRAM.3,7
TLR9, in response to binding unmethylated cytosine-phosphate-guanosine (CpG) motifs of DNA (CpG) within endosomes, only activates the MyD88-dependent pathway, using a MyD88 homodimer to activate downstream events similar to those described earlier in the “Introduction” for TLR4-induced MyD88-dependent events.3,5
By comparing the responses of SHIP+/+ and SHIP−/− BMmφs to first and second doses of CpG, dsRNA, and LPS, we demonstrate herein that TGF-β production and the subsequent up-regulation of SHIP occur through the MyD88-dependent pathway and that SHIP up-regulation is critical for CpG-induced tolerance and cross-tolerance with LPS. More importantly, we show that LPS-induced up-regulation of SHIP blocks a subsequent exposure to dsRNA or LPS from generating an enhanced IFN-β response. Using a chemically diverse panel of p110 isoform-specific phosphatidylinositol-3-kinase (PI3K) inhibitors, we show that this SHIP-induced inhibition of IFN-β is probably mediated via its hydrolysis of the critical second messenger in the PI3K pathway, phosphatidylinositol-3,4,5-trisphosphate (PIP3).
Methods
Reagents
Escherichia coli LPS serotype O127:B8 was from Sigma-Aldrich (St Louis, MO). High performance liquid chromatography-purified phosphorothioate-modified CpG, 5′-tccatgacgttcctgacgtt-3′ was from Invitrogen (Burlington, ON). The dsRNA analog, polyinosine:cytosine, was from Sigma-Aldrich. LY294002 (LY) and wortmannin (W) were from Calbiochem (San Diego, CA). The isoform-specific PI3K inhibitors, PIK90,8 PIK-93,8 PIK-103,8 TGX-221,9 SW18, and SW30 (O.W., M.E.F., K.M.S., manuscript in preparation), and AS605240,10 were synthesized according to literature-described methods. Murine TGF-β was from StemCell Technologies (Vancouver, BC). The anti–TGF-β blocking antibody was from R&D Systems (Minneapolis, MN). Anti-SHIP P1C1 (sc-8425), anti-PTEN, and anti-Shc (sc-967) were from Santa Cruz Biotechnology (Santa Cruz, CA), anti-SHIP2 from StemCell Technologies, and anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from Research Diagnostics (Flanders, NJ). Tissue-culture reagents were from StemCell Technologies, except for monothioglycerol (MTG), which was from Sigma-Aldrich.
Mice
MyD88−/− mice on a C57BL/6 × 129/Ola background were from S. Akira and B. Beutler (Scripps Research Institute, La Jolla, CA). These mice were backcrossed onto a C57BL/6 background for one generation to generate heterozygotes, which were bred to generate MyD88+/+ and MyD88−/− littermates. SHIP heterozygotes, derived from an F2 generation of C57BL/6 × 129Sv mice, were bred to generate SHIP+/+ and SHIP−/− littermates. Mice were housed in a conventional barrier facility, and experimentation was done in accordance with institutional and Canadian Council on Animal Care (CCAC) guidelines at the British Columbia Cancer Research Centre.
BMmφ and Pmφ stimulations
SHIP+/+ and SHIP−/− BMmφs and peritoneal mφs (Pmφs) were derived as described previously1 and were removed from flasks by incubating 3 minutes at 21°C with Cell Dissociation Buffer (Invitrogen). Dissociated cells (0.5 × 106 cells/mL) in complete medium (Iscove modified Dulbecco medium + 10% fetal calf serum + 150 μM MTG + Pen/Strep + 5 ng/mL macrophage colony-stimulating factor [M-CSF]) were plated in 12-well plates for stimulation. For inhibitor studies, cells were preincubated with 14 μM LY, 50 nM W, or 0.1% dimethyl sulfoxide (DMSO) for 15 minutes in complete medium without M-CSF before stimulation with 3 nM CpG, 5 μM dsRNA, or 10 ng/mL LPS for 3 or 24 hours. Cell supernatants were stored at −20°C for enzyme-linked immunosorbent assays (ELISAs). For pretreatment followed by stimulation studies, BMmφs or Pmφs were pretreated with the same concentrations of CpG, dsRNA, or LPS for 24 hours in complete medium without M-CSF and then treated with a second dose of CpG, dsRNA, or LPS for 24 hours before harvesting supernatants. For gene expression analyses, BMmφs were stimulated, and cells harvested into 0.5 mL TRIzol (Invitrogen) after 24 hours for TLR3 mRNA analysis or after 3 hours for IFN-β mRNA analysis.
SHIP siRNA transfection of RAW264.7 cells
Opti-MEM (300 μL) plus 4.5 μL of 20 μM SHIP siRNA or nonsilencing siRNA (QIAGEN, Mississauga, ON) plus 18 μL Hiperfect were vortexed, incubated at 21°C for 10 minutes, and added to RAW cells (0.5 × 106 cells/well in 24 well plates) after the cell medium was aspirated and incubated for 6 hours at 37°C in a CO2 incubator. RPMI plus 10% fetal calf serum (450 μL) were then added, and the cells incubated for another 24 hours and then treated with or without TLR ligand.
SDS-PAGE and Western blotting
Whole-cell lysates were prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis by washing cells once with Iscove modified Dulbecco medium plus MTG, lysing in 2× Laemmli buffer, boiling for 1 minute, and loading onto 10% polyacrylamide gels as described previously.11
ELISAs
ELISAs for TNF-α, IL-6 (BD Biosciences, San Jose, CA), and TGF-β (R&D Systems) were performed per the manufacturer's instructions. The ELISA for IFN-β used rat anti–mouse IFN-β mAb 7F-D3 (Seikagaku America, Rockville, MD) as the capture antibody, rabbit anti–mouse IFN-β polyclonal antibody (R&D Systems) as the detection antibody, and goat anti–rabbit-IgG-HRP for colorimetric detection (Jackson ImmunoResearch Laboratories, West Grove, PA); 1 mg of the IFN-β standard (Sigma-Aldrich) = 2.42 × 107 units.
Quantitative PCR
RNA was isolated from 0.5 × 106 BMmφs in 0.5 mL TRIzol (Invitrogen) as per the manufacturer's instructions. GAPDH for 5′-tgaggccggtgctgagta-3′ and GAPDH reverse, 5′-ccacagtcttctgggtgg-3′ were used for a no RT control (data not shown). cDNA synthesis was performed using oligo (dT)20-40 and Superscript II (Invitrogen) per the manufacturer's instructions. Quantitative polymerase chain reaction (PCR) was performed using the ABI Prism 5700 Q-PCR machine using 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Primer sequences used were: IFN-β for 5′-agctccaagaaaggacgaacat-3′, IFN-β reverse 5′-gccctgtaggtgaggttgatct-3′, TLR3 for 5′-tcacttgctcattctccctt-3′, TLR3 reverse 5′-gcctctccattcctggc-3′, GUS for 5′-tcggagagctcatctggaat-3′ and GUS reverse 5′-tctctggcgagtgaagatcc-3′. Oligodeoxynucleotide primers were synthesized and desalted by Invitrogen.
Mouse model of endotoxin tolerance
SHIP+/+ and SHIP−/− mice (12-14 weeks old) were given 1 mg/kg LPS intraperitoneally and challenged with 1 mg/kg LPS 24 hours later. Mouse temperatures were measured using a mouse rectal thermometer before and 1, 2, 4, 8, 12, and 24 hours after injection. Blood was collected from the tail vein at 4 hours after the first dose or 4 hours after the second dose of LPS (28 hours). After death, blood was collected via heart puncture, allowed to clot at 37°C for 30 minutes, and serum isolated by centrifugation at 1200g for 5 minutes for TNF-α and IFN-β ELISAs.
Statistical analyses
Two-tailed Student t tests were used to evaluate changes in cytokine and gene expression. One-way analysis of variance was used to compare mouse temperature data. Data were assumed to be significantly different at P values less than .05.
Results
CpG, unlike dsRNA, increases SHIP protein via the production of autocrine-acting TGF-β
Having established previously that LPS triggers an increase in SHIP and this increase is critical for endotoxin tolerance,1 we tested whether CpG and dsRNA also increased SHIP levels in SHIP+/+ BMmφs. As shown in Figure 1A, SHIP levels increased within 4 hours of CpG treatment, peaked at 24 hours to levels similar to that elicited by LPS, and remained above control levels for at least 48 hours. However, levels of 2 other PIP3 phosphatases, SHIP2 and PTEN, were unaffected by either CpG or LPS. Dose-response studies revealed that 3 nM CpG was sufficient to markedly increase SHIP levels and 30 nM gave the highest levels (Figure 1A). In contrast, dsRNA did not increase SHIP and did not affect SHIP2 or PTEN levels (Figure 1B).
CpG, like LPS, increases SHIP via the production of autocrine-acting TGF-β, whereas dsRNA does not. (A) Wild-type BMmφs were treated with 30 nM CpG for the indicated times or with 100 ng/mL LPS for 24 hours as a positive control (top panel) or the indicated concentrations of CpG for 24 hours (bottom panel) and whole-cell lysates subjected to immunoblot analyses for SHIP, SHIP2, PTEN, or GAPDH. Results shown are typical of 3 separate experiments. (B) Wild-type BMmφs were treated with 5 μg/mL dsRNA for the indicated times or with 100 ng/mL LPS for 24 hours and whole-cell lysates subjected to immunoblot analyses for SHIP, SHIP2, PTEN, and GAPDH. Results shown are typical of 3 separate experiments. Although SHIP frequently appears as multiple bands because of the presence of alternate splice forms, it appears as a singlet here because of poor gel resolution. (C) SHIP+/+ (□) or SHIP−/− (■) BMmφs were treated with CpG, dsRNA, or LPS for 24 hours and cell supernatants assessed for TGF-β by ELISA. TGF-β produced by unstimulated cells was consistently less than 20 pg/mL and has been subtracted. Data are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .05 comparing SHIP−/− to SHIP+/+. (D) Wild-type BMmφs were untreated, treated with 100 ng/mL LPS, or 30 nM CpG for 24 hours in the absence (0) or presence of a blocking antibody to TGF-β (αTGF-β) or an irrelevant control antibody (irrel). Whole-cell lysates were subjected to immunoblot analyses for SHIP, SHIP2, PTEN, and Shc. Results shown are typical of 3 separate experiments.
CpG, like LPS, increases SHIP via the production of autocrine-acting TGF-β, whereas dsRNA does not. (A) Wild-type BMmφs were treated with 30 nM CpG for the indicated times or with 100 ng/mL LPS for 24 hours as a positive control (top panel) or the indicated concentrations of CpG for 24 hours (bottom panel) and whole-cell lysates subjected to immunoblot analyses for SHIP, SHIP2, PTEN, or GAPDH. Results shown are typical of 3 separate experiments. (B) Wild-type BMmφs were treated with 5 μg/mL dsRNA for the indicated times or with 100 ng/mL LPS for 24 hours and whole-cell lysates subjected to immunoblot analyses for SHIP, SHIP2, PTEN, and GAPDH. Results shown are typical of 3 separate experiments. Although SHIP frequently appears as multiple bands because of the presence of alternate splice forms, it appears as a singlet here because of poor gel resolution. (C) SHIP+/+ (□) or SHIP−/− (■) BMmφs were treated with CpG, dsRNA, or LPS for 24 hours and cell supernatants assessed for TGF-β by ELISA. TGF-β produced by unstimulated cells was consistently less than 20 pg/mL and has been subtracted. Data are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .05 comparing SHIP−/− to SHIP+/+. (D) Wild-type BMmφs were untreated, treated with 100 ng/mL LPS, or 30 nM CpG for 24 hours in the absence (0) or presence of a blocking antibody to TGF-β (αTGF-β) or an irrelevant control antibody (irrel). Whole-cell lysates were subjected to immunoblot analyses for SHIP, SHIP2, PTEN, and Shc. Results shown are typical of 3 separate experiments.
We then examined the ability of BMmφs to secrete TGF-β in response to CpG and dsRNA because LPS-induced SHIP induction is mediated by autocrine-acting TGF-β.1 We found that CpG, but not dsRNA, induced TGF-β and did so to levels induced by LPS (Figure 1C). To confirm that CpG-induced TGF-β was actually required for SHIP induction, we stimulated SHIP+/+ BMmφs with CpG for 24 hours with or without anti–TGF-β blocking antibody and found, by Western analysis, that CpG-induced TGF-β was essential for increased SHIP levels (Figure 1D).
SHIP induction is responsible for CpG-induced tolerance and cross-tolerance between CpG and LPS
We then asked whether SHIP affects proinflammatory cytokine production in response to CpG, dsRNA, or LPS by stimulating SHIP+/+ and SHIP−/− BMmφs with CpG, dsRNA, or LPS and assaying for 24 hours cell supernatants for TNF-α and IL-6 levels. SHIP−/− BMmφs produced significantly more TNF-α and IL-6 in response to all doses of CpG and LPS tested (Figure 2A). dsRHA stimulated substantially less TNF-α and IL-6, and the differences between SHIP+/+ and SHIP−/− BMmφs were only significant at 5 μM dsRNA (Figure 2A). To determine whether SHIP was involved in CpG-induced tolerance as well as CpG- and LPS-induced cross-tolerance,12,13 we pretreated SHIP+/+ and SHIP−/− BMmφs for 24 hours with or without CpG, dsRNA, or LPS, challenged with CpG or LPS, and measured cytokine levels after 24 hours. Pretreatment of SHIP+/+ BMmφs with CpG or LPS dramatically blunted cytokine production in response to challenge with either CpG or LPS (Figure 2B). In contrast, pretreatment of SHIP−/− BMmφs with CpG did not inhibit either CpG- or LPS-induced cytokine production (Figure 2B). As well, pretreatment of SHIP−/− BMmφs with LPS did not inhibit LPS-induced (Figure 2B) and only modestly inhibited CpG-induced TNF-α or IL-6 production (Figure 2B), probably because of LPS-induced up-regulation/activation of non-SHIP negative regulators.14 These results demonstrate a critical role for SHIP in CpG-induced tolerance and cross-tolerance between CpG and LPS. Worthy of note, pretreatment of SHIP+/+ BMmφs with dsRNA, which does not increase SHIP levels, did not induce tolerance to a subsequent exposure to either CpG or LPS (Figure 2B).
SHIP is induced via the MyD88 pathway and is responsible for CpG-induced tolerance and cross-tolerance between CpG and LPS. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were treated with the indicated concentrations of CpG, dsRNA, or LPS and 24 hours cell supernatants assessed for TNF-α (top panels) and IL-6 (bottom panels) by ELISA. Results shown are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .01 compared with SHIP+/+. NS indicates not significant. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated (pre) or not (0) with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours and then challenged with either 30 nM CpG or 10 ng/mL LPS. At 24 hours, supernatants were assessed for TNF-α and IL-6. Unstimulated cells released less than 50 pg/mL TNF-α or IL-6, and these levels were subtracted. Results shown are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .02 compared with untolerized cells. **P < .02 compared with untolerized cells. NS indicates not significant. (C) MyD88+/+ or MyD88−/− BMmφs were treated or not (0) with 20 ng/mL TGF-β for 8 hours or 100 ng/mL LPS for 24 hours. Whole cell lysates were subjected to immunoblot analyses for SHIP or Shc. Results are typical of 3 independent experiments. (D) MyD88+/+ (□) or MyD88−/− (■) BMmφs were treated with CpG or LPS for 24 hours and cell supernatants assessed for TGF-β by ELISA. TGF-β levels detected from unstimulated cells were less than 20 pg/mL and were subtracted. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .001 compared with MyD88+/+ cells.
SHIP is induced via the MyD88 pathway and is responsible for CpG-induced tolerance and cross-tolerance between CpG and LPS. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were treated with the indicated concentrations of CpG, dsRNA, or LPS and 24 hours cell supernatants assessed for TNF-α (top panels) and IL-6 (bottom panels) by ELISA. Results shown are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .01 compared with SHIP+/+. NS indicates not significant. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated (pre) or not (0) with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours and then challenged with either 30 nM CpG or 10 ng/mL LPS. At 24 hours, supernatants were assessed for TNF-α and IL-6. Unstimulated cells released less than 50 pg/mL TNF-α or IL-6, and these levels were subtracted. Results shown are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .02 compared with untolerized cells. **P < .02 compared with untolerized cells. NS indicates not significant. (C) MyD88+/+ or MyD88−/− BMmφs were treated or not (0) with 20 ng/mL TGF-β for 8 hours or 100 ng/mL LPS for 24 hours. Whole cell lysates were subjected to immunoblot analyses for SHIP or Shc. Results are typical of 3 independent experiments. (D) MyD88+/+ (□) or MyD88−/− (■) BMmφs were treated with CpG or LPS for 24 hours and cell supernatants assessed for TGF-β by ELISA. TGF-β levels detected from unstimulated cells were less than 20 pg/mL and were subtracted. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .001 compared with MyD88+/+ cells.
To confirm it was the lack of SHIP that prevented CpG and LPS pretreatment from inducing tolerance and cross-tolerance and not a secondary event in the SHIP−/− BMmφs, we treated RAW264.7 cells with or without a SHIP or nonsilencing siRNA. As shown in Figure S1A (available on the Blood website; see the Supplemental Materials link at the top of the online article), these murine macrophages, like SHIP+/+ BMmφs, increased their SHIP levels in response to CpG or LPS and treatment with a SHIP siRNA, but not a nonsilencing siRNA, blocked this increase. In addition, CpG or LPS pretreatment of parental or nonsilencing siRNA-treated RAW cells induced tolerance to a subsequent treatment with CpG or LPS, whereas SHIP siRNA-treated RAW cells did not display tolerance (Figure S1B).
SHIP induction and TGF-β production are MyD88-dependent
Because both CpG and LPS initiate their inflammatory programs via the MyD88-dependent pathway, whereas dsRNA does not,5 we asked whether SHIP induction and TGF-β production were dependent on MyD88 by stimulating MyD88+/+ and MyD88−/− BMmφs with LPS or TGF-β. We found that MyD88+/+ BMmφs dramatically increased SHIP in response to TGF-β or LPS, whereas MyD88−/− BMmφs increased SHIP in response to TGF-β but not LPS (Figure 2C). This demonstrated that MyD88−/− BMmφs could respond to exogenous TGF-β by increasing SHIP but were incapable of inducing SHIP in response to LPS, probably because they failed to produce TGF-β. Indeed, MyD88+/+ BMmφs secreted TGF-β in response to CpG or LPS, but MyD88−/− BMmφs did not (Figure 2D).
LPS- or CpG-induced increases in SHIP inhibit IFN-β production triggered by a subsequent exposure to dsRNA or LPS
We next asked whether SHIP was capable of negatively regulating the MyD88-independent pathway by treating SHIP+/+ and SHIP−/− BMmφs with dsRNA, LPS, or CpG and comparing IFN-β production. We found that SHIP−/− produced more IFN-β than SHIP+/+ BMmφs in response to dsRNA or LPS (Figure 3A). Although the difference was more pronounced in LPS-treated cells, probably because of the increased SHIP levels, the basal levels of SHIP in dsRNA-induced SHIP+/+ BMmφs were still capable of inhibiting IFN-β production. CpG, however, at 3 to 300 nM, did not induce IFN-β from either SHIP+/+ or SHIP−/− BMmφs (data not shown), consistent with CpG acting solely through the MyD88-dependent pathway.
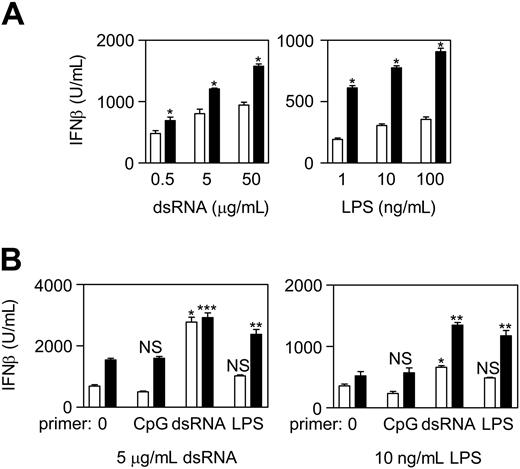
SHIP−/− BMmφs produce high levels of IFN-β after a first dose of dsRNA or LPS and markedly elevated IFN-β levels in response to a second dose. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were primed with dsRNA or LPS and 24 hours cell supernatants assessed for IFN-β by ELISA. Results are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .05 compared with SHIP+/+ cells. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (0) or primed with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours and then stimulated with either 5 μg/mL dsRNA (left panel) or 10 ng/mL LPS (right panel). Cell supernatants were harvested 24 hours later and assessed for IFN-β by ELISA. Unstimulated cells did not produce detectable IFN-β levels. Results are the mean plus or minus SEM of 3 or 4 independent experiments assayed in duplicate. *P < .001 compared with untolerized cells. **P < .02 compared with untolerized cells and compared with SHIP+/+ cells. ***P < .01 compared with untolerized cells but not significantly different from SHIP+/+ cells. NS indicates not significant.
SHIP−/− BMmφs produce high levels of IFN-β after a first dose of dsRNA or LPS and markedly elevated IFN-β levels in response to a second dose. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were primed with dsRNA or LPS and 24 hours cell supernatants assessed for IFN-β by ELISA. Results are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .05 compared with SHIP+/+ cells. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (0) or primed with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours and then stimulated with either 5 μg/mL dsRNA (left panel) or 10 ng/mL LPS (right panel). Cell supernatants were harvested 24 hours later and assessed for IFN-β by ELISA. Unstimulated cells did not produce detectable IFN-β levels. Results are the mean plus or minus SEM of 3 or 4 independent experiments assayed in duplicate. *P < .001 compared with untolerized cells. **P < .02 compared with untolerized cells and compared with SHIP+/+ cells. ***P < .01 compared with untolerized cells but not significantly different from SHIP+/+ cells. NS indicates not significant.
In contrast to the tolerizing effect that LPS and CpG have on proinflammatory cytokine production induced by a second exposure to these TLR ligands,13 a first dose of type I IFN-inducing ligand actually amplifies (ie, primes) the IFN response elicited by a second dose of type I IFN-inducing ligand, and this results in enhanced antiviral activity.15 Because SHIP negatively regulated IFN-β production, we asked whether SHIP had any impact on priming by pretreating SHIP+/+ and SHIP−/− BMmφs for 24 hours with or without CpG, dsRNA, or LPS and then stimulating with dsRNA or LPS. As shown in Figure 3B, priming with CpG, which increases SHIP levels, slightly reduced subsequent IFN-β production triggered by either dsRNA or LPS in SHIP+/+ BMmφs, but not in SHIP−/− BMmφs. In contrast, priming with dsRNA, which does not increase SHIP levels, caused a significant increase in IFN-β production in response to a subsequent exposure to dsRNA or LPS in both SHIP+/+ and SHIP−/− BMmφs. However, the enhanced response to LPS was far less dramatic in SHIP+/+ cells, probably because of the increase in SHIP levels during the subsequent 24-hour exposure to LPS. Interestingly, although LPS did not significantly prime SHIP+/+ BMmφs for a subsequent response to dsRNA or LPS, it dramatically primed SHIP−/− BMmφs (similar to that primed by dsRNA) for a subsequent response to dsRNA or LPS. This is consistent with LPS-induced SHIP limiting the ability of a second dose of LPS from enhancing a type I IFN response.
To confirm that SHIP was responsible for dampening down the effect of LPS priming on subsequent dsRNA- or LPS-induced IFN-β production, we first compared the ability of MyD88+/+ and MyD88−/− BMmφs, primed for 24 hours with LPS, to produce IFN-β in response to CpG, dsRNA, or LPS. As shown in Figure 4A, MyD88−/− but not MyD88+/+ BMmφs displayed dramatically enhanced IFN-β production in response to dsRNA or LPS if cells were primed with LPS, confirming the importance of the MyD88 pathway in limiting LPS-induced priming of subsequent dsRNA- or LPS-induced IFN-β production.
LPS-induced SHIP induction prevents an enhanced antiviral response to a second dose of LPS. (A) MyD88+/+ (□) and MyD88−/− (■) BMmφs, tolerized with 10 ng/mL LPS for 24 hours, were untreated (0) or stimulated with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours. Cell supernatants were then assessed for IFN-β by ELISA. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .01 compared with wild-type cells and comparing tolerized cells to untolerized cells. NS indicates not significant. Unstimulated cells did not produce detectable IFN-β. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (C) or treated with 10 ng/mL LPS with or without a blocking antibody to TGF-β (αTGF-β) or an irrelevant isotype control (irrel) for 24 hours and then stimulated with 5 μg/mL dsRNA (left panel) or 10 ng/mL LPS (right panel) for 24 hours. Cell supernatants were assessed for IFN-β by ELISA. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .01 compared with LPS tolerized cells or cells tolerized in the presence of the isotype control antibody. NS indicates not significantly different from tolerized cells in the absence of antibody. (C) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (C) or treated with 20 ng/mL TGF-β for 8 hours followed by stimulation with either 5 μg/mL dsRNA or 10 ng/mL LPS for 24 hours. Cell supernatants were assessed for IFN-β by ELISA. Results are the mean plus or minus SEM of 4 independent experiments assayed in duplicate. *P < .001 compared with untreated cells. NS indicates not significant.
LPS-induced SHIP induction prevents an enhanced antiviral response to a second dose of LPS. (A) MyD88+/+ (□) and MyD88−/− (■) BMmφs, tolerized with 10 ng/mL LPS for 24 hours, were untreated (0) or stimulated with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours. Cell supernatants were then assessed for IFN-β by ELISA. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .01 compared with wild-type cells and comparing tolerized cells to untolerized cells. NS indicates not significant. Unstimulated cells did not produce detectable IFN-β. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (C) or treated with 10 ng/mL LPS with or without a blocking antibody to TGF-β (αTGF-β) or an irrelevant isotype control (irrel) for 24 hours and then stimulated with 5 μg/mL dsRNA (left panel) or 10 ng/mL LPS (right panel) for 24 hours. Cell supernatants were assessed for IFN-β by ELISA. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .01 compared with LPS tolerized cells or cells tolerized in the presence of the isotype control antibody. NS indicates not significantly different from tolerized cells in the absence of antibody. (C) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (C) or treated with 20 ng/mL TGF-β for 8 hours followed by stimulation with either 5 μg/mL dsRNA or 10 ng/mL LPS for 24 hours. Cell supernatants were assessed for IFN-β by ELISA. Results are the mean plus or minus SEM of 4 independent experiments assayed in duplicate. *P < .001 compared with untreated cells. NS indicates not significant.
To determine whether the MyD88-mediated increase in TGF-β was important for preventing LPS from priming IFN-β production, we pretreated SHIP+/+ and SHIP−/− BMmφs with LPS with or without a neutralizing anti–TGF-β for 24 hours and then added dsRNA or LPS for a further 24 hours and assessed IFN-β levels. We found that LPS priming in the presence of anti–TGF-β elevated IFN-β production from SHIP+/+ BMmφs to that seen from SHIP−/− BMmφs in response to dsRNA or LPS (Figure 4B). Similar results were obtained with SHIP+/+ and SHIP−/− BMmφs pretreated with CpG + anti-TGF-β for 24 hours (data not shown). Thus, the MyD88-mediated increase in TGF-β is critical for blunting IFN-β production seen with LPS (or CpG) pretreatment.
To confirm that the TGF-β–induced increase in SHIP and not the increase in TGF-β itself was important, SHIP+/+ and SHIP−/− BMmφs were treated with TGF-β for 8 hours, which induces SHIP protein to maximal levels in SHIP+/+ BMmφs1, and then stimulated for 24 hours with either dsRNA or LPS. dsRHA- or LPS-treated SHIP+/+ BMmφs produced lower levels of IFN-β when primed with TGF-β but SHIP−/− BMmφ did not (Figure 4C), demonstrating the negative regulatory effect of TGF-β is dependent on SHIP.
We also confirmed the critical role that SHIP plays in blunting LPS-induced priming of subsequent dsRNA- or LPS-induced IFN-β secretion using RAW cells pretreated with or without SHIP or nonsilencing siRNA (Figure S2).
Up-regulation of SHIP reduces dsRNA- or LPS-induced IFN-β transcription and probably does so by hydrolyzing PIP3
To understand how the LPS-induced increase in SHIP was restraining subsequent LPS- or dsRNA-induced IFN-β production, we asked whether this was via the ability of SHIP to hydrolyze PIP3 and thus inhibit PI3K-mediated events. However, because the role of the PI3K pathway in TLR-induced signaling is very controversial, with several groups reporting a negative regulatory role for PI3K in TLR-induced cytokines and others reporting a positive role,16 we first asked whether the PI3K pathway positively or negatively regulated CpG or LPS-induced TNF-α and IL-6 production. To test this, we stimulated SHIP+/+ and SHIP−/− BMmφs with CpG or LPS for 3 hours with or without the PI3K inhibitors LY or W and found that they dramatically reduced TNF-α and IL-6 production (Figure 5A) and did so in a dose-dependent fashion (Figure S3A). dsRHA did not induce detectable levels of TNF-α or IL-6 during the 3-hour stimulation period (data not shown). Given that we observed higher CpG- and LPS-induced TNF-α and IL-6 production from SHIP−/− BMmφs (Figure 2A), this was consistent with SHIP inhibiting production of these inflammatory cytokines via inhibition of the PI3K pathway. However, both LY and W also inhibit PI3K-like kinases to various degrees,8 making interpretation difficult. Because genetic approaches to assess the role of class IA and B PI3Ks have not been very informative, in part because of compensatory effects,8 we instead tested a chemically diverse panel of p110 isoform-specific PI3K inhibitors.8 Specifically, we stimulated SHIP+/+ and SHIP−/− BMmφs with CpG or LPS with or without the α-, γ-, δ-specific PIK-90, PIK-93, and PI-103, the p110β-specific inhibitor TGX-221, the p110δ-specific inhibitor SW30, the p110γδ-dual inhibitor, SW18, and AS605240, a p110γ-directed inhibitor with some p110α activity. The structures of the inhibitors are shown in Figure S4 and their IC50 values, obtained using pure p110 preparations, are shown in Table 1. As shown in Figure 5B, they all inhibited, at 10 μM, CpG-induced TNF-α production. As well, apart from PI-103 and TGX-221, they all inhibited LPS-induced TNF-α production, albeit to a lesser extent. The more dramatic effect seen with PIK-93 may be partly the result of its inhibition of PI4KIIIβ (IC50 = 19 nM).8,17 Nevertheless, the effects seen with PIK-90, SW18, and SW30 implicate p110δ and p110γ. CpG- and LPS-induced IL-6 production was also inhibited by all isoform-specific inhibitors, except for the p110β-specific inhibitor, TGX-221. Further studies with these isoform-specific inhibitors demonstrated that they acted in a dose-dependent fashion and confirmed that TGX-221 stimulated both CpG- and LPS-induced IL-6 production (Figure S3B). This enhancement of IL-6 production with p110β inhibition could explain some of the discrepancies in the literature.
The class I p110 isoforms of PI3K positively regulate CpG- and LPS-induced TNF-α and IL-6 production except for p110β, which negatively regulates IL-6 production. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 14 μM LY, 50 nM W, or 0.1% DMSO (C) for 30 minutes before stimulation with 30 nM CpG or 10 ng/mL LPS and 3 hours cell supernatants assessed for TNF-α and IL-6 by ELISA. Results are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .02 compared with vehicle. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 10 μM PI3K p110 isoform specific inhibitors 1 through 7 or with 0.1% DMSO (sol) or without (C) vehicle control for 30 minutes before stimulation with 30 nM CpG or 10 ng/mL LPS and 24 hours cell supernatants assessed for TNF-α and IL-6 by ELISA. Results are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .05. (C) 5 × 104/mL SHIP+/+ BMmφs were exposed to 14 μM LY (●), 50 nM W (■), or 10 μM of p110 isoform specific inhibitor (1 = ▴, 2 = ▾, 3 = ♦, 4 = š, 5 =‖≤, 6 = Δ, 7 =■) for the times indicated and viable cell counts determined using trypan blue and a hemocytometer.
The class I p110 isoforms of PI3K positively regulate CpG- and LPS-induced TNF-α and IL-6 production except for p110β, which negatively regulates IL-6 production. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 14 μM LY, 50 nM W, or 0.1% DMSO (C) for 30 minutes before stimulation with 30 nM CpG or 10 ng/mL LPS and 3 hours cell supernatants assessed for TNF-α and IL-6 by ELISA. Results are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .02 compared with vehicle. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 10 μM PI3K p110 isoform specific inhibitors 1 through 7 or with 0.1% DMSO (sol) or without (C) vehicle control for 30 minutes before stimulation with 30 nM CpG or 10 ng/mL LPS and 24 hours cell supernatants assessed for TNF-α and IL-6 by ELISA. Results are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .05. (C) 5 × 104/mL SHIP+/+ BMmφs were exposed to 14 μM LY (●), 50 nM W (■), or 10 μM of p110 isoform specific inhibitor (1 = ▴, 2 = ▾, 3 = ♦, 4 = š, 5 =‖≤, 6 = Δ, 7 =■) for the times indicated and viable cell counts determined using trypan blue and a hemocytometer.
IC50 values of the p110 isoform-specific PI3K inhibitors with pure p110 enzymes
| Inhibitor . | Abbreviation . | IC50, μM . | |||
|---|---|---|---|---|---|
| p110α . | p110β . | p110γ . | p110δ . | ||
| PIK-90 | 1 | 0.011 | 0.35 | 0.018 | 0.058 |
| PIK-93 | 2 | 0.039 | 0.59 | 0.016 | 0.12 |
| PI-103 | 3 | 0.008 | 0.088 | 0.015 | 0.048 |
| TGX-221 | 4 | 5.0 | 0.005 | > 10.0 | 0.1 |
| SW18 | 5 | 6.7 | 2.4 | 0.038 | 0.005 |
| SW30 | 6 | 85 | 0.74 | 1.3 | 0.007 |
| AS605240 | 7 | 0.06 | 0.27 | 0.008 | 0.3 |
| Inhibitor . | Abbreviation . | IC50, μM . | |||
|---|---|---|---|---|---|
| p110α . | p110β . | p110γ . | p110δ . | ||
| PIK-90 | 1 | 0.011 | 0.35 | 0.018 | 0.058 |
| PIK-93 | 2 | 0.039 | 0.59 | 0.016 | 0.12 |
| PI-103 | 3 | 0.008 | 0.088 | 0.015 | 0.048 |
| TGX-221 | 4 | 5.0 | 0.005 | > 10.0 | 0.1 |
| SW18 | 5 | 6.7 | 2.4 | 0.038 | 0.005 |
| SW30 | 6 | 85 | 0.74 | 1.3 | 0.007 |
| AS605240 | 7 | 0.06 | 0.27 | 0.008 | 0.3 |
The IC50 values using intact cells are 10 to 100 times higher.30
To rule out that the effects observed with LY, W, or isoform-specific inhibitor were the result of cytotoxicity, we carried out cell viability studies and found that, apart from PIK90 (1) and PIK93 (2), which caused minor cell loss (ie, 9.3% and 9.9%, respectively, at 24 hours, the maximum exposure time for these inhibitors), there was no toxicity seen (Figure 5C).
We then investigated the role of the PI3K pathway in IFN-β production by stimulating SHIP+/+ and SHIP−/− BMmφs with dsRNA or LPS with or without LY or W and found that both significantly reduced IFN-β production (Figure 6A) and did so in a dose-dependent manner (Figure S5A). The isoform-specific inhibitors all markedly inhibited dsRNA-induced IFN-β production and also inhibited, to a lesser extent, LPS-induced IFN-β production (Figure 6A). Interestingly, p110β did not appear to play a role in LPS-induced IFN-β production. Further studies with these inhibitors confirmed that their inhibition was dose dependent (Figure S5B).
LPS-induced up-regulation of SHIP reduces, probably via inhibition of the PI3K pathway, subsequent dsRNA- or LPS-induced IFN-β mRNA levels. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 14 μM LY, 50 nM W, or 0.1% DMSO (C) for 30 minutes before stimulation with 5 μg/mL dsRNA (top) or 10 ng/mL LPS (bottom) and 3 hours cell supernatants assessed for IFN-β by ELISA. Data are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .02 compared with vehicle. In the right panels, SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 10 μM PI3K p110 isoform-specific inhibitors 1 to 7 for 30 minutes before stimulation with 5 μg/mL dsRNA or 10 ng/mL LPS and 24 hours cell supernatants assessed for IFN-β by ELISA. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .05. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (0) or treated with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours and then stimulated with either 5 μg/mL dsRNA or 10 ng/mL LPS. Cells were harvested 3 hours after stimulation for RNA isolation and relative IFN-β mRNA levels assessed by quantitative PCR. Relative gene expression is normalized to gene expression in unstimulated cells. Results are the mean plus or minus SEM for 4 independent experiments assayed in duplicate. *P < .03 compared with untolerized cells. **P < .01 compared with untolerized cells. ***P < .002 compared with untolerized cells and SHIP+/+ cells. ****P < .01 compared with untolerized cells but not significantly different from SHIP+/+ cells. NS indicates not significant.
LPS-induced up-regulation of SHIP reduces, probably via inhibition of the PI3K pathway, subsequent dsRNA- or LPS-induced IFN-β mRNA levels. (A) SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 14 μM LY, 50 nM W, or 0.1% DMSO (C) for 30 minutes before stimulation with 5 μg/mL dsRNA (top) or 10 ng/mL LPS (bottom) and 3 hours cell supernatants assessed for IFN-β by ELISA. Data are the mean plus or minus SEM of 3 independent experiments assayed in duplicate. *P < .02 compared with vehicle. In the right panels, SHIP+/+ (□) and SHIP−/− (■) BMmφs were pretreated with 10 μM PI3K p110 isoform-specific inhibitors 1 to 7 for 30 minutes before stimulation with 5 μg/mL dsRNA or 10 ng/mL LPS and 24 hours cell supernatants assessed for IFN-β by ELISA. Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .05. (B) SHIP+/+ (□) and SHIP−/− (■) BMmφs were untreated (0) or treated with 30 nM CpG, 5 μg/mL dsRNA, or 10 ng/mL LPS for 24 hours and then stimulated with either 5 μg/mL dsRNA or 10 ng/mL LPS. Cells were harvested 3 hours after stimulation for RNA isolation and relative IFN-β mRNA levels assessed by quantitative PCR. Relative gene expression is normalized to gene expression in unstimulated cells. Results are the mean plus or minus SEM for 4 independent experiments assayed in duplicate. *P < .03 compared with untolerized cells. **P < .01 compared with untolerized cells. ***P < .002 compared with untolerized cells and SHIP+/+ cells. ****P < .01 compared with untolerized cells but not significantly different from SHIP+/+ cells. NS indicates not significant.
To confirm that the effects observed with the PI3K inhibitors also occurred with in vivo-derived mφs, we isolated SHIP+/+ and SHIP−/− Pmφs and carried out similar studies (Figure S6). Although, as reported previously,18 SHIP−/− Pmφs possess an M2 phenotype and thus secreted less inflammatory cytokines than their WT counterparts, these cells displayed a very similar response to the PI3K inhibitors, with dramatic inhibition by LY and W of CpG- and LPS-induced TNF-α (Figure S6A), and with enhanced IL-6 production with TGX-221 in response to CpG and LPS (Figure S6B).
To determine at what level SHIP was inhibiting IFN-β production, we examined the effect of SHIP on the transcriptional up-regulation of TLR3 because up-regulation of this receptor has been shown to play a role in IFN-β–induced priming.19 We found that, whereas TLR3 mRNA was higher in SHIP−/− BMmφs, suggesting that SHIP negatively regulates TLR3 expression, TLR3 mRNA was increased to the same extent by LPS (which increases SHIP) as by dsRNA (which does not; Figure S7A). This suggests that the LPS-induced increase in SHIP does not restrain subsequent IFN-β production by reducing TLR3 expression.
We also looked at the ability of dsRNA and LPS to prime BMmφs for IFN-β production in response to subsequent CpG stimulation because dsRNA and LPS can up-regulate IRF7 via the MyD88-independent pathway in BMmφs and IRF7 can then be activated to promote IFN-β transcription via the MyD88-dependent pathway.20 We found that dsRNA and LPS did indeed prime SHIP+/+ BMmφs for CpG-induced IFN-β production and did so to a similar extent, although LPS induces SHIP protein and dsRNA does not (Figure S7B). Nonetheless, this priming for responsiveness to CpG was more effective in SHIP−/− BMmφs, suggesting that it is normally restricted by SHIP.
We then asked whether SHIP up-regulation was restraining LPS- or dsRNA-induced IFN-β production at the level of transcription by priming SHIP+/+ and SHIP−/− BMmφs with or without CpG, dsRNA, or LPS for 24 hours, stimulating with dsRNA or LPS for 3 hours, and measuring relative IFN-β mRNA levels. We found that CpG priming actually reduced dsRNA- or LPS-induced IFN-β mRNA levels in SHIP+/+ but not SHIP−/− BMmφs (Figure 6B). dsRHA priming, on the other hand, dramatically enhanced dsRNA- or LPS-induced IFN-β mRNA in both SHIP+/+ and SHIP−/− BMmφs. In contrast, LPS priming actually reduced IFN-β in SHIP+/+ but dramatically enhanced it in SHIP−/− BMmφs. These differences in relative IFN-β mRNA levels between LPS-primed SHIP+/+ and SHIP−/− BMmφs were far more dramatic than at the protein level (Figure 3B) and suggest that SHIP exerts its negative regulatory effects by dramatically affecting the transcription or stability of IFN-β mRNA.
SHIP−/− mice exhibit an antiviral response to LPS
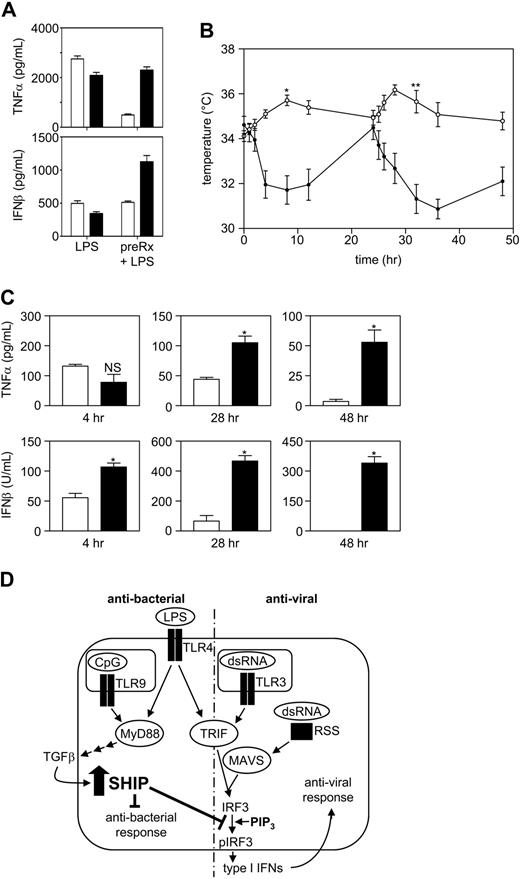
In humans, bacterial and viral infections are characterized by the production of the potent fever inducers, TNF-α and type I IFNs, respectively. In small rodents, however, bacterial infections or low doses of LPS cause fever, whereas viral infections cause a protective type I IFN–induced hypothermia.21,22 Before assessing whether the priming effect of LPS on IFN-β production that occurs within SHIP−/− BMmφs could be observed in vivo, we first carried out in vitro studies with SHIP+/+ and SHIP−/− Pmφs to confirm that they displayed similar tolerance properties as in vitro–derived BMmφs. Specifically, we stimulated SHIP+/+ and SHIP−/− Pmφs with LPS for 24 hours with or without pretreatment for 24 hours with LPS and found that LPS pretreatment both blunted TNF-α levels and prevented the priming of IFN-β secretion in SHIP+/+ but not in SHIP−/− Pmφs, consistent with our results with BMmφs (Figure 7A). We then monitored the temperatures and serum cytokine levels of SHIP+/+ and SHIP−/− mice after a first and second low dose of LPS (1 mg/kg). As expected, wild-type mice developed a transient fever in response to both doses, whereas SHIP−/− mice developed severe hypothermia after each dose of LPS with a nadir between 4 and 12 hours after the first dose and between 8 and 24 hours after the second dose (Figure 7B). This profound difference in core temperature between SHIP+/+ and SHIP−/− mice could not be explained by differences in serum TNF-α levels because levels of this cytokine were not significantly different 4 hours after the first dose of LPS (Figure 7C), even though a profound temperature drop was observed at this time. This lack of difference in serum TNF-α levels at 4 hours was probably because, although there are more mφs in SHIP−/− mice, they possess an M2 phenotype18 and thus secrete less TNF-α/cell, consistent with our results with LPS-stimulated Pmφs. Serum TNF-α levels became higher in SHIP−/− than in SHIP+/+ mice at later times (Figure 7C), consistent with SHIP−/− mice being unable to induce endotoxin tolerance. IFN-β levels, in contrast, were significantly higher in SHIP−/− mice at 4 hours after a first dose of LPS and dramatically higher at 4 and 24 hours after a second dose of LPS. These results are consistent with elevated type I IFN levels being responsible for the temperature drop in SHIP−/− mice and suggest that SHIP−/− mice display an inappropriately robust antiviral response to LPS, unchecked because of the absence of SHIP.
SHIP−/− mice have an inappropriate and robust antiviral response to LPS. (A) SHIP+/+ (□) and SHIP−/− (■) Pmφs with or without 24 hours pretreatment with 10 ng/mL LPS were challenged with 10 ng/mL LPS. At 24 hours, supernatants were assessed for IL-6 (left panel) and TNF-α (right panel). Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .01 compared with LPS tolerized cells. (B) SHIP+/+ (open symbols) and SHIP−/− (closed symbols) mice were injected IP with 1 mg/kg LPS at time 0 and 24 hours. Mouse core temperature was monitored using a rectal thermometer at time 0, 1, 2, 4, 8, 12, and 24 hours after each injection. Values are the mean plus or minus SEM for 6 mice of each genotype. *P < .001, **P < .001, SHIP+/+ vs SHIP−/− mice. (C) SHIP+/+ (□) and SHIP−/− (■) mouse blood was collected from the tail vein 4 hours after the first or second injection (28 hours) of LPS and by cardiac puncture after death 24 hours after the second dose of LPS (48 hours) and sera assessed for TNF-α and IFN-β by ELISA. Results are the mean plus or minus SEM for 3 mice at 4 and 28 hours and for 6 mice at 48 hours. *P < .01 compared with SHIP+/+ serum. NS indicates not significant. (D) A model to explain why Gram-negative bacteria do not trigger an antiviral response whereas viruses do, even though both stimulate the TRIF pathway. LPS triggers the production and secretion of TGF-β via the MyD88-dependent pathway. TGF-β then acts in an autocrine manner to up-regulate SHIP. The up-regulation of SHIP is critical to block a subsequent exposure to LPS or dsRNA from amplifying the transcription of IFN-β.
SHIP−/− mice have an inappropriate and robust antiviral response to LPS. (A) SHIP+/+ (□) and SHIP−/− (■) Pmφs with or without 24 hours pretreatment with 10 ng/mL LPS were challenged with 10 ng/mL LPS. At 24 hours, supernatants were assessed for IL-6 (left panel) and TNF-α (right panel). Results are the mean plus or minus SEM for 3 independent experiments assayed in duplicate. *P < .01 compared with LPS tolerized cells. (B) SHIP+/+ (open symbols) and SHIP−/− (closed symbols) mice were injected IP with 1 mg/kg LPS at time 0 and 24 hours. Mouse core temperature was monitored using a rectal thermometer at time 0, 1, 2, 4, 8, 12, and 24 hours after each injection. Values are the mean plus or minus SEM for 6 mice of each genotype. *P < .001, **P < .001, SHIP+/+ vs SHIP−/− mice. (C) SHIP+/+ (□) and SHIP−/− (■) mouse blood was collected from the tail vein 4 hours after the first or second injection (28 hours) of LPS and by cardiac puncture after death 24 hours after the second dose of LPS (48 hours) and sera assessed for TNF-α and IFN-β by ELISA. Results are the mean plus or minus SEM for 3 mice at 4 and 28 hours and for 6 mice at 48 hours. *P < .01 compared with SHIP+/+ serum. NS indicates not significant. (D) A model to explain why Gram-negative bacteria do not trigger an antiviral response whereas viruses do, even though both stimulate the TRIF pathway. LPS triggers the production and secretion of TGF-β via the MyD88-dependent pathway. TGF-β then acts in an autocrine manner to up-regulate SHIP. The up-regulation of SHIP is critical to block a subsequent exposure to LPS or dsRNA from amplifying the transcription of IFN-β.
Discussion
In this study, we have shown that SHIP restrains TLR9-induced TNF-α and IL-6 production and that a first exposure of wild-type BMmφs to CpG triggers autocrine-acting TGF-β production and subsequent up-regulation of SHIP. We also show that this up-regulation of SHIP markedly reduces the ability of a subsequent dose of CpG or LPS from triggering the production of TNF-α and IL-6. We have also looked at the role SHIP plays in regulating the response to dsRNA, which is present during the replication of RNA and DNA viruses.23 Interestingly, a critical difference between recognition of bacteria versus viruses by pathogen recognition receptors (PRRs) is that viral exposure does not lead to hyporesponsiveness to a subsequent challenge but rather amplification of responsiveness and protection against subsequent viral challenge,15 ie, the resolution of sepsis caused by bacterial infection leads to a state of immunoparalysis where patients are at increased risk of acquiring a secondary bacterial or viral infection, whereas viral infections typically protect against subsequent viral infections by production of autocrine and paracrine-acting type I IFNs. The results presented herein suggest that SHIP is a major negative regulator of type I IFN, especially after bacterial infections. Specifically, our data suggest a model to explain why Gram-negative bacteria do not elicit a potent antiviral response in macrophages whereas viruses do, even though both stimulate the TRIF pathway (Figure 7D). In this model, Gram-negative bacteria first activate TLR4 via LPS at the cell surface of macrophages and, subsequently, activate TLR9 via CpG, once phagocytosed into endosomes. These interactions trigger the production and secretion of TGF-β via the MyD88-dependent pathway. TGF-β then acts in an autocrine manner to, among other things, up-regulate SHIP. It is this up-regulation of SHIP, and not the other anti-inflammatory effects elicited by TGF-β,24 that blocks a subsequent exposure to LPS or dsRNA from increasing IFN-β mRNA levels. Of note, LPS-treated MyD88−/− BMmφs do not induce SHIP and thus have a dramatically enhanced ability to produce IFN-β in response to a subsequent exposure to dsRNA or LPS.
Our results also suggest that SHIP blocks this priming for IFN-β production by restraining the PI3K pathway (Figure 6A,B). There is a heated debate about whether class I PI3Ks are positive or negative regulators of TLR-induced cytokine production, with the majority of reports suggesting either a negative role or no role.16,25-27 LY and W are not ideal inhibitors to resolve this because of their off-target effects.16 Genetic approaches have also proved problematic because of the complex makeup of the class I PI3K family28 and the incompletely understood effects of knocking out one or more catalytic or regulatory subunits on the others.29 Because of this, we used a chemically diverse panel of isoform-specific class I PI3K inhibitors that have been used successfully to elucidate the role of specific isoforms in various biologic processes.25,30 These inhibitors do not affect class III PI3Ks so will not affect endocytic vesicle formation (where dsRNA and CpG interact with their receptors)31 or colocalization of these ligands with their TLR within these endocytic vesicles.32 Using these inhibitors, we found that p110α, -β, -γ, and -δ positively regulate TNF-α production triggered by CpG, whereas p110γ and -δ positively regulate TNF-α production triggered by LPS. PI3K p110α, -γ, and -δ also positively regulate IL-6 production downstream of both LPS and CpG. Intriguingly, however, we found that a highly specific inhibitor of p110β causes an increase in IL-6 production downstream of both LPS and CpG, and this may explain, to some extent, the controversy in the literature, ie, if the relative contribution of p110β is substantial, a nonspecific PI3K inhibitor might increase proinflammatory cytokine production. Importantly, we also found that these inhibitors inhibit cytokine production to a greater degree in the SHIP−/− BMmφs, reinforcing that the augmented cytokine production seen in the SHIP−/− BMmφs is the result of enhanced class I PI3K activity. Taken together with our results showing that SHIP−/− produce more IFN-β than SHIP+/+ BMmφs in response to LPS or dsRNA (Figure 3A) and that higher IFN-β mRNA levels are obtained when SHIP is not induced (Figure 6B), we propose that SHIP reduces IFN-β transcription or mRNA stability by lowering PIP3 levels. This is consistent with a report suggesting that PI3K binds to a tyrosine phosphorylated residue in the cytoplasmic domain of the activated TLR3 and promotes optimal phosphorylation of IRF3.33
Whereas a coordinated response by PRRs is important to combat infectious diseases, negative regulation of PRRs is also critically important to prevent hyperproduction of proinflammatory cytokines5 by nonpathogenic microorganisms and to avoid unchecked responses to pathogenic microorganisms that could lead to septic shock.5,34 At least 15 negative regulators of the MyD88-dependent pathway have been described,1,34,35 and those induced by TLR ligation (MyD88s, IRAKM, ST2, SOCS, and SHIP) have been shown to play a role in the development of endotoxin tolerance. As well, 6 negative regulators of type I IFN production have been reported, with 5 of them, SHP-2,36 Pin1,37 LGP2,15,37 SARM,38 and β-arrestin,39 being constitutively expressed, whereas A20,40 which is induced in response to TLR3 or TLR4 stimulation, probably limits type I IFN production.15 The dramatic antiviral response and concomitant hypothermia we observe in our SHIP−/− mice may be responsible, in part, for the increased susceptibility of these mice to LPS-induced death.1 Interestingly, A20 and SHP-2 knockout mice also display an enhanced susceptibility to LPS, and this too may be the result of an inability to limit antiviral IFN-β production.36,40,41
SHIP is unique in that it is induced downstream of MyD88 activation and not via TRIF and is thus uniquely poised to limit type I IFN production (and induction of antiviral activity) downstream of MyD88-activating pathogens. This is the first report of a negative regulator induced by the MyD88-dependent pathway that limits MyD88-independent type I IFN production, and SHIP may therefore serve to prevent an inappropriately potent antiviral response to LPS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christine Kelly for preparing the manuscript and Zachary Knight for synthesis of PI-103 and PIK93.
This work was supported by the Canadian Cancer Society with core support from the British Columbia Cancer Foundation and the British Columbia Cancer Agency as well as the National Institutes of Health (3R01EB0001987-12S1; B.T.H., K.M.S.). M.J.H. holds an MSFHR Trainee Award and a CIHR Studentship. V.W.H. and F.L.A. hold NSERC Trainee Awards. O.W. is a GREAT Fellowship Awardee. D.F. is an Ernst Schering Foundation Fellow. O.W. is a National Science Foundation Graduate Research Fellowship Awardee.
National Institutes of Health
Authorship
Contribution: L.M.S. and G.K. were responsible for the content, style, and composition of the manuscript; L.M.S., M.J.H., E.K., V.W.H., F.L.A., S.L.O., C.J.v.N.-T., D.W., and H.K.B. conducted all the experiments; and O.W., M.E.F., B.T.H., D.F., and K.M.S. contributed reagents, study design, and data analysis.
Conflict-of-interest disclosure: G.K. is a founding member and CSO of Aquinox Pharmaceuticals Inc, which is dedicated to identifying small molecule modulators of SHIP. The remaining authors declare no competing financial interests.
Correspondence: Gerald Krystal, British Columbia Cancer Research Centre, 675 West 10th Ave, Vancouver, BC, Canada V5Z 1L3; e-mail: gkrystal@bccrc.ca.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal