Abstract
Regulatory T cells (Tregs) play a pivotal role in preventing autoimmunity, graft-versus-host disease (GVHD), and organ graft rejection. We previously showed that either germline or induced SH2 domain–containing inositol 5-phosphatase (SHIP) deficiency in the host abrogates GVHD. Here we show that SHIP deficiency promotes an increase of CD4+CD25+FoxP3+ Tregs and CD4+CD25−FoxP3+“naive” T cells in the periphery that display increased CD103, glucocorticoid-induced tumor necrosis factor receptor–related protein (GITR), OX40, and FcγRII/III expression. SHIP deficiency does not compromise Treg function because SHIP-deficient CD3+CD4+CD25+ Tregs are as suppressive as wild-type (WT) CD3+CD4+CD25+ Treg. Interestingly, like conventional Tregs, SHIP−/− CD4+CD25− T cells are unresponsive to major histocompatibility complex (MHC)–mismatched stimulators and suppress allogeneic responses by T cells in vitro. In addition, SHIP−/− CD4+CD25− T cells mediate reduced lethal GVHD on adoptive transfer to MHC-mismatched hosts. Furthermore, hosts with induced SHIP deficiency exhibit delayed rejection of MHC-mismatched cardiac grafts. Thus, SHIP is required for robust graft-versus-host and host-versus-graft responses by CD4+ T cell and limits their immunoregulatory capacity. These findings further define the immunosuppressive mechanisms that result from SHIP deficiency and provide additional justification for targeting SHIP in clinical transplantation.
Introduction
Regulatory T cells (Tregs) actively mediate self-tolerance and thus control autoimmunity.1,2 Tregs also limit antitumor T-cell responses and deleterious allogeneic T-cell responses that cause graft-versus-host disease (GVHD)3,4 and solid organ allograft rejection,5 making them valuable therapeutic targets. We previously found that donor and host allogeneic responses are compromised in SH2 domain–containing inositol 5-phosphatase (SHIP)–deficient hosts, which exhibit significantly reduced acute rejection of MHC-mismatched bone marrow grafts and GVHD.6,7 Thus, an immunosuppressive environment prevails in SHIP-deficient hosts. We consistently observe a profound expansion of myeloid suppressor cells (MySC) in SHIP-deficient mice.6-9 Because host and donor Tregs limit GVHD4,10 and Mac1+Gr1+ cells, similar to SHIP−/−MySC, expand Tregs in tumor and GVHD models11,12 ; we considered that the Treg compartment in SHIP-deficient hosts may also be expanded. In addition, SHIP deficiency could intrinsically effect Treg homeostasis and function. SHIP can oppose PI3K signaling pathways triggered by engagement of costimulatory and cytokine receptors critical for the suppressive function, survival, and expansion of Tregs, such as CD25 (interleukin-2 receptor α), IL-7R, and OX40.13-15
Because Tregs were initially characterized as CD4+ T cells coexpressing CD25, most Treg studies focus on this phenotype, which is shared with activated CD4+ T cells.16 To distinguish between Tregs and activated T cells, molecular markers correlated with or obligate for Treg function have been identified, such as the transcription factor FoxP3, as well as surface markers CD103, glucocorticoid-induced tumor necrosis factor receptor–related protein (GITR) and OX40, among others. FoxP3 functions as the master regulator in the development and suppressive ability of Tregs.17 CD103 expression among CD4+CD25+ Tregs distinguishes an effector/memory-like subset that displays an inflammation-seeking phenotype and exhibits greater suppressive capacity.18,19 In addition, CD103, which binds E-cadherin, mediates the retention of CD103+ lymphocyte in epithelial compartments,19 which are major sites of GVHD. Thus, CD103+ Tregs might have a prominent role in the prevention against GVHD. GITR and OX40, members of the tumor necrosis factor receptor superfamily of receptors, are costimulatory molecules known to play key roles in promoting the homeostasis, expansion, and suppressive capability of Tregs.20,21 Furthermore, CD4+CD25+OX40+ Tregs represent a mature population that does not require preactivation or stimulation to suppress antigen-specific T-cell responses.22
Analysis of CD103, GITR, and FoxP3 expression has allowed the identification of a Treg population among “naive” CD4+CD25− T cells. Specifically, CD4+CD25−CD103+ T cells display regulatory activity in both an in vitro proliferation assay and in vivo disease models, such as colitis and antigen-induced arthritis.18,23 Additionally, CD4+CD25−GITR+ T cells express IL-10, TGF-β, and intracellular CTLA-4, are anergic, suppress T-cell proliferation, and can prevent wasting disease, colitis, autoimmune myocarditis, diabetes, and multiorgan inflammation.24,25 Thus, when assessing the entire Treg compartment, one should also consider these immunoregulatory subsets within the “naive” CD4+CD25− T-cell compartment.
Using 3 murine genetic models of SHIP deficiency, we show here that the frequency of CD4+CD25+FoxP3+ Treg and CD4+CD25−FoxP3+ T cells is increased in SHIP-deficient mice. We find that the suppressive capacity of SHIP-deficient CD3+CD4+CD25+ Tregs (CD25+ Tregs) is equal to that of wild-type (WT) CD25+ Treg. Interestingly, the SHIP-deficient CD3+CD4+CD25− T-cell (CD25− T-cell) compartment displays significant immunosuppressive capacity in vitro and in vivo, possibly because of the increase in FoxP3+ T cells. Furthermore, the surface expression of CD103, GITR, OX40, and FcγRII/III is significantly increased in SHIP-deficient CD25− and CD25+ CD4+ T-cell compartments. These qualitative changes possibly increase the survival and immunosuppressive capacity of the SHIP-deficient T-cell compartment and contribute to the reduced host-versus-graft and graft-versus-host responses observed in SHIP-deficient hosts.
Methods
Mice
SHIP−/− mice were created previously in our laboratory6 and maintained by intercrossing SHIP+/− mice (F10 to the C57BL/6J background). The creation of SHIPΔIP/ΔIP mice is described in Karlsson et al.26 SHIP-deficient mice and WT littermates used were between 6 and 9 weeks of age. Mice with germline transmission of a SHIPflox allele were previously created in our laboratory6 and are maintained by intercrossing SHIPflox/flox mice (F10 to the C57BL/6J background). MxCre transgenic mice (The Jackson Laboratory, Bar Harbor, ME) were intercrossed with SHIPflox/flox mice to generate MxCreSHIPflox/flox on a C57BL/6J background. MxCreSHIPflox/flox and SHIPflox/flox littermates were generated by intercrossing MxCreSHIPflox/flox and SHIPflox/flox mice. Rag2−/−γc−/− mice on an H2d background were obtained from Hergen Spits27 (Netherlands Cancer Institute, Amsterdam, The Netherlands) and propagated in the Moffitt Stabile Vivarium. Severe combined immunodeficient (SCID) mice (C57BL/6J background) were purchased from The Jackson Laboratory. Studies were performed in accordance with the guidelines and approval of the Institutional Animal Certification and Use Committee at the University of South Florida.
Conditional deletion of SHIP
SHIP was conditionally deleted in MxCreSHIPflox/flox mice by intraperitoneal injection of 625 μg polyinosinic-polycytidylic acid (polyI/C; Sigma-Aldrich, St Louis, MO) on days 0, 3, and 6 as described by Desponts et al.28
Cell purification
Whole splenocytes from SHIP−/−, SHIPΔIP/ΔIP, MxCreSHIPflox/flox, and respective littermate controls were magnetically enriched for CD3+ T cells using anti–CD3-phycoerythrin (PE), Miltenyi anti-PE microbeads, and an Automacs (Miltenyi Biotec, Auburn, CA) per the manufacturer's instructions. The positive fraction was stained for CD8, CD4, CD25, and viability (4′,6-diamidino-2-phenylindole dihydrochloride [DAPI]), then sorted for CD3+CD4+CD25−CD8− and CD3+CD4+CD25+CD8− T cells using a BD FACSAria cell sorter. Population purity was more than 95% as determined by postsort analysis. Sorted cells were used for Western blot analysis, in vitro mixed leukocyte reactions (MLRs), or adoptive transfer.
Flow cytometry
For phenotypic analysis of viable T cells, splenocytes, mesenteric lymph node (LN) cells, or thymocytes were Fc-blocked and stained using fluorescent-conjugated antibodies against the following surface markers: CD3, CD4, CD25, CD103, GITR, OX40, CD127, and CD16/32 (R&D Systems, Minneapolis, MN), along with 4,6-diamidino-2-phenylindole, a viability dye. For intracellular FoxP3 expression, cells were stained as mentioned above, then permeabilized and fixed using the eBioscience Fixation/Permeabilization kit (eBioscience, San Diego, CA), and stained with anti-FoxP3 (FJK-16a). All samples were analyzed on an LSRII (BD Biosciences, San Jose, CA). All antibodies except CD16/32 were purchased from BD Biosciences PharMingen (San Diego, CA) or eBioscience.
Western blotting
Fluorescence-activated cell sorter (FACS)–sorted CD3+CD4+CD25−CD8− and CD3+CD4+CD25+CD8− T cells were lysed for 30 minutes on ice in a modified TNE buffer (50 mM Tris-HCl, 1% Nonidet P-40, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaOV, 1 mM NaF, and protease inhibitors). Equal cell equivalents were resolved on a 4% to 12% Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred to a Hybond-ECL nitrocellulose membrane (GE Healthcare, Little Chalfont, United Kingdom). Blots were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE), probed with antibodies against SHIP1 (P1C1, 1:200) or FoxP3 (eBio7979, 1:500) and β-actin (C-11, 1:500) followed by a fluorochrome-tagged secondary. Probed blots were developed on a LI-COR Odyssey imager to quantitate and normalize SHIP levels or FoxP3 levels to β-actin, displayed as arbitrary fluorescence units (AFU).
Mixed leukocyte reaction
A total of 105/well irradiated (2000 rad) BALB/C splenocytes (stimulators) were plated in quadruplicate with 105/well WT or SHIP-deficient CD3+CD4+CD25−CD8− T cells (responders) in 96-well U-bottom plates (Corning Life Sciences, Acton, MA) containing RPMI 1640 complete media. After 3 days of culture, proliferation of responder T cells was determined by quantifying overnight incorporation of [3H] thymidine (1.0 μCi per well; MP Biomedicals, Irvine, CA). Results are expressed as the mean counts per minute (cpm) of quadruplicate wells plus or minus SEM. To assess suppressive ability, WT CD3+CD4+CD25+CD8− Tregs, SHIP-deficient CD3+CD4+CD25+CD8− Tregs, or SHIP-deficient CD3+CD4+CD25−CD8− T cells were added at the indicated ratios to each MLR well containing 105 irradiated BALB/C splenocytes and 105 WT CD3+CD4+CD25−CD8− T cells.
Adoptive transfer of T cells for colitis and GVHD induction
In the syngeneic colitis model, to assess Treg function, C57BL/6J SCID hosts received 4 × 105 sorted CD3+CD4+CD25−CD8− T cells from WT C57BL/6J donors along with or without 7 × 104 sorted CD3+CD4+CD25+CD8− Tregs from WT or SHIP−/− C57BL/6J donors by intraperitoneal injection on day 1. In parallel, a control group of C57BL/6J SCID hosts received a phosphate-buffered saline (PBS) injection. In the allogeneic GVHD colitis model, to assess allogeneic T-cell response, Rag2−/−γc−/− mice (on an H2d background) received 105 sorted CD3+CD4+CD25−CD8− T cells from WT or SHIP−/− C57BL/6J donors by retro-orbital injection. In parallel, a control group of Rag2−/−γc−/− mice hosts received a PBS injection.
Clinical and histologic examination of colitis
Recipient mice were weighed and monitored for colitis-associated appearance 3 times per week. Recipient mice were kept in a pathogen-free barrier room for 8 to 12 weeks after cell transfer. When premoribund (lost ≥ 10% or more of its starting body weight) or after 8 to 12 weeks after cell transfer, recipient mice were killed and given a disease activity index (DAI). The colon was obtained from each mouse and fixed in 10% phosphate-buffered formalin. Paraffin-embedded sections were cut and stained with hematoxylin and eosin for histologic examination and scoring. The DAI represents the sum of 2 scores: the clinical assessment score (CAS) and the histopathologic score (HPS). The CAS score is determined on a scale from 0 to 4 according to the occurrence of bristled fur, hunched posture, reduced activity, change in stool consistency, and rectal prolapse. The HPS is determined by scoring the most affected area of the proximal colon on a scale of 0 to 4 according to the degree of inflammatory cell infiltration, goblet cell depletion, reactive mucosal epithelial hyperplasia, and thickness of the colon wall. Histopathology grading was performed in a blinded fashion by R.W.E.
Vascularized heart rejection model
C57BL/6J, polyI/C-treated MxCreSHIPflox/flox and SHIPflox/flox mice received hearts from adult BALB/c donors. Fourteen days after the initiation of SHIP deletion, we performed vascularized heart transplantations following the procedure of Corry et al.29 Monitoring of transplant function is assessed by daily palpation of the graft. Moderate rejection is detected by a sclerotic graft, final rejection by missing heartbeats.
Statistics
In vitro experiments are representative of at least 3 independent analyses. MLR, flow cytometry results, weight change, and HPS results were analyzed with the 2-tailed Student t test using Prism 4 software (GraphPad Software, San Diego, CA). Differences were considered significant at P values less than .05. Comparisons of graft or mouse survival were done using the Kaplan-Meier log-rank test (Prism 4 software).
Results
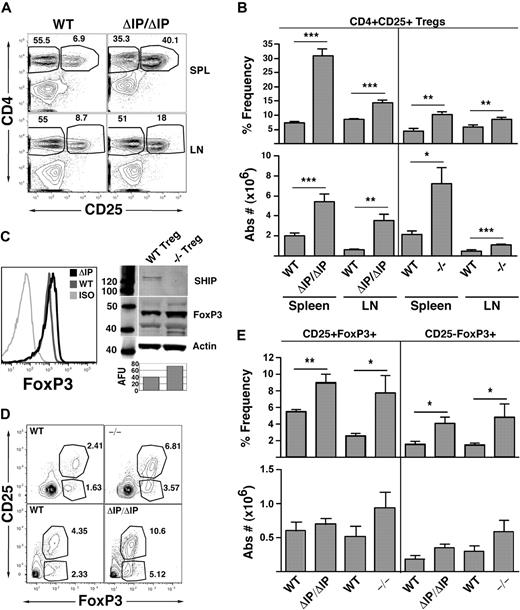
Mice with germline SHIP deficiency have increased numbers of conventional regulatory T cells
The expansion of Tregs in SHIP-deficient mice would be consistent with the relative resistance of these mice to GVHD.6,7 Thus, we examined the Treg compartment in the peripheral lymphoid organs of mice from 2 different genetic models of SHIP deficiency (Figure 1). Both models are germline SHIP-deficient, one having the promoter and first exon of SHIP deleted (SHIP−/−) and the other having the exon encoding the enzymatic domain of SHIP deleted (SHIPΔIP/ΔIP). Consistent with our hypothesis, we observed a significantly increased frequency and absolute number of CD25+FoxP3+ Tregs in the spleen and LN of both SHIP-deficient strains relative to their WT littermates (Figure 1A,B). In neither mutant strain is the frequency of total CD3+ T cells increased in the spleen or LN. In fact, the frequency of splenic CD3+ T cells is significantly decreased in both strains, whereas LN CD3+ T-cell frequency remain unchanged (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Interestingly, FoxP3 expression levels appear to be greater in splenic SHIP-deficient Tregs compared with their WT counterparts as determined by flow cytometry and Western blot analysis of sorted splenic CD25+ Tregs (Figure 1C). Thus, germline SHIP deficiency promotes a preferential expansion and/or accumulation of conventional Tregs that have increased expression of FoxP3.
Mice with germline SHIP deficiency have an expanded CD25+ Treg compartment in peripheral lymphoid organs and higher percentages of thymic CD4+FoxP3+ Tregs than WT counterparts. Spleens, mesenteric LN, and thymuses of SHIP−/−, SHIPΔIP/ΔIP, and their respective WT controls were harvested from 6- to 9-week-old mice and processed into single-cell suspensions. In spleens and LN, CD3+CD4+CD25+ Tregs were quantified using a CD3, CD4, CD25, and FoxP3 multicolor stain. In thymuses, CD4+CD8−CD25−FoxP3+ T cells and CD4+CD8−CD25+FoxP3+ Tregs were quantified using a CD4, CD8, CD25, and FoxP3 multicolor stain. (A) Representative CD4 versus CD25 staining after gating on CD3+ T cells for spleen and LN of SHIPΔIP/ΔIP and WT littermates. (B) Percentage frequency of CD4+CD25+FoxP3+ Tregs after gating on CD3+ T cells, and total absolute CD3+CD4+CD25+FoxP3+ Treg numbers, respectively, in the spleen and LN of the indicated genotype. For SHIPΔIP/ΔIP mice: n = 12 and littermate control: n = 10. For SHIP−/− mice: n = 6 and littermate control: n = 6. (C) Representative FACS analysis of FoxP3 expression CD3+CD4+CD25+ Tregs in the spleen of the indicated genotype. Western blot analysis of SHIP, FoxP3, and β-actin expression in lysates prepared from sorted CD3+CD4+CD25+ Tregs of the indicated genotype. AFU values (“Western blotting”) for FoxP3 expression in −/− and WT Tregs are displayed below the corresponding band in the bar graph. (D) Representative CD25 versus FoxP3 staining after gating on CD4+CD8− thymic cells from SHIPΔIP/ΔIP or SHIP−/− and WT littermate controls. (E) Percentage frequency of CD25+FoxP3+ and CD25−FoxP3+ Tregs after gating on CD4+CD8− T cells and total absolute number of CD4+CD25+FoxP3+ and CD4+CD25−FoxP3+ Tregs in the thymus of the indicated genotype. For SHIPΔIP/ΔIP mice: n = 5 and littermate control: n = 5. For SHIP−/− mice: n = 4 and littermate control: n = 4. *P < .05. **P < .01. ***P < .001.
Mice with germline SHIP deficiency have an expanded CD25+ Treg compartment in peripheral lymphoid organs and higher percentages of thymic CD4+FoxP3+ Tregs than WT counterparts. Spleens, mesenteric LN, and thymuses of SHIP−/−, SHIPΔIP/ΔIP, and their respective WT controls were harvested from 6- to 9-week-old mice and processed into single-cell suspensions. In spleens and LN, CD3+CD4+CD25+ Tregs were quantified using a CD3, CD4, CD25, and FoxP3 multicolor stain. In thymuses, CD4+CD8−CD25−FoxP3+ T cells and CD4+CD8−CD25+FoxP3+ Tregs were quantified using a CD4, CD8, CD25, and FoxP3 multicolor stain. (A) Representative CD4 versus CD25 staining after gating on CD3+ T cells for spleen and LN of SHIPΔIP/ΔIP and WT littermates. (B) Percentage frequency of CD4+CD25+FoxP3+ Tregs after gating on CD3+ T cells, and total absolute CD3+CD4+CD25+FoxP3+ Treg numbers, respectively, in the spleen and LN of the indicated genotype. For SHIPΔIP/ΔIP mice: n = 12 and littermate control: n = 10. For SHIP−/− mice: n = 6 and littermate control: n = 6. (C) Representative FACS analysis of FoxP3 expression CD3+CD4+CD25+ Tregs in the spleen of the indicated genotype. Western blot analysis of SHIP, FoxP3, and β-actin expression in lysates prepared from sorted CD3+CD4+CD25+ Tregs of the indicated genotype. AFU values (“Western blotting”) for FoxP3 expression in −/− and WT Tregs are displayed below the corresponding band in the bar graph. (D) Representative CD25 versus FoxP3 staining after gating on CD4+CD8− thymic cells from SHIPΔIP/ΔIP or SHIP−/− and WT littermate controls. (E) Percentage frequency of CD25+FoxP3+ and CD25−FoxP3+ Tregs after gating on CD4+CD8− T cells and total absolute number of CD4+CD25+FoxP3+ and CD4+CD25−FoxP3+ Tregs in the thymus of the indicated genotype. For SHIPΔIP/ΔIP mice: n = 5 and littermate control: n = 5. For SHIP−/− mice: n = 4 and littermate control: n = 4. *P < .05. **P < .01. ***P < .001.
We then considered whether peripheral expansion of the Treg compartment might be partly the result of increased thymic production of CD25+ Tregs. To examine this, we assessed the thymic content of CD25+FoxP3+ Tregs in both SHIP-deficient strains compared with their WT littermates. We found a significant increase in the frequency of thymic CD25−FoxP3+ and CD25+FoxP3+ Tregs in both SHIP-deficient models relative to their WT littermates (Figure 1D,E). However, because SHIP-deficient mice have smaller thymuses, the absolute numbers of these FoxP3+ Tregs are not significantly different from their WT littermates. Thus, increased thymic output does not account for the expanded peripheral Treg compartment. On a side note, the ratio of CD4+CD8−, CD4+CD8+, and CD4−CD8+ T cells in the thymus of SHIP-deficient mice is not significantly different from that seen in WT mice (data not shown).
SHIP regulates Treg cell compartment size during normal adult physiology
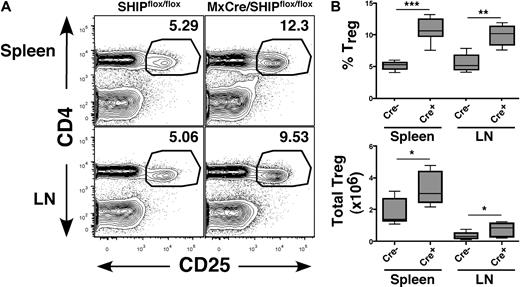
We previously found that, when SHIP deficiency is induced in adulthood, the MySC compartment expands rather rapidly, indicating that SHIP regulates MySC numbers in peripheral lymphoid tissues in response to homeostatic signals present in adult physiology.7 To test whether this is also the case for Tregs, we examined the T-cell compartment in peripheral lymphoid organs in adult MxCreSHIPflox/flox mice after induction of SHIP deficiency. The results obtained are strikingly similar to that observed for germline SHIP deficiency. The frequency and absolute number of CD25+FoxP3+ Tregs are significantly increased in both the spleen and LN of MxCreSHIPflox/flox mice with induced SHIP deficiency compared with SHIPflox/flox mice (Figure 2A,B). Thus, SHIP deficiency induced during normal adult physiology promotes the abnormal accumulation of CD25+FoxP3+ Tregs in the periphery, leading to a pronounced bias in the T-cell compartment toward immunosuppressive cells.
Induction of SHIP deficiency expands the CD25+FoxP3+ Treg compartment in peripheral lymphoid organs. Spleen and mesenteric LN of polyI/C-treated MxCreSHIPflox/flox (Cre+, n = 6) and SHIPflox/flox (Cre−, n = 9) were harvested and processed into single-cell suspensions. Frequency and absolute numbers of CD4+CD25+FoxP3+ Tregs were assessed by flow cytometry, as described in Figure 1. (A) Representative CD4 versus CD25 staining in spleen and LN from MxCreSHIPflox/flox and SHIPflox/flox mice after poly(I/C) administration. (B) Percentage frequency of CD4+CD25+FoxP3+ Tregs after gating on CD3+ T cells, and total absolute CD3+CD4+CD25+FoxP3+ Treg numbers in the spleen and LN of the indicated genotype. *P < .05. **P < .01. ***P < .001.
Induction of SHIP deficiency expands the CD25+FoxP3+ Treg compartment in peripheral lymphoid organs. Spleen and mesenteric LN of polyI/C-treated MxCreSHIPflox/flox (Cre+, n = 6) and SHIPflox/flox (Cre−, n = 9) were harvested and processed into single-cell suspensions. Frequency and absolute numbers of CD4+CD25+FoxP3+ Tregs were assessed by flow cytometry, as described in Figure 1. (A) Representative CD4 versus CD25 staining in spleen and LN from MxCreSHIPflox/flox and SHIPflox/flox mice after poly(I/C) administration. (B) Percentage frequency of CD4+CD25+FoxP3+ Tregs after gating on CD3+ T cells, and total absolute CD3+CD4+CD25+FoxP3+ Treg numbers in the spleen and LN of the indicated genotype. *P < .05. **P < .01. ***P < .001.
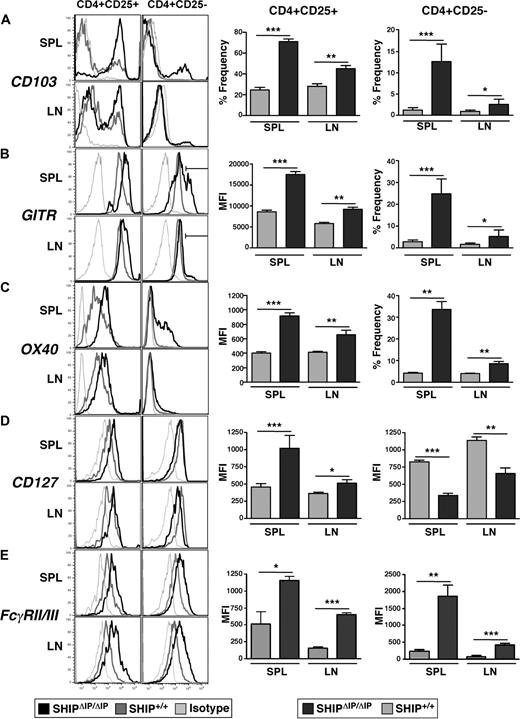
SHIP deficiency alters the expression of key receptors in the CD4+ T-cell compartment
The expression of specific receptors by Tregs, such as CD103, GITR, and OX40, has been associated with their regulatory function, activation status, trafficking, and retention in specific organs. We find that the percentage of CD103+ cells in CD25− and CD25+ T-cell subsets is significantly increased in the spleen and LN of SHIPΔIP/ΔIP mice compared with WT littermates (Figure 3A). Examination of GITR expression in the CD25+ Treg compartment in SHIPΔIP/ΔIP mice shows that the surface density, as determined by mean fluorescent intensity obtained from flow cytometric analysis, is also significantly greater than that seen on WT CD25+ Tregs in the spleen and LN (Figure 3B). In addition, SHIPΔIP/ΔIP mice exhibit a significantly larger representation of CD25−GITRhi T cells in the spleen and LN compared with WT littermates (Figure 3B). Similar to GITR, in SHIPΔIP/ΔIP mice, there is an increased surface density of OX40 on CD25+ Tregs (Figure 3C). In addition, the frequency of OX40+ cells among CD25− T cells is significantly higher in the spleen and LN of SHIPΔIP/ΔIP mice compared with WT littermates (Figure 3C).
SHIP deficiency promotes the alteration of surface marker expression in the CD4+ T-cell compartment. Spleens and mesenteric LN of SHIPΔIP/ΔIP (n = 6) and WT (n = 6) littermates were harvested from 6- to 9-week-old mice and prepared into single-cell suspensions. Cells were then stained with and analyzed using fluorescently conjugated antibodies against CD3, CD4, CD25 with CD103, GITR, OX40, CD127, and FcγRII/III. (A) Representative histogram of CD103 expression levels on viable CD3+CD4+CD25+ (CD25+) or CD3+CD4+CD25− (CD25−) T cells from spleen and LN of the indicated genotype. Bar graph representing percentage frequency of CD103+ T cells among viable CD25+ or CD25− T cells from the spleen and LN of the indicated genotype. (B) Representative histogram of GITR expression on viable T cells as in panel A. Bar graph representing mean fluorescence intensity of GITR expression on viable CD25+ T cells from spleen and LN of the indicated genotype. Bar graph representing percentage frequency of GITRhi (as determined by depicted gate in histogram) T cells among viable CD25− T cells. (C) Same as in panel B, but for OX40 expression. (D) Representative histogram of CD127 (IL-7R) expression on viable T cells as in panels A to C. Bar graph representing mean fluorescence intensity of CD127 expression on viable CD25+ or CD25− T cells from spleen and LN of the indicated genotype. (E) Same as in panel D, but for FcγRII/FcγRIII expression. *P < .05. **P < .01. ***P < .001.
SHIP deficiency promotes the alteration of surface marker expression in the CD4+ T-cell compartment. Spleens and mesenteric LN of SHIPΔIP/ΔIP (n = 6) and WT (n = 6) littermates were harvested from 6- to 9-week-old mice and prepared into single-cell suspensions. Cells were then stained with and analyzed using fluorescently conjugated antibodies against CD3, CD4, CD25 with CD103, GITR, OX40, CD127, and FcγRII/III. (A) Representative histogram of CD103 expression levels on viable CD3+CD4+CD25+ (CD25+) or CD3+CD4+CD25− (CD25−) T cells from spleen and LN of the indicated genotype. Bar graph representing percentage frequency of CD103+ T cells among viable CD25+ or CD25− T cells from the spleen and LN of the indicated genotype. (B) Representative histogram of GITR expression on viable T cells as in panel A. Bar graph representing mean fluorescence intensity of GITR expression on viable CD25+ T cells from spleen and LN of the indicated genotype. Bar graph representing percentage frequency of GITRhi (as determined by depicted gate in histogram) T cells among viable CD25− T cells. (C) Same as in panel B, but for OX40 expression. (D) Representative histogram of CD127 (IL-7R) expression on viable T cells as in panels A to C. Bar graph representing mean fluorescence intensity of CD127 expression on viable CD25+ or CD25− T cells from spleen and LN of the indicated genotype. (E) Same as in panel D, but for FcγRII/FcγRIII expression. *P < .05. **P < .01. ***P < .001.
Recent studies attempting to define surface markers that improve purification of viable Tregs showed that Tregs express lower levels of the IL-7 receptor α chain, CD127, than do activated effector T cells.30 Although IL-7R signaling, like IL-2R and IL-15R signaling, is required for Treg development. In SHIPΔIP/ΔIP mice, we observed that CD25+ Tregs in spleen and LN express higher levels of CD127 (∼ 2-fold higher mean fluorescent intensity value) compared with WT littermates (Figure 3D).
Furthermore, we examined the expression levels of these markers (CD103, GITR, OX40, and IL7R) among CD4+CD8−CD25− and CD4+CD8−CD25+ T cells in the thymus. We found no significant difference comparing SHIPΔIP/ΔIP thymocytes to WT thymocytes (data not shown), suggesting that the increased expression of these markers is acquired in the periphery.
As seen in other cell lineages, such as NK cells, receptors that recruit SHIP and whose activity is regulated by SHIP are often deregulated in SHIP-deficient T cells. For example, SHIP is recruited to Ly49B, a receptor found primarily on myeloid cells but not on NK cells in a WT mouse. In contrast, SHIP-deficient NK cells express Ly49B at high levels (K.H.T.P., C. Brooks, and W.G.K., unpublished data, November 28, 2006). Similarly, T cells are thought to not express Fcγ receptors, such as FcγRIIb and FcγRIIIa, which are also regulated by SHIP and are expressed by most other hematopoietic cells.31 When examining the expression levels of FcγRIIb (CD32b) and FcγRIIIa (CD16c) by flow cytometry using an antibody that recognizes both, we find that WT CD25− T cells and CD25+ Tregs express these FcγRs at low levels compared with appropriate isotype controls (Figure 3E). Interestingly, in spleen and LN of SHIP-deficient mice, we observed that both CD25− T cells and CD25+ Tregs express significantly elevated levels of FcγRIIb/FcγRIIIa compared with WT controls.
SHIP deficiency promotes the accumulation of CD4+CD25− “naive” T cells that express FoxP3 and have suppressive function
Analysis of surface marker expression, such as CD103, GITR, and of intracellular FoxP3 identifies an immunosuppressive subpopulation within the CD25− T-cell compartment. Consistent with the increase in CD25+FoxP3+ Tregs numbers, we find a significant expansion of CD25−FoxP3+ T cells in the periphery of SHIP−/−, SHIPΔIP/ΔIP, and polyI/C-treated MxCreSHIPflox/flox mice, as detected by flow cytometry and Western blot analysis (Figure 4A-C). As shown in Figure 3, when analyzing the expression of surface markers associated with regulatory T cells, such as CD103, GITR, and OX40, we found an enrichment of CD25− T cells that expressed these markers in SHIP−/− spleen and lymph nodes compared with WT littermates (Figure 3A-C). The majority of these cells also coexpressed FoxP3 (data not shown). Thus, these CD25−FoxP3+ T cells may represent an immunoregulatory subset that contributes to the immunosuppressive environment in SHIP−/− mice.
Consistent with their increased FoxP3 expression, CD25− T cells from either SHIP-deficient strain are unresponsive to MHC-mismatched stimulators, and also demonstrate significant suppressive capacity on other CD4+ T cells (Figure 4D,E). When placed in an MLR with WT effector CD25− T cells at a 1:1 ratio, the suppressive capacity of SHIP-deficient CD25− T cells was comparable with that of conventional CD25+ Tregs placed in an MLR with WT effector CD25− T cells at a 1:8 ratio (Figure 4E). This coincides with the fact that 15% to 20% of SHIP-deficient CD25− T cells are FoxP3+. Thus, the FoxP3+ cells in the SHIPΔIP/ΔIP CD25− T-cell population are at approximately a 1:8 ratio with WT effector CD25− T cells, suggesting that SHIPΔIP/ΔIP CD25−FoxP3+ T cells have suppressive capacity comparable with that of conventional WT Treg cells.
SHIP-deficient CD25− T cells exhibit multiple molecular features of an immunoregulatory cell and have profound immunosuppressive capacity. Single-cell suspensions were prepared from spleens and mesenteric LN of 6- to 9-week-old SHIP−/−, SHIPΔIP/ΔIP, and their respective WT controls, and from polyI/C-treated MxCreSHIPflox/flox and SHIPflox/flox mice. (A) Representative histogram of FoxP3 expression levels in fixed CD25− T cells from spleen and LN of the indicated genotype. (B) Western blot analysis of FoxP3 protein expression in FACS-purified CD25− T cells from the indicated SHIP-deficient strain and WT counterpart. (C) Bar graph representing the percentage frequency of FoxP3+ T cells among CD25− T cells and absolute numbers of CD25−FoxP3+ T cells in the spleen and LN of the indicated genotype. (D,E) FACS-purified C57BL/6J SHIP-deficient CD25− T cells (labeled CD25−), SHIP-deficient or WT CD25+ Tregs (labeled CD25+) were mixed with FACS-purified C57BL/6J WT effector CD25− T cells at the indicated ratios (suppressors/effectors) in a one-way MLR where irradiated BALB/C splenocytes were used as stimulators. This is representative of 3 independent MLR assays conducted at the indicated cell ratios. *P < .05. **P < .01. ***P < .001.
SHIP-deficient CD25− T cells exhibit multiple molecular features of an immunoregulatory cell and have profound immunosuppressive capacity. Single-cell suspensions were prepared from spleens and mesenteric LN of 6- to 9-week-old SHIP−/−, SHIPΔIP/ΔIP, and their respective WT controls, and from polyI/C-treated MxCreSHIPflox/flox and SHIPflox/flox mice. (A) Representative histogram of FoxP3 expression levels in fixed CD25− T cells from spleen and LN of the indicated genotype. (B) Western blot analysis of FoxP3 protein expression in FACS-purified CD25− T cells from the indicated SHIP-deficient strain and WT counterpart. (C) Bar graph representing the percentage frequency of FoxP3+ T cells among CD25− T cells and absolute numbers of CD25−FoxP3+ T cells in the spleen and LN of the indicated genotype. (D,E) FACS-purified C57BL/6J SHIP-deficient CD25− T cells (labeled CD25−), SHIP-deficient or WT CD25+ Tregs (labeled CD25+) were mixed with FACS-purified C57BL/6J WT effector CD25− T cells at the indicated ratios (suppressors/effectors) in a one-way MLR where irradiated BALB/C splenocytes were used as stimulators. This is representative of 3 independent MLR assays conducted at the indicated cell ratios. *P < .05. **P < .01. ***P < .001.
SHIP-deficient CD4+CD25+ Tregs are as suppressive as WT Tregs
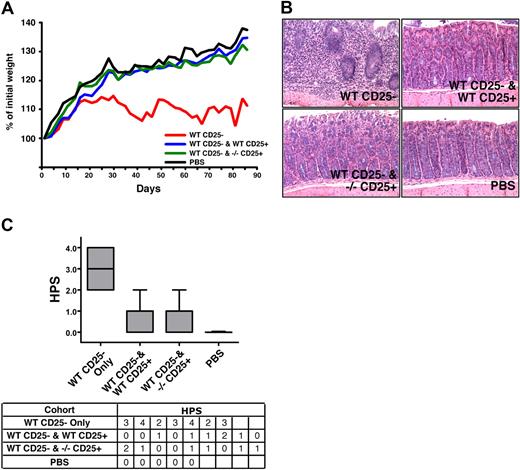
The altered phenotype described herein suggests that SHIPΔIP/ΔIP Tregs may be more suppressive than WT Tregs. To test this hypothesis in vitro, we directly compared the suppressive capacity of conventional SHIP-deficient and WT CD25+ Tregs at different ratios. Multiple comparisons indicate SHIP-deficient Tregs are equally potent at suppressing an MLR compared with WT Tregs on a per-cell basis (Figure 4D). To confirm that SHIP-deficient Tregs are as suppressive as WT Tregs, we used the in vivo syngeneic colitis model, which assesses the ability of Tregs to control autoreactive T cells. We found that SHIP−/− CD25+ Tregs can protect C57BL/6J SCID hosts from WT CD25− T cell–induced colitis just as well as WT CD25+ Tregs, as assessed by weight change and colon histopathology (Figure 5). Weight change analysis showed hosts receiving either WT or SHIP−/− Tregs, along with the WT effector CD25− T cells, gained approximately 35% more of their initial weight by the end of the study, similar to the control group that received PBS only (Figure 5A). Hosts receiving WT effector CD25− T cells gained approximately 10% of their initial weight, significantly less than the hosts receiving PBS or SHIP−/− or WT Tregs. Comparison of the colon histopathology for hosts in each cohort further supports that those hosts receiving either SHIP−/− or WT Tregs were equally protected from colitis (Figure 5B). The appearance and health of the colon in these hosts were very similar to that observed for hosts injected with PBS (Figure 5C). The colon histopathology of hosts injected with WT effector CD25− T cells only was the most severe compared with the hosts of the other cohorts (Figure 5B,C). Thus, SHIP−/− Tregs appear to have comparable regulatory capacity to their WT counterparts.
SHIP-deficient CD4+CD25+ Tregs exhibit normal immunoregulatory capacity in vivo. C57BL/6J SCID hosts received 4 × 105 CD3+CD4+CD25− T cells from WT C57BL/6J donors by intraperitoneal injection on day 1 (n = 7). Where indicated, the WT effector CD3+CD4+CD25− T cells were coinjected with 7 × 104 CD3+CD4+CD25+ Tregs from WT or SHIP−/− C57BL/6J donors into C57BL/6J SCID hosts (n = 9). In parallel, a control group of C57BL/6J SCID hosts received a PBS injection (n = 5). Disease and weight were monitored every other day. (A) Analysis of the rate of weight change over the course of the study (3 months) in the cohorts that received WT effector CD3+CD4+CD25− T cells only (labeled WT CD25−), or effector WT CD3+CD4+CD25− T cells with WT or SHIP−/−CD3+CD4+CD25+ Tregs (labeled WT CD25− and WT CD25+ or WT CD25− and −/− CD25+, respectively), or PBS. The weight change depicted was determined by converting each actual weight to a percentage of that mouse's initial weight. Each line depicts the average weight change for the specified cohort (P < .001, WT CD25− vs WT CD25− and WT CD25+, WT CD25− vs WT CD25− and SHIP−/− CD25+, and for WT CD25− vs PBS; P > .1 for WT CD25− and WT CD25+ vs WT CD25− and SHIP−/− CD25+, WT CD25− and WT CD25+ vs PBS, and for WT CD25− and SHIP−/− CD25+ vs PBS). (B) Histologic appearance of the colon (hematoxylin and eosin, ×200) from a mouse in the WT CD25− (top left; HPS = 3), WT CD25− and WT CD25+ (top right; HPS = 1), WT CD25− and −/− CD25+ (bottom right; HPS = 1), and PBS (bottom left; HPS = 0) cohorts. Histology micrographs were taken using a Leica DMLB microscrope (N PLAN 20×/0.40, total magnification ×200, at room temperature), and a SPOT Insight QE Model 42 camera with Spot Advanced acquisition software (Diagnostic Instruments, Sterling Heights, MI). (C) Box and whisker plots and table summarizing the histopathology scores (HPS) given to the hosts within each cohort. The HPS was determined by grading the histologic appearance of the colon using the following criteria: grade 0 indicates unaffected proximal colon; grade 1, mild leukocyte infiltration of the lamina propria (not shown); grade 2, moderate leukocyte infiltration of the lamina propria, mild reduction of goblet cells, and mild crypt epithelial regenerative hyperplasia; grade 3, marked leukocyte infiltration beyond the muscularis mucosa into a thickened submucosa, goblet cell depletion, and epithelial regenerative hyperplasia with atypia; grade 4, marked transmural leukocyte infiltration deep into a thickened submucosa and tunica muscularis with increased vascular density, marked goblet cell loss, and epithelial regenerative hyperplasia with atypia used to determine histopathologic score (HPS). P < .001, WT CD25− versus WT CD25− and WT CD25+, WT CD25− versus WT CD25− and SHIP−/− CD25+, and for WT CD25− versus PBS; P > .5 for WT CD25− and WT CD25+ versus WT CD25− and SHIP−/− CD25+.
SHIP-deficient CD4+CD25+ Tregs exhibit normal immunoregulatory capacity in vivo. C57BL/6J SCID hosts received 4 × 105 CD3+CD4+CD25− T cells from WT C57BL/6J donors by intraperitoneal injection on day 1 (n = 7). Where indicated, the WT effector CD3+CD4+CD25− T cells were coinjected with 7 × 104 CD3+CD4+CD25+ Tregs from WT or SHIP−/− C57BL/6J donors into C57BL/6J SCID hosts (n = 9). In parallel, a control group of C57BL/6J SCID hosts received a PBS injection (n = 5). Disease and weight were monitored every other day. (A) Analysis of the rate of weight change over the course of the study (3 months) in the cohorts that received WT effector CD3+CD4+CD25− T cells only (labeled WT CD25−), or effector WT CD3+CD4+CD25− T cells with WT or SHIP−/−CD3+CD4+CD25+ Tregs (labeled WT CD25− and WT CD25+ or WT CD25− and −/− CD25+, respectively), or PBS. The weight change depicted was determined by converting each actual weight to a percentage of that mouse's initial weight. Each line depicts the average weight change for the specified cohort (P < .001, WT CD25− vs WT CD25− and WT CD25+, WT CD25− vs WT CD25− and SHIP−/− CD25+, and for WT CD25− vs PBS; P > .1 for WT CD25− and WT CD25+ vs WT CD25− and SHIP−/− CD25+, WT CD25− and WT CD25+ vs PBS, and for WT CD25− and SHIP−/− CD25+ vs PBS). (B) Histologic appearance of the colon (hematoxylin and eosin, ×200) from a mouse in the WT CD25− (top left; HPS = 3), WT CD25− and WT CD25+ (top right; HPS = 1), WT CD25− and −/− CD25+ (bottom right; HPS = 1), and PBS (bottom left; HPS = 0) cohorts. Histology micrographs were taken using a Leica DMLB microscrope (N PLAN 20×/0.40, total magnification ×200, at room temperature), and a SPOT Insight QE Model 42 camera with Spot Advanced acquisition software (Diagnostic Instruments, Sterling Heights, MI). (C) Box and whisker plots and table summarizing the histopathology scores (HPS) given to the hosts within each cohort. The HPS was determined by grading the histologic appearance of the colon using the following criteria: grade 0 indicates unaffected proximal colon; grade 1, mild leukocyte infiltration of the lamina propria (not shown); grade 2, moderate leukocyte infiltration of the lamina propria, mild reduction of goblet cells, and mild crypt epithelial regenerative hyperplasia; grade 3, marked leukocyte infiltration beyond the muscularis mucosa into a thickened submucosa, goblet cell depletion, and epithelial regenerative hyperplasia with atypia; grade 4, marked transmural leukocyte infiltration deep into a thickened submucosa and tunica muscularis with increased vascular density, marked goblet cell loss, and epithelial regenerative hyperplasia with atypia used to determine histopathologic score (HPS). P < .001, WT CD25− versus WT CD25− and WT CD25+, WT CD25− versus WT CD25− and SHIP−/− CD25+, and for WT CD25− versus PBS; P > .5 for WT CD25− and WT CD25+ versus WT CD25− and SHIP−/− CD25+.
Evidence for enhanced T lymphoid immunoregulation of allogeneic responses in vivo
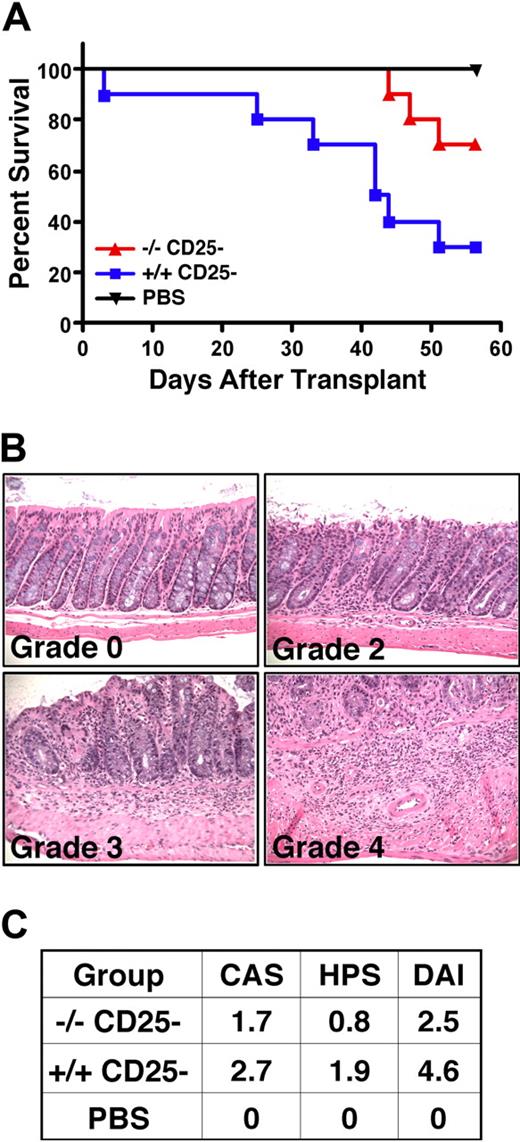
SHIP-deficient CD25− T cells exhibit reduced allogeneic T-cell responses in vitro and approximately 15% of them express FoxP3 (Figure 4). Thus, we assessed their capacity to mediate allogeneic responses in an in vivo model of GVHD-induced colitis using MHC-mismatched (H2d) Rag2−/−γc−/− hosts.27 As expected, WT CD25− T cells mediate robust colitis and lethal GVHD in MHC-mismatched hosts, resulting in only 30% survival, whereas 70% of Rag2−/−γc−/− hosts survived that received an equivalent number of SHIP-deficient CD25− T cells. This indicates that SHIP-deficient CD25− T cells have significantly less capacity for lethal GVHD (Figure 6A), consistent with their reduced response in the one-way MLR assay (Figure 4). Mice receiving SHIP-deficient CD25− T cells also have less evidence of colitis based on both the assessment of clinical symptoms (CAS) and histopathology (HPS) in the colon summarized in the DAI (Figure 6C). Specifically, the severity of colitis was approximately 2-fold less in mice receiving SHIP-deficient CD4+ T cells (average DAI, 2.5) than mice that received WT CD4+ T cells (average DAI, 4.6). Thus, SHIP deficiency reduces allogeneic CD4+ T-cell responses that can mediate colitis, GVHD, BM graft rejection, and organ graft rejection.
Reduced alloreactivity of SHIP-deficient CD4+CD25− effector T cells in vivo. Rag2−/−γc−/− hosts on an H2d background received 4 × 105 CD3+CD4+CD25− T cells from SHIP−/− or WT C57BL6 (H2b) donors by r.o. injection on day 1. In parallel, a control group of Rag2−/−γc−/− hosts received a PBS injection. Disease was monitored on a daily basis. Data represent 2 separate studies that were combined, where n = 5 per treatment group, resulting in an n = 10 per each treatment group. (A) Kaplan-Meier step functions that show survival for the indicated Rag2−/−γc−/− cohorts (n = 10). P = .01 for SHIP−/− versus WT CD25− T-cell-injected cohorts. (B) Histopathologic appearance (hematoxylin and eosin, × 200) of the proximal colon of Rag2−/−γc−/− mice after transfer of SHIP−/− or WT CD4+CD25− T cells. These are representative examples that show grading to determine the HPS, using the criteria described in Figure 5. Histology micrographs were taken as described in Figure 5. (C) Table summarizing the assessment of disease in Rag2−/−γc−/− mice cohorts receiving CD25− T cells from the indicated genotype or sterile PBS control based on the clinical assessment score (CAS), the HPS and the disease activity index (DAI = CAS + HPS). The CAS is determined as follows: 0 indicates no signs; 1, bristled fur; 2, bristled fur with hunched posture, and/or reduced activity; 3, all of the above and change in stool consistency (eg, soft, sticky); 4, rectal prolapse.
Reduced alloreactivity of SHIP-deficient CD4+CD25− effector T cells in vivo. Rag2−/−γc−/− hosts on an H2d background received 4 × 105 CD3+CD4+CD25− T cells from SHIP−/− or WT C57BL6 (H2b) donors by r.o. injection on day 1. In parallel, a control group of Rag2−/−γc−/− hosts received a PBS injection. Disease was monitored on a daily basis. Data represent 2 separate studies that were combined, where n = 5 per treatment group, resulting in an n = 10 per each treatment group. (A) Kaplan-Meier step functions that show survival for the indicated Rag2−/−γc−/− cohorts (n = 10). P = .01 for SHIP−/− versus WT CD25− T-cell-injected cohorts. (B) Histopathologic appearance (hematoxylin and eosin, × 200) of the proximal colon of Rag2−/−γc−/− mice after transfer of SHIP−/− or WT CD4+CD25− T cells. These are representative examples that show grading to determine the HPS, using the criteria described in Figure 5. Histology micrographs were taken as described in Figure 5. (C) Table summarizing the assessment of disease in Rag2−/−γc−/− mice cohorts receiving CD25− T cells from the indicated genotype or sterile PBS control based on the clinical assessment score (CAS), the HPS and the disease activity index (DAI = CAS + HPS). The CAS is determined as follows: 0 indicates no signs; 1, bristled fur; 2, bristled fur with hunched posture, and/or reduced activity; 3, all of the above and change in stool consistency (eg, soft, sticky); 4, rectal prolapse.
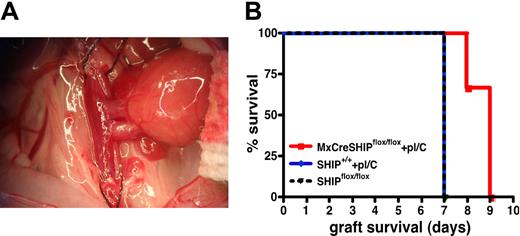
To further assess immunoregulatory T-cell function in vivo in SHIP-deficient hosts, we tested whether induction of SHIP deficiency in adults could delay or prevent rejection of allogeneic organ grafts, a known function of Treg cells. We induced SHIP deficiency in MxCreSHIPflox/flox recipients by polyI/C injection. Fourteen days after the first polyI/C injection, vascularized heart grafts from BALB/C donors were placed in MxCreSHIPflox/flox and SHIP+/+ C57BL/6J cohorts that received the same polyI/C regimen and unmanipulated SHIPflox/flox controls (Figure 7A). As expected with such cardiac allografts,29 the SHIPflox/flox and C57BL/6J control cohorts both rejected the BALB/C grafts within 7 days (Figure 7B). However, most MxCreSHIPflox/flox recipients demonstrated a significant delay in rejection, specifically within 9 days (P < .01; Figure 7B). These findings suggest that SHIP deficiency induced in the adult transplantation host can delay allogeneic organ graft rejection consistent with the expanded number and function of immunoregulatory T cells in SHIP-deficient hosts.
Induced SHIP deficiency delays rejection of MHC-mismatched, vascularized heart allografts. (A) Example of an anastomosed heart early after reperfusion. The heart is located in the right lower abdomen of the recipient mouse, and contractions can be palpated through the abdominal wall after closure. (B) Kaplan-Meier step-functions for graft survival in MxCreSHIPflox/flox mice after induction of SHIP deficiency (red), SHIPflox/flox controls (dashed black) and C57BL/6J mice (blue). The latter group was treated with an identical polyI/C (labeled pI/C) regimen as that given to MxCreSHIPflox/flox mice. P < .01 for MxCreSHIPflox/flox versus SHIPflox/flox graft survival. P < .05 for MxCreSHIPflox/flox versus C57BL/6J graft survival.
Induced SHIP deficiency delays rejection of MHC-mismatched, vascularized heart allografts. (A) Example of an anastomosed heart early after reperfusion. The heart is located in the right lower abdomen of the recipient mouse, and contractions can be palpated through the abdominal wall after closure. (B) Kaplan-Meier step-functions for graft survival in MxCreSHIPflox/flox mice after induction of SHIP deficiency (red), SHIPflox/flox controls (dashed black) and C57BL/6J mice (blue). The latter group was treated with an identical polyI/C (labeled pI/C) regimen as that given to MxCreSHIPflox/flox mice. P < .01 for MxCreSHIPflox/flox versus SHIPflox/flox graft survival. P < .05 for MxCreSHIPflox/flox versus C57BL/6J graft survival.
Discussion
Here we provide evidence that germline or induced systemic SHIP deficiency promotes an increased frequency of CD25+FoxP3+ T cells and CD25−FoxP3+ Tregs in secondary lymphoid tissues. Furthermore, we find that SHIP deficiency promotes a significant increased expression or representation of CD103, GITR, and OX40, markers associated with Tregs, and FcγRII/III among CD25+ Tregs and CD25− T cells. SHIP deficiency does not compromise Treg function because SHIP-deficient Tregs are as suppressive as WT Tregs. Finally, SHIP-deficient CD25− T cells are unresponsive to allogeneic stimulus in vitro and in vivo and suppress allogeneic T-cell responses in vitro. Because FoxP3 expression in murine T cells confers suppressive capacity,17 it is probable that the FoxP3+ subset among SHIP-deficient CD25− T cells is responsible for the observed immunosuppressive capacity of SHIP-deficient CD25− T cells in vitro and reduced GVHD in vivo.
The increased representation of FoxP3+ Treg populations within CD25+ and CD25− T-cell compartments could be because of several extrinsic or intrinsic effects resulting from SHIP deficiency. SHIP deficiency in T-lineage cells could alter intracellular signaling pathways important in thymic selection or peripheral differentiation or survival. Alternatively, SHIP deficiency could promote an immunosuppressive environment that preferentially promotes the generation, expansion, and/or survival of Tregs. Tarasenko et al32 found that T cell–specific SHIP deficiency does not promote the increased development or representation of FoxP3+ Tregs in the thymus or in peripheral lymphoid tissues. Furthermore, they showed that SHIP does not regulate signaling through the T-cell receptor. These results, when paired with our findings, suggest that SHIP deficiency promotes Treg expansion via a mechanism that is extrinsic to CD4+ T cells, although the possibility exists that both a SHIP-deficient environment and a SHIP-deficient T cell are required for the increased accumulation of Tregs that we observe.
Here we show that the SHIP-deficient environment promotes the expression of receptors, CD103, GITR, and OX40, which have been associated with Treg function as well as with activated T cells. Because of this, others have concluded that T cells exist in an activated state in SHIP-deficient mice.33 The data presented in this study further characterize T cells in SHIP-deficient mice, suggesting that expression of these receptors may be representative of an activated/effector T cell that also has immunosuppressive behavior. Although Tregs can suppress in an antigen nonspecific manner, Tregs must undergo antigen-specific activation to suppress.34 Thus, SHIP-deficient Tregs in both CD25+ and CD25− compartments may have an increased probability of suppressing other T cells because they exist in larger numbers and a larger proportion of them exist in an activated state. Although we found that SHIP-deficient Tregs are as equally potent at suppressing effector CD4+ T cells as are WT Tregs, SHIP-deficient Tregs may still have more potent suppressive activity when in a SHIP-deficient environment. In addition, as done by Lehmann et al,18 one can titrate the number of Tregs coinjected along with WT effector CD25− T cells to compare the smallest ratio of either SHIP-deficient or WT Tregs to effector T cells at which protection from colitis is compromised or lost. Regardless, we show that SHIP-deficient Tregs are at least as suppressive as WT Tregs as assayed in vitro and in vivo.
In WT mice, GITR expression in the CD25− and CD25+ T-cell compartments represents immunoregulatory T cells capable of preventing autoimmune myocarditis, multiorgan inflammation, and murine inflammatory bowel disease.24 These studies propose that GITR expression may be a better Treg surface marker than CD25. Similarly, in WT mice, CD25− and CD25+ T cells that express CD103 also represent a Treg subset capable of protecting mice from colitis in the SCID model in vivo.18 Coincidentally, most GITR+ and CD103+ T cells within CD25+ and CD25− CD4+ T-cell compartments in SHIP-deficient mice also coexpress FoxP3. When using GITR or CD103 in addition to FoxP3, instead of CD25 and FoxP3, as the markers to determine the frequency of Tregs, there is a more pronounced increase in the representation of CD4+GITR+FoxP3+ Tregs or CD4+CD103+FoxP3+ Tregs than of CD4+CD25+FoxP3+ Tregs in the peripheral lymphoid organs of SHIP-deficient mice compared with that in WT mice.
As mentioned, both GITR and CD103 expression identifies a regulatory T-cell subset within the CD25− T-cell compartment, although OX40 has not been shown to do so. Streeter et al demonstrated that CD4+CD25−OX40+ T cells are proliferative on alloantigen stimulation whereas CD4+CD25+OX40+ T cells are suppressive.35 In SHIP-deficient mice, even though there is approximately a 6-fold increase in the representation of CD25−OX40+ T cells compared with WT littermates, the SHIP-deficient CD25− T-cell compartment as a whole was not more proliferative on alloantigen stimulation as demonstrated in vitro and in vivo. Indeed, almost half of the CD25−OX40+ T cells in SHIP-deficient mice are also FoxP3+, whereas only approximately one-fourth of WT CD25−OX40+ T cells are FoxP3+. Thus, in SHIP-deficient mice, the expression of OX40 among CD25− T cells is more closely associated with T cells with immunoregulatory capacity instead of T cells capable of alloantigen-induced proliferation.
Engagement of costimulatory and cytokine receptors, such as OX40, GITR, CD103, IL-2R, and IL-7R, has been shown to confer survival and proliferative signals.21,36-38 The increased density and expression levels of these markers on CD25− and CD25+ Tregs in SHIP-deficient mice may thus provide a survival advantage. SHIP is known to oppose the activation of the PI3K pathway that can be activated by IL-2R, IL-7R, or OX40 signaling.36,39 Indeed, inhibition of PI3K signaling prevents IL-2 and other common gamma chain cytokines, IL-7 and IL-15, from supporting maximal suppression by Tregs.39 Thus, the survival and proliferative signals emanating from these receptors may be stronger when SHIP is not present to oppose PI3K. The possibility of enhanced signaling from these receptors in combination with their increased surface expression could contribute to the increased representation of Tregs in peripheral lymphoid organs of SHIP-deficient mice.
SHIP is also known to regulate signals emanating from both FcγRIIb, an inhibitory receptor, and FcγRIIIa, an activating receptor.40,41 FcγR family members recognize the immunoglobulin (Ig) Fc portion of antibodies, allowing free antibodies, immune complexes, and/or opsonized cells to fine-tune decisions between activation and suppression of an immune response. FcγRs are expressed by almost all types of hematopoietic cells. Previous studies examining whether T cells express FcγRs or not have been contradictory.31 Here we show that T cells in WT mice do express low levels of FcγRIIb/FcγRIIIa. Intriguingly, SHIP-deficient CD25− T cells and CD25+ Tregs express FcγRIIb/FcγRIIIa at significantly higher levels than their WT counterparts. This suggests that SHIP-deficient CD4+ T cells may be responsive to antibody, immune complexes and/or opsonized cells as SHIP is known to limit signals from both of these FcγRs.40,41 Further analysis is needed to distinguish whether unopposed signals from these FcγRs occur in SHIP-deficient T cells and promote the preferable expansion of CD25−FoxP3+ T cells and CD25+FoxP3+ Tregs. Consistently, serum levels of certain IgG isotypes are increased in SHIP−/− mice, providing increased ligands for deregulated FcγRs present on SHIP−/− CD4+ T cells.42
In addition to the increased expression of costimulatory molecules on Tregs, a SHIP-deficient environment promotes other immunologic changes that promote the accumulation of Tregs as well as protect against GVHD, specifically increased MySC numbers,7,8 and increased granulocyte colony-stimulating factor (G-CSF) expression.43 G-CSF promotes an immunosuppressive environment and thus protects against GVHD via many mechanisms. Importantly, one study showed that in vivo G-CSF exposure promotes the acquisition of Treg properties by naive CD4+ T cells after T-cell receptor ligation in vitro.44 Consistently, donors treated with pegylated G-CSF exhibited an increased generation of IL-10–producing Tregs and transplantation tolerance.45 Furthermore, MacDonald et al showed that G-CSF and derivatives protected against GVHD by promoting the expansion of a Mac1+Gr1+ subset, similar to MySC, that mediate the expansion of IL-10–secreting Tregs.11 This mechanism is plausible in a SHIP-deficient host, which is protected from GVHD, where increased G-CSF levels promote the expansion of MySC that in turn mediate expansion of the peripheral Treg compartment. Thus, SHIP may play both intrinsic and extrinsic roles to limit the accumulation of Tregs.
The in vivo models described here provide further support that targeting SHIP could facilitate transplantation across MHC barriers. We show that, in addition to the MySC expansion, SHIP deficiency promotes the accumulation of Tregs, which are known to play a pivotal role in transplantation immunology.4,46 In a model of GVHD-induced colitis, we show that isolated SHIP-deficient CD25− T cells inefficiently mount an immune response to cause colitis. This outcome can be mediated by one of the following mechanisms. First, because SHIP-deficient CD25− T cells could not cause colitis in a Rag2−/−γc−/− mouse, which is not SHIP-deficient, SHIP may play an intrinsic role in T cells that allows robust participation in allogeneic responses. Alternatively, the SHIP-deficient environment could have led to the irreversible differentiation of some or all of the SHIP-deficient CD25− T cells into an unresponsive and/or immunosuppressive cell. Consistently, 2 important observations can also be made from the vascularized heart transplantation model. First, a significant increase in Treg and MySC accumulation is observed in adult mice after only 8 days of induced SHIP deficiency (data not shown). Second, induced SHIP deficiency in adulthood promotes a significant delay in graft rejection. This study further characterizes the cells that make up the immunosuppressive environment promoted by SHIP deficiency, providing further support for targeting of SHIP to improve allogeneic transplantation procedures.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Gjertsen, Michelle Smith, and Robert W. Brooks for technical assistance, discussion, and support and Nathan Watts for genotyping.
This work was supported in part by academic development funds from Moffitt Cancer Center and the University of South Florida and grants from the National Institutes of Health (RO1 HL72523). W.G.K. was the Newman Scholar of the Leukemia and Lymphoma Society of America during much of this study.
National Institutes of Health
Authorship
Contribution: M.M.C. performed experiments, analyzed and interpreted data, and made the figures; D.W. aided in performing experiments and analyzed and interpreted data for Figures 1 and 2; K.H.T.P. aided in performing experiments for Figures 1, 2, and 4 and in proofreading the manuscript; E.L. aided in performing experiments and collecting data for Figure 6; R.W.E. analyzed and interpreted data for Figure 6; C.-T.L. and K.K. performed experiments and analyzed and interpreted data for Figure 7; D.S. contributed ideas to analyze FcγR expression for Figure 3; M.M.C. and W.G.K. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William G. Kerr, PhD, SUNY Upstate Medical University, 750 E Adams St, 2204 Weiskotten Hall, Syracuse, NY 13210; email:kerrw@upstate.edu.
References
Author notes
*K.K. and W.G.K. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal