Abstract
CD4+CD25+ regulatory T cells (Treg) play the critical role in maintenance of peripheral immune tolerance. However, the numbers of naturally occurring Treg (nTreg) that can be isolated from periphery are far too small to be clinically effective. The isolation and expansion of nTreg for treatment of autoimmune diseases encounter great difficulties. Whether autoantigen-specific Treg could be converted from CD4+CD25− T cells in patients with autoimmune diseases has not been reported. Here, we demonstrated that platelet glycoprotein (GP)–specific induced Treg (GP-iTreg) could be generated de novo from nonregulatory CD4+CD25−CD45RA+ cells in patients with idiopathic thrombocytopenic purpura and induced both antigen-specific and linked suppression. GP-iTreg mediated regulatory effects via modulating the T cell–stimulatory capacity of dendritic cells. By investigating the gene expression profile of iTreg-modulated dendritic cells, we provided a genome-wide assessment of the changes induced by antigen-specific iTreg and identified that the Toll-like receptor, Notch and transforming growth factor-β signaling pathways were related to the GP-specific tolerance, with the Toll-like receptor pathway being dominant. The findings in patients with idiopathic thrombocytopenic purpura will facilitate our understanding of the mechanisms of induction and maintenance of autoantigen-specific tolerance and highlight the considerable potential of antigen-specific iTreg for targeted immunotherapy in human auto-immune diseases.
Introduction
Chronic idiopathic thrombocytopenic purpura (ITP) is an immune-mediated disease in which platelets are destroyed by antiplatelet autoantibodies.1 Autoreactive T cells to platelet glycoprotein (GP) have been isolated not only from peripheral blood of patients with chronic ITP2 but also from healthy persons.3 Approximately 75% of the platelet antigenic determinants lie on the GP complex GPIIb/IIIa and GPIb/IX.4 Autoantibody production is under the control of autoantigen-specific helper T cells.5 This suggests that peripheral regulatory mechanisms that control these GP-reactive T cells are necessary for preventing autoimmunity
CD4+CD25+ regulatory T cells (Treg) play a critical role in maintenance of peripheral immune tolerance. They are generally divided into 2 subtypes, naturally occurring (nTreg) and induced (iTreg), based on their ontogeny and mode of action. nTreg are generated in the thymus, constitutively express cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), and transcription factor forkheadbox p3 (Foxp3).6 They are naturally anergic to antigenic stimulation and inhibit the proliferation of CD4+ conventional T cells via a direct T-T cell interaction. However, the numbers of nTreg that can be isolated from periphery are far too small to be clinically effective. They constitute only 3% of CD4+ T cells in the blood in humans. The isolation and expansion of nTreg for immunosuppressive therapy encounter great difficulties. Moreover, decreased numbers7 or function8,9 of nTreg have been reported in many autoimmune diseases, including ITP, suggesting that nTreg are not efficient in suppressing the proliferation of autoreactive T cells.
The iTreg are generated in the periphery and share the phenotypes and suppressive activities of nTreg. Extensive data in mice have demonstrated that antigen-specific iTreg can be induced from previously nonregulatory CD4+CD25− cells.10–13 In humans, despite that a regulatory phenotype could be induced from CD4+CD25− T cells,14 there are few functional studies showing acquisition of suppressive activity by stimulated CD4+CD25− T cells in healthy subjects.15–17 Whether autoantigen-specific Treg could be converted from CD4+CD25− T cells in patients with autoimmune diseases has not been reported. Although previous studies indicated that GP-reactive T cells were a part of T-cell repertoire in ITP patients as well as healthy donors, it has not been addressed whether these cells derived from a CD45RA+ or CD45RO+ pool.3,5 Conversion of different subsets of T cells may have different requirements for stimulatory mode, signaling intensity, and duration. Therefore, in the present study, we first investigated the potential CD45RA+ versus CD45RO+ origin of circulating CD4+ GP-reactive T cells from ITP patients and controls, and then generated GP-specific iTreg (GP-iTreg) from CD4+CD25−CD45RA+ T cells from ITP patients and explored their molecular mechanisms of immunosuppression. The results provided a genome-wide assessment of the changes in dendritic cells (DCs) induced by antigen-specific iTreg and formed a base for generating autoantigen-specific iTreg for the cellular biotherapy of autoimmune diseases.
Methods
Patients and antigens
Forty-one newly diagnosed patients in the active phase of chronic ITP were enrolled in this study (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The diagnosis in all the patients was based on the criteria for chronic ITP.18 Nineteen healthy volunteers were examined as normal controls. Signed and informed consent approved by the medical ethical committee of Qilu Hospital of Shandong University was granted by all study participants, in accordance with the Declaration of Helsinki. Human GPIIb/IIIa and GPIb/IX were purified from outdated platelet concentrates using affinity chromatography and were modified by treatment with porcine trypsin (0.1 μg/mL) as described previously.19
Detection of the frequency of GP-reactive T cells
The precursor frequency of GPIIb/IIIa-reactive T cells was estimated by a split-well method as described by Muraro et al20 with some modifications. CD4+CD45RA+CD45RO− and CD4+CD45RA−CD45RO+ T cells were isolated from the peripheral blood mononuclear cells (PBMCs) by negative selection with immunomagnetic beads. In brief, PBMCs were incubated with a cocktail of biotin-conjugated antibodies, including anti-CD8, anti-CD14, anti-CD16, anti-CD56, and anti-CD19, together with anti-CD45RA or anti-CD45RO (Genemay, San Diego, CA). Antigen-bound cells were subsequently removed by incubation with streptavidin-conjugated magnetic beads (Genemay). The purity of isolated CD4+CD45RA+CD45RO− and CD4+CD45RA−CD45RO+ T cells was generally greater than 95% as determined by fluorescence-activated cell sorter analysis. From 19 healthy donors and the 25 patients with detectable GPIIb/IIIa autoantibodies, the purified CD45RA+ or CD45RO+ T cells (2 × 104/well) were seeded into 96-well plates and cultured with autologous irradiated PBMCs (105/well) and GPIIb/IIIa (10 μg/mL) for 7 days. During the last 16 hours of incubation, the cells were pulsed with 1 μCi [3H]thymidine/well. For negative controls, cells were seeded without antigen. Wells stimulated with antigens that had cpm greater than 3 times the mean cpm of negative control wells were identified as responders. The frequency of GP-specific T cells was calculated by dividing the number of responding wells by the number of seeded wells, multiplied by the number of cells per well.
Induction of GP-specific Treg
CD4+CD45RA+ T cells were isolated by negative selection using magnetic beads from PBMCs from the 16 patients who were positive for both anti-GPIIb/IIIa and anti-GPIb/IX autoantibodies as described in “Detection of the frequency of GP-reactive T cells.” Subsequently, CD25+ T cells were removed using CD25 magnetic beads (Miltenyi Biotec, Auburn, CA). Purity was determined to be more than 98% CD25−. DCs from patients with ITP were prepared as described previously,21 which is described in Document S1.
For induction of GP-specific iTreg, the isolated CD4+CD25−CD45RA+ T cells were stimulated, respectively, with mature DCs (mDCs) prepulsed with trypsin-digested GPIIb/IIIa or GPIb/IX in complete medium supplemented with interleukin-2 (IL-2; 20 U/mL) in the presence or absence of transforming growth factor-β1 (TGF-β1; 5 ng/mL; R&D Systems, Minneapolis, MN). Seven days later, CD4+CD25+ GPIIb/IIIa- or GPIb/IX-specific iTreg were purified by positive selection using CD25 magnetic beads (Miltenyi Biotec) and analyzed for their phenotypes before suppression assays. In some experiments, after 7-day stimulation the purified cells were rested in IL-2 (20 U/mL) for an additional 1 or 3 weeks and tested for Foxp3 expression, anergic state, or suppressive activity. For phenotyping analysis, the iTreg were stained with fluorescein isothiocyanate- or phycoerythrin-labeled anti-CD4, anti-Foxp3 (BioLegend, San Diego, CA), anti-CD25, anti–CTLA-4, or anti-GITR monoclonal antibodies (BD PharMingen, San Diego, CA). Flow cytometric analyses were performed on a FACSCalibur. Only CD4+ cells expressing CD25 with higher intensities than the CD8+ cells were included in the gate for CD25high cells because CD8+ T cells do not express the CD25high population.22 For anergy assays, the rested CD4+CD25+ cells (2 × 105) were restimulated with GP-loaded mDCs (irradiated at 30 Gy) at a 10:1 ratio for 3 days. During the last 16 hours of incubation, the cells were pulsed with 1 μCi [3H]thymidine/well. The [3H]thymidine incorporation was determined with a β-scintillation reader.
T-cell suppression assays
GPIIb/IIIa or GPIb/IX-reactive T-cell lines were generated according to the methods by Kuwana et al5 and used as effector T cells. Briefly, PBMCs from ITP patients were stimulated with modified GPIIb/IIIa (10 μg/mL) or GPIb/IX (10 μg/mL). Fresh medium with IL-2 (30 U/mL) was added to the culture system on day 3. Cells were restimulated with modified GPIIb/IIIa or GPIb/IX and PBMCs (irradiated at 30 Gy) for 7 days in the presence of IL-2 (50 U/mL). After 2 or 3 rounds of stimulation, the GPIIb/IIIa or GPIb/IX-specific T-cell lines were selected using limiting dilution. For assessment of regulatory properties of GPIIb/IIIa-specific iTreg, the GP-reactive T-cell lines were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) at a final concentration of 5 μM for 10 minutes at 37°C. The labeling reaction was quenched by adding cold fetal bovine serum, and the cells were washed 3 times with phosphate-buffered saline extensively.
GPIIb/IIIa-specific iTreg (2 × 105) were cultured with 2 × 104 irradiated immature DCs (imDCs) loaded with GPIIb/IIIa and/or GPIb/IX in the presence of CFSE-labeled GPIIb/IIIa- or GPIb/IX-reactive T cells (2 × 105). In some experiments, the GPIIb/IIIa-specific iTreg or non–TGF-β–treated T cells sorted after 1 week of stimulation followed by resting in IL-2 for another 7 days were cultured with GPIIb/IIIa-reactive T cells and GPIIb/IIIa-loaded imDCs.
The cell-free supernatants were also harvested from stimulated cultures after 72-hour incubation to test for concentrations of IL-10 and active TGF-β1 using the ELISA-Quantikine kit (R&D Systems) according to the manufacturer's instructions.
For blocking experiments, other cultures were performed in the presence of neutralizing antibodies against IL-10 (10 μg/mL) and/or TGF-β1 (40 μg/mL, both from R&D Systems). After 3 days of coculture, the fluorescence intensity of GP-reactive T cells was determined by flow cytometry.
Transwell experiments
A total of 106 CFSE-labeled GPIIb/IIIa-reactive CD4+ T cells were stimulated with 105 GPIIb/IIIa-pulsed DCs in the lower chamber of a transwell plate (0.4 μm pore size) (Corning Life Sciences, Acton, MA). GPIIb/IIIa-specific iTreg (106) activated with 105 GPIIb/IIIa-pulsed DCs were either added directly to the lower chamber or were placed in the upper chamber. After 3 days of culture, CFSE-labeled GPIIb/IIIa-reactive T cells in the lower chamber were harvested. The fluorescence intensity of GPIIb/IIIa-reactive T cells was determined by flow cytometry.
Measurement of stimulatory capacity of GP-iTreg–modulated DCs
To detect the tolerogenic properties of GP-iTreg–modulated DCs, GPIIb/IIIa-prepulsed imDCs were cultured alone (unmodulated DCs), with GPIIb/IIIa-specific iTreg, or with conventional CD4+ GPIIb/IIIa-reactive T cells (Tconv) for 2 days. Then T cells were removed using CD4 magnetic beads (Miltenyi Biotec). The isolated iTreg- or Tconv-modulated DCs or unmodulated DCs were used to stimulate CFSE-labeled GPIIb/IIIa-reactive T cells at a 1:10 ratio. After 3 days, proliferation of GPIIb/IIIa-reactive T cells was determined by flow cytometry. All tests were carried out in triplicates. In parallel experiments, after overnight coculture with GPIIb/IIIa-specific iTreg, GPIIb/IIIa-prepulsed DCs were negatively selected by depleting iTreg with CD4 microbeads and analyzed by microarray analysis (Document S1). The results of microarray analysis were validated using quantitative reverse-transcribed polymerase chain reaction (Table S2). The microarray data described in this report have been deposited in NCBIs Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through Gene Expression Omnibus Series accession number GSE7387.
Statistical analysis
All analyses of cell frequencies, proliferations, and cytokine concentrations for statistically significant differences were performed with the Student t test for analysis of completely randomized 2-group design. A P value less than .05 was considered significant.
Results
GP-specific autoreactive T cells originate predominantly from the CD4+CD45RA+ T-cell subset
In preliminary experiments, we attempted to investigate which T-cell subset (CD45RA+ vs CD45RO+ T cells) was the potential origin of GP-reactive T cells. We initially stimulated highly purified CD4+CD45RA+ and CD4+CD45RO+ T cells with Staphylococcal enterotoxin A at concentrations ranging from 10−2 to 102 ng/mL to compare their primary responses to superantigens, in which maximum proliferation was observed. We found that the reactivity of T cells from both subsets to superantigens was very similar in both ITP patients and healthy donors. In addition, tetanus toxoid (TT) was used as a control antigen for memory response. As expected, the recall immune response to TT mainly resided in the CD45RO+ T-cell population (data not shown).
The precursor frequency of T cells responding to GPIIb/IIIa was compared between the 2 isolated subsets of CD4+CD45RA+ and CD4+CD45RO+ T cells in ITP patients as well as healthy donors. The results showed that the T-cell response to GP antigens originated predominantly from the CD45RA+ subset, in both ITP patients and healthy controls. The estimated precursor frequency of GP-reactive T cells in the CD45RA+ subset was on average 2-fold higher than in the CD45RO+ subset in ITP patients (18.9 ± 6.7 × 10−6 vs 10.2 ± 5.2 × 10−6; P < .01; Figure 1). Similar results were obtained from healthy donors (16.2 ± 6.5 × 10−6 vs 7.1 ± 3.1 × 10−6; P < .01). In addition, the precursor frequency of GP-reactive T cells originating from the CD45RA+ T-cell pool was identical between patients and controls (P > .05), although the patients had a higher frequency of autoreactive T cells originating from the CD45RO+ T-cell pool compared with controls (P < .05). These studies suggested that GP-reactive CD4+ T cells in ITP predominantly derived de novo from the CD45RA+ T-cell pool.
Precursor frequency of GPIIb/IIIa-reactive T cells in CD45RA+ or CD45RO+ T-cell subsets from ITP patients and controls. From 19 healthy donors (HD) and 25 ITP patients, the purified CD4+CD45RA+ and CD4+CD45RO+ T cells (2 × 104/well) were seeded into 96-well plates along with autologous irradiated PBMCs (1 × 105/well) and stimulated with trypsin-digested GPIIb/IIIa for 7 days. During the last 16 hours of incubation, the cells were pulsed with 1 μCi [3H]thymidine/well. Wells stimulated with antigens that had cpm greater than 3 times the mean cpm of negative control wells were identified as responders. The frequency of GP-specific T cells was calculated by dividing the number of responding wells by the number of seeded wells, multiplied by the number of cells per well. Data shown are the mean number of GPIIb/IIIa-reactive T cells per 106 CD45RA+ versus CD45RO+ T cells plus or minus SD.
Precursor frequency of GPIIb/IIIa-reactive T cells in CD45RA+ or CD45RO+ T-cell subsets from ITP patients and controls. From 19 healthy donors (HD) and 25 ITP patients, the purified CD4+CD45RA+ and CD4+CD45RO+ T cells (2 × 104/well) were seeded into 96-well plates along with autologous irradiated PBMCs (1 × 105/well) and stimulated with trypsin-digested GPIIb/IIIa for 7 days. During the last 16 hours of incubation, the cells were pulsed with 1 μCi [3H]thymidine/well. Wells stimulated with antigens that had cpm greater than 3 times the mean cpm of negative control wells were identified as responders. The frequency of GP-specific T cells was calculated by dividing the number of responding wells by the number of seeded wells, multiplied by the number of cells per well. Data shown are the mean number of GPIIb/IIIa-reactive T cells per 106 CD45RA+ versus CD45RO+ T cells plus or minus SD.
DCs induce differentiation of GP-specific CD4+CD25+Foxp3+ Treg from CD4+CD25−CD45RA+ T cells
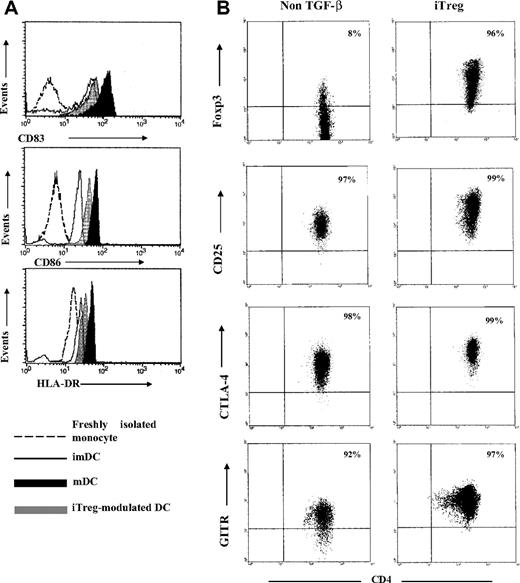
Autologous DCs were used to induce and expand GP-specific CD4+CD25+ iTreg in patients with chronic ITP. The surface phenotypes of imDCs and mDCs were characterized (Figure 2A). For induction of GP-specific T cells with regulatory function, the negatively selected CD4+CD25−CD45RA+ T cells from ITP patients were stimulated, respectively, with mDCs prepulsed with trypsin-digested GPIIb/IIIa or GPIb/IX in complete medium supplemented with IL-2 in the presence or absence of TGF-β1. Seven days later, CD4+CD25+ GPIIb/IIIa- or GPIb/IX-specific iTreg were purified by positive selection using CD25 magnetic beads. Foxp3 has been regarded as a specific marker for Treg, and the induced expression of Foxp3 was considered a conversion of Treg from non-Treg. To determine whether the presence of TGF-β during the priming promoted the generation of iTreg in vitro, the expression of Foxp3 was examined in purified CD4+CD25+ T cells induced from CD4+CD25−CD45RA+ T cells in the presence or absence of TGF-β. Consistent with previous studies,10,23 our data demonstrated that the presence of TGF-β significantly increased the level of Foxp3 in T cells cultured with TGF-β compared with T cells without it (Figure 2B), implying a requirement of TGF-β for induction of Foxp3. In 8 randomly selected patients, the induced CD25+ cells were rested in IL-2 for another 7 days after 1 week of stimulation, and Foxp3 expression was subsequently analyzed by flow cytometry on day 14. It was observed that the percentage of Foxp3-expressing T cells was stable over a 14-day period in T cells cultured in the presence of TGF-β (Figure S1A). Moreover, immunophenotyping of TGF-β–induced GP-specific CD4+CD25+ T cells exhibited other special phenotypes indicative of regulatory function, including CD25high, CTLA-4+, and GITR+ (Figure 2B). Therefore, CD4+CD25highFoxp3+ iTreg could be induced from CD4+CD25−CD45RA+ T cells in the presence of DCs and TGF-β.
Phenotypes of DCs and induced GP-specific CD4+CD25+Treg. (A) Freshly isolated monocytes cultured for 1 hour (day 0), immature DCs (day 5), and mature DCs (day 7) were analyzed by flow cytometry to determine the levels of expression of CD83, CD86, and HLA-DR. To detect the tolerogenic properties of GP-iTreg-modulated DCs, GPIIb/IIIa-prepulsed immature DCs were cocultured with GPIIb/IIIa-specific iTreg for 2 additional days (day 7). Then DCs were negatively selected by depleting iTreg with CD4 microbeads and analyzed by flow cytometry. Results were representative of 16 independent experiments. (B) Phenotypes of the induced GP-specific CD4+CD25+ Treg from ITP patients. CD4+CD25−CD45RA+ T cells isolated from the PBMCs of ITP patients were stimulated with mDCs prepulsed with trypsin-digested GPIIb/IIIa or GPIb/IX in the presence or absence of TGF-β1 (5 ng/mL). Seven days later, the CD4+CD25+ cells were purified by positive selection and analyzed for the expression of Foxp3, CTLA-4, GITR, and CD25.
Phenotypes of DCs and induced GP-specific CD4+CD25+Treg. (A) Freshly isolated monocytes cultured for 1 hour (day 0), immature DCs (day 5), and mature DCs (day 7) were analyzed by flow cytometry to determine the levels of expression of CD83, CD86, and HLA-DR. To detect the tolerogenic properties of GP-iTreg-modulated DCs, GPIIb/IIIa-prepulsed immature DCs were cocultured with GPIIb/IIIa-specific iTreg for 2 additional days (day 7). Then DCs were negatively selected by depleting iTreg with CD4 microbeads and analyzed by flow cytometry. Results were representative of 16 independent experiments. (B) Phenotypes of the induced GP-specific CD4+CD25+ Treg from ITP patients. CD4+CD25−CD45RA+ T cells isolated from the PBMCs of ITP patients were stimulated with mDCs prepulsed with trypsin-digested GPIIb/IIIa or GPIb/IX in the presence or absence of TGF-β1 (5 ng/mL). Seven days later, the CD4+CD25+ cells were purified by positive selection and analyzed for the expression of Foxp3, CTLA-4, GITR, and CD25.
Mechanism of suppression by GP-iTreg
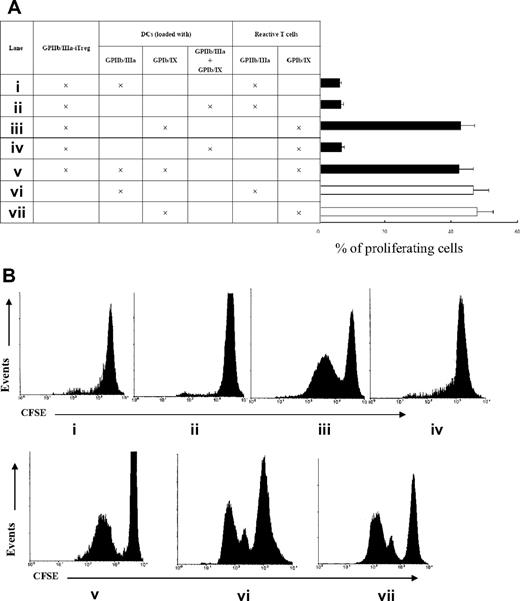
To further assess whether the GP-iTreg were functionally suppressive and determine the antigen specificity of the tolerance induced by GP-iTreg, GP-iTreg were cocultured with GP-reactive T cells and stimulated with DCs prepulsed with GPIIb/IIIa and/or GPIb/IX. As shown in Figure 3, GPIIb/IIIa-specific iTreg induced GPIIb/IIIa-reactive T-cell tolerance in the presence of DCs preloaded with GPIIb/IIIa or combination of GPIIb/IIIa and GPIb/IX. Conversely, GPIIb/IIIa-specific iTreg showed no inhibitory effect on the proliferation of GPIb/IX-reactive T cells stimulated with DCs preloaded with GPIb/IX. This observation indicated that the GP-iTreg lack constitutively suppressive activities but need specific antigenic stimulation through TCR to exert suppressive effects. In addition, when GPIIb/IIIa-specific iTreg were cocultured with GPIb/IX-reactive T cells and stimulated with DCs preloaded concurrently with GPIIb/IIIa and GPIb/IX, the proliferation of GPIb/IX-reactive T cells was inhibited. However, when GPIIb/IIIa-specific iTreg were cocultured with GPIb/IX-reactive T cells and stimulated with DCs preloaded separately with GPIIb/IIIa and GPIb/IX, they could not inhibit the proliferation of GPIb/IX-reactive T cells. These functional analyses demonstrated that GP-iTreg could suppress the GP-reactive T-cell proliferation in an antigen-specific manner. And the GP-iTreg specific for one antigen could inhibit the response of nearby effector cells to another antigen. This linked suppression required copresentation of the 2 antigens by the same DC. Because there were reports of human activated nonregulatory T cells expressing Foxp3 but not possessing regulatory activity,24,25 we addressed whether the GP-iTreg were anergic and maintained long-lasting suppressive function in 8 randomly selected patients. After 1 week of resting, the induced CD4+CD25+ cells were restimulated with GP-loaded mDC for 3 days. The results demonstrated that the induced iTreg lacked proliferative activity, indicating that they were in an anergic state. In contrast, the non–TGF-β–treated T cells showed high proliferating activity (Figure S1B). The suppression assays showed that the GPIIb/IIIa-specific iTreg could suppress the responder GPIIb/IIIa-reactive T cells even after the 7-day rest, whereas the non–TGF-β–treated T cells showed no suppressive activity (Figure S1C). Interestingly, in 2 randomly selected patients, the induced iTreg that had been rested for 3 weeks could still act as suppressor cells, corresponding with their persistence of Foxp3 expression (Figure S1D). Together, the data demonstrated that the induced iTreg were anergic and functionally suppressive.
Detection of antigen specificity of the tolerance induced by GP-iTreg. (A) A total of 2 × 105 GPIIb/IIIa-specific iTreg from 16 ITP patients were cocultured with CFSE-labeled GPIIb/IIIa-reactive T cells (2 × 105) and stimulated with 2 × 104 autologous imDCs (irradiated at 30 Gy) loaded with (i) GPIIb/IIIa, or (ii) both GPIIb/IIIa and GPIb/IX. Meanwhile, GPIIb/IIIa-specific iTreg (2 × 105) were cocultured with CFSE-labeled GPIb/IX-reactive T cells (2 × 105) and stimulated with 2 × 104 DCs loaded (iii) with GPIb/IX, (iv) concurrently with GPIIb/IIIa and GPIb/IX, or (v) separately with GPIIb/IIIa and GPIb/IX. For control purposes, CFSE-labeled (vi) GPIIb/IIIa- or (vii) GPIb/IX-reactive T cells (2 × 105) were cultured with autologous DCs (2 × 104) loaded with the respective antigen. After 3 days of culture, proliferation was measured as the percentage of proliferating GP-reactive T cells and shown as mean plus or minus SD with error bars representing SD. (B) A representative flow cytometric diagram was shown.
Detection of antigen specificity of the tolerance induced by GP-iTreg. (A) A total of 2 × 105 GPIIb/IIIa-specific iTreg from 16 ITP patients were cocultured with CFSE-labeled GPIIb/IIIa-reactive T cells (2 × 105) and stimulated with 2 × 104 autologous imDCs (irradiated at 30 Gy) loaded with (i) GPIIb/IIIa, or (ii) both GPIIb/IIIa and GPIb/IX. Meanwhile, GPIIb/IIIa-specific iTreg (2 × 105) were cocultured with CFSE-labeled GPIb/IX-reactive T cells (2 × 105) and stimulated with 2 × 104 DCs loaded (iii) with GPIb/IX, (iv) concurrently with GPIIb/IIIa and GPIb/IX, or (v) separately with GPIIb/IIIa and GPIb/IX. For control purposes, CFSE-labeled (vi) GPIIb/IIIa- or (vii) GPIb/IX-reactive T cells (2 × 105) were cultured with autologous DCs (2 × 104) loaded with the respective antigen. After 3 days of culture, proliferation was measured as the percentage of proliferating GP-reactive T cells and shown as mean plus or minus SD with error bars representing SD. (B) A representative flow cytometric diagram was shown.
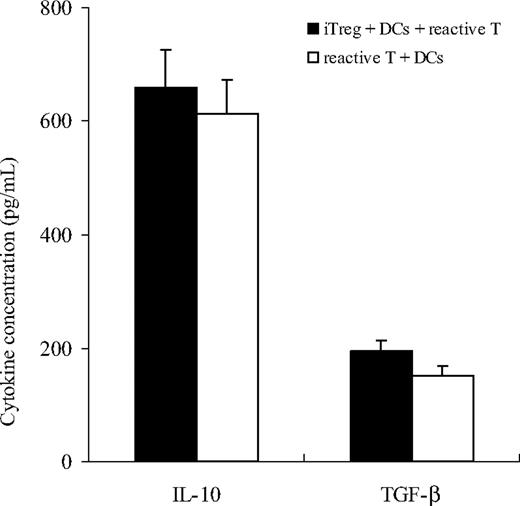
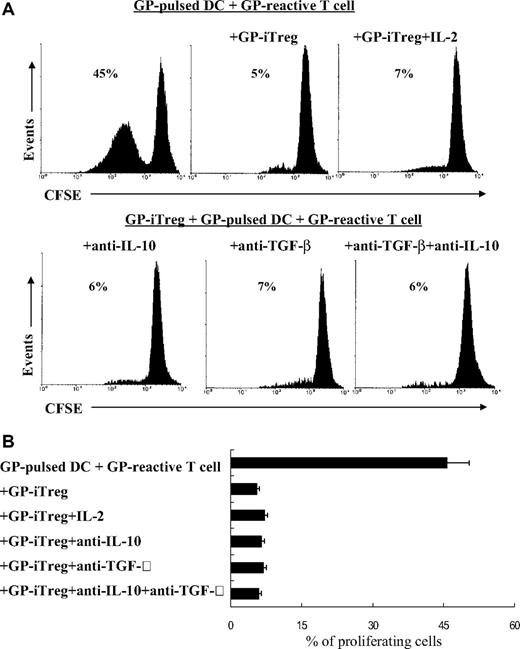
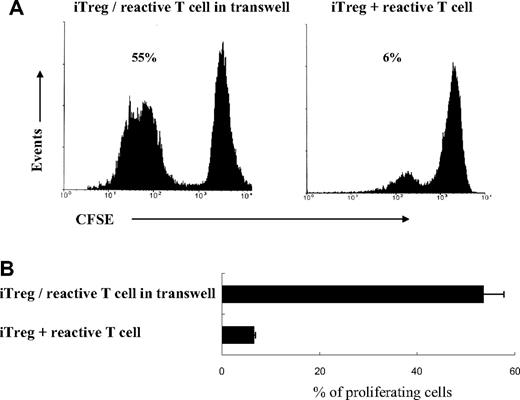
The role of cell-to-cell contact versus suppressive cytokines as the means by which GP-iTreg exert suppressive effects was determined by performing blocking and transwell experiments. The concentrations of the immunosuppressive cytokines IL-10 and TGF-β1 in the supernatants of culture system were measured (Figure 4). Although high levels of soluble IL-10 and TGF-β1 were detected in the cell coculture system, the addition of neutralizing anti–IL-10, anti–TGF-β1, or a combination of both antibodies into the in vitro cultures failed to reverse the blocking of GP-reactive T-cell proliferation (Figure 5). Moreover, exogenous IL-2 could not abrogate the suppression of effector T cells, suggesting that the suppressed proliferation of T cells was not the result of the consumption of IL-2 (Figure 5). We further investigated whether the suppressive function was mediated by direct cell-to-cell contact. As shown in Figure 6, separation of GP-iTreg and GPIIb/IIIa-reactive T cells by the transwell partition abolished the suppressive effects of GP-iTreg, demonstrating that the inhibition of GP-reactive T-cell proliferation was largely dependent on cell-to-cell contact between the T cells mediating the autoreactive responses and the GP-iTreg, and not mediated by soluble IL-10 or TGF-β1.
Cytokine production of GP-iTreg. GPIIb/IIIa-reactive T cells (2 × 105) were stimulated with GPIIb/IIIa-pulsed DCs (2 × 104) in the presence or absence of GPIIb/IIIa-specific iTreg (2 × 105). Cell-free supernatants were harvested from stimulated cultures after 72-hour incubation to assess for IL-10 and active TGF-β1 concentrations by ELISA. Results represent the mean plus or minus SD for 16 independent experiments.
Cytokine production of GP-iTreg. GPIIb/IIIa-reactive T cells (2 × 105) were stimulated with GPIIb/IIIa-pulsed DCs (2 × 104) in the presence or absence of GPIIb/IIIa-specific iTreg (2 × 105). Cell-free supernatants were harvested from stimulated cultures after 72-hour incubation to assess for IL-10 and active TGF-β1 concentrations by ELISA. Results represent the mean plus or minus SD for 16 independent experiments.
Inhibition of proliferation of GP-reactive T cells is independent on IL-10 or TGF-β. GPIIb/IIIa-specific iTreg (2 × 105) were cocultured with GPIIb/IIIa-pulsed DCs (2 × 104) and CFSE-labeled GPIIb/IIIa-reactive T cells (2 × 105) in the presence of neutralizing antibodies against IL-10 (10 μg/mL) and TGF-β (40 μg/mL), alone or in combination. In some experiments, IL-2 (100 μg/mL) was added to the culture system. After 3 days of coculture, the fluorescence intensity of CFSE-labeled GP-reactive T cells was determined by flow cytometry. Proliferation was measured as the percentage of proliferating GP-reactive T cells. Results are representative of 16 independent experiments (A) and shown as mean plus or minus SD with error bars representing SD (B).
Inhibition of proliferation of GP-reactive T cells is independent on IL-10 or TGF-β. GPIIb/IIIa-specific iTreg (2 × 105) were cocultured with GPIIb/IIIa-pulsed DCs (2 × 104) and CFSE-labeled GPIIb/IIIa-reactive T cells (2 × 105) in the presence of neutralizing antibodies against IL-10 (10 μg/mL) and TGF-β (40 μg/mL), alone or in combination. In some experiments, IL-2 (100 μg/mL) was added to the culture system. After 3 days of coculture, the fluorescence intensity of CFSE-labeled GP-reactive T cells was determined by flow cytometry. Proliferation was measured as the percentage of proliferating GP-reactive T cells. Results are representative of 16 independent experiments (A) and shown as mean plus or minus SD with error bars representing SD (B).
Requirement for cell-to-cell contact for suppressor activity of GP-iTreg. Transwell experiments were performed in 24-well plates. A total of 106 CFSE-labeled GPIIb/IIIa-reactive CD4+ T cells were stimulated with 105 GPIIb/IIIa-pulsed DCs in the lower chamber. A total of 106 GPIIb/IIIa-specific iTreg activated with 105 GPIIb/IIIa-pulsed DCs were either added directly to the lower chamber or placed in the upper chamber. After 3 days of culture, CFSE-labeled GPIIb/IIIa-reactive T cells in the lower chamber were harvested. The fluorescence intensity of GPIIb/IIIa-reactive T cells was determined by flow cytometry. Proliferation was measured as the percentage of proliferating GP-reactive T cells. Results are representative of 16 independent experiments (A) and shown as mean plus or minus SD with error bars representing SD (B).
Requirement for cell-to-cell contact for suppressor activity of GP-iTreg. Transwell experiments were performed in 24-well plates. A total of 106 CFSE-labeled GPIIb/IIIa-reactive CD4+ T cells were stimulated with 105 GPIIb/IIIa-pulsed DCs in the lower chamber. A total of 106 GPIIb/IIIa-specific iTreg activated with 105 GPIIb/IIIa-pulsed DCs were either added directly to the lower chamber or placed in the upper chamber. After 3 days of culture, CFSE-labeled GPIIb/IIIa-reactive T cells in the lower chamber were harvested. The fluorescence intensity of GPIIb/IIIa-reactive T cells was determined by flow cytometry. Proliferation was measured as the percentage of proliferating GP-reactive T cells. Results are representative of 16 independent experiments (A) and shown as mean plus or minus SD with error bars representing SD (B).
GP-specific iTreg mediate their regulatory effects via modulation of the stimulatory capacity of DCs
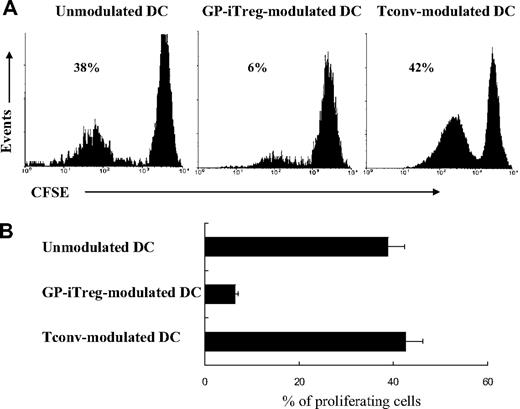
To investigate whether GP-iTreg mediated their regulatory effects via modulating the T-cell stimulatory capacity of antigen-presenting cells (APCs), GPIIb/IIIa-specific iTreg or Tconv were incubated with autologous DCs loaded with GPIIb/IIIa for 2 days. After that T cells were depleted using CD4 magnetic beads, either iTreg- or Tconv-modulated DCs were examined for their ability to induce tolerance on autologous GPIIb/IIIa-reactive T cells. As shown in Figure 7, after exposure to GPIIb/IIIa-specific iTreg, the iTreg-modulated DCs failed to stimulate GPIIb/IIIa-reactive T cells. In contrast, the unmodulated DCs or Tconv-modulated DCs were efficient to induce the proliferation of GPIIb/IIIa-reactive T cells. This result might reflect that DCs were rendered tolerogenic by GP-iTreg. Moreover, phenotypic analysis showed that GP-iTreg–modulated DCs turned out to display a semimature phenotype, as they expressed higher levels of CD86 and HLA-DR than unmodulated imDCs but limited levels of the markers compared with mDCs (Figure 2A). The data suggested that GP-iTreg mediated regulatory effects via modulation of the stimulatory capacity of DCs.
GP-specific iTreg down-regulated the T cell–stimulating capacity of DCs. GPIIb/IIIa-pulsed DCs were cultured alone (unmodulated DCs) or in the presence of GPIIb/IIIa-specific iTreg or conventional CD4+ GPIIb/IIIa-reactive T cells (Tconv) for 2 days. Then T cells were depleted using CD4 magnetic beads, and the isolated iTreg- or Tconv-modulated DCs (2 × 104) or unmodulated DCs (2 × 104) were used to stimulate CFSE-labeled GPIIb/IIIa-reactive T cells at a 1:10 ratio. After 3 days of culture, proliferation was measured as the percentage of proliferating GPIIb/IIIa-reactive T cells by flow cytometry. Results are representative of 16 independent experiments (A) and shown as mean plus or minus SD with error bars representing SD (B).
GP-specific iTreg down-regulated the T cell–stimulating capacity of DCs. GPIIb/IIIa-pulsed DCs were cultured alone (unmodulated DCs) or in the presence of GPIIb/IIIa-specific iTreg or conventional CD4+ GPIIb/IIIa-reactive T cells (Tconv) for 2 days. Then T cells were depleted using CD4 magnetic beads, and the isolated iTreg- or Tconv-modulated DCs (2 × 104) or unmodulated DCs (2 × 104) were used to stimulate CFSE-labeled GPIIb/IIIa-reactive T cells at a 1:10 ratio. After 3 days of culture, proliferation was measured as the percentage of proliferating GPIIb/IIIa-reactive T cells by flow cytometry. Results are representative of 16 independent experiments (A) and shown as mean plus or minus SD with error bars representing SD (B).
Analysis of global gene expression in DCs after exposure to GP-iTreg
To further define the molecular changes induced by iTreg in DCs, we performed microarray experiments to select relevant target genes in the gene expression profile of DCs treated with GP-iTreg. We attempted to describe and illustrate the relationship and specific transcriptional clusters between DCs treated or untreated with GP-iTreg with focus on immune response–associated gene families. The experiments were reproduced in the culture cells derived from 5 independent patients, and relevant genes were selected, which represented the specifically expressed genes in DCs modulated by GP-iTreg. Hierarchical clustering emphasized the similarities between different samples and visualized the transcriptional relationship between the DCs. We validated the microarray data for 11 genes using quantitative reverse-transcribed polymerase chain reaction (Table S2). Analysis of the mRNA microarray profile induced by GP-iTreg in DCs revealed characteristic changes in the level of transcription of genes. Approximately 150 genes were up-regulated or down-regulated in their expression levels during tolerance induction by 2-fold or greater in all the paired hybridization experiments. These genes encoded products that belonged to cellular and nuclear components, cell surface molecules, cytokines, cytokine receptors and growth factors, signal transduction, transcription, cell cycle, apoptosis, and cell metabolism (Table S3). Microarray analysis indicated up-regulation of genes associated with the tolerogenicity of DCs, such as TGF-β receptor III (TGFBR3), SKI-like (SKIL), DLL1 (Delta 1), Jagged2 (JAG2), and Notch1, and down-regulation of genes related to the maturation or immunogenicity of DCs, for example, Toll-like receptor (TLR) 3, TLR8, myeloid differentiation factor 88 (MyD88), Toll-IL-1R-containing adaptor molecule 2 (TICAM2), TIR domain-containing adapter protein (TIRAP), CD83, and TGF-β–activated kinase-binding protein 1 (TAB1). Our findings were in support of the aforementioned observation that DCs pulsed with cognate antigen obtained tolerogenicity after coculture with GP-iTreg.
To identify tolerance-associated genes, obtained genes were analyzed based on the BIOCARTA database to characterize the cellular and regulatory process pathways in which they function. The pathway analysis revealed that modulated expression of genes was involved in immune tolerance, such as TLR (TLR3, TLR8, MyD88, TICAM2, TIRAP, and TAB1), Notch (DLL1, JAG2, and Notch1), and TGF-β1 (TGFBR3, SKIL and TAB1) signaling pathways. The Fisher exact test led to discernment of pathways having P values less than .05 according to the website statistic analysis. TLR signaling was influenced significantly so that this pathway was dominant with a P value less than .001.
Discussion
In the present study, we first examined the GP-specific responses in terms of their origins in the CD45RA+ versus CD45RO+ compartments. Although the frequency of GP-reactive T cells originating from the CD45RO+ T-cell pool was increased in ITP patients, the detection of GP-specific responses from the CD45RA+ subset prevalent in both ITP patients and healthy controls pointed to this subpopulation as the major source of autoreactive T cells, which were consistently available to elicit autoimmune responses under appropriate stimulation. Our results were keeping with the findings of Muraro et al20 that the CD45RA+ T-cell population originated predominant responses to human self-Ag myelin basic protein in multiple sclerosis patients and healthy controls, suggesting a major role of the CD45RA+ T-cell subset in the origin and maintenance of autoreactive effector CD4+ T-cell repertoire. These findings offered important clues to the induction of autoantigen-specific iTreg from CD4+CD45RA+ T cells.
We demonstrated that GP-specific CD4+CD25+ iTreg could be induced and expanded from CD4+CD25−CD45RA+ T cells with GP-loaded autologous DCs in the presence of TGF-β1 and IL-2 in patients with ITP. These iTreg were anergic and exhibited phenotypes of nTreg because they expressed high levels of Foxp3, CTLA-4, and GITR and displayed a stable regulatory T-cell phenotype and suppressive function in long-term cultures. The use of DCs instead of mitogenic stimuli to differentiate Treg de novo from CD4+CD25− T cells has the advantage of maintenance of antigen specificity.26 Whereas Passerini et al27 reported that the STAT5-signaling cytokine IL-2 could up-regulate Foxp3 expression in CD4+CD25− T cells, our results demonstrated an essential role of TGF-β in IL-2–mediated Foxp3 induction. This was in accordance with the recent finding of TGF-β dependency of Foxp3 expression in human Treg.28
It was controversial whether stimulated human CD4+CD25− T cells could acquire regulatory function. Although our results that platelet GP stimulation in the presence of TGF-β and IL-2 conferred regulatory activity to CD4+CD25−CD45RA+ T cells in ITP patients was in disagreement with that by Tran et al,28 which reported that TGF-β–induced Foxp3 expression did not give rise to a suppressive population of T cells, other reports in humans showed that antigen-specific CD4+CD25+ Treg could be converted from nonregulatory CD4+CD25− cells.15–17 Similar to our findings, in one study of anti–tumor necrosis factor-α antibody (infliximab) in rheumatoid arthritis, the addition of infliximab to purified active rheumatoid arthritis CD4+CD25− T cells in vitro resulted in a substantial increase in Foxp3+ cells, which possessed suppressor activity, whereas no such effect was seen when CD4+CD25− T cells were isolated from healthy persons.29 Alternatively, it could not be excluded that prolonged culture (7 days) in our study with selective survival of Foxp3+ cells could eventually confer a suppressive function to nonregulatory T cells, as suggested by Passerini et al.27 The present data demonstrate that the GP-iTreg could mediate both antigen-specific and linked suppression. Induction of iTreg specific for one or few of self-epitopes on GPs involved in ITP might lead to the formation of GP-iTreg-APC clusters. This would induce a regulatory form of tolerance to other epitopes on GPs that are constitutively presented by the same APC as the putative culprit epitope(s). The linked suppression is particularly important given the findings that there is variation and spreading of the T-cell response to target epitopes on GP during the course of chronic ITP.5,30 Because there was more than one epitope on each GP molecule, a limitation of this study was that the efficiency of antigen-specific Treg induction was not assessed at the monoclonal level. Thus, in more strict terms, the induced GP-iTreg represented an antigen-reactive Treg cell population. Careful screening of antigenic peptides to generate oligoclonal or monoclonal GP-specific iTreg and enhance their in vivo suppressive function in ITP might open the door to a novel strategy of “therapeutic vaccination” for the treatment of human autoimmune diseases.31
Our study further demonstrated that cell-cell contact was the primary means by which GP-iTreg exerted their suppressive effects in the in vitro setting. However, this could not exclude completely the potential role of inhibitory cytokines in mediating Treg function. Recently, an exciting new inhibitory cytokine, IL-35, has been described that is preferentially expressed by Treg and contributes significantly to Treg function.32,33 Whether IL-35 may mediate the suppressive effects of GP-iTreg remains to be defined. Moreover, there were reports of membrane-tethered TGF-β mediating suppression by Treg in a cell contact–dependent manner.34,35 It was also probable that membrane-tethered TGF-β was partially relevant for the dependence on direct cellular contact of the GP-iTreg suppressive function. The GP-iTreg induced GP-specific tolerance and blocked the proliferation of pathogenic T cells through rendering DCs tolerogenic. The suppression was mediated via modulation of the T cell activating capacity of DCs. This reflected that DCs were indispensable for facilitating iTreg in inducing GP-specific tolerance. Our results were consistent with the findings by Manavalan et al36 that the prior interaction with CD8+CD28− suppressor T cells generated tolerogenic DCs that were competent for the induction of antigen-specific CD4+CD25+CD45RO+ iTreg.
It should be noted that imDCs were used to stimulate GP-reactive T cells in our study. The imDCs readily take up sources of autoantigens. Many studies have reported that the maturation status of DCs seems to determine the immune response toward tolerance or immunity when confronting effector T cells. However, imDCs were used on the basis of the findings that, if in a proper ratio between imDCs and effector T cells, imDCs were more efficient to stimulate T-cell proliferation than mDCs.37 True to our observations, imDCs could efficiently induce GP-reactive T-cell proliferation at a 1:10 ratio. On the other hand, this system would better mimic the environment in vivo as well, in which most DCs were immature. Interestingly, we observed that GP-iTreg-modulated DCs from patients with ITP failed to stimulate GP-reactive T cells. Together with the phenotypic analysis showing that GP-iTreg could modify DCs to become semimature DCs, we confirmed that DCs were rendered tolerogenic after coculture with GP-iTreg. According to our observations that had not been shown, a direct cell-cell contact between DCs and iTreg of 12 hours was sufficient to render DCs tolerogenic. In light of the recent finding that the antigen-presenting capacity of DCs from ITP patients was increased compared with healthy donors, which contributed to the abnormal activation of autoreactive T cells,38 our result of functional suppression by CD4+CD25+ iTreg through direct contact with DCs seemed particularly important for the down-regulation of autoimmune responses in ITP.
Although the direct modulation of DCs by iTreg was required for the licensing of DCs with full T-cell-regulatory activity, the molecular mechanisms responsible for the tolerogenicity of DCs were still a matter of speculation. Therefore, we first analyzed the gene-expression profile of DCs modulated by iTreg using microarray to investigate the underlying mechanisms involved in this process.
The pathway analysis based on the BIOCARTA database indicated that inhibition of the TLR pathway by GP-iTreg played an important role in the suppression process. Among genes involved in this pathway, TLR3, TLR8, MyD88, TICAM2, TIRAP, and TAB1 were down-regulated remarkably in DCs after exposure to GP-iTreg. TLRs transduce signaling via adaptor proteins MyD88, TICAM2, and TIRAP to induce the translocation of nuclear factor-κB, which allows DCs to mature and initiate an immune response.39 TLRs played a critical role in stimulating innate immunity by recognizing pathogen-associated molecular patterns on invading microbial pathogens.40 Several studies illustrated that TLRs could also be activated by a variety of endogenous ligands, such as heat shock proteins and certain contents of necrotic cells, leading to autoimmunity in systemic lupus erythematosus and multiple sclerosis models.41 Overexpression of TLR has been reported in ITP.42 Recently, it was demonstrated that, in ITP patients, platelets showed increased propensity to undergo apoptosis and their DCs had enhanced capability of presenting apoptotic platelets to T cells.38 Future studies will be required to address whether or what endogenous ligands on the destructed or apoptotic platelets are involved in the stimulation of the immune system in ITP. Whatever the endogenous ligands may be, this is the first description of suppressing the TLR pathway as a mechanism by which Treg render DCs tolerogenic. More recent studies43,44 showed that platelet-bound lipopolysaccharide enhanced Fc receptor–mediated phagocytosis of IgG-opsonized platelets via TLR4 expressed on platelets, which might at least be responsible for the aggravated destruction of platelets in patients with ITP during infections. It would be an interesting question whether the GP-iTreg could also have effect on the platelet phagocytosis via modulating TLRs on platelets and hence reduce the destruction of platelets.
The molecular targets of TGF-β1–mediated suppression in DCs are still ill defined. Our findings of overexpression of TGFBR3, SKIL, and TAB1 in GP-iTreg–modulated DCs suggested that TGFBR3 also played an essential role in TGF-β signaling in DCs. Recently, several studies have suggested that Notch ligands expressed on APCs could deliver immunomodulatory signals to engaged T cells and promote development of regulatory T cells.45–47 Our results were in line with those previous findings and demonstrated that gene expression of Notch1 and the Notch ligands DLL1 and JAG2 were up-regulated in iTreg-treated DCs. The present study uncovers some of the signaling pathways and up- or down-regulated genes associated with induction of tolerance in DCs after exposure to Treg. Identifying the tolerance mechanisms of Treg-modulated DCs has important implications for determining how iTreg exert regulatory effect in vivo and for developing novel molecular targets of immunotherapy in ITP patients. We think that these findings in ITP will facilitate our understanding of the mechanisms of induction and maintenance of autoantigen-specific tolerance and highlight the considerable potential of antigen-specific iTreg for targeted immunotherapy in human autoimmune diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Xian-Jun Ma for sample collection, Dr Liang Zhang and Jian-Qing Zhao for technical assistance, Yong-Hong Ren for help with data analysis, and Alice Shih for critical reading of the manuscript.
This work was supported by grants from National Natural Science Foundation of China (30570779, 30300312, 30600259, 30770922, 30671976, and 30628015), 973 Program (2006 CB 503803), Foundation for the Author of National Excellent Doctoral Dissertation of PR China (200561), Program for New Century Excellent Talents in University (NCET-07-0514), Key Clinical Research Project of Public Health Ministry of China 2007-2009, Commonweal Trade for Scientific Research (200802031), the 1020 Program from Health Department of Shandong Province, and Taishan scholar project funding.
Authorship
Contribution: X.-L.Z. and J.P. designed and performed research, analyzed data, and wrote the paper; J.-Z.S., C.-S.G., J.-J.L., Z.-G.W., and Y.Y. performed research, contributed clinical samples/data, and analyzed data; Y.S., P.Q., and S.-G.L. contributed new reagents or analytic tools, and contributed clinical samples/data; and L.-N.Z. and M.H. designed research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Peng, Department of Hematology, Qilu Hospital, Shandong University, Jinan, Shandong, 250012, P R China; e-mail: junpeng88@sina.com.cn.
References
Author notes
*X.-L.Z. and J.P. contributed equally to this work.

![Figure 1. Precursor frequency of GPIIb/IIIa-reactive T cells in CD45RA+ or CD45RO+ T-cell subsets from ITP patients and controls. From 19 healthy donors (HD) and 25 ITP patients, the purified CD4+CD45RA+ and CD4+CD45RO+ T cells (2 × 104/well) were seeded into 96-well plates along with autologous irradiated PBMCs (1 × 105/well) and stimulated with trypsin-digested GPIIb/IIIa for 7 days. During the last 16 hours of incubation, the cells were pulsed with 1 μCi [3H]thymidine/well. Wells stimulated with antigens that had cpm greater than 3 times the mean cpm of negative control wells were identified as responders. The frequency of GP-specific T cells was calculated by dividing the number of responding wells by the number of seeded wells, multiplied by the number of cells per well. Data shown are the mean number of GPIIb/IIIa-reactive T cells per 106 CD45RA+ versus CD45RO+ T cells plus or minus SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-03-148288/5/m_zh80080931360001.jpeg?Expires=1769841787&Signature=AT~~~orn95fJJdJgpNww2mgcO2u7cWn4u5ch5HgL0OPg3LZrgO0D96tauntb9Ne4cladMXbJ3zMz~9wflAFkKcAReAO5XfXy1NloLzLCXCjT0jNdQ-tFZFSG6dj~GJJEBr5qUbkkUwzN28ylbGoBKTGzZh086kelwtbltsVbDPUCj8R-qNfvEMGe-dnz0jzKBfapP2LmKxbM6mNtgOfwQLdxu4agHFA6X6net~Aa0Az~H8amWVn-5bXuHVTg8xxPlasGOVPamehTBJbV1M6QdZewqHyGofxiXOLPIx2P9NKkOXtst4zEpvZajlcRFWAbL~xiJ7o~TMPC~dtJlK2HvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal