Detoxification and clearance of extracellular hemoglobin (Hb) have been attributed to its removal by the CD163 scavenger receptor pathway. However, even low-level hydrogen peroxide (H2O2) exposure irreversibly modifies Hb and severely impairs Hb endocytosis by CD163. We show here that when Hb is bound to the high-affinity Hb scavenger protein haptoglobin (Hp), the complex protects Hb from structural modification by preventing α-globin cross-links and oxidations of amino acids in critical regions of the β-globin chain (eg, Trp15, Cys93, and Cys112). As a result of this structural stabilization, H2O2-exposed Hb-Hp binds to CD163 with the same affinity as nonoxidized complex. Endocytosis and lysosomal translocation of oxidized Hb-Hp by CD163-expressing cells were found to be as efficient as with nonoxidized complex. Hp complex formation did not alter Hb's ability to consume added H2O2 by redox cycling, suggesting that within the complex the oxidative radical burden is shifted to Hp. We provide structural and functional evidence that Hp protects Hb when oxidatively challenged with H2O2 preserving CD163-mediated Hb clearance under oxidative stress conditions. In addition, our data provide in vivo evidence that unbound Hb is oxidatively modified within extravascular compartments consistent with our in vitro findings.
Introduction
When Hb is released into the extracellular space during hemolysis or tissue injury it can be toxic to the environment.1 Hb cytotoxicity has been attributed to oxidative processes driven by the heme group. The primary binding protein for extracellular Hb is haptoglobin (Hp), which binds Hb irreversibly with a rate constant of 5.5 × 105 M−1s−1.2 Following complex formation, Hb-Hp is removed by circulating blood monocytes and tissue macrophages via the Hb scavenger receptor CD163.3,,,–7 However, we have previously shown that even in the absence of Hp a lower affinity interaction between Hb and CD163 allows for efficient endocytosis and degradation of Hb by macrophages.7 The precise role of Hp in the CD163-mediated Hb clearance pathway remains therefore elusive
Exposure of Hb to H2O2 induces structural modifications, which include altered heme protein product formation, extensive cross-linking of α-globin chains, and irreversible oxidative modifications of specific amino acids within the CD163 binding region of the Hb β-globin chain.8,9 These structural alterations result in reduced CD163 binding, and severely impair endocytosis of oxidized Hb by CD163.10 Therefore, failure of Hb clearance by endogenous mechanisms could aggravate tissue injury.
The ubiquitous oxidant molecule H2O2 is generated as a result of superoxide (O2·−) dismutation and is the major reactive oxygen species produced during metabolic processes.11 Highly elevated tissue concentrations of H2O2 occur during inflammation and tissue injury as a consequence of the oxidative burst activity of granulocytes and macrophages.12 Within diseased tissue, H2O2 concentrations in excess of 100 μM have been measured, though 1 to 15 μM appears to be the upper limit of a healthy physiologic range.11 In the presence of H2O2, extracellular Hb is susceptible to heme iron, and protein oxidation associated with pseudoperoxidase activity of Hb. H2O2 oxidizes ferrous Hb (Fe2+) to generate the oxo-ferryl (Fe4+ = O2−) state and, in the case of the reaction with ferric Hb (Fe3+), a protein radical (Hb·+ Fe4+ = O2−) is formed as follows: (1) Hb (Fe2+) + H2O2→Hb (Fe4+ = O2−) + H2O; (2) Hb (Fe2+) + Hb (Fe4+ = O2−) + H + →Hb (Fe3+) + OH−; (3) Hb (Fe3+) + H2O2 → Hb·+ Fe4+ = O2− + H2O.
Whereas classic peroxidases stabilize the radical on the porphyrin, the radical formed during the pseudoperoxidase cycles of Hb typically migrates away from the heme group, yielding a radical cation on an amino acid residue.13 Although strong oxidative conditions are usually required to irreversibly damage nonheme proteins,14,15 the radical formation during these cycles of H2O2 reaction renders Hb highly susceptible to oxidative modification in the presence of very low concentrations of H2O2.8,16
Our previous work defined specific amino acids within the β-globin chain as a target of H2O2-induced heme radical formation and migration. βCys93 and βCys112 underwent irreversible oxidation to cysteic acid, whereas βTrp15 was found to oxidize to oxyindolyl and kynureinyl products. Extensive protein-protein cross-linking and heme adduct formation characterize the oxidative modification of α-globin subunits.8 Beyond the impaired CD163 binding and clearance of oxidatively altered Hb products, these modifications may result in the occurrence and accumulation of potential neoantigens or inflammatory by-products.17
Hp is supposed to serve as an antioxidant through control of Hb-mediated oxidative side reactions and subsequent tissue damage.18 However, it has not been established whether Hp could also specifically stabilize Hb during oxidative insult and whether it could prevent H2O2-induced structural Hb modifications. Hp is released in large quantities by activated granulocytes at local sites of inflammation and tissue injury where also free Hb typically accumulates as a result of red blood cell lysis.19 Hb complexation by Hp seems thus to play a particularly important role during inflammation and tissue injury—conditions intimately associated with enhanced oxidative stress.20
The present study confirms that the in vitro characterized oxidative modifications do also occur in Hb exposed to oxidative stress in vivo. All H2O2-induced modifications are shown to be prevented when Hb is scavenged by Hp before it is exposed to H2O2. As a result, CD163 receptor binding, cellular uptake, and lysosomal sequestration were preserved even after Hb-Hp exposure to the most severe oxidative conditions tested. Taken together, these data suggest that induction and/or administration of Hp may have therapeutic potential in several acute and chronic hemolytic disease states.
Methods
Hemoglobin and haptoglobin sample preparation
We used highly purified human Hb (HbA0), which was a gift from Hemosol (Mississauga, ON). The purity of this Hb is 99.99%21 and has been confirmed by extensive characterizations.22,23 Human Hp 1-1 and 2-2 were from Sigma-Aldrich (St Louis, MO) and are 99% pure. Hemoglobinuria in beagle dogs was induced by continuous infusion of stroma-free HbA0 over 8 hours (a gift from Sangart, San Diego, CA) targeted to a constant plasma concentration of approximately 100 μM (heme). The dog Hb infusion experiments were approved by the animal research commission of the Kanton of Zurich, Switzerland.
Oxidation of hemoglobin and hemoglobin-haptoglobin complexes
Hb (250 μM, heme) was treated either with 5, 10, and 40 mU/mL glucose oxidase for 2 and 16 hours generating approximately 1.5 μM H2O2/min at 10 mU/mL glucose oxidase as previously reported by our laboratory24 or with stoichiometric to suprastoichiometric boluses of H2O2 in a 1:1 and 1:10 molar excess of oxidant over heme as previously reported by our laboratory.8 Steady-state H2O2 concentrations in the GOX system were measured as 10 to 50 μM. Oxidized Hb-Hp (1-1) was prepared as Hb (250 μM, heme) with an equivalent molar protein concentration of Hp to allow for 1:1 binding followed by oxidation as described previously.8 To prove complex formation and to exclude effects of nonbound Hb or Hp, we have analyzed the Hb-Hp complex by high-performance liquid chromatography (HPLC) before oxidation (data not shown). Although glucose oxidase generated H2O2 is thought to be the most physiologically relevant method for continuous exposure, a clear advantage of bolus additions of H2O2 in our circumstance is the simplicity of the reaction mixture for the mechanistic studies described here and that modifications induced by the glucose on the Hb protein are avoided prior to structural analysis. The same patterns of amino acid oxidations in Hb were observed with all oxidation procedures, therefore figures represent the most severe stoichiometric exposures evaluated (10-fold molar excess H2O2) to emphasize the protective influence of Hp.
Reverse-phase chromatography
In vitro and in vivo (renal filtrate) samples were separated on a Zobax SB 300 C3 (Agilent Technologies, Wilmington, DE) 250 × 4.6-mm column attached to a Water-626 pump and Waters-2487 dual-wavelength detector, controlled by a Waters-600s controller using Millenium32 software (Waters, Milford, MA). The mobile phase consisted of (A) 0.1% trifluoroacetic acid (TFA) in water and (B) 0.1% TFA in acetonitrile. A gradient was programmed to deliver 65% (A)/35% (B) over 10 minutes, 63% (A)/37% (B) after 10 minutes, 60% (A)/40% (B) after 15 minutes, 57% (A)/43% (B) after 16 minutes, then increased to 90% B over the remaining 40 minutes of the run. Solvents were mixed and run at a rate of 1 mL/min and absorbencies monitored at 280 nm and 405 nm.25
Mass spectrometric analysis
Samples were reduced and alkylated. Trypsin was added in a 1:25 enzyme-substrate wt/wt ratio. The samples were incubated overnight (∼ 12 hours) at 37°C. The reaction was quenched by acidifying with 0.1% formic acid. Before introduction into the mass spectrometer (liquid chromatography/mass spectrometry/mass spectrometry [LC-MS/MS]), samples were desalted using C18 zip-tips (Millipore, Billerica, MA). Tryptic peptides (2 μL) were mixed in a 10-μL solution of alpha-cyano-4-hydroxycinnamic acid (CHCA) in 50:50 acetonitrile water mixture containing 0.1% formic acid. After spotting and drying on the target plate, matrix-assisted laser desorption/ionization (MALDI)–MS analysis was performed using a 4800 MALDI TOF/TOF mass spectrometer (Applied Biosystems, Foster City, CA). Data were acquired with Xcalibur 2.0 software (Thermo Scientific, San Jose, CA) and processed using Bioworks (Thermo Scientific). Peptide searching was performed using MASCOT26 (detailed description is provided in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Surface plasmon resonance analyses
Surface plasmon resonance (SPR) was performed using a Biacore 3000 instrument (GE Healthcare, Biacore, Uppsala, Sweden) with purified CD163 immobilized on CM5 Biacore sensor chips. Detailed protocols of receptor immobilization and data acquisition are given in Document S1.
Kinetic measurements of peroxidase activity of Hb with or without haptoglobin
The slower kinetic processes after mixing H2O2 (in molar ratios of 1:1, 1:2, 1:5, and 1:10) with ferrous Hb (50 μM heme) with or without Hp (1:1 molar ratio) were monitored in a thermostated cell of a rapid scanning diode array spectrophotometer (HP-8453; Agilent Technologies). All experiments were run at 37°C, in 50 mM phosphate buffer (pH 7.4) that had been previously treated with Chelex resin (Bio-Rad, Hercules, CA). All spectra were recorded as a function of time, and representative spectra of ferrous Hb in the beginning, ferric Hb at the end of the reactions, as well as the intermediate spectra of ferryl Hb were recorded. Ferric Hb treated with excess amount of H2O2 was measured in an Applied Photophysics SF-17 stopped-flow spectrophotometer (Surrey, United Kingdom) in 50 mM phosphate buffer (pH 7.4) at room temperature as previously reported.27 Several spectra were captured as a function of time using an Applied Photophysics photodiode array accessory. A minimum of 200 spectra were collected in a given time period with a resolution of 2.38 ms per spectrum for each reaction. The whole set of spectral data was then subjected to global analysis and curve fitting (Applied Photophysics software). Spectra of major reaction species from ferric Hb to ferryl Hb were reconstructed, and the reaction rate constants were obtained from the analysis. The experiments were carried out with increasing H2O2 concentrations in the presence or absence of Hp.
Hb endocytosis and competitive cellular uptake assay
Culture of CD163-transduced HEK293 cells (CD163-HEK293) and the fluorescent Hb-Hp uptake assay were performed as described.7 To quantify Hb endocytosis by fluorescence microscopy, cells were incubated with the indicated ligands, washed, fixed with paraformaldehyde, permeabilized with Triton X-100, and stained with polyclonal rabbit anti-Hb (1:1000; Abcam, Cambridge, United Kingdom) and Alexa 568 goat anti–rabbit (1:1000; Molecular Probes, Eugene, OR) antibodies. We confirmed that the anti-Hb antibody reacted equally with nonoxidized and oxidized Hb by Western blot (data not shown). Samples were examined with a Carl Zeiss epifluorescence Axioskope-2 equipped with an AxioCam-MR digital camera and Axio-Vision software (Carl Zeiss, Feldbach, Switzerland). For quantification, red channel fluorescence of at least 10 microphotographs per coverslip was quantified using SigmaScan software (Systat Software, Chicago, IL). Values were corrected for the number of DAPI-stained nuclei to get arbitrary fluorescence per cell values.28 For subcellular localization of Hb-Hp cells were additionally stained with mouse anti–LAMP-1 and Alexa 488 goat anti–mouse antibodies. Samples were analyzed as 0.2-μm-thick optical sections using a Leica SP2-AOBS-UV confocal laser-scanning microscopy system (CLSM; Leica, Heidelberg, Germany) with an optical magnification of 630×. Images were processed using Imaris (Bitplane, Zurich, Switzerland).
Quantification of HO-1 mRNA and protein by quantitative real-time PCR and Western blot
HO-1 mRNA abundance was quantified and normalized to GAPDH levels with LightCycler (Roche Diagnostics, Basel, Switzerland) polymerase chain reaction (PCR) system as described.7 SDS–polyacrylamide gel electrophoresis (PAGE) of total cellular extracts and Western blot detection of HO-1 were performed using a polyclonal antibody to HO-1 (Stressgen, Victoria, BC) and goat anti–rabbit HRP-conjugated secondary reagent (Amersham, Arlington Heights, IL). Blots were developed with enhanced chemiluminescence (ECL Plus; Amersham) and analyzed on a Chemi Doc XRS system (Bio-Rad, Hercules, CA). For direct staining of native and H2O2-reacted Hb in PAGE gels, gels were fixed and stained with the Silver-Plus kit (Bio-Rad).29
Results
Identification of oxidatively altered Hb in vivo
We recently demonstrated that exposing Hb to a range of H2O2 concentrations in vitro results in a highly reproducible pattern of amino acid oxidations, heme protein adducts, and globin chain cross-linking.8,10 We have now considered a pathophysiologic condition where Hb is potentially exposed to peroxides in vivo. During severe hemolysis non–Hp-bound Hb is readily filtered by the kidney and it has previously been shown that hemoproteins such as myoglobin do redox cycle H2O2 and lipid peroxides in the kidney.30 We have therefore evaluated a dog model of free Hb infusion with hemoglobinuria to study the structural changes imparted to Hb when it escapes the antioxidant plasmatic compartment and enters an acidic pro-oxidant environment. The largely protein-depleted urine further offers the unique possibility to study the excreted Hb in a low background environment with the same technical approaches that we previously used to characterize oxidative Hb modifications in vitro. Compared with the HPLC profile of native Hb, the HPLC profile of dog urine collected during infusion of stroma-free Hb revealed altered globin chains and altered heme protein products that elute at 22.0 minutes (Figure 1A). Previous work has demonstrated the same oxidation-induced altered heme protein product in CSF following subarachnoid hemorrhage.31 We have shown that high-mass MALDI-MS offers a convenient and specific approach to identify covalently cross-linked Hb subunits.10 Figure 1B shows representative MALDI-MS spectra of renally filtered Hb that closely resembles the spectrum obtained with in vitro–oxidized Hb. A prominent peak of covalently cross-linked dimers (30.9 kDa) occurs in addition to smaller trimer (46.7 kDa) and tetramer (62.6 kDa) peaks. No high-molecular masses can be found in native Hb. In addition, whereas only the nonoxidized β-globin Cys112 can be detected in plasma, the same amino acid is almost completely converted to its triple oxidation product (cysteic acid) upon passage through the renal compartment (Figure 1C). Of note, within plasma the nonoxidized cell-free Hb was found mainly to circulate bound within the Hb-Hp complex, whereas the noncomplexed Hb is rapidly excreted by the kidney.
Identification of oxidatively modified hemoglobin in vivo. Panels A throuch C demonstrate oxidative modification to Hb following hemoglobinuria (Hb-U). (A) The reverse-phase HPLC of hemoglobin within dog urine. During the initial elution time (0-10 minutes) altered heme is shown. At approximately 23 minutes, a heme-altered protein product elutes, consistent with an identical HPLC analysis of CSF.31 Neither altered heme nor heme protein adducts are observed in native highly purified stroma-free hemoglobin from plasma (right panel). (B) α-Globin chain cross-links ([M + H] = 30.9 m/z) and stabilized tetramer ([M + H] = 62.6 m/z) from a representative Hb-U sample. This spectra is identical to a representative oxidized Hb sample (1:10, HbA0/H2O2) (OxHb) and demonstrates a similar αα cross-link ion ([M + H] = 30.2 m/z) found in αXLHb. The high-mass ions are absent in nonmodified Hb, which demonstrates only single α- and β-globin chain ions. (C) The full scan spectra of the reduced and alkylated Cys112 peptide (L105LGNVLVC112 [CH2CONH2]VLAHHFGK120 [M + 3H] = 593.14, M = 1719.95 + 56 = 1775.95) shown to be nonoxidized in circulating plasma Hb. Within urine, the same Hb peptide is almost exclusively found as a trioxidation product on Cys112 (cysteic acid) (L105LGNVLVC112[O3]VLAHHFGK120 [M + 3H] = 590.2, M = 1719.95 + 48 [3 oxygens], = 1767.95).
Identification of oxidatively modified hemoglobin in vivo. Panels A throuch C demonstrate oxidative modification to Hb following hemoglobinuria (Hb-U). (A) The reverse-phase HPLC of hemoglobin within dog urine. During the initial elution time (0-10 minutes) altered heme is shown. At approximately 23 minutes, a heme-altered protein product elutes, consistent with an identical HPLC analysis of CSF.31 Neither altered heme nor heme protein adducts are observed in native highly purified stroma-free hemoglobin from plasma (right panel). (B) α-Globin chain cross-links ([M + H] = 30.9 m/z) and stabilized tetramer ([M + H] = 62.6 m/z) from a representative Hb-U sample. This spectra is identical to a representative oxidized Hb sample (1:10, HbA0/H2O2) (OxHb) and demonstrates a similar αα cross-link ion ([M + H] = 30.2 m/z) found in αXLHb. The high-mass ions are absent in nonmodified Hb, which demonstrates only single α- and β-globin chain ions. (C) The full scan spectra of the reduced and alkylated Cys112 peptide (L105LGNVLVC112 [CH2CONH2]VLAHHFGK120 [M + 3H] = 593.14, M = 1719.95 + 56 = 1775.95) shown to be nonoxidized in circulating plasma Hb. Within urine, the same Hb peptide is almost exclusively found as a trioxidation product on Cys112 (cysteic acid) (L105LGNVLVC112[O3]VLAHHFGK120 [M + 3H] = 590.2, M = 1719.95 + 48 [3 oxygens], = 1767.95).
Haptoglobin prevents peroxide-induced hemoglobin amino acid oxidation
Addition of Hp to Hb prior to H2O2 oxidation protects against β-globin chain amino acid modification(s) and limits α-globin chain cross-linking. Figure 2 shows a representative HPLC chromatogram of Hb with β- and α-globin chains eluting at 22.0 and 23.0 minutes, respectively. H2O2 oxidation caused a decrease in single globin chain absorbance with altered α- and β-globin chains eluting at 24 minutes, demonstrating that the addition of Hp 1-1 prior to H2O2 oxidation preserves α- and β-globin chains. Heme alone in the oxidized Hb-Hp (oxHb-Hp) chromatogram elutes at 18.0 minutes, whereas oxidized Hb (oxHb) heme and nonoxidized Hb heme elute at 19.0 minutes and 16.0 minutes, respectively. We have previously established that heme is internalized within Hb and involved in globin chain cross-linking following Hb exposure to H2O2, hence the observed change in heme retention times.8 In an extensive mass spectrometry evaluation of H2O2-exposed Hb-Hp, we did not find any Hb modifications indicating heme cross-links or globin chain–bound heme groups. The absence of these findings in oxidized Hb-Hp may therefore suggest heme transfer and binding to Hp instead of Hb during oxidation of the complex. The additional peaks in the oxidized Hb-Hp chromatogram likely originate from oxidative modifications within the Hp protein.
Representative reverse-phase HPLC chromatograms of oxidized (ox) and nonoxidized Hb-Hp products. Gray lines indicate spectra at 280 nm (protein) and black lines indicate spectra at 405 nm (heme). The chromatogram of nonoxidized Hb demonstrates a typical elution pattern of β- and α-globin chains at 22.0 and 23.0 minutes, respectively. H2O2 causes decreased absorbance of chains at 280 and coelution with heme at approximately 24.0 minutes. Hp(1-1) elutes at approximately 6.2 minutes (shown as dashed gray and dashed black lines). When complexed with Hb and exposed to H2O2, several newly formed Hp-related oxidation products elute between 7 and 8 minutes and at 22.5 minutes. In oxidized Hb-Hp (oxHb-Hp), the Hb β and α chains retain their elution times similar to nonoxidized Hb at 280 nm, indicating intact undamaged protein. Red indicates Hb's β- and α-globin chains, tan indicates heme, and blue indicates Hp.
Representative reverse-phase HPLC chromatograms of oxidized (ox) and nonoxidized Hb-Hp products. Gray lines indicate spectra at 280 nm (protein) and black lines indicate spectra at 405 nm (heme). The chromatogram of nonoxidized Hb demonstrates a typical elution pattern of β- and α-globin chains at 22.0 and 23.0 minutes, respectively. H2O2 causes decreased absorbance of chains at 280 and coelution with heme at approximately 24.0 minutes. Hp(1-1) elutes at approximately 6.2 minutes (shown as dashed gray and dashed black lines). When complexed with Hb and exposed to H2O2, several newly formed Hp-related oxidation products elute between 7 and 8 minutes and at 22.5 minutes. In oxidized Hb-Hp (oxHb-Hp), the Hb β and α chains retain their elution times similar to nonoxidized Hb at 280 nm, indicating intact undamaged protein. Red indicates Hb's β- and α-globin chains, tan indicates heme, and blue indicates Hp.
Verification of amino acid oxidations
The protective influence of Hp on H2O2-mediated Hb amino acid oxidation is summarized in Table 1. βCys93 oxidation to the trioxidation product cysteic acid and the protective role of Hp are demonstrated in the MALDI-TOF mass spectra shown in Figure 3 as a representative example for other amino acids. In the absence of previous modification to cysteine residues, reduction and alkylation of sulfhydryl groups prior to trypsin digestion add 56.0 mass units to cysteine as (CH2CONH2). This is observed in Figure 3A through D where monoisotopic masses (m/z = 2585.2 [M + H]) of the alkylated peptide G83TFATLSELC93 (CH2CONH2) DKLHVDPENFR104 are observed at m/z = 2529.2 (M + H). In the event of cysteine oxidation, the equivalent mass of one or more oxygen atoms is bound to the cysteine as seen in panel B where a mass equivalent to 3 oxygen atoms was observed on βCys93. These data clearly demonstrate the protective influence of Hp on the irreversible oxidation of one of several amino acids within the Hb β-globin chain. Amino acid sequences for all oxidized peptides were verified by LCMS/MS analysis (Figure S1).
Hemoglobin amino acid oxidations following hydrogen peroxide incubations
| Beta globin peptides* . | Hb . | oxHb . | Hb-Hp . | oxHb-Hp . |
|---|---|---|---|---|
| GTFATLSELHC93(-CH2CONH2)†DKLHVDPENFR | + | − | + | + |
| GTFATLSELHC93(-O3H)DKLHVDPENFR | − | + | − | − |
| LLGNVLVC112(-CH2CONH2)*VLAHHFGK | + | − | + | + |
| LLGNVLVC112(-O3H)VLAHHFGK | − | + | − | − |
| FFESFGDLSTPDAVM55(O)GNPK | + | + | + | + |
| SAVTALW15(NFK)GK | − | + | − | − |
| LLGNVLVCVLAHH117(O)FGK | − | + | − | − |
| VVAGVANALAHKY145(OH)H146(O) | − | + | − | − |
| Beta globin peptides* . | Hb . | oxHb . | Hb-Hp . | oxHb-Hp . |
|---|---|---|---|---|
| GTFATLSELHC93(-CH2CONH2)†DKLHVDPENFR | + | − | + | + |
| GTFATLSELHC93(-O3H)DKLHVDPENFR | − | + | − | − |
| LLGNVLVC112(-CH2CONH2)*VLAHHFGK | + | − | + | + |
| LLGNVLVC112(-O3H)VLAHHFGK | − | + | − | − |
| FFESFGDLSTPDAVM55(O)GNPK | + | + | + | + |
| SAVTALW15(NFK)GK | − | + | − | − |
| LLGNVLVCVLAHH117(O)FGK | − | + | − | − |
| VVAGVANALAHKY145(OH)H146(O) | − | + | − | − |
Listed peptides showing candidate amino acids that have been identified to undergo oxidation (+) or the lack of it (−) in the presence of Hp. These amino acids are C93 (Cys93), M55 (Met55), W15 (Trp15), H117 (His117), and Y145 (Tyr145).The mechanism of amino acid oxidation under our experimental conditions has previously been described.14
Represent 2-iodoacetamide alkylated sulfhydryl groups in nonoxidized Hb cysteine–containing peptides.
Representative mass spectra of Hb β-globin peptides that contain cysteine (βCys93) in the presence and absence of Hp. (A) Nonoxidized Hb peptide (G83TFATLSELC93 [CH2CONH2] DKLHVDPENFR104), showing the 56.0 mass unit alkylated Cys93 consistent with the absence of oxidation (m/z = 2585.2 [M + H]). (B) Oxidized Hb showing a mass addition of 47 to Cys93 (G83TFATLSELC93 [O3] DKLHVDPENFR104) consistent with the trioxidation product, cysteic acid (m/z = 2576.2 [M + H]). Both panels C and D demonstrate the absence of significant oxidation on the Cys93 tryptic peptide before or after oxidation, consistent with spectra (A). LC/MS/MS amino acid sequence data for all oxidized peptides are included in Figure S1.
Representative mass spectra of Hb β-globin peptides that contain cysteine (βCys93) in the presence and absence of Hp. (A) Nonoxidized Hb peptide (G83TFATLSELC93 [CH2CONH2] DKLHVDPENFR104), showing the 56.0 mass unit alkylated Cys93 consistent with the absence of oxidation (m/z = 2585.2 [M + H]). (B) Oxidized Hb showing a mass addition of 47 to Cys93 (G83TFATLSELC93 [O3] DKLHVDPENFR104) consistent with the trioxidation product, cysteic acid (m/z = 2576.2 [M + H]). Both panels C and D demonstrate the absence of significant oxidation on the Cys93 tryptic peptide before or after oxidation, consistent with spectra (A). LC/MS/MS amino acid sequence data for all oxidized peptides are included in Figure S1.
To gain further insight into the potential mechanism by which Hb amino acids (βTrp15, βCys93, βCys112, βHis117, βTyr145, and βHis146) could be protected within the Hb-Hp complex, distances were calculated for each of these residues to proposed Hp binding/contact sites32,,,,,–38 (Figure S1). βTrp15, βHis117, βTyr145, and βHis146 are located within 1 of the 3 binding regions, whereas βCys93 and βCys112 display close proximity to one or more of the proposed binding sites (Figure S1). The proximity to Hb-Hp contact sites might suggest reduced surface accessibility of susceptible amino acids within the Hb-Hp complex and thus could provide a shielding effect from oxidative attack during H2O2 treatment. Alternatively, proximity to free radical stabilizing amino acids, such as Tyr or Trp, within Hp may provide oxidative protection.39,,,–43
Haptoglobin prevents peroxide-induced hemoglobin cross-linking and polymerization
We confirmed our previous observation that H2O2 treatment of Hb resulted in the formation of oligomeric and polymeric species that are detectable by reducing SDS-PAGE (Figure 4A). An α-globin–specific Western blot (Figure 4B) suggests that the dimer/polymer formation is largely attributable to α-globin cross-linking. Hp markedly reduced formation of these large molecular size Hb species. There was no visible alteration of free α-globin subunits upon treatment of Hb-Hp complexes with H2O2. MALDI-MS (Figure 4D) analysis confirmed that free α-globin chain mass ions at m/z = 15 255.86 (M + H) disappear in oxidized Hb but not in oxidized Hb-Hp complex. Together, these data suggest that Hp complex formation prevents the extensive α chain modifications that occur when Hb alone is exposed to H2O2. From our previous work, we knew that Hb that is chemically cross-linked between the α-globin chains cannot bind to Hp although its peroxidative activity remains unchanged compared with native Hb.44 Oxidative modification of αXLHb was thus considered an ideal control to dissect nonspecific effects of Hp (ie, as a H2O2) from the protective effects specifically mediated through the high-affinity interaction between the 2 proteins. In contrast to the protection of native Hb, Hp prevented neither cross-linking (Figure 4C) nor oxidative modification of specific amino acids (data not shown) within αXLHb when exposed to H2O2.
Distribution of molecular size Hb cross-links and polymers after H2O2 treatment. (A) Silver-stained SDS-PAGE analysis of nonoxidized and H2O2-treated Hb/Hb-Hp (5 μg Hb per lane). Hb was mixed with different concentrations of Hp (phenotype 1-1) to get Hp/Hb ratios between 2:1 and 0.25:1. The concentration of Hb was equal in all samples (250 μM in heme). Oxidation was performed with a 10-fold excess concentration of H2O2 over heme where indicated (H2O2+) for 60 minutes. (B) Western blot analysis of nonoxidized and H2O2-treated Hb and Hb-Hp with an anti–α-globin–specific monoclonal antibody (α-globin) and with a polyclonal anti-Hp antibody (Hp). Nonoxidized αα DBBF cross-linked Hb was used as a control to prove α-globin specificity of the anti-Hb antibody. With αα cross-linked Hb there appears a strong signal at approximately 30 kDa that represents αα dimers, but no signal can be observed at 15 kDa where fully dissociated α-globin chains (monomer) appear in HbA0. (C) In contrast to HbA0, α-α cross-linked Hb that does not bind to Hp is not protected from polymerization when exposed to H2O2 (left panel: [1] marker, [2] HbA0 + H2O2, [3] HbA0-Hp + H2O2; right panel: [1] α-α Hb + H2O2 [2/3] α-α Hb-Hp + H2O2 [2 independent reactions]). (D) MALDI-MS analysis of H2O2-treated Hb and Hb-Hp. As observed in the α-globin Western blot, α-chains at m/z approximately 15 290 disappear during H2O2 treatment of Hb but do not disappear if Hb is oxidized in the presence of equimolar concentrations of Hp.
Distribution of molecular size Hb cross-links and polymers after H2O2 treatment. (A) Silver-stained SDS-PAGE analysis of nonoxidized and H2O2-treated Hb/Hb-Hp (5 μg Hb per lane). Hb was mixed with different concentrations of Hp (phenotype 1-1) to get Hp/Hb ratios between 2:1 and 0.25:1. The concentration of Hb was equal in all samples (250 μM in heme). Oxidation was performed with a 10-fold excess concentration of H2O2 over heme where indicated (H2O2+) for 60 minutes. (B) Western blot analysis of nonoxidized and H2O2-treated Hb and Hb-Hp with an anti–α-globin–specific monoclonal antibody (α-globin) and with a polyclonal anti-Hp antibody (Hp). Nonoxidized αα DBBF cross-linked Hb was used as a control to prove α-globin specificity of the anti-Hb antibody. With αα cross-linked Hb there appears a strong signal at approximately 30 kDa that represents αα dimers, but no signal can be observed at 15 kDa where fully dissociated α-globin chains (monomer) appear in HbA0. (C) In contrast to HbA0, α-α cross-linked Hb that does not bind to Hp is not protected from polymerization when exposed to H2O2 (left panel: [1] marker, [2] HbA0 + H2O2, [3] HbA0-Hp + H2O2; right panel: [1] α-α Hb + H2O2 [2/3] α-α Hb-Hp + H2O2 [2 independent reactions]). (D) MALDI-MS analysis of H2O2-treated Hb and Hb-Hp. As observed in the α-globin Western blot, α-chains at m/z approximately 15 290 disappear during H2O2 treatment of Hb but do not disappear if Hb is oxidized in the presence of equimolar concentrations of Hp.
Autoxidative and peroxidative activities of hemoglobin and hemoglobin-haptoglobin complex
The peroxidative processes associated with heme ultimately drive free radical formation, radical protein migration, and amino acid oxidation. Hb spontaneously oxidizes to ferric and ferryl species in the absence of antioxidative enzymes, such as superoxide dismutase and catalase.45 The rate of autoxidation in the absence of Hp (kauto = 4.165 × 10−2 h−1) was very close to the rate calculated in the presence of Hp (kauto = 3.498 × 10−2 h−1). The lack of effect of Hp on the so-called “pseudoperoxidase activity” of Hb was also demonstrated (Figure 5). When ferrous Hb is treated with equimolar or higher concentrations of H2O2, a decrease in the absorption peaks at 540 and 577 nm is accompanied by spectral shifts indicative of the presence of a ferryl species (peaks at 545 and 580 and a flattened region between 600-700 nm), which occurs 2 to 3 minutes after the initiation of the oxidation reaction. As the reaction progresses, a significant portion of the ferryl iron becomes reduced back to the ferric form, with characteristic peaks at 550, 554, and 630 nm according to the model that we have previously described46 and summarized in equations 1 to 3 (“Introduction”). The formation and the presence of the ferryl Hb were further evaluated by dervatization of this species to sulfHb with sodium sulfide (not shown). In the presence of Hp (Hb-Hp ratio 1:1), no changes were observed in the pseudoperoxidase activity of Hb (Figure 5A,B). To investigate the effects of Hp on the true “peroxidase” activity of Hb, the conversion of ferric to ferryl Hb was measured. We followed the transient kinetics of ferric Hb within a rapid mixing stopped-flow spectrophotometer in the presence and absence of Hp. Formation of ferryl Hb following rapid mixing of the ferric Hb with H2O2 was monitored in the stopped flow using a diode array detector (Figure 5C). Spectral changes in the Soret and visible regions were recorded as a function of time as shown in Figure 5D. Two major spectral intermediates of Hb (ferric and ferryl) were deconvoluted and reconstructed (Figure 5E). The observed rates of the reaction are linearly dependent on H2O2 concentration under our pseudo first-order reaction conditions with excess peroxide (Figure 5F). The second-order rate constants from the slopes of the plots are shown for both sets of experiments in the presence and absence of Hp and demonstrate reaction rates of 42 M−1s−1 and 43.6 M−1s−1, respectively.
Spectral changes during the oxidation of Hb by H2O2. Spectral changes during the oxidation of Hb by H2O2. (A,B) Hb (50 μM in heme) was incubated with 250 μM H2O2 at 37°C in 50 mM phosphate buffer, pH 7.4. Spectra were collected in a fast scanning diode-array spectrophotometer. The representative spectra of oxy Hb in the beginning, intermediate ferryl Hb, and ferric Hb at the end of the reaction were shown. (A) Hb without Hp and (B) Hb preincubated with 50 μM Hp. (C-F) The rate of reaction of ferric Hb with H2O2 to form ferryl Hb. The reaction rate was evaluated using a stopped-flow equipped with a photodiode array detector (Applied Photophysics). Spectral changes in the Soret and visible regions were recorded as a function of time and H2O2 concentration. (C) Representative spectral changes recorded after mixing Hb (4 μM) with excess H2O2. (D) Reconstructed spectra of the main reaction species after the global fitting computation of the data set. (E) Concentration changes of the main reaction species over time obtained by the global analysis. The reactions by ferric Hb only or by ferric Hb preincubated with slight molar excess of haptoglobin were carried out as a dependent of [H2O2], and plotted in panel F. (F) Second-order rate constants obtained under our experimental conditions for met HbA0 (●) and met HbA0 with haptoglobin (○). The derived rate constants are 42 M−1s−1 and 43.6 M−1s−1, respectively.
Spectral changes during the oxidation of Hb by H2O2. Spectral changes during the oxidation of Hb by H2O2. (A,B) Hb (50 μM in heme) was incubated with 250 μM H2O2 at 37°C in 50 mM phosphate buffer, pH 7.4. Spectra were collected in a fast scanning diode-array spectrophotometer. The representative spectra of oxy Hb in the beginning, intermediate ferryl Hb, and ferric Hb at the end of the reaction were shown. (A) Hb without Hp and (B) Hb preincubated with 50 μM Hp. (C-F) The rate of reaction of ferric Hb with H2O2 to form ferryl Hb. The reaction rate was evaluated using a stopped-flow equipped with a photodiode array detector (Applied Photophysics). Spectral changes in the Soret and visible regions were recorded as a function of time and H2O2 concentration. (C) Representative spectral changes recorded after mixing Hb (4 μM) with excess H2O2. (D) Reconstructed spectra of the main reaction species after the global fitting computation of the data set. (E) Concentration changes of the main reaction species over time obtained by the global analysis. The reactions by ferric Hb only or by ferric Hb preincubated with slight molar excess of haptoglobin were carried out as a dependent of [H2O2], and plotted in panel F. (F) Second-order rate constants obtained under our experimental conditions for met HbA0 (●) and met HbA0 with haptoglobin (○). The derived rate constants are 42 M−1s−1 and 43.6 M−1s−1, respectively.
Haptoglobin preserves hemoglobin clearance by the CD163 scavenger pathway following peroxide oxidation
We next investigated whether the structural stabilization by Hp may also preserve the interaction between the Hb-Hp complex and CD163. Figure 6A confirms our previous observation that Hb endocytosis by CD163 is abrogated by treatment of Hb with H2O2. In contrast, Hb-Hp is avidly taken up by CD163-HEK cells even after exposure to a 10-fold excess of H2O2. Neither native nor oxidized Hb-Hp was taken up by CD163− cells. Unaltered binding of both oxidized and nonoxidized Hb-Hp complexes to CD163 was confirmed by Biacore SPR analysis as well as with our competitive Hb-Hp uptake assay in CD163-HEK cells (Figure 6B,C).
CD163 interaction and endocytosis of peroxide-treated and untreated Hb and Hb-Hp. (A) CD163-HEK cells were incubated with 20 μg/mL native or H2O2-treated Hb or Hb-Hp. After 30 minutes, cells were washed, fixed, and stained with an anti-Hb antibody (red fluorescence, Alexa 568). Nuclei were stained with DAPI (blue). Images were taken with a Carl Zeiss epifluorescence Axioskope-2 (20×/0.43 objective) equipped with an AxioCam-MR digital camera and Axio-Vision software. Results of digital image analysis and quantification of red fluorescence (per cell) that indicates cellular Hb uptake are shown in the graph. Bars represent mean plus or minus SD of 3 independent experiments performed in triplicates with at least 10 images quantified per coverslip. (B) Equal binding of nonoxidized Hb-Hp (top) and oxidized Hb-Hp (bottom) complexes was confirmed by Biacore analysis over a range of concentrations (from 2.5-200 nM) normalized to Hb. (C) CD163-HEK cells were incubated with 3 μg/mL fluorescent Hb-Hp633 in the presence or absence of different concentrations of nonfluorescent competitors (● = Hb-Hp [1-1]; ○ = oxidized Hb-Hp [1-1]). After 30 minutes, cells were washed, trypsinized, and fluorescence was determined by fluorescence-activated cell sorting (FACS). Mean channel fluorescence values are given as percentage of the Hb-Hp633 fluorescence in the absence of any competitor. (Mean ± SD from 3 independent experiments.)
CD163 interaction and endocytosis of peroxide-treated and untreated Hb and Hb-Hp. (A) CD163-HEK cells were incubated with 20 μg/mL native or H2O2-treated Hb or Hb-Hp. After 30 minutes, cells were washed, fixed, and stained with an anti-Hb antibody (red fluorescence, Alexa 568). Nuclei were stained with DAPI (blue). Images were taken with a Carl Zeiss epifluorescence Axioskope-2 (20×/0.43 objective) equipped with an AxioCam-MR digital camera and Axio-Vision software. Results of digital image analysis and quantification of red fluorescence (per cell) that indicates cellular Hb uptake are shown in the graph. Bars represent mean plus or minus SD of 3 independent experiments performed in triplicates with at least 10 images quantified per coverslip. (B) Equal binding of nonoxidized Hb-Hp (top) and oxidized Hb-Hp (bottom) complexes was confirmed by Biacore analysis over a range of concentrations (from 2.5-200 nM) normalized to Hb. (C) CD163-HEK cells were incubated with 3 μg/mL fluorescent Hb-Hp633 in the presence or absence of different concentrations of nonfluorescent competitors (● = Hb-Hp [1-1]; ○ = oxidized Hb-Hp [1-1]). After 30 minutes, cells were washed, trypsinized, and fluorescence was determined by fluorescence-activated cell sorting (FACS). Mean channel fluorescence values are given as percentage of the Hb-Hp633 fluorescence in the absence of any competitor. (Mean ± SD from 3 independent experiments.)
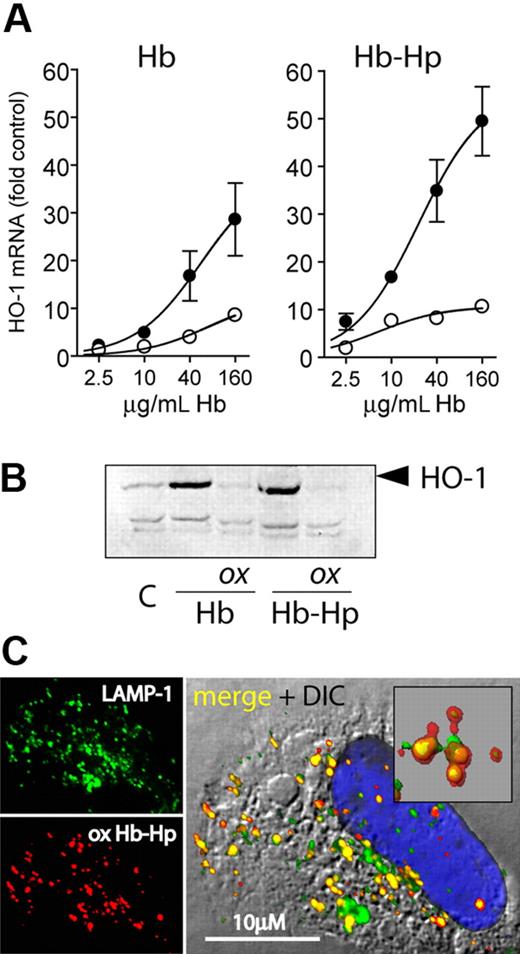
Hydrogen peroxide exposure of hemoglobin-haptoglobin results in reduced heme oxygenase induction
Induction of the heme breakdown enzyme heme oxygenase-1 (HO-1) is the major response of CD163-expressing cells to Hb exposure47,48 and is an important mechanism against Hb-associated oxidative stress. Therefore, we evaluated whether oxidative damage to Hb and Hb-Hp affected the HO-1 response. Both nonoxidized Hb and Hb-Hp induced strong expression of HO-1 mRNA and protein, respectively, in CD163-HEK but not in CD163− HEK293 cells (Figure 7A,B). However, H2O2 treatment of Hb in the absence and presence of Hp almost completely abrogated the CD163-dependent HO-1 induction. To confirm that the reduced HO-1 induction was not a result of altered cellular uptake or subcellular trafficking of the oxidized complex, we confirmed that oxidized Hb-Hp complexes were colocalized with LAMP-1–positive lysosomes 30 minutes after endocytosis (Figure 7C), as recently reported for nonoxidized Hb-Hp.29 To exclude that the lack of HO-1 induction by oxidized Hb-Hp was a result of altered/delayed Hb breakdown, we performed similar experiments with exposure times of 8, 16, and 24 hours. At each of these time points, we found only low HO-1 induction by the oxidized complex compared with the induction by nonoxidized Hb-Hp. During oxidation by H2O2, heme might be released from Hb and might induce HO-1 by a nonspecific, CD163-independent mechanism. To avoid this potentially confounding effect, we have removed the traces of free heme after H2O2 reaction by filtration. However, the equally sized heme peaks found in the HPLC profiles of nonoxidized as well as of oxidized and filtered Hb and Hb-Hp, respectively, suggest that only a very minor fraction of heme is actually released during oxidation and removed in the subsequent purification (Figure 2). Increased intracellular heme concentration resulting from cellular Hb/Hb-Hp degradation is the main stimulus for HO-1 induction upon Hb endocytosis and was shown to act independent of potential CD163 receptor signaling.48 Since no free heme-associated oxidations were observed by mass spectrometry, we suspect that covalent heme protein association with Hp (as suggested in Figure 1) may impair cellular heme sensing. This is supported by previous work that demonstrated that heme transfer from ferric Hb to LDL and its subsequent oxidation were inhibited by approximately 80% in the presence of Hp 1-1 compared with ferric Hb alone, suggesting a high affinity of Hp 1-1 for oxidized heme.49
HO-1 expression following cell exposure to peroxide-treated and untreated Hb-Hp. (A) LightCycler quantification of HO-1 mRNA expression induced by oxidized and nonoxidized Hb and Hb-Hp in CD163-HEK cells after 8-hour incubation. Data were normalized for GAPDH expression and represent mean plus or minus SD from 3 independent experiments (● = nonoxidized; ○ = H2O2 treated). (B) HO-1 Western blot of CD163-HEK cell lysates after 12-hour incubation with oxidized (ox) or nonoxidized Hb/Hb-Hp. (c = nontreated cells.) (C) Subcellular localization of Hb 30 minutes after endocytosis of oxidized Hb-Hp (1-1) in CD163-HEK cells. Cells were stained with anti-Hb + Alexa 594 secondary antibody (red) and anti–LAMP-1 + Alexa 488 secondary antibody (green); nuclei were stained with DAPI (blue). The insert in the merged image demonstrates extensive colocalization of Hb with LAMP-1–positive lysosomes (colocalization appears yellow). Images were acquired with a Leica SP2-AOBS-UV confocal laser-scanning microscopy system (objective 40×/1.25, oil).
HO-1 expression following cell exposure to peroxide-treated and untreated Hb-Hp. (A) LightCycler quantification of HO-1 mRNA expression induced by oxidized and nonoxidized Hb and Hb-Hp in CD163-HEK cells after 8-hour incubation. Data were normalized for GAPDH expression and represent mean plus or minus SD from 3 independent experiments (● = nonoxidized; ○ = H2O2 treated). (B) HO-1 Western blot of CD163-HEK cell lysates after 12-hour incubation with oxidized (ox) or nonoxidized Hb/Hb-Hp. (c = nontreated cells.) (C) Subcellular localization of Hb 30 minutes after endocytosis of oxidized Hb-Hp (1-1) in CD163-HEK cells. Cells were stained with anti-Hb + Alexa 594 secondary antibody (red) and anti–LAMP-1 + Alexa 488 secondary antibody (green); nuclei were stained with DAPI (blue). The insert in the merged image demonstrates extensive colocalization of Hb with LAMP-1–positive lysosomes (colocalization appears yellow). Images were acquired with a Leica SP2-AOBS-UV confocal laser-scanning microscopy system (objective 40×/1.25, oil).
Discussion
Hb is vulnerable to oxidation and subsequent structural damage as a result of the reaction of oxidants with its reactive heme groups. We have recently identified patterns of irreversible oxidation of β- chain amino acids that occur in the presence of H2O2.8 In addition, H2O2 reaction induces the formation of heme adducted protein as well as extensive cross-linking and polymerization of Hb α-globin subunits. Accumulation of these potentially immunogenic and inflammatory Hb oxidation products is a potential mechanism of exaggerated tissue damage during oxidative stress.10
Evidence of oxidation-induced structural Hb damage has been found in different in vivo conditions.50 However, the structural characterization of these modifications remained poorly defined compared with the in depth structural characterization of the Hb modifications occurring during in vitro H2O2 exposure.8,51,52 We have therefore extended our mechanistic in vitro studies by providing evidence that similar structural oxidative modifications can be observed during specific pathological conditions. We have previously demonstrated that exposure to a non–Hp-binding chemically modified Hb with a long circulating half-life results in significant heme iron as well as globin chain oxidative processes within the circulation of guinea pigs.50,53 Based on the short circulating half life of native extracellular Hb (< 30 minutes) and a relatively high short-term antioxidant capacity of the circulation, it is likely that acute exposure to free Hb results in its oxidation following distribution to extravascular tissue sites and not directly within the circulation. Hemoglobinuria and myoglobinuria have been causally linked to kidney damage following severe hemolysis and rhabdomyolysis.30 The rationale for us to study Hb oxidation products in hemoglobinuria is based on the fact that it has been confirmed that heme proteins do redox cycle peroxides within the kidney (ie, in myoglobinuric animals).30 It was therefore realistic to expect that specific Hb protein modifications related to its redox cycling activity could be observed in an experimental model of hemolysis/hemoglobinuria. Furthermore, the usually very low protein concentration in urine offers unique conditions for sensitive and specific analysis of modified Hb in a low background environment using the same analytic approach as for our in vitro model. Oxidative modifications to Hb isolated from urine were consistent with our in vitro findings. These oxidative modifications included the identification of altered heme protein products, oxidative induction of cross-linked globin chains, and specific amino acid oxidations (eg, trioxidation of β-globin Cys112 to cysteic acid).
Endogenous mechanisms of Hb scavenging are critical to oxidative protection and detoxification of free Hb. This concept gained biologic relevance when Hp knockout mice were shown to be more vulnerable than wild-type mice to oxidative kidney and lung injury.18,54,55 In addition to CD163, alternative pathways such as the proposed liver parenchymal cell Hb/Hb-Hp receptor might participate in systemic Hb clearance, whereas specific scavengers such as hemopexin bind and detoxify free heme.51,52 The pivotal importance of the CD163 scavenger receptor pathway became evident in patients treated with the monocyte/macrophage-depleting immunotoxin gemtuzumab ozogamicin. In the case of severe intravascular hemolysis, some of these patients experienced severe systemic toxicity as a result of delayed Hb clearance.56 The macrophage scavenger pathway might be even more relevant at sites of local Hb accumulation following hemorrhage, tissue damage, or inflammation and it thus seems plausible that this pathway must be protected from functional failure even in the most severe oxidative conditions encountered during inflammation and tissue injury.48
During the course of this investigation, the following observations were made: (1) Hp prevents H2O2-induced amino acid oxidation in the β chain of bound Hb and prevents α-globin cross-linking/polymerization. (2) Hb retains its ability to consume H2O2 when complexed with Hp but appears to redirect the radical chemistry initiated at the heme iron toward Hp. (3) CD163-mediated cellular uptake, and lysosomal sequestration of the complex exposed to as high as a 10-fold excess of H2O2, was nearly identical to the uptake of non–H2O2-exposed Hb-Hp, indicating that Hb can withstand extreme oxidative insult when shielded within the complex and still be removed effectively from the extracellular environment. Thereby, the intimate proximity of vulnerable amino acids to putative Hb-Hp binding sites may suggest that either Hp physically blocks the primary oxidizable amino acids in Hb, or it may interfere with protein electron pathways that allow for free radical transfer from heme to the protein. We also carried out several experiments to verify the accessibility of the heme in Hb when complexed with Hp by monitoring its reaction with ligands. We found that oxygen binding kinetics were only slightly altered and NO binding kinetics were unchanged, in agreement with earlier reports.57,58
Endocytosis of unprotected Hb after reaction with H2O2 is severely compromised as a result of impaired interaction of the damaged Hb with Hp and CD163.10 However, whereas Hp cannot restore the CD163 binding of already damaged Hb, we found that reaction of H2O2 with the Hb-Hp complex did not reduce the high-affinity binding to CD163. Beyond the structural protection of Hb, Hp seems to have a crucial role in preserving cellular uptake and lysosomal sequestration of extracellular Hb within oxidative environments. This novel function of Hp might explain why neutrophil granulocytes store large quantities of preformed Hp within their granules that can be released to immediately protect Hb after leukocyte extravasation into damaged tissues.19 Although we were able to confirm delivery of oxidized Hb-Hp to lysosomes, the HO-1 induction by oxidized Hb-Hp was only weak, compared with the expression observed after incubation of CD163+ cells with nonoxidized Hb-Hp. Since increased intracellular heme concentration is the principal signal for enhanced HO-1 expression, this observation is likely explained by impaired heme sensing after oxidized Hb-Hp uptake. Impaired heme sensing may be a result of the high affinity of oxidized heme toward Hp and potential nonspecific heme protein product formation.49
In conclusion, we report here that Hp complex formation protects Hb against structural damage when exposed to H2O2. Perhaps most intriguing is that Hp preserves the CD163 Hb clearance pathway and thus allows lysosomal sequestration of extracellular Hb under strong oxidative conditions. Although it remains unclear how the oxidized complex is metabolized once in the lysosome, the oxidative protection of cell-free Hb by Hp affords a specific and unique role for Hp in the CD163 Hb scavenger receptor pathway under oxidative stress conditions. The antioxidant protection afforded by Hp-bound Hb suggests that early administration of Hp in acute hemolysis or induction of endogenous Hp production in chronic hemolytic diseases may be a therapeutic modality in the prevention of vascular and extravascular toxicity induced by cell-free Hb exposures.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy.
This study was supported by the Swiss National Science Foundation (Bern, Switzerland, grant 31-120658) and the Helmut Horten Foundation (Lugano, Switzerland; both to D.J.S.).
Authorship
Contribution: P.W.B. and D.J.S. performed experiments, analyzed data, and wrote the paper; B.A., C.P.P., F.V., C.L., J.F.C., Y.J., F.S.B., and M.M. performed experiments; and G.S. and A.I.A. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominik J. Schaer, Medical Clinic Research Unit, University Hospital, CH-8091 Zurich, Switzerland; e-mail: dominik.schaer@usz.ch; or Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, MD 20892; e-mail: abdu.alayash@fda.hhs.gov.

![Figure 1. Identification of oxidatively modified hemoglobin in vivo. Panels A throuch C demonstrate oxidative modification to Hb following hemoglobinuria (Hb-U). (A) The reverse-phase HPLC of hemoglobin within dog urine. During the initial elution time (0-10 minutes) altered heme is shown. At approximately 23 minutes, a heme-altered protein product elutes, consistent with an identical HPLC analysis of CSF.31 Neither altered heme nor heme protein adducts are observed in native highly purified stroma-free hemoglobin from plasma (right panel). (B) α-Globin chain cross-links ([M + H] = 30.9 m/z) and stabilized tetramer ([M + H] = 62.6 m/z) from a representative Hb-U sample. This spectra is identical to a representative oxidized Hb sample (1:10, HbA0/H2O2) (OxHb) and demonstrates a similar αα cross-link ion ([M + H] = 30.2 m/z) found in αXLHb. The high-mass ions are absent in nonmodified Hb, which demonstrates only single α- and β-globin chain ions. (C) The full scan spectra of the reduced and alkylated Cys112 peptide (L105LGNVLVC112 [CH2CONH2]VLAHHFGK120 [M + 3H] = 593.14, M = 1719.95 + 56 = 1775.95) shown to be nonoxidized in circulating plasma Hb. Within urine, the same Hb peptide is almost exclusively found as a trioxidation product on Cys112 (cysteic acid) (L105LGNVLVC112[O3]VLAHHFGK120 [M + 3H] = 590.2, M = 1719.95 + 48 [3 oxygens], = 1767.95).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-08-174466/5/m_zh80130932720001.jpeg?Expires=1765905167&Signature=3LqxUnUuNzhcjqKQHEgLJxg5Xq6n~DHyxklT5vIKyBb0fB~MIOcbF2ir4AqYiD4utP8e0NO8rZdMkAgWEzpZnkcqslErK-hIvdDBqlfY~C4yl9DVU6aGZG7kYYxkn8hlcsxNvlDJgRc5jwAaDDMVa4P8obRTdlwjn8oNEWzkJJ3DXT9fnE7to90rCk7ZMwgmEeWyuVzsQyCVeTV9NwvYQdQj6NE8Sl-LOWA4EOJvlcqyv7-2L~7YqSqfC5mc3i6LL0pl-Oi1zR8mYeGwN1h05vr-1pM~e6h~H3K7eNyxhL3TK5R9Nf4l5u7aD7aA0IufZ7QZgeSTBtWmLRhfpVpYag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Representative mass spectra of Hb β-globin peptides that contain cysteine (βCys93) in the presence and absence of Hp. (A) Nonoxidized Hb peptide (G83TFATLSELC93 [CH2CONH2] DKLHVDPENFR104), showing the 56.0 mass unit alkylated Cys93 consistent with the absence of oxidation (m/z = 2585.2 [M + H]). (B) Oxidized Hb showing a mass addition of 47 to Cys93 (G83TFATLSELC93 [O3] DKLHVDPENFR104) consistent with the trioxidation product, cysteic acid (m/z = 2576.2 [M + H]). Both panels C and D demonstrate the absence of significant oxidation on the Cys93 tryptic peptide before or after oxidation, consistent with spectra (A). LC/MS/MS amino acid sequence data for all oxidized peptides are included in Figure S1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-08-174466/5/m_zh80130932720003.jpeg?Expires=1765905167&Signature=YaqAdklR0M9QmSLUcnBzaN-sSs36m4sDFD6cN2GPIjrI5V0t0JCBU-IhJpeiynfLt4MxUrm9zD4~JDtwvR3HSJO~glI4Bld15IM127Ee9~syzsA-r74myJsHbCehS7LlTR4tDtdHJPoZeNzna0T2wQmNK732K~ANvwudFWkLguM99Ux~Sb~c0ZsJiLfoeMErVqUgkyoU3Zfkc~2Ln3AeOhWtaBl58EKQYQhZm3ux8FgS9mwA1Wv7mXLi0J7ZKzLum5Fh595Nm~0aS7dYIpZnRMzWK~fVZ9dMPK8yYgrdnEsJKwOO4dLPWxOPdC6I6KfafMMdgw6ZKhaj5kGY08bOEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Distribution of molecular size Hb cross-links and polymers after H2O2 treatment. (A) Silver-stained SDS-PAGE analysis of nonoxidized and H2O2-treated Hb/Hb-Hp (5 μg Hb per lane). Hb was mixed with different concentrations of Hp (phenotype 1-1) to get Hp/Hb ratios between 2:1 and 0.25:1. The concentration of Hb was equal in all samples (250 μM in heme). Oxidation was performed with a 10-fold excess concentration of H2O2 over heme where indicated (H2O2+) for 60 minutes. (B) Western blot analysis of nonoxidized and H2O2-treated Hb and Hb-Hp with an anti–α-globin–specific monoclonal antibody (α-globin) and with a polyclonal anti-Hp antibody (Hp). Nonoxidized αα DBBF cross-linked Hb was used as a control to prove α-globin specificity of the anti-Hb antibody. With αα cross-linked Hb there appears a strong signal at approximately 30 kDa that represents αα dimers, but no signal can be observed at 15 kDa where fully dissociated α-globin chains (monomer) appear in HbA0. (C) In contrast to HbA0, α-α cross-linked Hb that does not bind to Hp is not protected from polymerization when exposed to H2O2 (left panel: [1] marker, [2] HbA0 + H2O2, [3] HbA0-Hp + H2O2; right panel: [1] α-α Hb + H2O2 [2/3] α-α Hb-Hp + H2O2 [2 independent reactions]). (D) MALDI-MS analysis of H2O2-treated Hb and Hb-Hp. As observed in the α-globin Western blot, α-chains at m/z approximately 15 290 disappear during H2O2 treatment of Hb but do not disappear if Hb is oxidized in the presence of equimolar concentrations of Hp.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-08-174466/5/m_zh80130932720004.jpeg?Expires=1765905167&Signature=QOSzgw6VAIZRsDfV0oHTU-1dsae-NiMMoUsLn1jOwgk919CSjQhB7tfWMW58wKRR6X2p0UfwD2xqd5DrPejVRXCbtSE5wRrZYhOdmJ9lY3X5Clgu2Se7Z3B63PoAfhMpfoSBPOjxuWs6fsCxHKvlUZMrbTDyHb2tLRTK64vtsvqdk7tXKkSQOg0KbtezTSKIDyUhSUeIeMheFiHU3iOsgnHYol46Pna33pFkvZWfQH8oOUXNsvepMv~fWoGbAAJRJ7OecDeUhWrKwALwwyEuzAytg8Oo2S6uZw80NIPl1PJkC2-Q9jxwWlBiU4gegKewUecLx4063hDF0u708N~i-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Spectral changes during the oxidation of Hb by H2O2. Spectral changes during the oxidation of Hb by H2O2. (A,B) Hb (50 μM in heme) was incubated with 250 μM H2O2 at 37°C in 50 mM phosphate buffer, pH 7.4. Spectra were collected in a fast scanning diode-array spectrophotometer. The representative spectra of oxy Hb in the beginning, intermediate ferryl Hb, and ferric Hb at the end of the reaction were shown. (A) Hb without Hp and (B) Hb preincubated with 50 μM Hp. (C-F) The rate of reaction of ferric Hb with H2O2 to form ferryl Hb. The reaction rate was evaluated using a stopped-flow equipped with a photodiode array detector (Applied Photophysics). Spectral changes in the Soret and visible regions were recorded as a function of time and H2O2 concentration. (C) Representative spectral changes recorded after mixing Hb (4 μM) with excess H2O2. (D) Reconstructed spectra of the main reaction species after the global fitting computation of the data set. (E) Concentration changes of the main reaction species over time obtained by the global analysis. The reactions by ferric Hb only or by ferric Hb preincubated with slight molar excess of haptoglobin were carried out as a dependent of [H2O2], and plotted in panel F. (F) Second-order rate constants obtained under our experimental conditions for met HbA0 (●) and met HbA0 with haptoglobin (○). The derived rate constants are 42 M−1s−1 and 43.6 M−1s−1, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-08-174466/5/m_zh80130932720005.jpeg?Expires=1765905167&Signature=oZTo5wJOSf9JihJVQXi4yNQUSvxnCaEbTV7NIYD6CO8ax4o7QRAIBFK8~r~2LXeNduwDKIdC-4PMw7y50DcZNFffO5zEDIv1P3Cjy1y~s33YGLaBNBYLVHN2xwYbZkN3oL-1NEh10DR3r9165GPoqB0Qodqw1L~86YhZsHsI8LlcYcYRzMb1cjhKZm22Evz64wnp9wi5SE36ALiea3tL1YVxgWyX8dYp8RcTyprdbfq8dqFMVBRFywS~U5ygPoqQK9kTHTJoDZj4337zutAIt-pt0MGH4tYLdcEhAzaab42F4xocEnNY7uosTV2wEtE4M8QjKa8RdfhKXihkUHiTyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. CD163 interaction and endocytosis of peroxide-treated and untreated Hb and Hb-Hp. (A) CD163-HEK cells were incubated with 20 μg/mL native or H2O2-treated Hb or Hb-Hp. After 30 minutes, cells were washed, fixed, and stained with an anti-Hb antibody (red fluorescence, Alexa 568). Nuclei were stained with DAPI (blue). Images were taken with a Carl Zeiss epifluorescence Axioskope-2 (20×/0.43 objective) equipped with an AxioCam-MR digital camera and Axio-Vision software. Results of digital image analysis and quantification of red fluorescence (per cell) that indicates cellular Hb uptake are shown in the graph. Bars represent mean plus or minus SD of 3 independent experiments performed in triplicates with at least 10 images quantified per coverslip. (B) Equal binding of nonoxidized Hb-Hp (top) and oxidized Hb-Hp (bottom) complexes was confirmed by Biacore analysis over a range of concentrations (from 2.5-200 nM) normalized to Hb. (C) CD163-HEK cells were incubated with 3 μg/mL fluorescent Hb-Hp633 in the presence or absence of different concentrations of nonfluorescent competitors (● = Hb-Hp [1-1]; ○ = oxidized Hb-Hp [1-1]). After 30 minutes, cells were washed, trypsinized, and fluorescence was determined by fluorescence-activated cell sorting (FACS). Mean channel fluorescence values are given as percentage of the Hb-Hp633 fluorescence in the absence of any competitor. (Mean ± SD from 3 independent experiments.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-08-174466/5/m_zh80130932720006.jpeg?Expires=1765905167&Signature=Pq2sQRCsEowALmewbqZdEropQGZqlmDmKLwD9800-J4Y0kgzD7aVLtQSIoCsY4qS34zjc9hnhRFdLohDSjkkwQNJ2LuaGsNkD7PaUItgw0m8EuD-JG31oxT~20zdFDf04N1FE7VGnrxQD85uRDty-nixOiBoONaNbJav5jFn4mOpzXQptaCR9thk9Wk3Z9VIBsWdUPUIQzDKkJwLCshfw4TzzkKdUJcaWLuk~qfjHYirbQRDIwuEQcd6zCLHKzmsHaMtpVXcnpUB1tSkWDdLNj7LfGSYc47ApqVppJC2wuBB3niaIcriZuJnYjRPmX6aP9BAz9-Cf07hwzO4Gh3yoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)