Abstract
Overexpression of antiapoptotic members of the Bcl-2 family is observed in approximately 80% of B-cell lymphomas, contributing to intrinsic and acquired drug resistance. Nullifying the antiapoptotic influence of these proteins can potentially overcome this resistance, and may complement conventional chemotherapy. ABT-737 is a BH3-only mimetic and potent inhibitor of the antiapoptotic Bcl-2 family members Bcl-2, Bcl-XL, and Bcl-w. In vitro, ABT-737 exhibited concentration-dependent cytotoxicity against a broad panel of lymphoma cell lines including mantle cell lymphoma (MCL) and diffuse large B-cell lymphoma (DLBCL). ABT-737 showed synergism when combined with the proteasome inhibitors bortezomib or carfilzomib in select lymphoma cell lines and induced potent mitochondrial membrane depolarization and apoptosis when combined with either. ABT-737 plus bortezomib also induced significant apoptosis in primary samples of MCL, DLBCL, and chronic lymphocytic leukemia (CLL) but no significant cytotoxic effect was observed in peripheral blood mononuclear cells from healthy donors. In severe combined immunodeficient beige mouse models of MCL, the addition of ABT-737 to bortezomib enhanced efficacy compared with either drug alone and with the control. Collectively, these data suggest that ABT-737 alone or in combination with a proteasome inhibitor represents a novel and potentially important platform for the treatment of B-cell malignancies.
Introduction
Antiapoptotic proteins are key regulators of programmed cell death, and are known to be overexpressed in both solid tumors and hematologic malignancies.1-4 For example, Bcl-2 is known to be constitutively overexpressed in almost all follicular lymphomas and approximately 20% of diffuse B-cell lymphomas as a result of the t(14;18) translocation or gene amplification, respectively.5,6 Overexpression of antiapoptotic family members is associated with inhibition of apoptosis and chemotherapy resistance, and likely contributes to reduced clinical response rates and shortened survivals.7-11 Targeting antiapoptotic Bcl-2 family members with new small molecule inhibitors represents a new opportunity to affect this biology directly. The major advantage of these compounds lies in their ability to lower the threshold required to induce apoptosis, making them potentially complimentary to many conventional cytotoxic drugs used in the treatment of cancer. We have recently shown that AT-101, a small molecule inhibitor of Bcl-2, Bcl-XL, and Mcl-1, is able to synergize with conventional drugs in vitro and in vivo and with the new proteasome inhibitor carfilzomib in mantle cell lymphoma (MCL) and diffuse large B-cell lymphoma (DLBCL) in vitro.12
ABT-737 is a BH3-only mimetic capable of binding with high affinity to the prosurvival proteins Bcl-XL, Bcl-2, and Bcl-w, inducing Bax/Bak-dependent killing.13,14 Proteasome inhibitors are a new class of therapeutic agents with established activity against different hematologic malignancies including multiple myeloma, follicular lymphoma, and mantle cell lymphoma.15-17 Proteasome inhibition is known to affect a diverse array of intracellular signaling pathways, including effects on NF-κB (impaired degradation of IκB); cell-cycle regulation (accumulation of p21/p27); modulation of Bcl-2 family members (up-regulation of proapoptotic and BH3-only members); and accumulation of p53.18-23
Based on the rationale that these 2 classes of drugs may complement each other through different effects on the apoptotic cascade, we sought to establish a firm pharmacological basis for combining these agents in the treatment of lymphoma. These studies are among the first to demonstrate that the inhibition of antiapoptotic Bcl-2 family members with ABT-737 synergizes with proteasome inhibitors in vitro and in vivo. The combined effects on 2 distinct arms of the apoptotic cascade synergize to induce apoptosis in lymphoma, and could represent a novel platform for developing future therapeutic strategies to treat lymphoma.
Methods
Cells and cell lines
RL is a large B-cell lymphoma cell line harboring the t(14; 18) translocation; H9 is a cutaneous T-cell lymphoma cell line obtained from ATCC (Manassas, VA); SKI24,25 is a diffuse large B-cell lymphoma cell line; HBL-226 is a mantle cell lymphoma cell line, and JJN-3 is a multiple myeloma cell line.27
A total of 9 primary samples were obtained from patients diagnosed with chronic lymphocytic leukemia (CLL), MCL, DLBCL, or marginal zone lymphoma (MZL) according to the World Health Organization classification28 ; all patients were untreated with the exclusion of the MZL patient and one CLL patient who did not receive any treatments 6 months prior to the beginning of the study. Tumor cells were obtained from peripheral blood, spleen, or lymph nodes. Informed consent was obtained from each patient in accordance with the guidelines of the Institutional Review Board of Columbia University and the Declaration of Helsinki. CD19+ cells were isolated by an immunomagnetic method (AutoMACS) using anti-CD19 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Mononuclear cells from peripheral blood samples (PBMCs) of healthy donors were purchased from Allcells (Emeryville, CA). All cell lines were grown as previously described.29
Materials
All reagents for Western blotting were obtained from Bio-Rad Laboratories (Hercules, CA) and Pierce Biotechnology (Rockford, IL); dimethyl sulfoxide (DMSO) was obtained from Sigma-Aldrich (St Louis, MO). Drugs were obtained as follows: ABT-737, from Abbott Laboratories (Abbott Park, IL); 4-hydroxycyclophosphamide, from Niomech (Bielefeld, Germany); and carfilzomib, from Proteolix (San Francisco, CA); all other drugs were obtained from the institutional research pharmacy.
Cytotoxicity assays
For all in vitro assays, cells were counted, incubated, and processed as previously described.12 ABT-737 was diluted in DMSO that was maintained at a final concentration of less than 0.5%. ABT-737 was added at concentrations from 1 nM to 10 μM. For the scheduled administration of drugs, the final concentration of ABT-737 was 10 nM or 100 nM according to the cell line studied, whereas all other drugs concentrations were studied at 10 nM. These concentrations were selected to approximate the IC10-20 (inhibitory concentration of 10%-20% of cells) for each drug. Rituximab (R), etoposide (E), doxorubicin hydrochloride (D), pralatrexate (P), bortezomib (B), or carfilzomib (C) was either present from time 0 or added after 24 or 48 hours and incubated for an additional 24 hours (72-hour assays). Cell-Titer-Glo Reagent (Promega, Madison, WI) and a Synergy HT Multi-Detection Microplate Reader (Biotek Instruments, Winooski, VT) were used as previously described.12 Each experiment was performed in triplicate and repeated at least twice.
Flow cytometry
RL or HBL-2 cells were seeded at a density of 7 × 105/mL and incubated with concentrations of ABT-737 from 1 nM to 10 μM for 24 hours. For the transmembrane mitochondrial membrane potential (Δψm) determination, cells were stained with 1.25 μg/mL JC-1 dye (Invitrogen, Carlsbad, CA) for 30 minutes at 37° in normal growth media, then washed once in PBS, resuspended in a 200 μL media, and analyzed using a FACSCalibur Flow Cytometer (BD, Franklin Lakes, NJ). Carbonyl cyanide m-chlorophenylhydrazone (cccp; Sigma Immunochemicals, St Louis, MO) was used as a positive control for loss of membrane potential. A minimum of 105 events were acquired from each sample. Median values obtained from the gated FL1 and FL2 channels were used to calculate the normalized transmembrane mitochondrial membrane potential (Δψm). For detection of apoptosis, yo-pro-1 and propidium iodide (PI) were used (Invitrogen). Cells were incubated with the approximate IC10-20 of ABT-737 alone and in combination with the approximate IC10-20 of bortezomib for up to 48 hours. CD19+ cells from patients, or mononuclear cells from healthy donors, were plated at a density of 0.5 to 1 × 106 cells/mL with concentrations of ABT-737 ranging from 1 nM to 1 μM with or without bortezomib (ranging from 1 to 10 nM) for 24 hours. After incubation, cells were washed and resuspended in cold PBS. yo-pro-1 and PI (1 μL of each) were added to each 1 mL of cell suspension. The fluorescence signals were acquired by a FACSCalibur System. Each experiment was performed in triplicate. Data are presented as averages plus or minus standard deviation (SD).
Western blot analysis
RL and HBL2 cells were incubated with the approximate IC10-20 of each drug alone (ABT-737, bortezomib, and carfilzomib) and in combination (ABT-737 ± proteasome inhibitor) under normal growth conditions for 24 hours. Proteins from total cell lysates were resolved on 10% to 18% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. Membranes were blocked in phospho-buffered saline, 0.05% Triton X-100 containing 5% skim milk powder and were then probed overnight with specific primary antibodies. Antibodies were detected with the corresponding horseradish peroxidase–linked secondary antibodies. Blots were developed using SuperSignal West Pico chemiluminescent substrate detection reagents (Thermo Fisher Scientific, Waltham, MA). The membranes were exposed to x-ray films for various time intervals. The images were captured with a GS-800 calibrated densitometer (Bio-Rad Laboratories), and the ratios were quantified by densitometric analyses within the linear range of each captured signal. The following monoclonal and polyclonal antibodies were used: Bax (N20; Santa Cruz Biotechnology, Santa Cruz, CA), Bak (NT; GeneTex, San Antonio, TX), Puma (Biochain Institute, Hayward, CA), Noxa (Calbiochem, San Diego, CA), Mcl-1 (Rockland, Gilbertsville, PA), Bcl-2 (clone 100; Upstate, Lake Placid, NY), and beta-actin (clone AC-74; Sigma).
Confocal microscopic analysis
Cells were seeded at a density of 7 × 105/mL. After incubation with ABT-737 for 24 hours, alone or in combination with bortezomib, cells were exposed to 5 μg/mL Hoechst 33342, 250 nM MitoTracker Red, and 1 μM yo-pro-1, made in Hanks balanced salt solution supplemented with 10% FBS, for 20 minutes at room temperature. The membranes of apoptotic cells, but not the membranes of live cells, are permeant to the yo-pro-1; Hoechst 33342 is a specific stain for double-stranded DNA, whereas MitoTracker Red is concentrated by active mitochondria in living cells.30 Fluorescence of stained cells was detected with the use of a laser scanning confocal microscope (Leica TCS AOBS SP2, inverted stand; Leica Microsystems, Heidelberg, Germany). Image acquisition and analysis were performed with a semiautomated and design-based stereology system (MetaMorph version 6; Universal Imaging, Downingtown, PA). The percentage of apoptotic cells was calculated as previously described.12 Up to 20 images for each sample were acquired and analyzed in 2 different experiments.
Mouse xenograft models
In vivo experiments were performed as described previously.29 In brief, 5- to 7-week-old beige mice with severe combined immunodeficiency (SCID; Charles River Laboratories, Wilmington, MA) were injected with 107 HBL-2 cells in their flanks via a subcutaneous route. When tumor volumes approached 50 mm3 , mice were separated into treatment groups of 5 to 6 mice each. Tumors were assessed using the 2 largest perpendicular axes (l, length; w, width) as measured with standard calipers. Tumor volume was calculated using the formula 4/3Πr3, where r = (l + w)/4. Tumor-bearing mice were assessed for weight loss and tumor volume at least twice weekly. Animals were killed when 1-dimensional tumor diameter exceeded 2.0 cm, or after loss of more than 10% body weight in accordance with institutional guidelines. Complete response (CR) was defined as nonpalpable tumor. ABT-737 was given by intraperitoneal injection. In the combination experiments, ABT-737 was administered at a dose of 75 mg/kg per day for 10 days; bortezomib (B) was administered by intraperitoneal injection according to different schedules: at 0.5 mg/kg on days 1, 4, 11, and 14; at 1 mg/kg on days 1 and 8; at 0.5 mg/kg on day 1; and at 0.75 mg/kg on days 5 and 10. For intraperitoneal administration, ABT-737 was added to a mixture of 30% propylene glycol, 5% Tween 80, 65% D5W (5% dextrose in water), pH 4 to 5. Control groups were treated with the vehicle solution. All animal studies were conducted in agreement with Institutional Review Board requirements of Columbia University.
Statistical analysis
For different in vitro experimental groups, permutation tests were performed to determine whether any of the experimental groups was superior to a control group. The analysis entailed comparing groups based on repetitions (typically 3) using analysis of variance after a normalizing transformation. Since multiple hypotheses were simultaneously tested, all P values are adjusted using Dunnett method.31 For each cell line, the IC50 (inhibitory concentration of 50% of cells) and the drug-drug interactions in terms of synergism, additivity, or antagonism were computed using the Calcusyn software (Biosoft, Cambridge, United Kingdom) (combination index [CI] < 1 defines synergism; CI = 1, additivity; CI > 1, antagonism). In animal experiments, tumor volume is presented graphically as the mean at each time point for each treatment group. Each animal's time-tumor volume curve is represented using the area under the curve (AUC) that is interpreted as the total tumor burden of the animal. A logarithmic transformation to normalize the AUC is followed by an analysis of variance for group comparisons with an adjustment for multiple comparisons using resampling. All significance testing was done at the P less than .05 level, protecting the family-wise error rate.
Results
ABT-737 sensitizes drug-resistant diffuse large B-cell and mantle cell lymphoma to cytotoxic agents
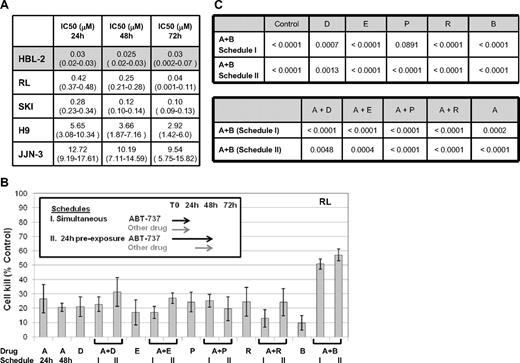
The IC50 to ABT-737 across a panel of different lymphoid malignancies ranged from less than 50 nM to more than 10 μM (Figure 1A). For example, the IC50's for the mantle cell (HBL-2) and T-cell lymphoma (H9) cell lines were 30 nM and 5.7 μM, respectively, at 24 hours, whereas the 2 diffuse large B-cell lymphoma cell lines (DLBCL, RL, and SKI) exhibited an intermediate IC50 of approximately 0.3 to 0.4 μM. These data suggest a broad spectrum of activity of ABT-737 across various lymphomas, and also underscore the possible variability that can be seen across different lymphoma cell lines. The duration of exposure to ABT-737 did not appear to be a major determinant of activity.
Cytotoxic effect of ABT-737 in cell lines of hematologic malignanices. (A) IC50 values (mM) to ABT-737. HBL2 cell line was the most sensitive through all time points explored; confidence intervals are shown between parenthesis. (B) Model of in vitro exposure to ABT-737 and other drugs is shown on the upper left. Percentage of cells killed compared to control for each treatment group (± SD) is shown as histograms. All drug concentrations used approximated the IC10-25. (C) Multiple comparison analysis for ABT-737 at 100 nM in combination with other drugs at 10 nM in RL (DLBCL). Probability values for all averages comparisons proved (P values). Where statistically significant (ie, P .05), bolded cohorts are superior. A = ABT-737, D = doxorubicin, E = etoposide, P = pralatrexate, R = rituximab, B = bortezomib.
Cytotoxic effect of ABT-737 in cell lines of hematologic malignanices. (A) IC50 values (mM) to ABT-737. HBL2 cell line was the most sensitive through all time points explored; confidence intervals are shown between parenthesis. (B) Model of in vitro exposure to ABT-737 and other drugs is shown on the upper left. Percentage of cells killed compared to control for each treatment group (± SD) is shown as histograms. All drug concentrations used approximated the IC10-25. (C) Multiple comparison analysis for ABT-737 at 100 nM in combination with other drugs at 10 nM in RL (DLBCL). Probability values for all averages comparisons proved (P values). Where statistically significant (ie, P .05), bolded cohorts are superior. A = ABT-737, D = doxorubicin, E = etoposide, P = pralatrexate, R = rituximab, B = bortezomib.
To explore the potential for favorable drug-drug interactions, a variety of schedules were explored as presented in Figures 1B and 2A. These models were used to determine the requirement for a “lead in” exposure to ABT-737. These studies demonstrated that at relatively low IC ranges (IC10-40), the combination of ABT-737 and either proteasome inhibitor (bortezomib or carfilzomib) was among the most favorable, with more than 50% cell killing. Using the 2 optimal schedules of ABT-737 plus bortezomib (schedules I and II in Figure 1B), this combination was compared with different cytotoxic agents alone or in combination with ABT-737 in a multiple comparison analysis model. These data suggest that the combination of ABT-737 plus bortezomib was superior to any conventional cytotoxic agent and all other ABT-737 combinations (at the IC10-25) in a diffuse large B-cell lymphoma cell line (RL) (Figure 1C). The coexposure of ABT-737 plus bortezomib for 24 hours resulted in a statistically significant advantage for this combination compared with either drug alone (with the exception of PDX) or in combination with AB-737. Importantly, a cytotoxicity assay exploring the simultaneous and 24-hour preexposure of ABT-737 and bortezomib revealed both schedules to be equivalent (P = .96), whereas the 48-hour preexposure was statistically inferior to both the simultaneous (P = .02) and 24-hour pre-exposure (P < .001, data not shown) schedules.
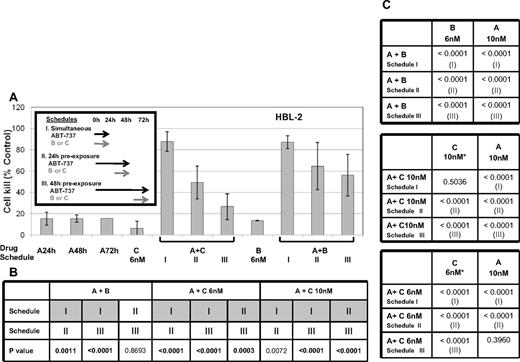
Enhanced cytotoxic effect of ABT-737 combined with a proteasome inhibitor in MCL (HBL-2). (A) Percentage of cells killed compared to control for each treatment group (± SD). ABT-737 was given for up to 72 hours. Model of in vitro exposure is shown on the upper left. (B) Multiple comparison analysis for ABT-737 at 10 nM in combination with bortezomib at 6 nM or carfilzomib at 6 nM or 10 nM for 24 hours; 3 different schedules for each drug combination are compared. Statistically superior group (P < .05) is shown in a gray box. Probability values for comparisons of all averages proved (P values). (C) Comparison of each combination to each drug alone; superior treatment group is shown in parentheses. A = ABT-737, B = bortezomib, C = carfilzomib.
Enhanced cytotoxic effect of ABT-737 combined with a proteasome inhibitor in MCL (HBL-2). (A) Percentage of cells killed compared to control for each treatment group (± SD). ABT-737 was given for up to 72 hours. Model of in vitro exposure is shown on the upper left. (B) Multiple comparison analysis for ABT-737 at 10 nM in combination with bortezomib at 6 nM or carfilzomib at 6 nM or 10 nM for 24 hours; 3 different schedules for each drug combination are compared. Statistically superior group (P < .05) is shown in a gray box. Probability values for comparisons of all averages proved (P values). (C) Comparison of each combination to each drug alone; superior treatment group is shown in parentheses. A = ABT-737, B = bortezomib, C = carfilzomib.
The addition of a proteasome inhibitor (bortezomib or carfilzomib) to ABT-737 in a mantle cell lymphoma cell line (HBL-2) produced a statistically significant advantage for the combination over ABT-737 or the proteasome inhibitor alone (Figure 2). For example, 10 nM ABT-737 (ie, the approximate IC10-20) with 6 nM bortezomib (approximately IC10-20) for 24 hours (schedule I in Figure 1A) resulted in a statistically significant difference (P < .001) for the combination compared with ABT-737 alone or with ABT-737 plus lower concentrations of bortezomib (1 nM and 3 nM). This activity seems to suggest a threshold effect for the concentration of bortezomib. When 10 nM ABT-737 was combined with bortezomib at 6 nM or carfilzomib at 6 nM or 10 nM, the combination was superior to either drug alone or control groups (P < .001) for all schedules at each time point considered (ie, 24, 48, and 72 hours), save one comparison with carfilzomib at 10 nM, which we attributed to the possibly greater IC values for this drug in these analyses. These experiments were designed to identify the lowest concentration of a proteasome inhibitor that produced consistent and reproducible synergy with ABT-737 under a variety of different schedules of exposure. A simultaneous exposure (schedule I in Figure 2A) with ABT-737 and bortezomib or carfilzomib produced a statistically significant advantage for the combinations compared with alternative schedules (schedule II and III; P ≤ .007). These data suggest that preexposure to ABT-737 provides no advantage, and may be possibly antagonistic in select circumstances.
ABT-737 interacts synergistically with bortezomib in a MCL and a DLBCL cell line
Formal synergy analyses were performed using RL and HBL-2 cells treated with ABT-737 and each of the proteasome inhibitors. In both cell lines, a synergistic cytotoxic effect was observed as follows: ABT-737 plus bortezomib showed a CI less than 0.3 in RL and less than 0.7 in HBL-2; whereas ABT-737 plus carfilzomib showed a CI less than 0.7 in HBL-2. These data support the mathematic contention that these drugs are synergistic in vitro.
ABT-737 disrupts the Δψm in a concentration-dependent manner
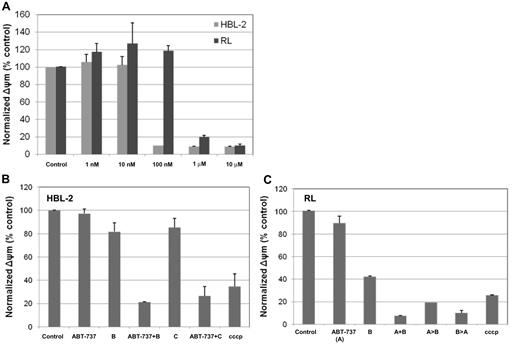
Changes in mitochondrial membrane potential (Δψm) are thought to represent an early event in the induction of apoptosis, and likely capture the effects of agents on various aspects of Bcl-2 family members. Treatment of RL and HBL-2 cells with ABT-737 decreased the normalized Δψm in a concentration-dependent manner (Figure 3). After incubation with ABT-737 for 24 hours, the HBL-2 cell line showed a more than a 10-fold decrease in Δψm compared with the RL line in the concentration range of 10 nM to 100 nM. When ABT-737 (10 nM) was combined with bortezomib (at 6 nM) or carfilzomib (at 10 nM), a statistically significant decrease in Δψm was observed in HBL-2 after 24 hours of incubation in both treatment groups compared with each drug alone, the control, and ABT-737 alone (P < .001). To understand the impact of a preexposure on the Δψm, RL cells were incubated both simultaneously and with a 24-hour preexposure to either ABT-737 (100 nM) or bortezomib (10 nM). These data suggest that all the combination groups exhibited the lowest Δψm (favoring induction of apoptosis), with a statistically significant difference compared with any single-agent treatment group and control (P < .001). There was no statistically significant difference between any of the different schedules analyzed (ie, simultaneous exposure vs A > B or B > A). These data are consistent with the cytotoxicity data that support the contention that a preexposure of ABT-737 prior to bortezomib does not appear to be a requisite for optimal activity.
The combination of ABT-737 and bortezomib or carfilzomib significantly changes the mitochondrial membrane potential (ΔΨm) in DLBCL (RL) and MCL (HBL-2). (A) HBL-2 and RL cells were incubated with ABT-737 from 1 nM to 10 μM for 24 hours. (B) HBL-2 cells were incubated with with ABT-737 (10 nM), bortezomib (B, 6 nM), carfilzomib (C, 10 nM) for 24 hours. Both combination groups were statistically significant compared to any of the single groups and controls (P < .001). (C) RL cells were incubated with ABT-737 (100 nM), bortezomib (B, 10 nM) for 48 hours. In the sequence explored (>) the second drug was added after 24 hours from the beginning. All combination groups were statistically significant compared each single group and the control (P < .001). No statistically significant difference among the 3 combination groups explored. Δψm was evaluated by cytofluorimetric analysis of JC-1. Results represent the means plus or minus SD. cccp indicater carbonyl cyanide m-chlorophenylhydrazone.
The combination of ABT-737 and bortezomib or carfilzomib significantly changes the mitochondrial membrane potential (ΔΨm) in DLBCL (RL) and MCL (HBL-2). (A) HBL-2 and RL cells were incubated with ABT-737 from 1 nM to 10 μM for 24 hours. (B) HBL-2 cells were incubated with with ABT-737 (10 nM), bortezomib (B, 6 nM), carfilzomib (C, 10 nM) for 24 hours. Both combination groups were statistically significant compared to any of the single groups and controls (P < .001). (C) RL cells were incubated with ABT-737 (100 nM), bortezomib (B, 10 nM) for 48 hours. In the sequence explored (>) the second drug was added after 24 hours from the beginning. All combination groups were statistically significant compared each single group and the control (P < .001). No statistically significant difference among the 3 combination groups explored. Δψm was evaluated by cytofluorimetric analysis of JC-1. Results represent the means plus or minus SD. cccp indicater carbonyl cyanide m-chlorophenylhydrazone.
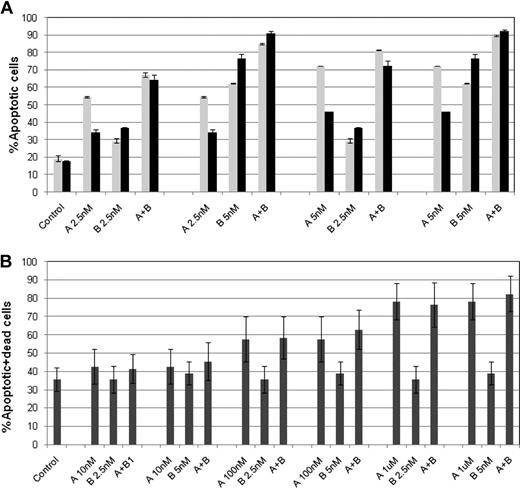
ABT-737 plus a proteasome inhibitor enhances apoptosis in diffuse large B-cell and mantle cell lymphoma cell lines
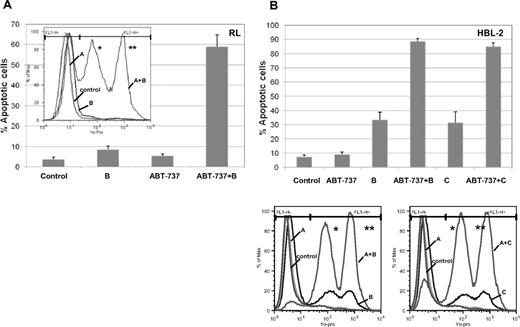
Treatment with ABT-737 and a proteasome inhibitor for 24 hours showed potent induction of apoptosis in both RL and HBL-2 cell lines. When RL cells were treated with ABT-737 (100 nM) and bortezomib (10 nM), more than 50% of the cells were apoptotic, compared with less than 10% for the individual drugs (Figure 4A). When HBL-2 was treated with ABT-737 (10 nM) plus bortezomib or carfilzomib (6 or 10 nM, respectively), the combination produced more than 80% apoptotic cells, compared with less than 10% of apoptotic cells for ABT-737 alone and approximately 30% for either of the proteasome inhibitors (Figure 4B). These data support earlier findings suggesting that there are likely class effects between proteasome inhibitors and ABT-737, which appear to be synergistic at both the biophysical and cellular levels.
Enhanced apoptosis of ABT-737 combined to a proteasome inhibitor in DLBCL (RL) and MCL (HBL-2). (A) Treatment of RL cells with ABT-737 at 100 nM and bortezomib (B) at 10 nM induces apoptosis in more than 50% of cells (P < .001). (B) Treatment of HBL-2 cells with ABT-737 at 10 nM and bortezomib (B) at 6 nM or carfilzomib (C) at 10 nM induces apoptosis in more than 80% of cells (P < .001). Apoptosis. was evaluated by cytofluorimetric analysis of yo-pro-1 and propidium iodide. Results represent the average plus or minus SD. * Early apoptotic: yo-pro-1–positive and PI–negative; ** late apoptotic: yo-pro-1–positive and PI–positive.
Enhanced apoptosis of ABT-737 combined to a proteasome inhibitor in DLBCL (RL) and MCL (HBL-2). (A) Treatment of RL cells with ABT-737 at 100 nM and bortezomib (B) at 10 nM induces apoptosis in more than 50% of cells (P < .001). (B) Treatment of HBL-2 cells with ABT-737 at 10 nM and bortezomib (B) at 6 nM or carfilzomib (C) at 10 nM induces apoptosis in more than 80% of cells (P < .001). Apoptosis. was evaluated by cytofluorimetric analysis of yo-pro-1 and propidium iodide. Results represent the average plus or minus SD. * Early apoptotic: yo-pro-1–positive and PI–negative; ** late apoptotic: yo-pro-1–positive and PI–positive.
Confocal microscopy confirms induction of apoptosis
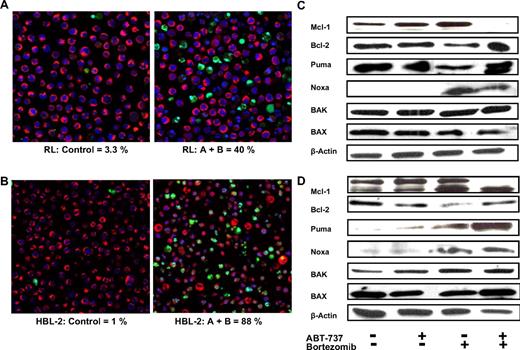
Confocal microscopy was used to directly study changes in the treated cell populations as a function of drug concentration. The incubation of RL and HBL2 cells with ABT-737 with or without bortezomib for 24 hours induced apoptosis as revealed by staining with YO-PRO-1, Hoechst 33342, and MitoTracker Red. RL cells revealed an apoptotic ratio of 40% for the combination group compared with only 3% for controls, 8.8% for the bortezomib group, and 19% for the ABT-737 group (Figure 5A). HBL-2 cells revealed an apoptotic ratio of 88% for the combination group compared with only 1% for controls, 12% for the bortezomib group, and 4% for the ABT-737 group (Figure 5B). In both cases, the combination of ABT-737 plus bortezomib resulted in a statistically significant difference compared with controls and any other treatment group (P < .001).
Enhanced apoptosis of ABT-737 combined to bortezomib in DLBCL (RL) and MCL (HBL-2) and effect on Bcl-2 family of proteins. (A,B) The combination induces significant apoptosis as shown by confocal microscopy, in RL (DLBCL, panel A) and HBL-2 (MCL, panel B) after 24 hours. ABT-737 (A) + bortezomib (B) showed statistically significant more apoptosis compared to any other treatment group (P .001). Mitotacker is red (mitochondria of live cells), Hoechst 33342 is blue (nuclei), and Yo-pro-1 is green (apoptotic cells). (C,D) Bcl-2, Mcl-1, BAX, BAK, Puma, and Noxa expression before and after treatment with ABT-737 at 100 nM (C, RL) or 10 nM (D, HBL-2) and bortezomib at 10 nM (RL) or 6 nM (HBL-2) was analyzed by Western blot. β-Actin was used to normalize protein loading.
Enhanced apoptosis of ABT-737 combined to bortezomib in DLBCL (RL) and MCL (HBL-2) and effect on Bcl-2 family of proteins. (A,B) The combination induces significant apoptosis as shown by confocal microscopy, in RL (DLBCL, panel A) and HBL-2 (MCL, panel B) after 24 hours. ABT-737 (A) + bortezomib (B) showed statistically significant more apoptosis compared to any other treatment group (P .001). Mitotacker is red (mitochondria of live cells), Hoechst 33342 is blue (nuclei), and Yo-pro-1 is green (apoptotic cells). (C,D) Bcl-2, Mcl-1, BAX, BAK, Puma, and Noxa expression before and after treatment with ABT-737 at 100 nM (C, RL) or 10 nM (D, HBL-2) and bortezomib at 10 nM (RL) or 6 nM (HBL-2) was analyzed by Western blot. β-Actin was used to normalize protein loading.
The influence of bortezomib on Bcl-2 family members
It has been established that Bak is regulated by both Mcl-1 and Bcl-XL, and that the association of Noxa with Mcl-1 can trigger Bak displacement and Mcl-1 degradation. The treatment of the HBL-2 and RL cell lines with ABT-737 plus bortezomib revealed changes in protein levels for some important Bcl-2 family members (Figure 5C,D). Following treatment with ABT-737 plus bortezomib, the expression of the antiapoptotic protein MCL-1 decreased in both cell lines. The expression of Bcl-2 seemed to decrease after treatment with bortezomib alone in both cell lines. After treatment with bortezomib alone and ABT-737 plus bortezomib, the expression of the BH3-only protein Noxa increased in both cell lines, whereas an increase in the expression of Puma was detected only in an MCL cell line after treatment with the combination. Total levels of Bax and Bak did not change significantly after treatment in both cell lines.
ABT-737 sensitizes primary CLL, MCL, and DLBCL to bortezomib without enhancing cytotoxicity to PBMCs from healthy donors
To verify these results, we tested the cytotoxic effect of ABT-737 combined with bortezomib in primary lymphoma cells from 9 patients with different types of non-Hodgkin lymphomas. In 4 of 5 CLL samples, the combinations of ABT-737 and bortezomib (both given at 2.5 or 5 nM) showed significant induction of apoptosis compared with the single agents and control (P ≤ .02). Figure 6A shows the results obtained in cells from 2 representative CLL patients treated with ABT-737 plus bortezomib.
Enhanced apoptosis of ABT-737 (A) combined with bortezomib (B) in CLL primary cells and lack of enhanced toxicity in PBMC. (A) Primary cells from 2 representative patients with CLL were treated with bortezomib and/or ABT-737 at 2.5 or 5 nM for 24 hours. Each combination group resulted statistically significant compared to the single drugs and control (P < .02). (B) PBMCs were treated with bortezomib at 2.5 or 5 nM and/or ABT-737 at 0.01, 0.1, or 1 μM ABT-737 for 24 hours. Each combination group was not significantly more cytotoxic then ABT-737 given alone (P < .36). Apoptosis was evaluated by cytofluorimetric analysis of yo-pro-1 and propidium iodide. Results represent the means plus or minus SD.
Enhanced apoptosis of ABT-737 (A) combined with bortezomib (B) in CLL primary cells and lack of enhanced toxicity in PBMC. (A) Primary cells from 2 representative patients with CLL were treated with bortezomib and/or ABT-737 at 2.5 or 5 nM for 24 hours. Each combination group resulted statistically significant compared to the single drugs and control (P < .02). (B) PBMCs were treated with bortezomib at 2.5 or 5 nM and/or ABT-737 at 0.01, 0.1, or 1 μM ABT-737 for 24 hours. Each combination group was not significantly more cytotoxic then ABT-737 given alone (P < .36). Apoptosis was evaluated by cytofluorimetric analysis of yo-pro-1 and propidium iodide. Results represent the means plus or minus SD.
In one MCL sample, the comparison of ABT-737 plus bortezomib trended toward significance compared with ABT-737 alone (P = .064), and it was statistically significant compared with bortezomib and the control (P ≤ .01). In a second MCL patient, ABT-737 at 2.5 or 5 nM plus bortezomib at 1, 2.5, or 5 nM was significantly superior to the single drugs and control (P ≤ .04). No significant activity was observed in a MZL patient, although the cells treated with the combination of ABT-737 and bortezomib again trended toward significance compared with bortezomib alone (P = .07). In primary DLBCL cells, the combination of ABT-737 at 100 nM (but not at 10 nM) with bortezomib at 5 or 10 nM showed statistically more apoptosis than either agent alone and control (P ≤ .008). Interestingly, the synergistic effect was specific to malignant cells, since the combination therapy showed no additional cytotoxic effect compared with ABT-737 alone in PBMCs from healthy donors (P ≥ .36; Figure 6B).
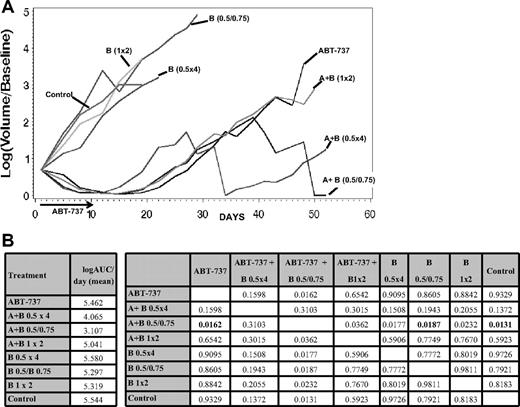
ABT-737 enhances the activity of bortezomib in vivo
The in vivo efficacy of ABT-737 was investigated in combination with bortezomib in a xenograft model of MCL (HBL-2, Figure 7). Starting from day 41 after treatment, the combination of ABT-737 and bortezomib given on days 1 (at 0.5 mg/kg), 5, and 10 (at 0.75 mg/kg) was statistically superior to bortezomib alone (P = .007), ABT-737 alone (P = .035), and the control (P = .005). This advantage retained significance beyond day 41 with 2 durable complete responses starting from day 8. Fifty percent of mice in the group receiving combination therapy experienced a significant weight loss (more than 10% of initial weight) by the end of the first week of treatment; all of them regained their weight by day 28. Alternative schedules of the same combination (ie, bortezomib given at 1 mg/kg on days 1 and 8 or given at 0.5 mg/kg on days 1, 4, 11, and 15) did not show significant activity compared with ABT-737 alone.
Enhanced activity of ABT-737 combined to bortezomib in a xenograft SCID-beige mouse model of MCL (HBL-2). The combination of intraperitoneal ABT-737 (A) at 75 mg/kg per day for 10 days plus intraperitoneal bortezomib (B) at 0.5 mg/kg on day 1 and 0.75 mg/kg on days 5 and 10 has shown the best activity, with 2 complete responses from day 8. (B) A multiple comparison analysis of the average AUCs (left panel) at day 52, showed statistical significant tumor shrinkage (starting from day 41) for the combination of ABT-737 plus bortezomib (0.5 mg/kg on day 1 and 0.75 mg/kg on days 5 and 10) compared to the single drugs and the control (right panel, P values for all comparisons are provided, P < .018, in bold). All significance testing is done at the P < .05 level.
Enhanced activity of ABT-737 combined to bortezomib in a xenograft SCID-beige mouse model of MCL (HBL-2). The combination of intraperitoneal ABT-737 (A) at 75 mg/kg per day for 10 days plus intraperitoneal bortezomib (B) at 0.5 mg/kg on day 1 and 0.75 mg/kg on days 5 and 10 has shown the best activity, with 2 complete responses from day 8. (B) A multiple comparison analysis of the average AUCs (left panel) at day 52, showed statistical significant tumor shrinkage (starting from day 41) for the combination of ABT-737 plus bortezomib (0.5 mg/kg on day 1 and 0.75 mg/kg on days 5 and 10) compared to the single drugs and the control (right panel, P values for all comparisons are provided, P < .018, in bold). All significance testing is done at the P < .05 level.
Discussion
Given the prominent role Bcl-2 family members play in normal lymphocyte ontogeny and in lymphomagenesis, there is a strong rationale for targeting Bcl-2 family members in lymphoma. At present, there are several different strategies for targeting both the intrinsic and extrinsic arms of these survival pathways, including antisense oligonucleotides, BH-3–only mimetic small molecules, monoclonal antibodies, and proteasome inhibitors.32-35 In response to cellular damage, some BH-3–only family members activate a cascade of events that lead to Bax and/or Bak activation, mitochondrial outer membrane permeabilization (MOMP), and release of cytochrome c and other proapoptotic factors.36-43
ABT-737 induces apoptosis by direct inhibition of Bcl-2, Bcl-XL, and Bcl-w, in a manner analogous to the proapoptotic BH3-only protein Bad. Bad has been shown to cooperate with Noxa to induce potent killing by inducing Bax/Bak activation.41 ABT-737 has potent single-agent efficacy against cell lines from lymphoid malignancies known to express high levels of Bcl-2, including follicular lymphoma, chronic lymphocytic leukemia, multiple myeloma, as well as small cell lung cancer.13,44-47
The experiments presented here support the potent activity of ABT-737 in a variety of B-cell lymphomas and lymphoma cell lines. The cytotoxicity assays suggest IC50 values in the nanomolar range for mantle cell lymphoma (HBL-2) and a drug-resistant large B-cell lymphoma (RL) cell lines. In general, the time of exposure to ABT-737 did not significantly affect the IC50, suggesting that the effects of Bcl-2 inhibition on the induction of apoptosis are rapid, probably due to the very high affinity of this compound for the target.
Another potentially critical pharmacologic determinant of this class of drugs pertains to their schedule of administration. Earlier experiences with the Bcl-2 antisense molecule and the small molecule AT-101 have suggested a requirement for preexposure to the anti–Bcl-2 drug prior to treatment with a conventional cytotoxic agent.12,29 This was not universally the case for ABT-737. For example, in the mantle cell lymphoma line, preexposure to ABT-737 before administering bortezomib or carlfizomib did not improve the activity of these agents. This observation has been confirmed by others as well.48 Using the mitochondrial membrane potential as an early surrogate marker, ABT-737 induced changes in the Δψm in a concentration-dependent manner. The observed effect on the mitochondrial membrane potential was particularly prominent after 24 hours of exposure in the concentration range of 10 to 100 nM for HBL-2 and 100 nM to 1 μM for RL, suggesting the possible existence of a threshold concentration required to trigger apoptosis in specific cell lines. These findings are not surprising, given the complexities of the apoptotic pathway and the different combination of proto-oncogenes and tumor suppressor genes deregulated in different B-cell non-Hodgkin lymphomas. Certainly, the panoply of different lymphomas can be characterized by a remarkable variation in these proteins from one type of lymphoma to another (or cell line to cell line), differences that may be best captured through the use of a “common” or terminal surrogate measure of their influence (Δψm).
To interrogate any possible “synergistic” interaction between ABT-737 and the proteasome inhibitors studied, intentional subtherapeutic concentrations of different drugs were studied. When used as single agents in the same concentrations as in the combination therapies, neither ABT-737 nor the proteasome inhibitors were associated with any significant change in Δψm or induction of apoptosis. Interestingly, the combination of drugs strongly induced mitochondrial membrane depolarization, as shown by flow cytometry with JC-1 dye and subsequent potent induction of apoptosis as shown by both flow cytometry and confocal microscopy.
It is becoming increasingly clear that proteasome inhibitors can have vast effects on the apoptotic regulatory machinery. Inhibition of the 26S proteasome can lead to programmed cell death through both direct and indirect influences, where some of the indirect effects are known to be mediated by NF-κB.49
Small molecules targeted against antiapoptotic family members offer the prospect of silencing prosurvival pathways, whereas proteasome inhibitors offer the possibility of modulating BH3-only and proapoptotic proteins in a prodeath fashion. For example, the combination of the Bcl-2–targeted antisense molecule oblimersen and bortezomib sensitized drug-resistant human B-cell lymphomas to nominal doses of cyclophosphamide.29 This effect was attributed in part to the increased half-life of the antisense molecule following treatment with bortezomib that resulted in a marked decrease in Bcl-2 levels in mice receiving the proteasome inhibitor. These observations were found to have remarkable schedule dependency with an absolute requirement for pre-exposure.
In models of MCL, where Mcl-1 plays a major prosurvival role, some investigators have demonstrated that proteasome inhibitors can increase the level of the protein, theoretically antagonizing the effects of other proapoptotic influences.49 Despite this increase in Mcl-1, many studies continue to report a favorable treatment effect with proteasome inhibitors in hematologic malignancies.16 Willis et al42 suggested that apoptosis is mediated by Bak liberation from Mcl-1 and Bcl-XL that was facilitated by Noxa. When both Mcl-1 and Bcl-XL are inactivated by BH3-only proteins, proapoptotic family members become liberated, inducing apoptosis. Interestingly, it has been shown that Noxa not only affects levels of free Bak, but also promotes the proteasome-dependent degradation of Mcl-1.42 Additional support for this model has recently been advanced by Perez-Galan et al.48,50 These authors have demonstrated that bortezomib potently activates the mitochondrial pathway of apoptosis in mantle cell lymphoma cell lines and synergizes with the Bcl-2–targeted drug GX15-070 by enhancing Noxa-mediated activation of Bak. Despite the presumed low affinity of ABT-737 for Mcl-1, we observed marked synergism when it was combined with a proteasome inhibitor in all cell lines studied.
Based on the Western blot data presented here, there seems to be a cooperation between ABT-737 and Noxa that serves to trigger apoptosis; Noxa accumulated in both lines following treatment with the proteasome inhibitor. The difference in the relative ratios of different protein members, as we alluded to earlier, could account for some of the differences between the selected cell lines. Interestingly, the antiapoptotic protein Mcl-1 showed some reduction after treatment with ABT-737 plus bortezomib in both cell lines. An additional and new observation that can account for these synergistic interactions pertains to the modulation of Puma. Puma, like Noxa, is a BH3-only protein capable of activating Bax and Bak that then induces cytochrome c release. Treatment with the combination of ABT-737 and bortezomib produced an increase of Puma in the MCL cell line. We speculate that Puma can cooperate with Noxa to induce Bak displacement, Bak/Bax activation, and potent induction of apoptosis.
The combination of ABT-737 and bortezomib also showed significant activity in a panel of primary malignant cells including CLL, MCL, and DLBCL (P < .02). Interestingly, MCL continued to be among the most sensitive diseases to ABT-737 and the combination with a proteasome inhibitor, whereas much higher concentrations of ABT-737 were required to show synergism in DLBCL. These findings are concordant with the in vitro activity seen in the secondary cell lines. CLL samples showed a pattern of sensitivity more similar to MCL, with concentrations of ABT-737 and bortezomib inducing significant apoptosis in the low nanomolar range. Importantly, when the same combination was tested on PBMCs, the ABT-737 plus bortezomib combination was not more cytotoxic than ABT-737 alone (P ≥ .36).
A xenograft experiment of MCL (HBL-2) in SCID-beige mice with ABT-737 combined with bortezomib according to different schedules alone showed a statistically significant advantage for one of the combinations (with bortezomib given at 0.5 mg/kg on day 1 and at 0.75 mg/kg on days 5 and 10) compared with the single-agent cohorts and the control (P ≤ .018 after 52 days), with 2 complete responses out of 6 mice. Interestingly, alternative combinations (with bortezomib given at 1 mg/kg on day 1 and 8 or at 0.5 mg/kg on days 1, 4, 11, and 15), delivering the same total dose of bortezomib, did not show any significant benefit compared with ABT-737 alone. This observation underscores the importance of clarifying the effective schedules of these 2 drugs to maximize activity while minimizing toxicity. In a pilot study, the combination of ABT-737 with bortezomib given at 0.5 mg/kg on days 1, 4, 8, and 11 resulted in excess toxicity, which led to alternative schedules. In conclusion, the combination of ABT-737 and a proteasome inhibitor has shown to be markedly active across a panel of B-cell malignancies, and these combinations do not produce excess toxicity in normal PBMCs. Experiments in primary patient samples and in vivo studies confirmed the in vitro observations made in B-cell lymphoma cell lines. Carefully designed phase 1 studies should explore this combination, with a focus on relevant pharmacokinetic and pharmacodynamic relationships.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Saul Rosenberg for his input into these experiments. We also thank Proteolix for its advice and supply of carfilzomib.
O.A.O. is the recipient of the Leukemia & Lymphoma Society (White Plains, NY) Scholar in Research Award. L.P. is the recipient of the American Italian Cancer Foundation Fellowship (New York, NY). M.L.H. is supported by the Naomi Rosenfeld Research Fund (New York, NY).
Authorship
Contribution: L.P. designed and performed all presented experiments, interpreted data, and wrote the paper; M.G. did the statistical analysis; G.B. and R.R.F. provided samples from patients and contributed to their processing; J.R.G. assisted with flow cytometric analysis; L.S. provided assistance with the Western blots; V.D.G. and K.M. provided assistance with the Western blots and the laser confocal microscopy; M.L.H. provided input regarding experiments measuring the transmembrane mitochondrial potential; and O.A.O. provided input regarding experimental design, interpretation of data, and editing of the final paper.
Conflict-of-interest disclosure: O.A.O. receives research support from Millennium and Proteolix. All other authors declare no competing financial interests.
Correspondence: Owen A. O'Connor, Lymphoid Development and Malignancy Program, Herbert Irving Comprehensive Cancer Center, Columbia University, 1130 St Nicholas Ave, New York, NY 10032; e-mail: oo2130@columbia.edu.