Abstract
The interleukin-12 (IL-12) receptor (R) B2 gene acts as tumor suppressor in human acute and chronic B-cell leukemias/lymphomas and IL-12rb2–deficient mice develop spontaneously localized plasmacytomas. With this background, we investigated the role of IL-12Rβ2 in multiple myeloma (MM) pathogenesis. Here we show the following: (1) IL-12Rβ2 was expressed in primary MM cells but down-regulated compared with normal polyclonal plasmablastic cells and plasma cells (PCs). IL-6 dampened IL-12Rβ2 expression on polyclonal plasmablastic cells and MM cells. (2) IL-12 reduced the proangiogenic activity of primary MM cells in vitro and decreased significantly (P = .001) the tumorigenicity of the NCI-H929 cell line in SCID/NOD mice by inhibiting cell proliferation and angiogenesis. The latter phenomenon was found to depend on abolished expression of a wide panel of proangiogenic genes and up-regulated expression of the antiangiogenic genes IFN-γ, IFN-α, platelet factor-4, and TIMP-2. Inhibition of the angiogenic potential of primary MM cells was related to down-regulated expression of the proangiogenic genes CCL11, vascular endothelial-cadherin, CD13, and AKT and to up-regulation of an IFN-γ–related antiangiogenic pathway. Thus, IL-12Rβ2 directly restrains MM cell growth, and targeting of IL-12 to tumor cells holds promise as new therapeutic strategy.
Introduction
Multiple myeloma (MM) is a monoclonal postgerminal center tumor that has phenotypic features of plasmablasts/long-lived plasma cells and usually localizes at multiple sites in the bone marrow (BM).1 MM is the second most common hematologic malignancy worldwide, and its prognosis remains grim despite advanced therapeutic protocols.2 Promising targeted therapies for MM have emerged, including proteasome inhibitors, heat shock protein 90 inhibitors, AKT inhibitors, and antiangiogenic molecules with immunostimulatory properties (eg, thalidomide), but treated patients still relapse.2 Thus, novel therapeutic strategies are warranted to improve MM prognosis.
Interleukin-12 (IL-12) is a cytokine that exerts potent antitumor activity through a combination of immunostimulatory and antiangiogenic mechanisms.3-6 The latter are related to induction of interferon-γ (IFN-γ), which in turn triggers the release of the antiangiogenic chemokines CXCL9, CXCL10, and CXCL11. In addition, IL-12 down-regulates the production of the proangiogenic molecules vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2).7-11
We have previously shown3,12 that the IL-12RB2 gene, encoding the IL-12R chain essential for IL-12 signal transduction, functions as a tumor suppressor in human neoplastic B cells from various chronic lymphoproliferative disorders and acute lymphoblastic leukemia. We13 have also demonstrated that IL-12rb2–deficient mice develop spontaneously multiorgan lymphoid infiltrates, systemic IL-6 up-regulation, and CD138+ plasma-cell hyperplasia.13 Finally, aged IL-12rb2 knockout animals develop localized lymph node plasmacytoma, which is exceedingly rare in humans,14 possibly in relation to IL-6 overexpression.13
MM progression is characterized by changes in the BM microenvironment, including overexpression of IL-6 and VEGF that support tumor growth through paracrine loops, induction of angiogenesis, and suppression of cell-mediated immunity.15,16 Here we have asked whether IL-12Rβ2 plays a role in human MM pathogenesis and investigated for the first time the expression and function of IL-12Rβ2 in MM cells and their normal counterparts. Next, we have performed functional studies to assess the direct antitumor activity of IL-12 on MM cells and to unravel the molecular mechanisms involved.
Methods
Patients
The study design was approved by the Ethical Committee of the University of Parma (Parma, Italy). Nineteen MM patients were studied (Table 1). Thirteen of them were male, 6 female. Patient age ranged from 49 to 92 years. Ten patients had stage IIIa, 3 had stage IIIb, 2 had stage IIa, and 4 had stage Ia disease, according to the Durie and Salmon staging system.17 The monoclonal serum component was IgGκ in 9 cases, IgGλ in 5 cases, IgAκ in 2 cases, IgAλ in 2 cases, and λ in the remaining case. BM infiltration with malignant plasma cells at diagnosis ranged from 27% to 98%. At study, all patients were untreated.
Aliquots of BM aspirates performed for clinical evaluation were obtained after informed consent at diagnosis in 13 cases and at relapse in the remaining 6. BM aspirates from 4 healthy donors were obtained after their informed consent. All informed consent was obtained in accordance with the Declaration of Helsinki.
Generation of normal polyclonal plasmablastic cells
Normal polyclonal plasmablastic cells (PPCs) were generated in vitro from peripheral blood samples of 12 healthy volunteers obtained after informed consent. CD19+ B cells were positively selected using MACS micro-beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and subjected to 2 different procedures.18-20 In some experiments, CD19+ B cells were cultured in the presence of CD40L (1 μg/mL), IL-2 (20 U/mL), IL-4 (50 ng/mL), IL-10 (50 ng/mL), and CpG 2006 oligonucleotide21 (2.5 μg/mL). After 4-day culture, B cells were washed in phosphate-buffered saline (PBS) and cultured with IL-2, IL-10, and IL-6 (5 ng/mL). On day 6 of culture, PPCs were purified by depletion of CD20+ cells using immunomagnetic bead manipulation. Alternatively, PPCs were obtained after 6-day culture of CD19+ and CD3+ T cells (ratio, 0.5:1), isolated by immunomagnetic beads, in the presence of 1 μg/mL pokeweed mitogen (Sigma-Aldrich, St Louis, MO), as previously described.18,19 PPCs were negatively selected using CD3 and CD20 monoclonal antibodies and antimouse Ig beads. PPCs obtained using the 2 procedures were CD19+CD20−CD38++CD138+/−,20 as assessed by flow cytometry. Their purity ranged from 90% to 95% in the different experiments.
In some experiments, tonsil plasmablasts were sorted as CD19+, CD38bright, IgDnegative/low, stained with IL-12rb2 monoclonal antibody (mAb), and analyzed by flow cytometry.
BM aspirates from patients with MM and from healthy donors were depleted of erythrocytes by osmotic lysis, and neoplastic cells were purified to homogeneity by positive selection using CD138-coated magnetic beads (Miltenyi Biotec).
Cell culture, antibodies, reagents, and flow cytometry
LP-1, U266, Karpas 620, RPMI 8226, H-Sultan, OPM-2, JJN3, XG-1, XG-6, and NCI-H929 MM cell lines were cultured in RPMI 1640 medium with 10% FCS (Seromed-BiochromKG, Berlin, Germany). Human recombinant (hr) IL-12 was provided by Wyeth (Cambridge, MA). Human recombinant IL-2, IL-10, IL-4, and IL-6 were from R&D Systems (Abingdon, United Kingdom). Trimeric CD40L was from Axxora Life Sciences (San Diego, CA). Normal PPCs generated in vitro, CD138+ MM cells, NCI-H929, and XG-1 cells were cultured in the presence of 50 ng/mL IL-6 and tested for IL-12Rβ2 surface expression by flow cytometry. The source of anti-CD3 mAb was the supernatant of OKT3 murine hybridoma (ATCC, Manassas, VA). Fluorochrome-conjugated anti–human IL-12Rβ2, CD19, CD20, CD38, CD138, anti-IL6, anti-IFN-γ, anti-Ig, and anti–Ki-67 mAbs were from BD Biosciences PharMingen (San Diego, CA). An anti–human IL-12Rβ2 goat IgG that detects an intracellular epitope of IL-12Rβ2 (Santa Cruz Biotechnology, Santa Cruz, CA) was used in some experiments. This antibody was tested using BD Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences PharMingen). Isotype-matched mAb of irrelevant specificity or nonimmune goat IgG (Caltag Laboratories, Burlingame, CA) were used as controls. Cells were scored using a FACSCalibur analyzer (BD Bioscences, San Jose, CA) and data processed using CellQuest software (BD Biosciences).
Cell proliferation and apoptosis assays
The NCI-H929 MM cell line was cultured for 48 hours in either the presence or absence of 20 ng/mL hrIL-12. Cells were then stained intracellularly with anti–Ki-67 mAb and analyzed by flow cytometry. Apoptosis was assessed using the rhAnnexin V/FITC kit from Bender MedSystems (Burlingame, CA).
Real-time PCR
Quantitative analysis of IL-12RB2 transcript was performed as follows; 1 μg total RNA was reverse-transcribed using the ReactionReady First Strand cDNA Synthesis kit (Superarray Bioscience, Frederick, MD). cDNA was subjected to real-time polymerase chain reaction (PCR) using the RT2 Real-Time SYBR Green PCR master mix, human GAPDH, and human IL-12RB2 primer sets purchased from Superarray Bioscience (proprietary primers, sequence not disclosed). PCR was performed in triplicate using an ABI PRISM 7700 sequence detector instrument (Applied Biosystems, Foster City, CA) in a final volume of 25 μL. A standard 2-step amplification with 60°C annealing temperature was used, as suggested by datasheet. Relative quantification of IL-12RB2 transcript was obtained using comparative Ct method.22 The copy number in the unknown samples was normalized to an endogenous reference (GAPDH gene) and expressed relative to a calibrator sample (positive control) using the 2−(ΔΔCt±SD) method.
RT-PCR and methylation assay
RNA was extracted from freshly isolated MM cells and MM cell lines using RNeasy Mini Kit from Qiagen (Hilden, Germany) and subjected to reverse transcription (RT)–PCR.23 Expression of IL-12RB2 mRNA was investigated by RT-PCR using the conditions and the primers published elsewhere.23
DNA was extracted using GenElute DNA miniprep kit from Sigma-Aldrich, and the methylation status of the target sequence was assessed by methylation-specific PCR as previously described.3
Mouse studies
Four- to 6-week-old SCID-NOD mice (Harlan Laboratories, Udine, Italy) were housed under specific pathogen-free conditions. All procedures involving animals were performed in accordance with national and international current regulations (D.l.vo 27/01/1992, n.116, European Economic Community Council Directive 86/609, OJL 358, December 1, 1987).
Two groups of 16 animals each were injected intraperitoneally with 8 × 106 NCI-H929 cells. One group of mice was treated with 3 weekly doses of hrIL-12 (1 μg/mouse per dose) starting 8 hours after injection of tumor cells. The other group of mice was injected with PBS following the same time schedule. Twenty-three days after tumor cell inoculation, mice were killed and autopsies were carried out. Tumor masses were measured as described.3
Morphologic and immunohistochemical analyses
For histologic evaluation, tissue samples were fixed in 4% neutral buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. For immunohistochemistry, formalin-fixed, paraffin-embedded sections were immunostained with rabbit anti–human/mouse laminin Ig (Biogenex, San Ramon, CA) or mouse anti-human proliferating cell nuclear antigen (PCNA) mAb (clone PC10; DakoCytomation Denmark, Glostrup, Denmark). After washing, sections were overlaid with goat anti–mouse/rabbit Ig conjugated to peroxidase-labeled dextran (EnVision + Peroxidase, rabbit/mouse; DakoCytomation, Glostrup, Denmark) for 30 minutes. Unbound immunoglobulin was removed by washing and slides were incubated with avidin-biotin complex/alkaline phosphatase (DakoCytomation) for 30 minutes; then sections were counterstained with hematoxylin and eosin.
CAM assay
Fertilized White Leghorn chicken eggs (20/group) were incubated at 37°C at constant humidity. On day 3, a square window was opened in the shell, and 2 to 3 mL of albumen was removed to allow detachment of the developing chorioallantoic membrane (CAM). The window was sealed with a glass, and the eggs were returned to the incubator. On day 8, eggs were treated with 1-mm3 sterilized gelatin sponges (Gelfoam Upjohn, Kalamazoo, MI) placed on top of the growing CAM, as reported,24 and loaded with 1 μL PBS (negative control); 1 μL PBS with 250 ng VEGF (R&D Systems) as positive control; 1 μL of medium from NCI-H929, XG-1, U266 cell lines, or purified MM cells from patients cultured 48 hours with or without hrIL-12; or 1 μL of medium containing hrIL-12. The viability of cells from primary MM samples and MM cell lines was checked before supernatant harvest by cell count using trypan blue dye exclusion test. All supernatants were tested in triplicate, and mean values plus or minus SD were calculated. CAMs were examined daily until day 12 and photographed in ovo with a stereomicroscope equipped with a camera and image analyzer system (Olympus Italia, Milan, Italy). On day 12, the angiogenic response was evaluated by the image analyzer system as the number of vessels converging toward the sponges.
Angiogenesis PCR array
RNA was extracted from tumors removed from SCID-NOD mice 23 days after injection of NCI-H929 cells and treatment with hrIL-12 or PBS, using Trizol from Invitrogen (Carlsbad, CA) and retrotranscribed by the ReactionReady First Strand cDNA Synthesis kit (Superarray Bioscience). In some experiments, CD138+ myeloma cells purified from BM of MM patients were cultured for 48 hours with medium in the presence or absence of hrIL-12 and RNA was extracted using Trizol. Contaminant genomic DNA was removed by Dnase treatment using Rneasy Micro Kit (Qiagen), and IL-12RB2 expression was tested by PCR before starting PCR array procedure.
Human Angiogenesis RT2 Profiler PCR Array and RT2 Real-Timer SyBR Green/ROX PCR Mix were purchased from Superarray Bioscience. PCR was performed on ABI Prism 7700 Sequence Detector (Applied Biosystems).
For data analysis, the ΔΔCt method was used; for each gene, fold changes were calculated as difference in gene expression between tumors formed by NCI-H929 cells in hrIL-12- or PBS-treated animals or MM primary cells treated or not with IL-12 in vitro. According to the manufacturer's instructions, a significant threshold is defined as a 4-fold change in gene expression. To render the assay more stringent, we elevated the threshold to 5-fold change.
Statistical methods
Results were calculated with 99% confidence interval. Data were analyzed using Student t test for analysis of the results of the CAM assay or Mann-Whitney test for analysis of the results of the remaining experiments. A P value less than .05 was considered statistically significant.
Results
Expression and function of IL-12rb2 in normal PPCs, plasma cells, and primary MM cells
In the following experiments, we first investigated expression and function of IL-12Rβ2 in normal PPCs generated in vitro from normal peripheral blood or isolated from tonsil, as well as in normal plasma cells (PCs) purified from BM or tonsil. Both PPCs and PCs represent potential counterparts of MM cells.
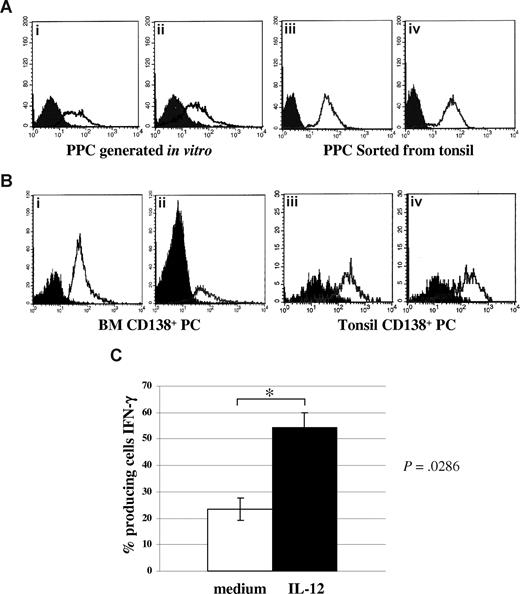
Figure 1Ai,ii shows 2 experiments, representative of the 12 performed with similar results, in which surface IL-12Rβ2 expression was consistently detected by flow cytometry using the mAb from BD Biosciences in PPCs generated in vitro from normal peripheral blood (mean percentage of IL-12Rβ2+ cells = 58.6%; range, 43%-75%). In 4 different experiments, IL-12Rβ2+ was found to be expressed also by PPCs sorted from tonsil (mean percentage of IL-12Rβ2+ cells = 97%; range, 95%-99%). Figure 1Aiii,iv shows 2 representative experiments.
Expression of IL-12Rβ2 in normal plasma cells. (A) IL-12Rβ2 surface expression in PPCs generated in vitro (i,ii) or sorted from tonsils (iii,iv), as assessed by flow cytometry using the BD mAb. Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched mAb staining. (B) IL-12Rβ2 surface expression in CD138+ PCs purified from normal BM (i,ii) or tonsil (iii,iv), as assessed by flow cytometry using the BD mAb. Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched mAb staining. (C) Up-regulation of IFN-γ production in PPCs generated in vitro on incubation with medium (□) or hrIL-12 (■) for 48 hours, as assessed by flow cytometry. Results represent median IFN-γ+ cells plus or minus SE from 4 different experiments.
Expression of IL-12Rβ2 in normal plasma cells. (A) IL-12Rβ2 surface expression in PPCs generated in vitro (i,ii) or sorted from tonsils (iii,iv), as assessed by flow cytometry using the BD mAb. Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched mAb staining. (B) IL-12Rβ2 surface expression in CD138+ PCs purified from normal BM (i,ii) or tonsil (iii,iv), as assessed by flow cytometry using the BD mAb. Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched mAb staining. (C) Up-regulation of IFN-γ production in PPCs generated in vitro on incubation with medium (□) or hrIL-12 (■) for 48 hours, as assessed by flow cytometry. Results represent median IFN-γ+ cells plus or minus SE from 4 different experiments.
Figure 1B shows that CD138+ PCs from normal BM (Figure 1Bi,ii) or tonsil (Figure 1Biii,iv) expressed IL-12Rβ2 on the cell surface using the mAb from BD Biosciences (BM CD138+ cells: mean percentage of IL-12Rβ2+ cells from 4 different experiments = 76.5%, range, 62%-84%; tonsil CD138+ cells: mean percentage of IL-12Rβ2+ cells from 4 different experiments = 80.3%; range, 71%-87%).
The ubiquitous IL-12Rβ1 chain was expressed in all PPC suspensions in vitro or in PPCs and PCs ex vivo (not shown).
To investigate whether IL-12R was functional in normal PPCs, 4 PPC suspensions generated in vitro were incubated with hrIL-12 or medium for 48 hours and subsequently tested for IFN-γ production by intracellular staining. As apparent from Figure 1C, IL-12 up-regulated significantly (P = .029) IFN-γ production, thus indicating that the IL-12R was functional.
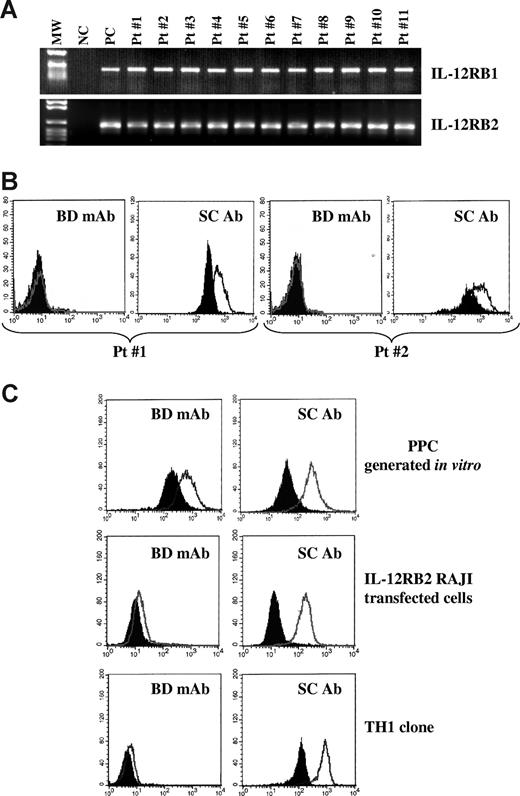
Next, we investigated the expression of the IL-12RB1 and B2 chains in primary neoplastic cells isolated from the BM of 19 MM patients. Primary MM cells from all patients expressed IL-12RB1 (top panel) and IL-12RB2 (bottom panel) mRNA, as assessed by RT-PCR (Figure 2A, 11 patients shown). Nonetheless, primary MM cells from the same 11 patients tested negative for IL-12Rβ2 surface expression, as assessed by flow cytometry using the mAb from BD Biosciences, indicating that IL-12Rβ2 was strongly down-regulated compared with normal PPCs or PCs. Figure 2B shows 2 representative profiles from patients 1 and 2 (BD mAb). However, the same MM cell suspensions still displayed evident expression of IL-12Rβ2 on staining with the anti IL-12Rβ2 antibody from Santa Cruz Biotechnology (Figure 2B). Figure 2C shows comparative staining of one representative normal PPC sample (top panels), of IL-12RB2–transfected RAJI Burkitt lymphoma cell line3 (middle panels), and of a normal T helper 1 clone (bottom panels) with the 2 anti–IL-12rb2 antibodies, clearly highlighting the superior performance of the Santa Cruz reagent.
Expression of IL-12Rβ2 in primary CD138+ MM cells. (A) IL-12RB1 and B2 expression in primary CD138+ MM cells, as assessed by RT-PCR. MW indicates molecular weight; NC, negative control (water in the place of cDNA); PC, positive control (total tonsil B cells); 11 MM cases (patients 1-11) are shown. (B) IL-12Rβ2 surface expression in primary CD138+ MM cells (patients 1 and 2), as assessed by flow cytometry using the mAb from BD Biosciences (BD mAb) or the Santa Cruz antibody (SC Ab). Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched Ab staining. (C) Flow cytometric analysis of IL-12Rβ2 expression in PPCs generated in vitro (top panels), IL-12RB2-transfected Raji Burkitt lymphoma cells (middle panels), and a normal T helper 1 clone using the BD Bioscences mAb (left panels) and the Santa Cruz antibody (right panels). Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched antibody staining.
Expression of IL-12Rβ2 in primary CD138+ MM cells. (A) IL-12RB1 and B2 expression in primary CD138+ MM cells, as assessed by RT-PCR. MW indicates molecular weight; NC, negative control (water in the place of cDNA); PC, positive control (total tonsil B cells); 11 MM cases (patients 1-11) are shown. (B) IL-12Rβ2 surface expression in primary CD138+ MM cells (patients 1 and 2), as assessed by flow cytometry using the mAb from BD Biosciences (BD mAb) or the Santa Cruz antibody (SC Ab). Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched Ab staining. (C) Flow cytometric analysis of IL-12Rβ2 expression in PPCs generated in vitro (top panels), IL-12RB2-transfected Raji Burkitt lymphoma cells (middle panels), and a normal T helper 1 clone using the BD Bioscences mAb (left panels) and the Santa Cruz antibody (right panels). Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched antibody staining.
By comparing the results of PPC (Figure 2C top panels) and primary MM cell (Figure 2B) staining with the Santa Cruz antibody, down-regulation of IL-12Rβ2 protein in tumor cells versus PPC is confirmed (mean percentage of IL-12Rβ2+ PPC from 3 different experiments = 92%; range, 89%-95%; mean percentage of IL-12Rβ2+ primary MM cells from 3 different experiments = 61.5%; range, 55%-68%).
Quantitative PCR performed with cDNA from 4 normal PPC preparations and 10 CD138+ MM cell suspensions revealed that IL-12RB2 mRNA was expressed at similar levels in all samples tested (data not shown), thus suggesting that down-regulation of IL-12Rβ2 protein on the cell surface of MM cells occurred through posttranscriptional mechanisms.
To gain more insight into this issue, we hypothesized that IL-12Rβ2 chain down-regulation on primary MM cells, compared with normal PPCs or PCs, was related to IL-6 overproduced in the BM microenvironment. IL-6 is a major paracrine myeloma growth factor both in vitro and in vivo, and high serum IL-6 levels were detected in MM patients with active disease.1 Thus, we cultured 4 different CD138+ MM cell suspensions for 48 hours in the presence or absence of hrIL-6 and subsequently tested them for IL-12Rβ2 expression by flow cytometry using the Santa Cruz antibody. Figure 3A shows that the IL-12Rβ2 chain was expressed on CD138+ MM cells that had been cultured for 48 hours with medium alone and down-regulated significantly (P = .002) on 48-hour incubation with hrIL-6. The same MM cell suspensions cultured with or without IL-6 displayed similar levels of IL-12RB2 mRNA, indicating that protein down-regulation induced by the latter cytokine occurred through posttranscriptional mechanisms (Figure 3B).
Down-regulation of IL-12Rβ2 by IL-6. (A) Down-regulation of IL-12Rβ2 expression in primary MM cells on incubation with medium (□) or IL-6 (■) for 48 hours, as assessed by flow cytometry using the Santa Cruz antibody. Results represent median IL-12Rβ2+ cells plus or minus SE from 4 different experiments. (B) Quantitative analysis of IL-12RB2 versus GAPDH transcript in 2 MM cell suspensions (patients 1 and 2) cultured with (□) or without (■) IL-6. (C) IL-12Rβ2 surface expression in normal PPCs before (open profile) and after (dashed line) treatment for 48 hours with hrIL-6, as assessed by flow cytometry using the BD Bioscience mAb. Dark profile indicates staining with isotype-matched mAb. Two different experiments of the 4 performed with superimposable results are shown.
Down-regulation of IL-12Rβ2 by IL-6. (A) Down-regulation of IL-12Rβ2 expression in primary MM cells on incubation with medium (□) or IL-6 (■) for 48 hours, as assessed by flow cytometry using the Santa Cruz antibody. Results represent median IL-12Rβ2+ cells plus or minus SE from 4 different experiments. (B) Quantitative analysis of IL-12RB2 versus GAPDH transcript in 2 MM cell suspensions (patients 1 and 2) cultured with (□) or without (■) IL-6. (C) IL-12Rβ2 surface expression in normal PPCs before (open profile) and after (dashed line) treatment for 48 hours with hrIL-6, as assessed by flow cytometry using the BD Bioscience mAb. Dark profile indicates staining with isotype-matched mAb. Two different experiments of the 4 performed with superimposable results are shown.
Figure 3C shows 2 representative experiments of the 4 performed in which IL-6 48-hour treatment strongly down-regulated surface expression of IL-12rb2 also in PPCs (P = .019).
hrIL-12 inhibits the proangiogenic activity of primary MM cells
MM cells are known to release several proangiogenic factors.25 We then asked whether this feature was affected by IL-12, which we have found to exert direct antitumor activity through inhibition of angiogenesis in other tumor models.3,7 We therefore incubated CD138+ neoplastic cells from 4 MM patients (patients 1, 5, 14, and 18) with hrIL-12 or medium alone and tested the angiogenic activity of culture supernatants in the CAM assay. We also checked the viability of primary tumor cells incubated with or without IL-12 before harvesting supernatants by trypan blue staining. There were no statistically significant differences in the proportions of viable cells between cultures performed in the presence or absence of IL-12.
CAM treated with sponges loaded with VEGF (positive control) or with supernatants from primary MM cells were surrounded by allantoic vessels developing radially toward the implant in a “spoked wheel” pattern. In the representative experiment shown in Figure 4A left panel, the mean number of vessels formed in the presence of supernatant from CD138+ MM cells (patient 1) was 30 plus or minus 3, whereas that formed in the presence of VEGF was 30 plus or minus 5 (not shown). No vascular reaction was detected around the sponges on exposure to hrIL-12 diluted in medium at the same final concentration used to treat tumor cells (mean number of vessels = 7 ± 3 in the presence or absence of hrIL-12, not shown). When the supernatants from hrIL-12–treated CD138+ cells from the same MM patient was tested in the CAM assay, a significant (P < .001) reduction of the angiogenic response was appreciable (mean number of vessels = 12 ± 2; Figure 4A right panel), compared with positive control. Similar results with the same statistical significance were obtained when supernatants from the 3 remaining CD138+ primary MM cell suspensions were tested (patient 5, mean number of vessels formed in the presence of untreated supernatant 32 ± 3, of IL-12–treated supernatant 14 ± 3; patient 14, mean number of vessels formed in the presence of untreated supernatant 35 ± 4, of IL-12–treated supernatant 16 ± 3; patient 18, mean number of vessels formed in the presence of untreated supernatant 28 ± 2, of IL-12–treated supernatant 12 ± 2). Taken together, these findings demonstrated that IL-12R was functional in primary MM cells and that IL-12 treatment damped their proangiogenic activity.
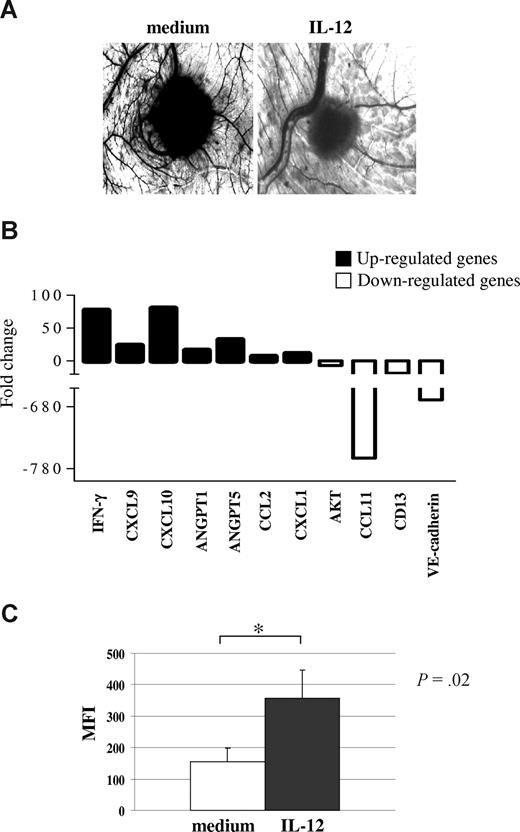
IL-12 activity on primary MM cells. (A) Angiogenic activity of supernatants from one representative CD138+ MM sample cultured in the presence or absence of hrIL-12. CAMs treated with sponges loaded with the conditioned medium from the untreated cells were surrounded by allantoic vessels developing radially toward the implant in a “spoked-wheel” pattern (left panel). When medium from the same MM sample cultured with hrIL-12 was tested, a significant reduction of the angiogenic response was evident (right panel) (original magnification ×50). (B) Results from human angiogenesis PCR array performed in one representative CD138+ MM sample cultured in the presence or absence of hrIL-12 are shown. (C) Purified CD138+ primary MM cells incubated with IL-12 for 48 hours significantly up-regulated expression of the IFN-γ protein. Results are median mean fluorescence intensity (MFI) values plus or minus SE.
IL-12 activity on primary MM cells. (A) Angiogenic activity of supernatants from one representative CD138+ MM sample cultured in the presence or absence of hrIL-12. CAMs treated with sponges loaded with the conditioned medium from the untreated cells were surrounded by allantoic vessels developing radially toward the implant in a “spoked-wheel” pattern (left panel). When medium from the same MM sample cultured with hrIL-12 was tested, a significant reduction of the angiogenic response was evident (right panel) (original magnification ×50). (B) Results from human angiogenesis PCR array performed in one representative CD138+ MM sample cultured in the presence or absence of hrIL-12 are shown. (C) Purified CD138+ primary MM cells incubated with IL-12 for 48 hours significantly up-regulated expression of the IFN-γ protein. Results are median mean fluorescence intensity (MFI) values plus or minus SE.
Next, we investigated expression of proangiogenic and antiangiogenic genes in primary MM cells incubated with IL-12 or medium. Purified CD138+ cells from 3 different MM patients (patients 4, 13, and 19) were cultured for 48 hours in the presence or absence of hrIL-12. RNA was extracted from cultured cells, reverse-transcribed, and tested by PCR array.
Figure 4B shows the pooled results from the 3 samples analyzed. IL-12 treatment down-regulated significantly mRNA of the proangiogenic factors CCL11 (P = .02), VE-cadherin (P = .013), AKT (P = .021), and CD13 (P = .05) but up-regulated mRNA of the angiogenesis inhibitors IFN-γ (P = .012), CXCL9 (P = .02), and CXCL10 (P = .015). Notably, also the transcripts of the proangiogenic CCL2 (P = .013), angiopoietin (ANGPT)–1 (P = .032), and ANGPT-5 (P = .029) genes were up-regulated in these cells (Figure 4B).
Because PCR array studies pointed to a major role of an IFN-γ driven pathway in angiogenesis inhibition, we investigated by flow cytometry whether purified CD138+ primary MM cells incubated with IL-12 up-regulated expression of the IFN-γ protein. Indeed, as shown in Figure 4C, constitutive production of IFN-γ by primary MM cells was significantly increased after culture with IL-12.
Expression and function of IL-12rb2 in MM cell lines
Figure 5A (top panel) shows that LP1, U266, JJN3, Karpas 620, RPMI 8226, H-Sultan, and OPM-2 MM cell lines did not express the IL-12Rβ2 chain, as assessed by RT-PCR. Lack of expression of IL-12RB2 gene in these cells was found to depend on methylation of the CpG island within exon 1 (Figure 5A bottom panel), as previously described for other human B-cell malignancies.3 In contrast, XG-1, XG-6, and NCI-H929 MM cell lines expressed IL-12RB2 mRNA (Figure 5A top panel) as well as the corresponding protein (Figure 5B and data not shown for XG-6 cell line), as assessed by staining with the Santa Cruz antibody.
Expression and function of IL-12R in MM cells. (A) Top panel: IL-12RB2 expression in MM cell lines as assessed by RT-PCR. MW indicates molecular weight; NC, negative control (water in place of cDNA); PC, positive control (total tonsil B cells); different MM cell lines (LP-1, U266, JJN3, Karpas 620, RPMI 8226, H-Sultan, OPM-2, XG-1, XG-6, and HCI-H929) are shown. Bottom panel: Methylation specific PCR analysis of MM cell lines. MM cell lines that do not express the IL-12RB2 mRNA (LP-1, U266, Karpas 620, RPMI8226, H-Sultan, and OPM-2) show the amplification band corresponding to the methylated target sequence, whereas MM cell lines that express IL-12RB2 mRNA (XG-1, XG-6, and NCI-H929) failed to amplify the methylated sequence. (B) IL-12RB2 expression in NCI-H929 and XG-1 MM cell lines, as assessed by flow cytometry using the anti IL-12Rβ2 antibody from Santa Cruz. Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched antibody staining. (C) Left panel: IL-12Rβ2 protein expression in NCI-H929 cells cultured with medium alone or with hrIL-6 for 48 hours, as assessed by flow cytometry using the Santa Cruz antibody. Right panel: Quantitative analysis of IL-12RB2 versus GAPDH transcript in the same NCI-H929 cell suspensions analyzed in the left panel, cultured with (□) or without (■) IL-6. (D) Angiogenic activity of supernatants from NCI-H929 (top panels), XG-1 (middle panels), and U266 (bottom panels) cells cultured in the presence or absence of hrIL-12, as assessed by CAM assay.
Expression and function of IL-12R in MM cells. (A) Top panel: IL-12RB2 expression in MM cell lines as assessed by RT-PCR. MW indicates molecular weight; NC, negative control (water in place of cDNA); PC, positive control (total tonsil B cells); different MM cell lines (LP-1, U266, JJN3, Karpas 620, RPMI 8226, H-Sultan, OPM-2, XG-1, XG-6, and HCI-H929) are shown. Bottom panel: Methylation specific PCR analysis of MM cell lines. MM cell lines that do not express the IL-12RB2 mRNA (LP-1, U266, Karpas 620, RPMI8226, H-Sultan, and OPM-2) show the amplification band corresponding to the methylated target sequence, whereas MM cell lines that express IL-12RB2 mRNA (XG-1, XG-6, and NCI-H929) failed to amplify the methylated sequence. (B) IL-12RB2 expression in NCI-H929 and XG-1 MM cell lines, as assessed by flow cytometry using the anti IL-12Rβ2 antibody from Santa Cruz. Open profile represents IL-12Rβ2 staining; dark profile, isotype-matched antibody staining. (C) Left panel: IL-12Rβ2 protein expression in NCI-H929 cells cultured with medium alone or with hrIL-6 for 48 hours, as assessed by flow cytometry using the Santa Cruz antibody. Right panel: Quantitative analysis of IL-12RB2 versus GAPDH transcript in the same NCI-H929 cell suspensions analyzed in the left panel, cultured with (□) or without (■) IL-6. (D) Angiogenic activity of supernatants from NCI-H929 (top panels), XG-1 (middle panels), and U266 (bottom panels) cells cultured in the presence or absence of hrIL-12, as assessed by CAM assay.
Figure 5C left panel shows that, similar to primary MM cells, incubation of the IL-6–independent NCI-H929 MM cell line with IL-6 down-regulated IL-12Rβ2 protein expression. Figure 5C right panel demonstrates that such down-regulation was not attributable to reduced gene expression because similar levels of IL-12RB2 mRNA were detected in cells that had been cultured with or without IL-6.
NCI-H929 cells were next cultured in the presence or absence of hrIL-12 for 48 hours and subsequently tested for proliferation or apoptosis by Ki-67 mAb or propidium iodide/annexin V staining, respectively. In 3 different experiments, IL-12 inhibited the proliferation and induced apoptosis of NCI-H929 cells by 5% to 10%. Likewise, IL-12 had minimal effects on apoptosis of 2 primary MM cell suspensions in 48-hour cultures (not shown).
The angiogenic activity of IL-12Rβ2+ NCI-H929 and XG-1 cells, and of IL-12Rβ2− U266 cells was subsequently investigated in the CAM assay using supernatants from cells cultured in the presence or absence of hrIL-12. Again, the proportions of viable cells did not differ significantly in cultures performed with or without IL-12 for all the cell lines tested.
In these experiments, the XG-1 cell line was cultured without IL-6 for 48 hours before treatment with hrIL-12.
In the representative experiment shown in Figure 5D top left panel, the mean number of vessels formed in the presence of supernatant from the NCI-H929 cells was 28 plus or minus 4. When the supernatant from the NCI-H929 cell line incubated with hrIL-12 was tested, a significant (P < .001) reduction of the angiogenic response was appreciable (mean number of vessels = 14 ± 5), compared with positive control (VEGF, mean number of vessels 31 ± 5). The mean number of vessels formed in the presence of supernatants from XG-1 or U266 cells was 27 plus or minus 4 and 28 plus or minus 3, respectively (Figure 5D left, middle, and bottom panels, respectively). When supernatant from the XG-1 cells incubated with hrIL-12 was tested, a significant inhibition of their angiogenic potential was observed (mean number of vessel 13 ± 4, P = .001, Figure 5D middle right panel). In contrast, supernatant from U266 cell line tested as negative control did not shown any significant reduction in vessel formation irrespective of incubation with hrIL-12 (mean number of vessels 25 ± 2, Figure 5D bottom right panel). Taken together, these results demonstrated unambiguously that the antiangiogenic activity of IL-12 on MM cell lines was absolutely dependent on IL-12R.
hrIL-12 strongly inhibits tumorigenicity of NCI-H929 cells in SCID-NOD mice
In subsequent experiments, the tumorigenicity of NCI-H929 cells was investigated in SCID-NOD mice. Two groups of 16 animals each were injected intraperitoneally with 8 × 106 cells, treated with hrIL-12 or PBS intraperitoneally, and killed after 23 days. By the end of the follow-up period, all mice developed tumors that grew in the peritoneal cavity in the absence of metastases at distant sites.
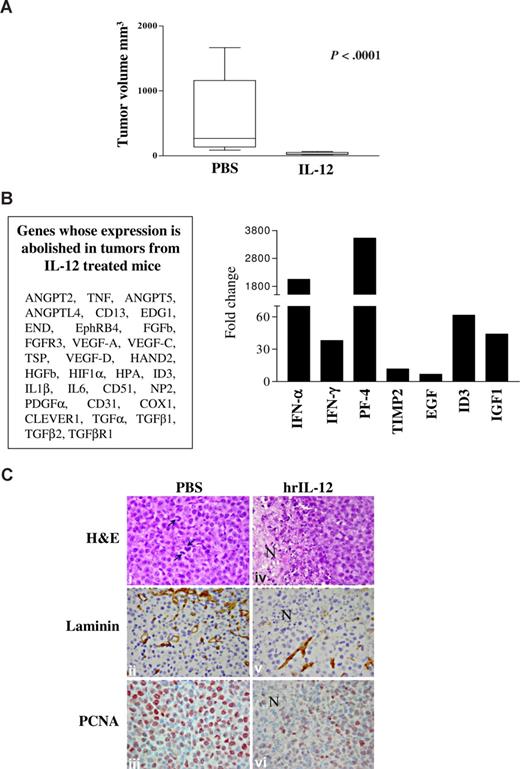
Mice injected with NCI-H929 cells and treated with hrIL-12 developed tumors significantly smaller (P < .001) than mice inoculated with the same cells and treated with PBS (n = 10 for both groups; IL-12–treated, median volume, 24 mm3; range, 18.5-65 mm3; PBS-treated, median volume, 268 mm3; range, 88-1668 mm3; Figure 6A).
IL-12 antitumor activity in vivo. (A) Volume of tumors grown intraperitoneally in PBS- and IL-12–treated animals 23 days after NCI-H929 cell inoculation. The differences in size between tumors removed from PBS- and IL-12–treated mice were evaluated by Mann-Whitney U test. Boxes represent values between the 25th and 75th percentiles; whisker lines, highest and lowest values for each group; horizontal lines, median values. (B) Human angiogenesis PCR array on tumors explanted from IL-12– versus PBS-treated animals 23 days after NCI-H929 cell inoculation. Left panel enlists the gene whose expression has been abolished in tumors from IL-12– versus PBC-treated mice. Histogram in right panel shows fold expression changes of genes up-regulated in tumors from IL-12– versus PBC-treated mice. (C) Histologic and immunohistochemical features of tumors developed in PBS-treated (i-iii) and hrIL-12–treated (iv-vi) SCID/NOD mice 23 days after NCI-H929 tumor cell injection. NCI-H929 tumors are mostly formed by undifferentiated, proliferating (mitotic features indicated by arrows) blast cells that are large and pleomorphic and sometimes binucleated or endowed with very prominent nucleoli (i). These tumors are supplied by a distinct network of mature microvessels, as assessed by laminin staining (ii), and show frequent PCNA expression (iii). In hrIL-12–treated mice, these morphologic features are frequently altered by the appearance of ischemic-hemorrhagic foci of necrosis (N; iv) associated with defective microvascularization (v) and decreased tumor cell proliferation (vi). Histologic and immunohistochemical analyses were performed under a Zeiss LSM 510 Meta laser scanning confocal microscope (Zeiss, Oberkochen, Germany). ×400 field (×40 objective and ×10 ocular lens; 0.180 mm2 per field).
IL-12 antitumor activity in vivo. (A) Volume of tumors grown intraperitoneally in PBS- and IL-12–treated animals 23 days after NCI-H929 cell inoculation. The differences in size between tumors removed from PBS- and IL-12–treated mice were evaluated by Mann-Whitney U test. Boxes represent values between the 25th and 75th percentiles; whisker lines, highest and lowest values for each group; horizontal lines, median values. (B) Human angiogenesis PCR array on tumors explanted from IL-12– versus PBS-treated animals 23 days after NCI-H929 cell inoculation. Left panel enlists the gene whose expression has been abolished in tumors from IL-12– versus PBC-treated mice. Histogram in right panel shows fold expression changes of genes up-regulated in tumors from IL-12– versus PBC-treated mice. (C) Histologic and immunohistochemical features of tumors developed in PBS-treated (i-iii) and hrIL-12–treated (iv-vi) SCID/NOD mice 23 days after NCI-H929 tumor cell injection. NCI-H929 tumors are mostly formed by undifferentiated, proliferating (mitotic features indicated by arrows) blast cells that are large and pleomorphic and sometimes binucleated or endowed with very prominent nucleoli (i). These tumors are supplied by a distinct network of mature microvessels, as assessed by laminin staining (ii), and show frequent PCNA expression (iii). In hrIL-12–treated mice, these morphologic features are frequently altered by the appearance of ischemic-hemorrhagic foci of necrosis (N; iv) associated with defective microvascularization (v) and decreased tumor cell proliferation (vi). Histologic and immunohistochemical analyses were performed under a Zeiss LSM 510 Meta laser scanning confocal microscope (Zeiss, Oberkochen, Germany). ×400 field (×40 objective and ×10 ocular lens; 0.180 mm2 per field).
The angiogenic phenotype of tumors formed in IL-12– versus PBS-treated animals was next investigated using PCR array. In 2 different experiments performed with superimposable results, expression of the following angiogenesis activators was virtually abolished by IL-12 (Figure 6B left panel): ANGPT-2, ANGPT-5, angiopoietin-like (ANGPTL)–4, CD13, endothelial differentiation gene (EDG)1, endoglin (END), ephrin (Eph) receptor B4, FGFb, FGF receptor 3 (ACH), VEGF-A, -C, and -D, heart and neural crest derivates expressed (HAND)2, HGFb, hypoxia inducible factor (HIF)1α, inhibitor of DNA binding (ID)3, IL-6, IL-1b, CD51, neuropilin (NP)2, PDGFα, CD31, COX1, stabilin (Clever)1, TGFα, TGFb1, TGFbR2, thrombospondin (TSP)1, and TNF. All these molecules are expressed during tumor neovascularization and promote organization, survival, or migration of endothelial cells.26-51 Conversely, IFN-α and IFN-γ, platelet factor (PF)–4, and tissue inhibitor of metalloproteinase (TIMP)-2, which are inhibitors of angiogenesis,10,11,52-55 were found to be up-regulated in tumors grown in IL-12– versus PBS-treated animals (Figure 6B right panel). Finally, expression of the proangiogenic ID3, IGF-1, and EGF was up-regulated (Figure 6B right panel)
We next investigated the histologic and immunohistochemical features of tumors formed by the NCI-H929 MM cell line in IL-12– versus PBS-treated mice (Figure 6C). Tumors from IL-12–treated animals displayed a wide focus of ischemic-coagulative necrosis (Figure 6Civ) compared with control tumors (Figure 6Ci). In the former tumors, microvessel density, as assessed by laminin staining, and proliferation index, as assessed by anti-PCNA staining (Figure 6Cv and 6Cvi, respectively), were strongly reduced in comparison to control tumors (Figure 6Cii and 6Ciii, respectively).
Discussion
Pathogenesis of MM is complex and dependent on the interactions between tumor cells and their microenvironment in the BM, the primary site of MM development.1,15,56 Different cytokines, chemokines, and proangiogenic factors released in the tumor microenvironment are known to promote MM cell growth and metastatic dissemination, especially to the skeleton.15,25,57,58 The most prominent of these molecules is IL-6, which is produced by stromal cells from tumor-infiltrated BM and supports survival and proliferation of MM cells.1,15 Other molecules that exert similar effects are VEGF and the chemokine CCL2.59,60
No information was so far available on the involvement of the IL-12/IL-12R system in MM pathogenesis, with the exception of the reported increase of serum IL-12 levels in a cohort of MM patients.61
We addressed the role of the IL-12/IL-12R system in MM based on our previous finding that aged IL-12rb2 mice, which produce but cannot use IL-12, develop spontaneously localized monoclonal plasmacytomas in the setting of a systemic autoimmune lymproliferative disorder characterized by IL-6 overproduction.13 Other studies from our group lend cogent support to the notion that IL-12 acts a negative regulator of the growth of both hematopoietic and nonhematopoietic tumors.3,7,12,13
In this study, we demonstrate that IL-12rb2 is expressed on the surface of normal PPCs and PCs but down-regulated in primary MM cells. Because quantitative PCR experiments disclosed similar levels of IL-12RB2 mRNA in normal PPC and primary MM cells, down-regulation of IL-12Rβ2 protein expression in the latter cells must depend on posttranscriptional events. Consistent with these findings, the CpG island in exon 1 of the IL-12RB2 gene3 was never found to be methylated in primary MM cells (not shown), at variance with that reported from our group in a large number of chronic B-cell malignancies, including B-cell chronic lymphocytic leukemia, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma,3 as well as in B-cell acute lymphoblastic leukemia.12
Many human MM cell lines did not express IL-12RB2 either at the protein or the mRNA level, and most of them displayed methylation of the CpG island in exon 1 of the gene. Differences in IL-12RB2 gene expression between primary MM cells and the MM cell lines here investigated may be related to the following: (1) these cell lines, like most MM cell lines, were derived from extramedullary sites where more aggressive tumors develop in comparison with MM confined to BM; and/or (2) long-term in vitro culture of MM cell lines may have caused epigenetic changes, including IL-12RB2 gene methylation.
Nonetheless, 3 MM cell lines (XG-1, XG-6, and NCI-H929) were found to express IL-12RB2 mRNA and protein. We focused on the NCI-H929 cell line because it grows independently of exogenous growth factors, whereas the 2 remaining cell lines are IL-6–dependent. NCI-H929 cells showed various analogies with primary MM cells that made them attractive candidates for in vivo studies, namely (1) expression of similar patterns of IL-12rb2 mRNA and protein and (2) comparable inhibition of the angiogenic potential of tumor cells in the CAM assay induced by IL-12.
We next investigated the in vivo effects of human IL-12 on tumorigenicity of the NCI-H929 cell line in SCID/NOD mice. This model allows assessment of the direct effects of human IL-12, which is species-specific and inactive in the mouse, on human tumor cells injected in severely immunodeficient animals. We injected NCI-H929 cells intraperitoneally to generate a tumor mass suitable for the investigation of IL-12–mediated antiangiogenic effects. Although this model does not closely mimic human disease that develops in the BM, the results obtained provide useful translational information.
These experiments demonstrated that MM growth was virtually abrogated by IL-12 treatment, primarily through impaired formation of laminin-lined mature blood vessels. The reduced MM cell proliferation detected in tumors was consequent to angiogenesis inhibition because IL-12 had minimal effects on the in vitro proliferation of NCI-H929 cells. This latter finding may depend on the intrinsic properties of NCI-H929 cells rather than on those of IL-12 because the cytokine was previously found to inhibit the in vitro proliferation of IL-12Rβ2 expressing RAJI B lymphoma cells.
Ex vivo PCR array analysis of tumor masses showed that IL-12 damped the expression of a wide set of proangiogenic genes, including VEGF-A, -C, and -D, FGFb, ANGPT-2 and -5, COX-1, PDGFα, and HIF-1α. Concomitant up-regulation of a limited number of antiangiogenic genes, including IFN-γ, IFN-α, PF-4, and TIMP-2, was also detected.
In addition, primary MM cells exposed to IL-12 in vitro showed down-regulated expression of proangiogenic genes and up-regulated expression of antiangiogenic genes. The former included AKT, a key component of the phosphatidylinositol 3 phosphate kinase pathway that is now being targeted for therapeutic purposes,62 VE-cadherin, an endothelial cell specific adhesion molecule whose soluble form correlated with tumor burden,63 and CCL11, a chemokine binding to CCR3 expressed by MM cells and driving their chemotaxis.64 IL-12 up-regulated antiangiogenic factors included IFN-γ and the IFN-γ inducible chemokines CXCL9 and CXCL10, pointing to the involvement of this pathway in angiogenesis inhibition. Expression of 4 proangiogenic genes, ie, ANGPT1, ANGPT5, CXCL1, and CCL2, was also increased by IL-12, but the functional significance of this finding remains to be established.
Differences in IL-12–induced expression profiles of angiogenesis-related genes between NCI-H929 cell tumors and primary MM cells may be related to intrinsic differences between the 2 cell types and/or to the different microenvironments to which they have been exposed in vivo.
Finally, we attempted to identify potential mechanisms involved in IL-12Rβ2 down-regulation on MM cells compared with normal PPCs and PCs. We pointed to IL-6 because it is overproduced in MM microenvironment and strongly up-regulated in IL-12rb2 knockout mice. Indeed, incubation of primary MM cells and the NCI-H929 cell line, as well as of normal PPCs, with IL-6 down-regulated significantly surface expression of IL-12Rβ2. These results suggest IL-6 as a major regulator of IL-12Rβ2 expression on MM cells in vivo.
The present findings have translational relevance because the prognosis of MM patients remains grim despite recent therapeutic improvements. IL-12 already has been tested as an investigational drug in patients with different malignancies,65-67 and its safety and pharmacokinetics profiles are well known. Thus, a clinical trial in MM patients appears to be feasible. IL-12 may be targeted directly to tumor cells and/or administered systemically to take advantage also of the well-known ability of this cytokine to activate antitumor cytotoxic T-lymphocyte and natural killer cell–mediated responses.5
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Chiara Bernardini for excellent secretarial assistance.
This work was supported by grants from AIRC, Milano, Italy (1429 to V.P. and 4014 to I.A.), Ministero della Salute, Ricerca Finalizzata 2006 (to V.P.), Fondazione CARIGE Genova (to V.P.), Fondazione Querci (to V.P.), and Fondazione Cassa di Risparmio della Provincia di Chieti (CariChieti), Italy (to E.D.C.). C.C. is the recipient of a fellowship from FIRC, Milano, Italy.
Authorship
Contribution: I.A. designed research, performed research, collected data, analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; C.C. performed research, collected data, performed statistical analysis, and analyzed and interpreted data; N.G. and S.C. performed research and provided reagents; M.F., V. Perfetti, and V.R. provided reagents; E.O., G.T., and G.C. performed research; E.DiC. and D.R. performed research and analyzed and interpreted data; and V. Pistoia designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irma Airoldi, Department of Experimental and Laboratory Medicine, G. Gaslini Institute, 16148 Genova, Italy; e-mail: irmaairoldi@ospedale-gaslini.ge.it.
References
Author notes
*I.A. and C.C. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal