Abstract
B-cell chronic lymphocytic leukemia (B-CLL) is a lymphoproliferative disorder characterized by the surface expression of CD20, CD5 antigens, as well as the receptor CD40. Activation of CD40 by its ligand (CD40L) induces proliferation and rescues the cells from spontaneous and chemotherapy-induced apoptosis. CD40 activation also induces secretion of cytokines, such as IL-6, IL-10, TNF-α, IL-8, and GM-CSF, which are involved in tumor cell survival, migration, and interaction with cells in the tumor microenvironment. Here we demonstrate that in primary B-CLL tumor cells, the novel antagonist anti-CD40 monoclonal antibody, HCD122, inhibits CD40L-induced activation of signaling pathways, proliferation and survival, and secretion of cytokines. Furthermore, HCD122 is also a potent mediator of antibody-dependent cellular cytotoxicity (ADCC), lysing B-CLL cells more efficiently than rituximab in vitro, despite a significantly higher number of cell surface CD20 binding sites compared with CD40. Unlike rituximab, however, HCD122 (formerly CHIR-12.12) does not internalize upon binding to the cells. Our data suggest that HCD122 may inhibit B-CLL growth by blocking CD40 signaling and by ADCC-mediated cell lysis.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the United States and is characterized by progressive accumulation of malignant B cells in the blood, bone marrow, and lymphoid organs. CLL has been considered a prime example of a malignancy involving defects in the regulation of cell death; the slow accumulation of B-cell CLL (B-CLL) cells is presumably the result of a low proliferative index coupled with an intrinsic defect in apoptosis. However, recent clinical data from B-CLL patients given deuterated water have shown that there is a considerably higher turnover of CLL cells than previously recognized,1 and that rates of B-CLL cell proliferation, as well as cell death, can vary widely among the lymphatic and extralymphatic compartments. Observations from other studies suggest that there are 2 types of malignant cells: quiescent and apoptosis-resistant cells in the blood, and actively dividing cells found in lymphatic aggregates in the lymph nodes and bone marrow.2 B-CLL cells in peripheral blood are arrested in the G0/G1 phase of the cell cycle3 and express high levels of the cell-cycle inhibitor p27.4 In contrast, Survivin- and Ki67-positive B-CLL cells,5 and those with low expression of p27,6 have been identified in proliferation centers of the lymph nodes and bone marrow. Thus, the goal of developing therapeutics to treat and cure CLL is to disrupt the pathologic conditions that promote malignant cell growth while accelerating tumor cell death and clearance.
Until recently, treatment of progressive CLL, with steroids and alkylating agents, was largely palliative, with no impact on the natural history of the disease.7 Introduction of purine analogs as single-agent therapies (fludarabine [F])8 and in combination with alkylators (fludarabine/cyclophosphamide [FC])9 has improved clinical responses and complete remission rates. Purine analogues have a high specificity for lymphoid cells and can induce death in both proliferating and resting cells. As a result, these agents are as effective as single agents for treating bulky CLL disease, and substantially reduce tumor burden with little extramedullary toxicity. Severe myelosuppression and immunosuppression are, however, associated with this class of drugs, and despite improvements in clinical responses, an increase in median survival time has not been demonstrated. Addition of the monoclonal antibody rituximab (anti-CD20, Rituxan; Genentech [South San Francisco, CA] and IDEC Pharmaceuticals [San Diego, CA]) to FC regimens has resulted in significantly higher overall response rates (ORRs), complete responses (CRs), molecular remissions, and importantly, longer median overall survival.10-12 Grades 3 to 4 myelosuppression, infection, and viral reactivation remain major morbidities.
Monoclonal antibodies have demonstrated activity in CLL as single agents, fueling interest in targeted therapeutic agents that engage the immune effector system to kill tumor cells. Alemtuzumab (CamPath; Millennium and ILEX Partners, Cambridge, MA) is effective when patients with less bulky disease13 are treated.13-15 However, the target of alemtuzumab, CD52, is present on T cells as well as malignant B cells, and immunosuppression is severe; reactivation of cytomegalovirus (CMV) occurs and requires treatment.16 Since rituximab depletes only B cells, it has a more favorable safety profile. However, when used to treat CLL, rituximab is a weak single agent compared with the single-agent efficacy observed in follicular lymphoma.17-19 Escalating the dose of rituximab20 or intensifying the dosing regimen21 raises response rates, although complete remissions are rare. Both pharmacokinetic18,22 and pharmacodynamic23-27 hypotheses have been proposed to explain the lack of response of patients with CLL to rituximab. It has also been proposed that CLL cells are resistant to apoptosis-inducing processes in general,28 and this may impact the ability of immune effectors to induce apoptosis via antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). New monoclonal antibody therapeutics that target cell surface receptors and signaling pathways that support disease progression, and efficiently mediate immune-effector killing and clearance of the B-CLL cells without increasing morbidity, would be desirable in the effort to increase remission rates and extend survival.
CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily, is an attractive target for therapeutic intervention in CLL. Cell surface expression of CD40 has been detected on most B-cell malignancies.29 CD40 expression has been found on 90% to 100% of CLL cells,30 suggesting that the receptor could serve as a binding substrate for ADCC-mediating monoclonal antibodies. In vitro, activation of CD40 by its natural ligand, CD40L, or by activating monoclonal antibodies, has been shown to support the survival and expansion of B-CLL cells,31,32 and to increase the resistance of B-CLL cells to fludarabine-induced apoptosis.31-35 There is histopathologic evidence that proliferating CLL cells may be exposed to CD40L-mediated survival and proliferation signals in vivo. In patient bone marrow and lymph nodes, T lymphocytes have been found to be interspersed with CLL cells in proliferative centers,2,36 and CD40L+ T cells have been detected in these “pseudofollicles” in lymph nodes.37 Therefore, disruption of this signaling pathway may provide clinical benefit for CLL patients.
We have generated a fully human anti-CD40 antagonist monoclonal antibody, HCD122 (formerly CHIR-12.12), which blocks CD40/CD40L-mediated signaling. In addition, the antibody efficiently mediates ADCC and clearance of tumor cells. The present study evaluates the in vitro antitumor efficacy of this antibody in primary CLL cells.
Methods
Patient samples
Peripheral blood mononuclear cells (PBMCs) from patients with CLL were obtained from 2 sources. Frozen PBMCs were obtained from Cureline (South San Francisco, CA). Fresh blood samples from patients with CLL who were either newly diagnosed or free from therapy for at least 3 weeks were collected at the M. D. Anderson Cancer Center (Houston, TX) after obtaining IRB-approved informed consent in accordance with the Declaration of Helsinki. Whole blood samples (20 mL) were collected in heparinized tubes, and mononuclear cells were separated using Histopaque 1077 (Sigma Diagnostics, St Louis, MO) or Ficoll-Hypaque (GE Healthcare, Little Chalfont, United Kingdom) according to the manufacturers' instructions. Clinical information from 11 donor patients is summarized in Table 1, and includes percentage CD5+/CD19+ and CD40+ cells as determined by flow cytometry. Primary tumor cells were maintained in culture using RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL, Gaithersburg, MD), 1% l-glutamine, and penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2.
Summary of clinical data for B-CLL patient samples, n = 11
| Donor no. . | Sex, age, y . | Rai stage . | Leukocyte/lymphocyte counts, ×109/L . | %CD5/CD19† . | %CD40† . |
|---|---|---|---|---|---|
| C4 | M, 43 | 2 | NA | 71 | 92 |
| C21 | M, 72 | 2 | NA | 53 | 78 |
| C22 | M, 59 | 4 | 77.8 | 88 | 86 |
| C27 | F, 54 | 2 | 98 | 83 | 90 |
| C30 | F, 41 | 2 | 150 | 80 | 84 |
| C31 | M, 43 | 2 | 30.1 | 84 | 85 |
| C32 | M, 45 | 2 | 31 | 88 | 84 |
| C33 | F, 58 | 0 | 34.4 | 66 | 76 |
| M9 | M, 50 | 1 | 73.7* | 88 | 92 |
| M10 | M, 76 | 0 | 38.2* | 90 | 92 |
| M11 | M, 64 | 4 | 39.2* | 86 | 93 |
| Donor no. . | Sex, age, y . | Rai stage . | Leukocyte/lymphocyte counts, ×109/L . | %CD5/CD19† . | %CD40† . |
|---|---|---|---|---|---|
| C4 | M, 43 | 2 | NA | 71 | 92 |
| C21 | M, 72 | 2 | NA | 53 | 78 |
| C22 | M, 59 | 4 | 77.8 | 88 | 86 |
| C27 | F, 54 | 2 | 98 | 83 | 90 |
| C30 | F, 41 | 2 | 150 | 80 | 84 |
| C31 | M, 43 | 2 | 30.1 | 84 | 85 |
| C32 | M, 45 | 2 | 31 | 88 | 84 |
| C33 | F, 58 | 0 | 34.4 | 66 | 76 |
| M9 | M, 50 | 1 | 73.7* | 88 | 92 |
| M10 | M, 76 | 0 | 38.2* | 90 | 92 |
| M11 | M, 64 | 4 | 39.2* | 86 | 93 |
Lymphocyte count.
Percentage of total peripheral blood mononuclear cells (PBMCs).
Antibodies and sources of CD40 ligand
The fully human anti-CD40 antagonist monoclonal antibody, HCD122, was generated in the transgenic XenoMouse expressing human IgG1 (Abgenix, Fremont, CA) after immunization with the extracellular domain of recombinant human CD40. Human IgG1 anti–keyhole limpet hemocyanin (KLH) monoclonal antibody derived from XenoMouse (Abgenix) was used as isotype control. HCD122 and control human IgG1 (huIgG1) were formulated in phosphate-buffered saline (PBS). The anti-CD20 monoclonal antibody, rituximab, was diluted in PBS before use.
Human CD40 ligand (CD40L)–expressing Chinese hamster ovary (CHO) cells were prepared by transfecting cells with a plasmid vector carrying the gene for human CD40L as described previously.38 Recombinant human soluble CD40L (rhsCD40L) and enhancer for ligands were purchased from Axxora (San Diego, CA) and repurified to remove endotoxin.
Flow cytometry
Binding of HCD122 to CLL patient cells was analyzed by flow cytometry using the FACSCalibur system (BD Biosciences, San Jose, CA). PBMCs from CLL patients were resuspended in staining buffer (PBS with 1% FBS and 0.1% sodium azide), incubated on ice with fluorescein isothiocyanate (FITC)–labeled HCD122 or human IgG1 isotype control for 30 minutes, washed, and analyzed by flow cytometry. Samples were analyzed on the FACSCalibur flow cytometer (BD Biosciences). Each sample represented 10 000 collected events; CellQuest software (Becton Dickinson, Mountain View, CA) was used for both the data acquisition and subsequent analysis. Only data from viable cells, as determined by negative staining with propidium iodide (PI; Roche Diagnostic, Indianapolis, IN), were included in the analysis.
In vitro ADCC assay
Purified human natural killer (NK) cells (AllCells, Emeryville, CA) were used as effector cells, and PBMCs from CLL patients were used as target cells. Target cells in R10 medium (phenol red–free RPMI1640 with 10% FBS) at a density of 106 cells/mL were incubated for 30 minutes at 37°C with 5 μM calcein AM (Molecular Probes, Eugene, OR). Purified human NK cells and washed calcein-labeled target cells were mixed in R10 medium at a 10:1 ratio (5 × 104 NK cells and 5 × 103 target cells in a total volume of 200 μL) and added to a 96-well round-bottom plate. HCD122, rituximab, or control huIgG1 monoclonal antibodies were serially diluted and added to triplicate wells containing the NK/target cell mixture. The detergent NP-40 (1%) was added to 3 wells for determination of maximal cell lysis. To measure spontaneous label release, target cells were also incubated in triplicate without NK cells or antibodies. After 4-hour incubation at 37°C, 100 μL culture supernatant (100 μL) was transferred into a 96-well black flat-bottom plate, and the released calcein was measured in arbitrary fluorescent units (AFU) at 485 nm excitation and 535 nm emission using a Tecan SpectraFluor Plus (Tecan US, Research Triangle, NC). The percentage specific lysis was calculated using the following formula: 100 × (AFU test − AFU spontaneous release)/(AFU maximal release − AFU spontaneous release).
Quantitation of CD20 and CD40 expression on patient B-CLL cells
B-CLL cells were first incubated with huIgG1 at 1 mg/mL at 4°C for 30 minutes to occupy all nonspecific binding sites. Various concentrations (0.1-100 μg/mL) of FITC-conjugated huIgG1, HCD122, or rituximab were added, and the cells were incubated at 4°C for an additional 30 minutes. CD40 quantitation was performed using the lymphoid gate, and geometric mean fluorescence (GMF) intensities were measured using the FACSCalibur system. Molecules of equivalent soluble fluorochrome (MESF) were calculated based on the standard curve established by calibrated FITC beads using the Quantum FITC Premixed MESF kits (Bangs Laboratories, Fishers, IN) and were reported as antibody binding sites per cell.
Internalization by flow cytometry and confocal microscopy
For flow cytometry experiments, CLL-PBMCs (5 × 105) were incubated with huIgG1, HCD122, or rituximab at 10 μg/mL for 3 hours at 4°C in staining buffer or at 37°C in staining buffer without sodium azide. Cells were washed with staining buffer, followed by incubation with FITC-conjugated Fc-specific goat anti–human IgG (Caltag Laboratories, Burlingame, CA) for an additional 30 minutes at 4°C. GMF intensity was measured using the FACSCalibur system. The percentage internalization was calculated using the following formula: 100 × {(GMF of test antibody at 4°C − GMF of isotype antibody at 4°C) − (GMF of test antibody at 37°C − GMF of isotype antibody at 37°C)}/(GMF of test antibody at 4°C − GMF of isotype antibody at 4°C).
For confocal microscopy experiments, CLL-PBMCs or normal human B cells (5 × 105) were incubated with Alexa488- or FITC-conjugated HCD122, rituximab, or huIgG1 control antibodies at 10 μg/mL for 3 hours at 4°C in staining buffer or 37°C in staining buffer without sodium azide, followed by washing and fixation with 2% formaldehyde for 5 minutes at room temperature. Then cells were washed and placed on Superfrost Plus Micro Slides (VWR, West Chester, PA) precoated with poly-l-lysine (Sigma, St Louis, MO), and then mounted and sealed for confocal imaging. Images were acquired using HCX PL APD 63×/1.4-0.60 oil BL objective with Leica TCS SP2 spectral confocal and multiphoton system (Leica Microsystem CMS, Mannheim, Germany).
Western blot analysis
Signaling pathway studies were performed using 2.0 × 106 CLL-PBMCs/mL incubated for either 20 minutes or 24 hours at 37°C, in culture medium only or with rhsCD40L cross-linked by enhancer. For 20-minute time points, HCD122 or human IgG1 isotype control antibody was added 2.5 hours prior to addition of CD40L, whereas for 24-hour time points the antibodies were added with the CD40L. Following treatment, cells were centrifuged (365g for 10 minutes at 4°C) and lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease (Roche Molecular Biochemicals, Indianapolis, IN) and phosphatase (Sigma Chemicals, St Louis, MO) inhibitors. Lysates were cleared by centrifugation (16 000g for 10 minutes at 4°C) and normalized for protein content using a BCA kit (Pierce, Rockford, IL). Protein (10 and 20 μg per lane for normal and phospho-proteins, respectively) was loaded onto 10% or 4% to 20% Tris-glycine gels (Invitrogen, Carlsbad, CA) and then transferred to nitrocellulose membrane (Invitrogen). Membranes were probed with antibodies against human cleaved PARP (cPARP), Bcl-xl, Mcl-1, phospho-Akt (Ser473), Akt, phospho-p38 MAPK, p38 MAPK, phospho-ERK 1/2, ERK 1/2, phospho-IKK α/β, and IKK α/β (Cell Signaling Technology, Beverly, MA; BD Pharmingen, San Diego, CA). To confirm equal loading and transfer efficiency, a mouse antihuman β-actin antibody (Sigma Chemicals) was used. Secondary HRP-labeled goat antirabbit and horse antimouse antibodies (Vector Laboratories, Burlingame, CA) in conjunction with the enhanced chemiluminescence (ECL) Plus kit (GE Healthcare) were used for protein detection.
In vitro cell proliferation, survival, and growth inhibition
PBMCs were seeded in 96-well plates at 5 × 104 to 10 × 104 cells/well in complete medium and incubated with serial dilutions (0.001 to 10 μg/mL) of HCD122 or human IgG1 isotype control antibody for 72 hours at 37°C in a humidified atmosphere of 5% CO2. To evaluate human PBMC proliferation in response to CD40L stimulation and inhibition by HCD122, CLL-PBMCs were incubated with recombinant soluble human CD40 ligand (1 μg/mL) in the presence of enhancer (2 μg/mL) alone, or simultaneously with HCD122 or human IgG1 isotype control antibody.
Cell viability and proliferation as indicated by luminescence intensity (RLU = relative light units) was assessed using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI) according to the manufacturer's instructions.
Cytokine detection assay
PBMCs (10 × 104) from 9 patients with CLL were cultured in complete medium in 96-well round-bottom plates with human IgG1 control antibody or HCD122 (10 μg/mL) in the presence or absence of formaldehyde-fixed CHO-CD40L cells at a 1:2 ratio for 24 hours at 37°C, 5% CO2. Culture supernatant was harvested and pooled together from 10 wells, then frozen immediately at − 70°C until assayed in triplicate. Human IL-10, TNF-α, IL-8, GM-CSF, and IL-6 levels were measured by the Meso Scale Discovery Multi-Array Platform (Gaithersburg, MD) per the manufacturer's instructions.
Statistical analysis
Paired t tests were used to compare the amount of CD20 and CD40 molecules on CLL patient cell surface, percentage maximum lysis, and ED50 of HCD122- and rituximab-mediated ADCC activities on cells from 9 CLL patients.
Results
HCD122 binds to patient B-CLL cells
To test for binding of HCD122 to B-CLL cells, PBMCs from 9 patients with CLL were stained with HCD122-FITC or isotype-matched control IgG1-FITC. HCD122 showed specific binding against all 9 CLL patient samples, binding an average of 85% (± 11%) of PBMCs. The 2 histograms (Figure 1A,B) represent the low and high staining of B-CLL cells by HCD122. The average geometric mean fluorescence (GMF) of HCD122 binding was 16 (± 4), whereas the average GMF of the isotype control was 2 (± 1). These results are consistent with previously published results29,30 showing that B-CLL cells express CD40 on their surface and confirm that HCD122 binds to CD40 on the surface of B-CLL cells.
Binding of HCD122 to patient B-CLL cells. B-CLL patient cells were incubated on ice for 30 minutes with FITC-labeled HCD122 or huIgG1 isotype control antibody, washed, and analyzed by flow cytometry. The histograms show staining of lymphocyte-gated cells by isotype control and HCD122 from 2 patient samples representing low (A) and high (B) binding from patients C30 and C2, respectively. Dashed line represents huIgG1 isotype control; solid line represents HCD122.
Binding of HCD122 to patient B-CLL cells. B-CLL patient cells were incubated on ice for 30 minutes with FITC-labeled HCD122 or huIgG1 isotype control antibody, washed, and analyzed by flow cytometry. The histograms show staining of lymphocyte-gated cells by isotype control and HCD122 from 2 patient samples representing low (A) and high (B) binding from patients C30 and C2, respectively. Dashed line represents huIgG1 isotype control; solid line represents HCD122.
HCD122 lyses target B-CLL cells by ADCC
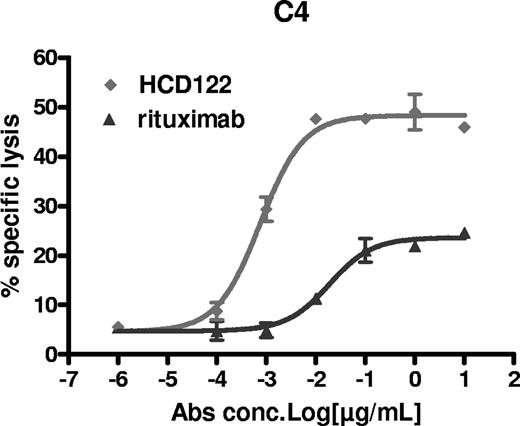
ADCC is considered to be one of the primary mechanisms of the antitumor effects of IgG1 antibodies, thus HCD122 was evaluated for its ability to mediate lysis of patient-derived primary B-CLL cells. Since B-CLL cells express both CD40 and CD20, the target for rituximab, the relative ADCC activity of HCD122 and rituximab was assessable. Purified human NK cells from healthy volunteers were used as effector cells at a fixed effector-target (E/T) ratio of 10:1 in the presence of various concentrations of HCD122, rituximab, or huIgG1 isotype control. As shown in Figure 2, both HCD122 and rituximab lysed patient B-CLL cells in a dose-dependent manner. However, both the maximum specific cell lysis and the potency (ED50) of HCD122-mediated ADCC were significantly higher than those of rituximab. The assay was performed using samples from multiple CLL patients (n = 8) to confirm that the observed HCD122-mediated ADCC activity was not limited to a single patient. The average maximal lysis of B-CLL cells by HCD122 was 49% with an ED50 of 14 pM, whereas the average maximal lysis of the same target cells by rituximab was 31% with an ED50 of 156 pM (Table 2).
Mediation of ADCC by HCD122 and rituximab in patient B-CLL cells. Freshly isolated human NK cells were used as the effector (E) cells in the ADCC assay. Calcein-labeled patient CLL target (T) cells were mixed with the NK cells at an E/T ratio of 10 to 1. Percentage specific lysis was calculated as described in “Statistical analysis.” Graph represents data from one representative CLL patient (patient C4 in Table 2). Values are mean (± SD) of triplicate measurements.
Mediation of ADCC by HCD122 and rituximab in patient B-CLL cells. Freshly isolated human NK cells were used as the effector (E) cells in the ADCC assay. Calcein-labeled patient CLL target (T) cells were mixed with the NK cells at an E/T ratio of 10 to 1. Percentage specific lysis was calculated as described in “Statistical analysis.” Graph represents data from one representative CLL patient (patient C4 in Table 2). Values are mean (± SD) of triplicate measurements.
Summary of ADCC data for CLL patient samples, n = 8
| Patient ID . | Maximal lysis, % . | ED50, pM . | ||
|---|---|---|---|---|
| HCD122 . | Rituximab . | HCD122 . | Rituximab . | |
| C4 | 46.35 | 20.15 | 5.12 | 127.64 |
| C21 | 32.90 | 32.80 | 14.86 | 272.68 |
| C22 | 46.65 | 31.08 | 3.24 | 62.08 |
| C27 | 79.87 | 56.04 | 0.99 | 71.54 |
| C30 | 37.60 | 21.33 | 8.87 | 170.34 |
| C31 | 63.40 | 27.27 | 3.23 | 64.59 |
| C32 | 56.92 | 49.97 | 3.14 | 16.58 |
| C33 (1)* | 27.71 | 12.33 | 49.53 | 473.28 |
| C33 (2)* | 48.57 | 28.63 | 37.85 | 141.03 |
| Average | 48.89 | 31.07 | 14.09 | 155.53 |
| P = .001 | P = .010 | |||
| Patient ID . | Maximal lysis, % . | ED50, pM . | ||
|---|---|---|---|---|
| HCD122 . | Rituximab . | HCD122 . | Rituximab . | |
| C4 | 46.35 | 20.15 | 5.12 | 127.64 |
| C21 | 32.90 | 32.80 | 14.86 | 272.68 |
| C22 | 46.65 | 31.08 | 3.24 | 62.08 |
| C27 | 79.87 | 56.04 | 0.99 | 71.54 |
| C30 | 37.60 | 21.33 | 8.87 | 170.34 |
| C31 | 63.40 | 27.27 | 3.23 | 64.59 |
| C32 | 56.92 | 49.97 | 3.14 | 16.58 |
| C33 (1)* | 27.71 | 12.33 | 49.53 | 473.28 |
| C33 (2)* | 48.57 | 28.63 | 37.85 | 141.03 |
| Average | 48.89 | 31.07 | 14.09 | 155.53 |
| P = .001 | P = .010 | |||
ADCC activity was determined for patient C33 using NK cells from 2 different donors and is therefore designated C33 (1) and C33 (2).
Expression levels of target molecules on the cell surface may affect the sensitivity of B-CLL cells to ADCC-mediated cell killing. To determine whether target expression levels contributed to the higher ADCC activity of HCD122 compared with rituximab, flow cytometric quantitation of CD20 and CD40 densities on B-CLL cells was performed. The number of CD40 and CD20 molecules on B-CLL cells was found to be variable among patients, ranging from 552 to 2932 (CD40) and 1455 to 28 494 (CD20) molecules per cell (Table 3). On average, the patient B-CLL cell expressed 5.9-fold more CD20 than CD40, suggesting that the greater ADCC activity mediated by HCD122 in these cells was not a result of higher CD40 expression.
Summary of expression of CD20 and CD40 molecules on B-CLL cells, n = 8
| Patient ID . | CD20 molecules/cell, mean ± SD . | CD40 molecules/cell, mean ± SD . | Ratio of CD20 to CD40 . |
|---|---|---|---|
| C27 | 1455 ± 126 | 1158 ± 57 | 1.26 |
| C30 | 9584 ± 44 | 684 ± 8 | 14.01 |
| C31 | 6838 ± 135 | 1590 ± 79 | 4.30 |
| C32 | 28494 ± 547 | 2860 ± 38 | 9.96 |
| C33 | 5799 ± 160 | 1421 ± 201 | 4.08 |
| M9 | 4518 ± 53 | 1305 ± 30 | 3.46 |
| M10 | 3693 ± 180 | 552 ± 25 | 6.69 |
| M11 | 11020 ± 58 | 2932 ± 89 | 3.76 |
| Patient ID . | CD20 molecules/cell, mean ± SD . | CD40 molecules/cell, mean ± SD . | Ratio of CD20 to CD40 . |
|---|---|---|---|
| C27 | 1455 ± 126 | 1158 ± 57 | 1.26 |
| C30 | 9584 ± 44 | 684 ± 8 | 14.01 |
| C31 | 6838 ± 135 | 1590 ± 79 | 4.30 |
| C32 | 28494 ± 547 | 2860 ± 38 | 9.96 |
| C33 | 5799 ± 160 | 1421 ± 201 | 4.08 |
| M9 | 4518 ± 53 | 1305 ± 30 | 3.46 |
| M10 | 3693 ± 180 | 552 ± 25 | 6.69 |
| M11 | 11020 ± 58 | 2932 ± 89 | 3.76 |
P = .03.
Modulation of HCD122 and rituximab levels on the surface of B-CLL cells
Antibodies can induce internalization upon binding to their target on the cell surface, which can have an impact on ADCC activity. We determined whether HCD122 and rituximab differ in their ability to induce internalization upon binding to their respective antigens on B-CLL cells. For flow cytometric analysis, the cells were stained with HCD122 or rituximab and incubated for 3 hours at either 37°C, a temperature permissive for internalization, or under control conditions in which internalization is minimized (4°C in the presence of sodium azide). There was no reduction of HCD122 levels on the cell surface after incubation at 37°C compared with control conditions (Figure 3; Table 4). In fact, slight increases in levels of HCD122 were detected at 37°C, resulting in negative values for the percentage internalization in Table 4. In contrast, rituximab levels on the cell surface were reduced after incubation at 37°C compared with control conditions (n = 8).
Evaluation of internalization of antibody bound to CD40 on patient B-CLL cells as determined by flow cytometry. Patient CLL cells were incubated with huIgG1 (10 μg/mL), HCD122 (10 μg/mL), or rituximab (10 μg/mL) on ice with 0.1% sodium azide to block internalization ( ) or 37°C without sodium azide (▨) for 3 hours. Geometric mean fluorescent (GMF) intensities were measured and used as an indicator of internalization. Graph represents data from one CLL patient.
) or 37°C without sodium azide (▨) for 3 hours. Geometric mean fluorescent (GMF) intensities were measured and used as an indicator of internalization. Graph represents data from one CLL patient.
Evaluation of internalization of antibody bound to CD40 on patient B-CLL cells as determined by flow cytometry. Patient CLL cells were incubated with huIgG1 (10 μg/mL), HCD122 (10 μg/mL), or rituximab (10 μg/mL) on ice with 0.1% sodium azide to block internalization ( ) or 37°C without sodium azide (▨) for 3 hours. Geometric mean fluorescent (GMF) intensities were measured and used as an indicator of internalization. Graph represents data from one CLL patient.
) or 37°C without sodium azide (▨) for 3 hours. Geometric mean fluorescent (GMF) intensities were measured and used as an indicator of internalization. Graph represents data from one CLL patient.
Summary of internalization data for HCD122 and rituximab in B-CLL cells, n = 8
| Patient ID . | % Internalization* . | |
|---|---|---|
| HCD122 . | Rituximab . | |
| C4 | −12 | 53 |
| C21 | 18 | 23 |
| C22 | −9 | 38 |
| C27 | −14 | 20 |
| C30 | −43 | 32 |
| C31 | −71 | 49 |
| C32 | −14 | 27 |
| C33 | 13 | 59 |
| Mean ± SD | −16.5 ± 28.8 | 37.6 ± 14.6 |
| Patient ID . | % Internalization* . | |
|---|---|---|
| HCD122 . | Rituximab . | |
| C4 | −12 | 53 |
| C21 | 18 | 23 |
| C22 | −9 | 38 |
| C27 | −14 | 20 |
| C30 | −43 | 32 |
| C31 | −71 | 49 |
| C32 | −14 | 27 |
| C33 | 13 | 59 |
| Mean ± SD | −16.5 ± 28.8 | 37.6 ± 14.6 |
A negative value for percentage internalization represents a GMF at 37°C that is higher than at 4°C.
Internalization was confirmed by confocal microscopy in both normal B cells and B-CLL cells (n = 2). Similar to the results obtained with flow cytometry, HCD122 remained uniformly distributed on the cell surface, even after the 3-hour incubation at 37°C, whereas rituximab was redistributed into caps and internalized (Figure 4A,B). These data suggest that the potent ADCC activity of HCD122 may be derived, at least in part, from its ability to display itself uniformly and continuously on the surface of target tumor cells, providing optimal conditions for interaction with effector cells.
Evaluation of internalization of antibody bound to CD40 after binding to patient CLL cells as confirmed by confocal microscopy. Normal B cells (A) and patient CLL cells (B) were incubated with FITC- and Alexa488-conjugated HCD122, rituximab, or huIgG1 (10 μg/mL for each), respectively, at 4°C with 0.1% sodium azide to block internalization or at 37°C without sodium azide for 3 hours. After washing and fixation, cells were imaged by confocal microscopy.
Evaluation of internalization of antibody bound to CD40 after binding to patient CLL cells as confirmed by confocal microscopy. Normal B cells (A) and patient CLL cells (B) were incubated with FITC- and Alexa488-conjugated HCD122, rituximab, or huIgG1 (10 μg/mL for each), respectively, at 4°C with 0.1% sodium azide to block internalization or at 37°C without sodium azide for 3 hours. After washing and fixation, cells were imaged by confocal microscopy.
HCD122 inhibits CD40L-induced signaling and viability of B-CLL cells
Engagement of CD40 by CD40L is known to be a survival signal in B-CLL cells.39 We first tested whether HCD122 can serve as a CD40 agonist in the absence of CD40L. B-CLL cells were cultured with various concentrations of huIgG1 isotype control or HCD122 (0.1-10 μg/mL), or with CD40L (1 μg/mL) as a positive control for 3 days, and the relative numbers of viable cells in each culture were determined by measuring luminescence (RLU). Multiple patient samples were analyzed (n = 8, summarized in Table 5); a representative example is shown in Figure 5A. Increased viability was detected in cultures containing rhsCD40L compared with cultures containing either the isotype control antibody or HCD122. These data demonstrate that CD40L serves as a survival/proliferation stimulus for B-CLL cells, and that HCD122 does not promote cell proliferation or survival upon binding CD40 on B-CLL cells. Comparison of the calculated stimulation indices (SIs) for huIgG1, HCD122, and rhsCD40L, summarized for the 8 patient samples in Table 5, further illustrates HCD122 lack of stimulatory activity at antibody concentrations ranging from 0.01 to 100 μg/mL. We next tested the ability of HCD122 to inhibit CD40L-induced viability. Various concentrations of either isotype control antibody or HCD122 were added simultaneously with rhsCD40L to B-CLL cultures. Addition of HCD122 caused a dose-dependent decrease in cell viability (representative data from one patient shown in Figure 5B). This effect was HCD122 specific, since the nonspecific IgG1 (isotype control) antibody did not act similarly. The degree of response to CD40L was variable among patients (n = 7), ranging from 1.5- to 4-fold (Table 6). Similar results were obtained when a cell-bound form of the CD40L (formaldehyde-fixed CHO cells expressing CD40L) was used instead of soluble recombinant form of CD40L (data not shown). These results show that HCD122 is a potent antagonist of CD40L-mediated proliferation/survival of B-CLL cells.
Stimulation index (SI) of soluble Abs on B-CLL patient PBMCs, n = 8
| Ab concentration, μg/mL . | huIgG1 . | HCD122 . | rhsCD40L . |
|---|---|---|---|
| 100 | 1.13 ± 0.05 | 1.15 ± 0.11 | |
| 10 | 1.14 ± 0.07 | 1.18 ± 0.09 | |
| 1 | 1.11 ± 0.14 | 1.11 ± 0.09 | 11.12 ± 17.15 |
| 0.1 | 1.1 ± 0.11 | 1.12 ± 0.17 | |
| 0.01 | 1.11 | 1.16 |
| Ab concentration, μg/mL . | huIgG1 . | HCD122 . | rhsCD40L . |
|---|---|---|---|
| 100 | 1.13 ± 0.05 | 1.15 ± 0.11 | |
| 10 | 1.14 ± 0.07 | 1.18 ± 0.09 | |
| 1 | 1.11 ± 0.14 | 1.11 ± 0.09 | 11.12 ± 17.15 |
| 0.1 | 1.1 ± 0.11 | 1.12 ± 0.17 | |
| 0.01 | 1.11 | 1.16 |
Stimulation index: (RLU of CLL patient PBMCs with HCD122 or huIgG1 or rhsCD40L)/(RLU of CLL patient PBMCs alone).
Inhibition of CD40L-induced B-CLL cell survival and proliferation. Responses of B-CLL cells to stimulation by CD40L, and inhibition of the responses by HCD122, are shown for a single representative patient. (A) B-CLL cells were cultured in concentrations of 0 to 100 μg/mL either HCD122 or huIgG1, or in 1 μg/mL rhsCD40L + 2 μg/mL enhancer for 72 hours. (B) B-CLL patient cells were cultured for 72 hours in 1 μg/mL rhsCD40L + 2 μg/mL enhancer with 0 to 10 μg/mL either HCD122 or huIgG1. Cell viability was measured as luminescence intensity (RLU = relative light units). Values represent mean (± SD) of triplicate measurements. (C) Effects of CD40L and HCD122 were evaluated on a number of signaling pathways and antiapoptotic proteins. As indicated by + signs above the figure, CLL cells were incubated in 10 μg/mL HCD122 or 10 μg/mL IgG1 isotype control and 2 μg/mL CD40L (rhsCD40L) for 20 minutes (α and β IKK and ERK) or 24 hours (p38, cPARP, Mcl-1, Bcl-xl). Western blot analysis was performed using antibodies as listed on left-hand side of the panel. β-Actin was used to ensure equal loading of protein in all lanes and is shown as an example for Bcl-xl. For each protein analyzed, representative samples are shown. p indicates phospho-protein; t, total protein.
Inhibition of CD40L-induced B-CLL cell survival and proliferation. Responses of B-CLL cells to stimulation by CD40L, and inhibition of the responses by HCD122, are shown for a single representative patient. (A) B-CLL cells were cultured in concentrations of 0 to 100 μg/mL either HCD122 or huIgG1, or in 1 μg/mL rhsCD40L + 2 μg/mL enhancer for 72 hours. (B) B-CLL patient cells were cultured for 72 hours in 1 μg/mL rhsCD40L + 2 μg/mL enhancer with 0 to 10 μg/mL either HCD122 or huIgG1. Cell viability was measured as luminescence intensity (RLU = relative light units). Values represent mean (± SD) of triplicate measurements. (C) Effects of CD40L and HCD122 were evaluated on a number of signaling pathways and antiapoptotic proteins. As indicated by + signs above the figure, CLL cells were incubated in 10 μg/mL HCD122 or 10 μg/mL IgG1 isotype control and 2 μg/mL CD40L (rhsCD40L) for 20 minutes (α and β IKK and ERK) or 24 hours (p38, cPARP, Mcl-1, Bcl-xl). Western blot analysis was performed using antibodies as listed on left-hand side of the panel. β-Actin was used to ensure equal loading of protein in all lanes and is shown as an example for Bcl-xl. For each protein analyzed, representative samples are shown. p indicates phospho-protein; t, total protein.
Percentage inhibition of HCD122 on CD40 ligand–induced CLL patient PBMC proliferation, n = 8
| Ab concentration, μg/mL . | huIgG1 . | HCD122 . |
|---|---|---|
| 10 | −49 ± 38 | 88 ± 10 |
| 1 | −53 ± 36 | 74 ± 25 |
| 0.1 | −46 ± 33 | 60 ± 29 |
| 0.01 | −65 ± 48 | 1 ± 23 |
| 0.001 | −48 ± 33 | −17 ± 26 |
| Ab concentration, μg/mL . | huIgG1 . | HCD122 . |
|---|---|---|
| 10 | −49 ± 38 | 88 ± 10 |
| 1 | −53 ± 36 | 74 ± 25 |
| 0.1 | −46 ± 33 | 60 ± 29 |
| 0.01 | −65 ± 48 | 1 ± 23 |
| 0.001 | −48 ± 33 | −17 ± 26 |
Percentage inhibition: 100 − (RLU with Abs-PBMCs alone)/(RLU of PBMCs with rhsCD40L-PBMCs alone) × 100%.
To confirm the specificity of HCD122's antagonist activity, we evaluated the ability of HCD122 to inhibit CD40L-mediated activation of multiple signaling and survival pathways. In each of the CLL samples analyzed (n = 7-9), CD40L was found to cause phosphorylation of p38, IKK α/β, and ERK 1/2 (representative data shown in Figure 5C); HCD122 alone did not effect phosphorylation of these proteins, but did block the effects of CD40L. Effects of CD40L on Akt phosphorylation were also evaluated, but were found to be more variable among the samples (not shown). In addition, in all samples evaluated, CD40L induced the antiapoptotic proteins Mcl-1 and Bcl-xl, and consistent with having an antiapoptotic effect, reduced levels of cPARP. Again, HCD122 alone had no effect, but effectively blocked the action of CD40L (Figure 5C).
HCD122 inhibits cytokine secretion by B-CLL cells
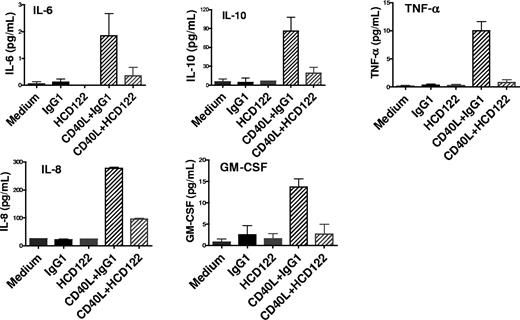
CLL cells are known to secrete a variety of cytokines that are involved in cell survival, migration, and interaction with cells in the tumor microenvironment.40 We determined whether HCD122 modulates cytokine secretion by B-CLL cells. PBMCs from 9 CLL patients were cultured at 37°C in the following conditions: media only, huIgG1 isotype control, HCD122, CHO-CD40L cells plus huIgG1, or CHO-CD40L cells plus HCD122. Culture supernatants collected at 24 hours were assayed for secretion of various cytokines. The CLL cells cultured in media alone or media containing huIgG1 produced very low levels of cytokines (Figure 6). When cultured with CHO-CD40L cells, secretion of substantially elevated levels of IL-6, IL-10, TNF-α, IL-8, and GM-CSF was observed. Addition of HCD122 to CHO-CD40L cell cultures inhibited the secretion of all cytokines measured, whereas addition of HCD122 in the absence of CD40L resulted in levels of cytokine secretion comparable with control. The data for all 9 CLL samples are summarized in Table 7. Plasma IL-8 levels predict for survival in CLL patients.41 Notably, IL-8 was induced by CD40L in all patient cells; however, the other cytokines were produced by only a subset of patient cells.
Inhibition of CD40L-induced cytokine secretion of primary CLL cells. Patient CLL cells were cultured with huIgG1 (10 μg/mL) or HCD122 (10 μg/mL) in the presence or absence of CHO-CD40L cells (1:2 ratio). Culture supernatants were collected at 24 hours and assayed for cytokine levels by the Meso Scale Discovery (MSD) Multi-Array Platform. Graphs represent data from one representative CLL patient. Values represent mean (± SD) of triplicate measurements.
Inhibition of CD40L-induced cytokine secretion of primary CLL cells. Patient CLL cells were cultured with huIgG1 (10 μg/mL) or HCD122 (10 μg/mL) in the presence or absence of CHO-CD40L cells (1:2 ratio). Culture supernatants were collected at 24 hours and assayed for cytokine levels by the Meso Scale Discovery (MSD) Multi-Array Platform. Graphs represent data from one representative CLL patient. Values represent mean (± SD) of triplicate measurements.
Summary of cytokine secretion data for all CLL samples,* n = 9
| Cytokine . | Patients responding to CD40L, % . | Inhibition by HCD122, %, range . |
|---|---|---|
| IL-6 | 67 (6/9) | 43-100 |
| IL-10 | 78 (7/9) | 50-100 |
| TNF-α | 67 (6/9) | 87-100 |
| IL-8 | 100 (9/9) | 32-100 |
| GM-CSF | 33 (3/9) | 85-100 |
| Cytokine . | Patients responding to CD40L, % . | Inhibition by HCD122, %, range . |
|---|---|---|
| IL-6 | 67 (6/9) | 43-100 |
| IL-10 | 78 (7/9) | 50-100 |
| TNF-α | 67 (6/9) | 87-100 |
| IL-8 | 100 (9/9) | 32-100 |
| GM-CSF | 33 (3/9) | 85-100 |
The percentage of monocytes ranges from 2.2% to 9%.
Discussion
We have generated a human IgG1 monoclonal antibody, HCD122, that targets and binds to the cell surface receptor CD40 and efficiently facilitates lysis of tumor cells by immune effector cells. HCD122 is also a strong antagonist of the CD40/CD40L signaling pathway,42 an activity that we propose will provide therapeutic benefit to patients with B-cell malignancies. Although there is no direct evidence that the activated receptor is essential to disease progression in vivo, studies performed ex vivo with freshly isolated tumor cells demonstrate that activation of CD40 by CD40L provides a growth and survival signal for neoplastic B cells.33,34,43-53 In this study, we show that HCD122 exerts antitumor activity against primary B-CLL cells ex vivo by at least 2 mechanisms: (1) lysing patient leukemia cells by potently and efficiently mediating ADCC, and (2) inhibiting CD40L-induced survival and proliferation of CLL cells and secretion of cytokines that are implicated in tumor growth.
The sensitivity of B-CLL cells to HCD122-mediated ADCC in this study was assessed relative to the monoclonal antibody rituximab for several reasons. It is widely agreed that rituximab's immune effector functions—ADCC, CDC, and opsonization/phagocytosis—contribute to its efficacy in low-grade follicular lymphomas.54 ADCC was implicated as an important antitumor mechanism in a study showing an association between the FCGR3A genotype of lymphoma patients (low-affinity Fc receptor CD16) and clinical responses to rituximab.55,56 As an approved therapeutic for follicular lymphoma that has demonstrated clinical efficacy, rituximab provides a benchmark for development of other therapeutic monoclonal antibodies. Just as important to the purposes of this study, rituximab is less active as a single-agent therapy for CLL. It is unclear to what degree rituximab's effector functions are impaired in CLL, or if so, whether the impairment is due to an intrinsic resistance of CLL cells to ADCC killing. In our assays, B-CLL cells were sensitive to lysis by HCD122 and rituximab. ADCC activity was evaluated for efficacy (maximal lysis) and potency (EC50), and by both measures HCD122 exhibited superior ADCC activity compared with rituximab. One explanation for this difference could be that CLL cells express fewer CD20 than CD40 molecules on the cell surface, since ADCC activity has been shown to correlate with the number of surface target molecules expressed.57 Several studies suggest that rituximab may be less effective in CLL because expression of CD20 is lower on CLL cells than on other B-cell malignancies.24,58,59 However, our data show that receptor number does not account for the difference, since CD20 expression was, on average, 5.9-fold higher than CD40 expression on B-CLL cells. Another possibility was that the degree of internalization upon binding to their respective target antigens differed for the 2 antibodies. Flow cytometric and confocal microscopic data confirm that HCD122 remains on the cell surface and is uniformly distributed, whereas levels of rituximab on the cell surface decrease after binding. Rituximab was also detected in surface caps and inside the cells, whereas this was not observed for HCD122. These data are consistent with a previous report showing downmodulation of CD20 by rituximab,25 and suggest that the efficiency with which HCD122 mediates ADCC ex vivo may be related to its uniform and consistent display on the CLL cell surface, thus providing more optimal conditions for interaction with NK or other effector cells.
Other factors affecting effector-target cell interactions, however, cannot be ruled out. Under the experimental conditions used in this study, the HCD122/CD40 complex remains stable on the cell surface, but under other conditions, this apparent stability may be altered. When cross-linked with a labeled secondary antibody, the murine anti-CD40 antibody, MAB89, was reported to internalize with bound CD40,60 whereas cross-linking the murine anti-CD40 antibody G28.5 bound to receptor resulted in shedding of CD40 from the cell surface.61 We have not observed internalization with HCD122 or MAB89 in our 3-hour assays, but when CLL cells were incubated with HCD122 or G28.5 for 72 hours, shed CD40 was detected in the supernatant (data not shown). It is possible that shedding could affect the efficiency of ADCC, although it may not be apparent in the 4-hour ADCC assay formats. We are currently conducting time course experiments to look at the kinetics of CD40 shedding subsequent to binding of HCD122.
Although we have shown that B-CLL cells are susceptible to killing by ADCC ex vivo, it remains to be determined clinically whether ADCC is a relevant therapeutic mechanism for treatment of CLL. The function of NK cells, the primary effectors of ADCC in vivo, has been described as defective in CLL patients,62,63 and the NK subset usually represents a minor percentage of the peripheral blood lymphocyte population. On the other hand, it has also been reported that the absolute numbers of NK cells in CLL patients and NK-cell function ex vivo are similar to that of healthy controls.64,65 It is possible that the effector cell populations may be functional, but in instances where tumor burden is high, the effector-target ratio may be too low for efficient killing. Reduction in tumor burden could explain, at least in part, the enhanced activity of rituximab/fludarabine combination therapies. Debulking with fludarabine may improve conditions for immune effector-target cell interaction, as absolute NK-cell numbers are not drastically reduced after treatment and rebound to pretreatment levels relatively quickly.65 The efficacy of an antibody, such as HCD122, that binds stably to the target cell surface, may be more evident in both the single-agent treatment setting, when effector-target ratios are suboptimal, as well as in combination with purine analogues and alkylators.
A second question that can be addressed only in the clinic is whether CD40 contributes significantly to CLL disease progression in vivo. Although we have confirmed that activation of CD40 on B-CLL cells results in phosphorylation of cellular p38, ERK 1/2, and IKK and up-regulation of the Mcl-1 and Bcl-xl; delays apoptosis; and stimulates proliferation ex vivo, and that HCD122 completely abrogates these responses, the evidence that CD40 plays a role in the growth and accumulation of B-CLL cells in vivo is circumstantial. Data from other studies suggest that cognate cellular interactions and soluble mediators in the tumor microenvironment play an important role in CLL cell survival and proliferation.36,66 Activated CD4+ T cells,36,67,68 as well as those expressing CD40L,37 have been observed concentrated in pseudofollicles in the BM and LN microenvironments. These T cells are a potential source of CD40L for cognate interactions with B-CLL cells. In vitro, a variety of cytokines have prosurvival and antiapoptotic activities in B-CLL cells, including IL-2, IL-4, IL-6, IL-8, IFN-α, IFN-γ, G-CSF, GM-CSF, TNF-α, and IL-10.69,70 In the tumor microenvironment, there are multiple sources of these cytokines, including activated T cells, stromal cells, and B-CLL cells themselves.66 In this study, we found that CD40L mediated B-CLL cell secretion of IL-6, IL-10, TNF-α, IL-8, and GM-CSF, and that HCD122 inhibited this effect. Reduction of prosurvival cytokine release by B-CLL cells may have a beneficial impact on CLL disease progression. This conclusion is supported by the promising clinical activities of the immunomodulatory analogues thalidomide and lenalidomide in CLL, which are believed to be mediated by down-regulation of cytokines including TNF-α and IL-6.71
As an antagonist of the CD40 signaling pathway, HCD122 may also demonstrate antitumor activity by disrupting other processes that support tumor growth. VEGF and angiogenesis are known to play a tumor-promoting role in CLL,72-74 and stimulation of CD40 expressed on endothelial cells, monocytes, fibroblasts, multiple myeloma cells, and CLL cells has been shown to up-regulate VEGF secretion by these cells.75-78 HCD122 is likely to have an inhibitory effect on CD40-associated angiogenesis since HCD122 blocks all CD40L-induced responses we have tested to date. The antiangiogenic activity of HCD122 is currently under investigation.
In summary, HCD122 is an anti-CD40 antagonist antibody with the potential to improve patient outcome in CLL. The dual mechanisms of action of this antibody may exert antitumor effects on both the proliferating compartment of malignant cells as well as the accumulating pool of apoptosis-defective B-CLL cells. The work presented here, combined with the safety profile and bioactivity observed in cynomolgus monkeys,79,80 supports the clinical development of HCD122. A phase 1 clinical trial in patients with CLL is ongoing.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Maria Cacia-Calderon, Asha Yabannavar, Julie Klinger, Mike Kavanaugh, Linda Masat, and Seema Kantak for their support and contributions to these studies. HCD122 is under codevelopment by Novartis Oncology and XOMA (Berkeley, CA).
Authorship
Contribution: M.L. is principal investigator for this project and was responsible for preparing this paper; S.K., K.L., A.C., J.H., C.G., X.X., E.E.K., S.H.L., S.L.A., B.J., and N.A. comprise the research team at Novartis Institutes for BioMedical Research that contributed to the design of the experiments and performed the assays; W.-K.W., A.Y., and G.V.G. also performed assays and provided consultation on interpretation of the data from all experiments; and S.O. and W.W. supplied primary patient materials, consultation on experimental design, and interpretation of experimental outcomes.
Conflict-of-interest disclosure: M.L., S.K., K.L., A.C., J.H., C.G., X.X., E.E.K., S.H.L., S.L.A., B.J., and N.A. are Novartis employees. S.O. received research support from Novartis. G.V.G., W.-K.W., W.W., and A.Y. declare no competing financial interests.
Correspondence: Mohammad Luqman, Novartis Institutes for BioMedical Research, 4560 Horton St, M/S 4.4, Emeryville, CA 94608; e-mail: mohammad.luqman@novartis.com.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal