Abstract
Waldenström macroglobulinemia (WM) is a B-cell malignancy characterized by an IgM monoclonal gammopathy and bone marrow (BM) infiltration with lymphoplasmacytic cells (LPCs). Excess mast cells (MCs) are commonly present in WM, and provide growth and survival signals to LPCs through several TNF family ligands (CD40L, a proliferation-inducing ligand [APRIL], and B-lymphocyte stimulator factor [BLYS]). As part of these studies, we demonstrated that WM LPCs secrete soluble CD27 (sCD27), which is elevated in patients with WM (P < .001 vs healthy donors), and serves as a faithful marker of disease. Importantly, sCD27 stimulated expression of CD40L on 10 of 10 BM MC samples and APRIL on 4 of 10 BM MC samples obtained from patients with WM as well as on LAD2 MCs. Moreover, the SGN-70 humanized monoclonal antibody, which binds to CD70 (the receptor-ligand partner of CD27), abrogated sCD27 mediated up-regulation of CD40L and APRIL on WM MCs. Last, treatment of severe combined immunodeficiency–human (SCID-hu) mice with established WM using the SGN-70 antibody blocked disease progression in 12 of 12 mice, whereas disease progressed in all 5 untreated mice. The results of these studies demonstrate a functional role for sCD27 in WM pathogenesis, along with its utility as a surrogate marker of disease and a target in the treatment of WM.
Introduction
Waldenström macroglobulinemia (WM) is a distinct B-cell lymphoproliferative disorder characterized primarily by bone marrow (BM) infiltration with lymphoplasmacytic cells (LPCs), along with demonstration of an IgM monoclonal gammopathy.1 This condition is considered to be lymphoplasmacytic lymphoma as defined by the Revised European-American Lymphoma (REAL) and World Health Organization (WHO) classification systems.2,3 An interesting feature of the disease is the finding of increased number of mast cells (MCs) in the BM of patients with WM, most typically in association with LPCs.4-7 This striking association has become characteristic of WM, and is often widely used as a supportive basis for making the diagnosis of WM.2,3,6
Recently, we demonstrated that BM MCs provide important growth and survival cues to WM LPCs through multiple TNF-family ligands, including CD40L (CD154), a proliferation-inducing ligand (APRIL), and B-lymphocyte stimulator factor (BLYS).8-10 Importantly, MC-induced expansion of WM LPCs was inhibited by use of blocking proteins to CD40L, APRIL, and BLYS. Moreover, direct therapeutic targeting of BM MCs with alemtuzumab and imatinib mesylate have also resulted in remissions among patients with WM.11,12
While these studies have shown that MCs can induce WM cells through multiple ligand-receptor signals, the mechanism(s) by which WM cells may potentially facilitate such supportive signaling through MCs remains to be clarified. One potential pathway for WM-MC signaling is via CD70, a TNF-family member which is found on activated lymphocytes, stromal cells of the thymic medulla, and mature dendritic cells, but which is absent from other normal tissues, including all vital organs.13 CD70 has been shown to play a role in B-lymphocyte regulation though binding to CD27, a TNF-family member expressed by thymocytes, natural killer, T, and B cells, including memory B cells from which WM LPCs may have derived.14-18 As such, we sought to establish the expression of CD27 and CD70 in WM, and delineate their interactions between WM LPCs, and MCs.
Methods
Approval for human studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board (IRB). Informed consent was obtained in accordance with the Declaration of Helsinki.
Cell lines and cultures
BCWM.1 and LAD2 cell lines were used in these studies. BCWM.1 is a cell line derived from an untreated patient with WM,19 whereas the LAD2 cell line is a MC line derived from a patient with untreated MC sarcoma.20 Cells were maintained as previously described.19,20 Sorted lymphoplasmacytic cells (CD19+) and mast cells (FcϵRI+, CD117+) were obtained from consenting patients with WM and isolated as previously described.9,18,21
RT-PCR analysis
Total RNA was extracted using RNase Mini Kit (QIAGEN, Valencia, CA). A total of 0.3 μg RNA was reverse transcripted in a 20 μL reaction by oligo-p-(dT)15 priming using Superscript III reverse transcriptase according to the protocol provided by Invitrogen (Carlsbad, CA). First-strand cDNA was synthesized using Superscript III reverse transcriptase according to the protocol provided by Invitrogen. A total of 2 μL first-strand cDNA was used as template for polymerase chain reaction (PCR) amplification. PCR was performed using the PTC-200 DNA Engine Thermal Cycler (MJ Research, Waltham, MA).
Flow cytometric analysis
Direct immunofluorescence flow cytometric analysis was performed using a Coulter Epics XL with data acquisition software (Cytomics FC500-CXP; Beckman Coulter, Fullerton CA) as described previously.9,18,21 Peripheral blood mononuclear cells (PBMCs) or natural killer (NK) cells treated with antibody alone or in combination were washed, followed by dual immunostaining with specific anti-FITC– or anti-phycoerythrin (PE)–conjugated mAbs to CD27, CD70 (Beckman Coulter), or APRIL, BLYS, or CD40L (R&D Systems, Minneapolis, MN).
ELISA analysis of WM patient serum
To evaluate cytokine production, 100 μL patient or age-matched healthy donor serum was collected and analyzed for soluble CD27 (sCD27) by an enzyme-linked immunosorbent assay (ELISA) kit per manufacturer's instructions (Bender Medsystems, Burlingame, CA).
Functional assays for sCD27
BCWM.1 LPCs and LAD2 MCs, as well as primary BM LPCs and MCs isolated from 10 patients with WM were cultured for 24 to 48 hours with recombinant human sCD27 (0.1-50 ng/mL). Proliferation and apoptosis were assessed by tritiated thymidine analysis and APO2.7 staining as before.9,18,21 Changes in cell-surface expression of APRIL, BLYS, and CD40L were determined by flow cytometric analysis following culture for 24 hours with sCD27 (10 μg/mL) in the presence or absence of the anti-CD70 human mAb SGN-70 (1 μg/mL; Seattle Genetics, Bothell, WA).13
ADCC assays
WM target cells labeled with calcein-AM were cocultured with normal donor NK cell–enriched PBMCs as before21 at various effector-target (E/T) ratios for 2 hours at 37°C in either medium alone or with SGN-70 (0-20 μg/mL). Following incubation with or without SGN-70, cells were centrifuged and absorbance in supernatants measured by spectrophotometry. Percentage of specific lysis was calculated using the equation Sp − S / M − S, where Sp to denote experimental lysis caused by antibody. S denotes spontaneous lysis, and M is maximum cell lysis in Triton X-100. {(M − medium control release) / (S − medium control release)} > 7 validated each experiment. Experiments were performed at least twice.
Mouse model
WM severe combined immunodeficiency–human (SCID-hu) mice were generated as previously described.22 The mice were housed and monitored in a VA animal research facility. The Institutional Animal Care and Use Committee approved all experimental procedures and protocols. Increasing levels of circulating human paraprotein in mice sera were used to monitor tumor engraftment and growth of BCWM.1 WM cells in SCID-hu mice. Mouse blood was collected from tail vein, and sera were serially tested for circulating IgM, IgG, and kappa light chains by ELISA (Bethyl, Montgomery, TX).
Results
Expression of CD27 and CD70 in WM LPCs and MCs
We first sought to establish CD27 and CD70 expression on WM BM LPCs and MCs. By reverse transcriptase (RT)–PCR analysis, CD27 transcripts were expressed in BM LPCs from 7 of 7 patients with WM and on the BCWM.1 WM cell line (Table 1). However, in contrast to the wide expression of CD27 transcripts by RT-PCR analysis, cell-surface expression of CD27 on BM LPCs was observed in only 5 (42%) of 12 patients with WM, and was absent on BCWM.1 WM cells. CD27 was less commonly expressed on BM MCs, with expression observed by RT-PCR and flow cytometric analysis in 4 (52%) of 7 and 2 (25%) of 8 patients with WM, respectively, but not in LAD2 MCs (Table 1). In contrast to the heterogeneous expression of CD27 on WM LPCs and MCs, CD70 was found to be widely expressed on BM LPCs and MCs, as well as on BCWM.1 WM and LAD2 MCs by both RT-PCR and multicolor flow cytometric analysis (Table 1).
CD27 and CD70 expressions on BM LPCs and MCs as assessed by flow cytometric and RT-PCR analyses
| . | Flow cytometry, no. (%) . | RT-PCR, no. (%) . | ||
|---|---|---|---|---|
| LPC . | MC . | LPC . | MC . | |
| CD27 | 5/12 (42) | 2/8 (25) | 7/7 (100) | 4/7 (52) |
| CD70 | 6/6 (100) | 10/11 (91) | 7/7 (100) | 7/7 (100) |
| . | Flow cytometry, no. (%) . | RT-PCR, no. (%) . | ||
|---|---|---|---|---|
| LPC . | MC . | LPC . | MC . | |
| CD27 | 5/12 (42) | 2/8 (25) | 7/7 (100) | 4/7 (52) |
| CD70 | 6/6 (100) | 10/11 (91) | 7/7 (100) | 7/7 (100) |
sCD27 in patients with WM and healthy donors
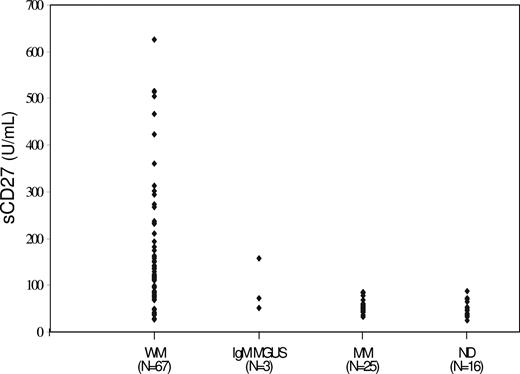
In view of these results demonstrating constitutive CD27 transcript expression in WM LPCs, but expression at the cell-surface level in only a subset of patients, we next sought to delineate if sCD27 was produced by WM LPCs. By ELISA, sCD27 was detectable in supernatants taken from BCWM.1 cells, which express CD27 transcripts by RT-PCR but are devoid of cell-surface CD27 expression (data not shown). Given these results, we next sought to determine if sCD27 levels were elevated in patients with WM, IgM monoclonal gammopathy of unknown significance (MGUS), and multiple myeloma (MM), as well as in healthy donors. As shown in Figure 1, significantly higher levels of sCD27 were observed in the serum of patients with active WM (n = 67; median, 120.1 U/mL; range, 27.2-626.2 U/mL) and IgM MGUS (n = 3; median, 71.3 U/mL; range, 51.3-156.7 U/mL) versus age-matched healthy donors (n = 16; median, 45.7 U/mL; range, 24.7-87.7 U/mL; P < .001 and P = .01 for comparison by Student t test of sCD27 levels in patients with WM and IgM MGUS versus healthy donors, respectively). In contrast, sCD27 levels for patients with MM with active disease (n = 25; median, 54.2 U/mL; range, 32.3-87.7 U/mL) did not differ from those observed in healthy donors (P = .2).
sCD27 levels in serum of patients with WM, IgM MGUS, MM, and healthy donors.
sCD27 as a marker of disease burden in WM
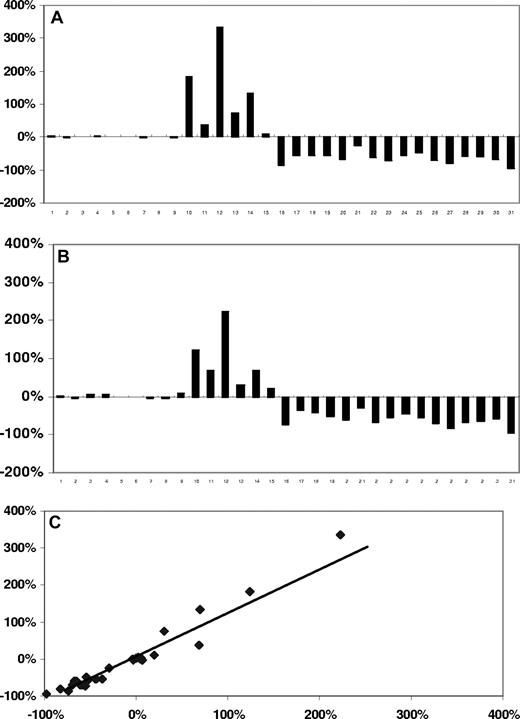
We next investigated if sCD27 served as a marker of disease burden in patients with WM. sCD27 levels were serially measured in 31 patients whose clinical outcomes (responder, stable disease, or progressive disease) were determined using consensus panel criteria based on concurrently measured changes in serum IgM levels.23 As shown in Figure 2, changes in sCD27 levels paralleled those of serum IgM levels in all outcome categories, and highly correlated to serial changes in serum IgM levels (r = .976; P < .001 by Pearson correlation test).
Temporal changes in serum IgM and sCD27 levels in WM patients. Serial (A,B) and correlative (C) changes in serum IgM and sCD27 levels in 31 patients with WM (WM 1-9, stable disease; WM 10-16, progressive disease; and WM 17-31, responders) whose outcomes were determined by consensus panel response criteria.23 (C) Diagonal line represents line of best fit.
Temporal changes in serum IgM and sCD27 levels in WM patients. Serial (A,B) and correlative (C) changes in serum IgM and sCD27 levels in 31 patients with WM (WM 1-9, stable disease; WM 10-16, progressive disease; and WM 17-31, responders) whose outcomes were determined by consensus panel response criteria.23 (C) Diagonal line represents line of best fit.
Sequence analysis of CD27 and CD70 in WM
As part of studies delineating CD27-CD70 interactions in WM, we sequenced CD27 and CD70 from genomic DNA isolated from BM LPCs and MCs isolated from 15 patients with WM. All exons with flanking intronic regions were sequenced on both strands. These studies demonstrated no novel sequence variants in CD27 and CD70 when compared with consensus sequences (National Center for Biotechnology Information [NCBI] human genome build 36.2), and all known polymorphisms appeared at their expected frequency with normal distribution.
Functional role of sCD27 in WM
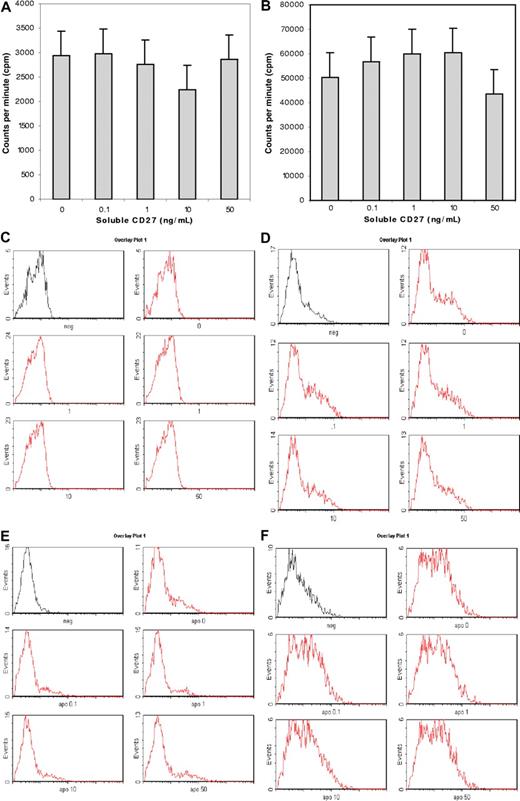
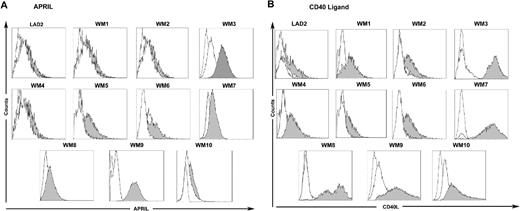
To define a functional role for sCD27-CD70 interactions in WM, we cultured BCWM.1 (CD27−CD70+) WM cells and LAD2 (CD27−CD70+) MCs, as well as primary BM LPCs and MCs (CD70+) isolated from patients with WM with recombinant human sCD27 (0.1-50 μg/mL). We observed no direct effect of sCD27 on the proliferation of BCWM.1 and LAD2 cells (as assessed by tritiated thymidine analysis) or on the induction of direct apoptosis (as assessed by APO2.7 staining) on BCWM.1 and LAD2 cells as well as BM LPCs and MCs from 10 patients with WM (Figure 3). However, as shown in Figure 3, culture of LAD2 MCs as well as primary MCs with sCD27 from 10 of 10 and 4 of 10 patients with WM resulted in marked up-regulation of CD40L and APRIL, respectively. Importantly, SGN-70 (1 μg/mL), a CD70-directed mAb that blocks CD27-CD70 signaling, abrogated sCD27 up-regulation of CD40L and APRIL (Figure 4). In contrast, we observed no changes in levels of BLYS on either WM MCs or LPCs, or in CD40L or APRIL levels on CD70 expressing WM LPCs following sCD27 stimulation (data not shown).
Effect of sCD27 on WM and MC proliferation and apoptosis. Effect of sCD27 (0-50 ng/mL) on proliferation of BCWM.1 LPCs (A) and LAD2 MCs (B) as assessed by tritiated thymidine uptake, as well as on induction of apoptosis of BCWM.1 LPCs (C) and LAD2 MCs (D), and on BM LPCs (E) and MCs (F) from a representative patient with WM as assessed by APO2.7 staining. (A,B) Results denote mean plus or minus 2 SD. (C-F) The first histogram represents unstained control cells, while histograms 2 through 5 represent cells stained with APO2.7 following treatment with sCD27 at 0, 0.1, 1, 10, and 50 ng/mL.
Effect of sCD27 on WM and MC proliferation and apoptosis. Effect of sCD27 (0-50 ng/mL) on proliferation of BCWM.1 LPCs (A) and LAD2 MCs (B) as assessed by tritiated thymidine uptake, as well as on induction of apoptosis of BCWM.1 LPCs (C) and LAD2 MCs (D), and on BM LPCs (E) and MCs (F) from a representative patient with WM as assessed by APO2.7 staining. (A,B) Results denote mean plus or minus 2 SD. (C-F) The first histogram represents unstained control cells, while histograms 2 through 5 represent cells stained with APO2.7 following treatment with sCD27 at 0, 0.1, 1, 10, and 50 ng/mL.
sCD27 induces APRIL and CD40L on WM patient BM MCs. Expression of APRIL (A) and CD40 ligand (B) on CD70-expressing LAD2 MCs, and primary BM MCs from 10 patients with WM following stimulation with sCD27 (10 μg/mL) alone (shaded histogram) or sCD27 (10 μg/mL) after preincubation with the CD70-directed SGN-70 (1 μg/mL) mAb (clear histogram, solid line). Dotted, clear histograms denotes isotype control.
sCD27 induces APRIL and CD40L on WM patient BM MCs. Expression of APRIL (A) and CD40 ligand (B) on CD70-expressing LAD2 MCs, and primary BM MCs from 10 patients with WM following stimulation with sCD27 (10 μg/mL) alone (shaded histogram) or sCD27 (10 μg/mL) after preincubation with the CD70-directed SGN-70 (1 μg/mL) mAb (clear histogram, solid line). Dotted, clear histograms denotes isotype control.
Direct targeting of WM LPCs by the CD70 directed human mAb SGN-70
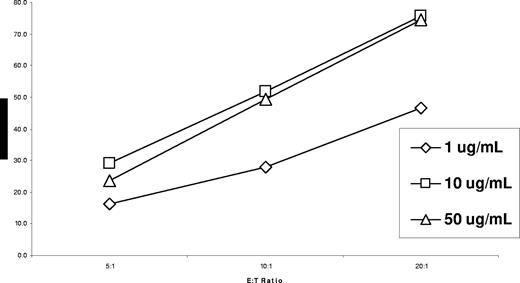
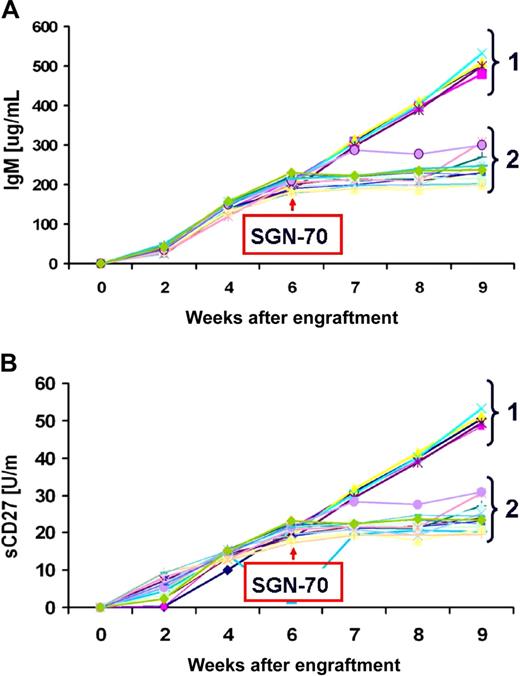
We next assessed the SGN-70 mAb for its ability to kill WM LPCs by direct induction of apoptosis, by complement-dependent cytotoxicity (CDC), and through antibody-dependent cell-mediated cytotoxicity (ADCC). We did not observe induction of apoptosis or CDC activity for SGN-70 against BCWM.1 LPCs at dose levels up to 20 μg/mL (data no shown). However, as shown in Figure 5, SGN-70 mediated robust dose-dependent ADCC activity against BCWM.1 cells. We further evaluated the therapeutic potential of SGN-70 in an in vivo WM model using SCID-hu mice bearing BCWM.1 cells. SCID-hu mice were engrafted with BCWM.1 cells and treated with the SGN-70 antibody after demonstrating progressive WM, as assessed by serial increases in human IgM and sCD27 serum levels. Treatment of WM SCID-hu mice every other day with SGN-70 (1 mg/kg, intraperitoneally) blocked disease progression, as assessed by serial human IgM and sCD27 serum levels in 12 of 12 mice, whereas disease continued to progress in all 5 of 5 untreated mice (P < .001 by Fisher exact test; Figure 6).
Specific lysis for SGN-70–mediated ADCC against CD70-expressing BCWM.1 WM LPCs at E/T ratios of 5:1 to 20:1.
Specific lysis for SGN-70–mediated ADCC against CD70-expressing BCWM.1 WM LPCs at E/T ratios of 5:1 to 20:1.
Treatment of WM SCID-hu mice with SGN-70. Effect of SGN-70 on disease progression as assessed by serial changes in serum IgM (A) and sCD27 (B) levels in SCID-hu mice engrafted with BCWM.1 WM LPCs. Group 1 denotes untreated mice (n = 5), and group 2 denotes mice treated with SGN-70 (n = 12).
Treatment of WM SCID-hu mice with SGN-70. Effect of SGN-70 on disease progression as assessed by serial changes in serum IgM (A) and sCD27 (B) levels in SCID-hu mice engrafted with BCWM.1 WM LPCs. Group 1 denotes untreated mice (n = 5), and group 2 denotes mice treated with SGN-70 (n = 12).
Discussion
The results of these studies demonstrate an important role for sCD27-CD70 signaling among BM LPCs and MCs in WM, and demonstrate a novel mechanism of action for sCD27 as a regulator of 2 principal TNF family members (APRIL and CD40L) whose role as growth and survival factors has previously been established by us and others in WM and other B-cell malignancies. In addition to its functional role, these studies also suggest that sCD27 may serve as a faithful marker of disease in patients with WM. Notably, our data also suggest that targeting CD70 and sCD27-CD70 interactions may produce important clinical benefits for patients with WM, and possibly other B-cell malignancies such as chronic lymphocytic leukemia (CLL) and Hodgkin disease (HD), as well as autoimmune-related disorders wherein either elevated sCD27 levels have been reported24-30 or the presence of excess MCs has been observed in association with lymphoid aggregates.31-34 Investigation, therefore, of a role for sCD27-CD70 interactions among lymphocytes and MCs in these disorders would appear warranted.
It remains unclear at present whether the high levels of sCD27 detected in patients with WM is a result of a malignant event, or part of a normal homeostatic mechanism for control of LPC expansion. Normally, CD27 is expressed on the cell surface of memory B cells from which WM is thought to derive. However, in WM, CD27 is heterogeneously expressed, and more often is absent on the cell surface of WM LPCs.7,13,17 Both APRIL and CD40L play important roles in normal LPC differentiation, and it remains plausible that their expression on MCs (and possibly other regulatory cells) are induced by sCD27 as a normal countermeasure to curtail LPC expansion by inducing their differentiation.
The release of sCD27 by CLL cells through the action of matrix metalloproteases (MMPs) has been suggested in one report.35 It remains possible that overexpression of MMP may contribute to the cell-surface loss of CD27 and subsequent release of sCD27 in WM. Investigation for a role of MMP in modulating sCD27 release is therefore warranted, as is also the potential therapeutic role of MMP inhibitors.
The lack of cell-surface CD27 observed in this and other studies of LPCs in most patients with WM7,14,18 may also have important implications for the deregulation of LPC homeostasis in WM. Upon binding of CD70 to CD27, the proapoptotic adaptor proteins SIVA-1 and SIVA-2 bind to the intercellular domain of CD27 and trigger apoptosis via a caspase-dependent mitochondrial pathway.36,37 The loss of cell-surface expression of CD27, inhibition of CD70 signaling by tumor-released sCD27, or loss of SIVA-1 and SIVA-2 may represent pathways by which normal homeostasis of LPCs is lost in WM.38 Our sequencing studies did not detect any variants in CD27 to suggest that a solubilized form of this molecule resulted from mutation inducing truncation of this protein. However, it does remain possible that posttranscriptional or posttranslational events may still be shifted as an epigenetic malignant phenomenon resulting in the overproduction of sCD27. Investigation into these potential mechanisms of WM pathogenesis is currently ongoing in our laboratory.
Additional investigation is also warranted to uncover the detailed molecular mechanisms underlying the up-regulation of CD40L and APRIL by sCD27 on MCs. Recent work has shown that CD70 activates the PtdIns-3 kinase, NFκB, protein kinase B, and Erk MAP kinase pathways.16,39 CD70 signaling affects cell-cycle entry and progression in activated B cells, and impedes plasma cell differentiation and IgG production in response to hapten-protein conjugates in vivo.39 Signaling through CD70 on WM MCs by sCD27 resulted in the up-regulation of CD40L and APRIL; however, this CD70 specific up-regulation was not observed in WM LPCs. This implies that there may be different signaling properties for CD70 between WM LPCs and MCs, or potentially an inhibitory network in WM LPCs. Moreover, our lack of observation of a proliferative or survival effect of sCD27 on CD70 expressing WM LPCs may also reflect the lack of other costimulatory signals such as CD40L as suggested by the studies of Arens et al.16 Further studies to characterize a direct functional role for sCD27 on WM LPCs are under way in our laboratory.
Last, the results of this study suggest that disruption of the CD27-CD70 signaling pathway may yield important clinical benefits for patients with WM, and possibly in patients with other malignancies and autoimmune disorders in which sCD27-CD70 interactions may have a pathogenic role. Use of the SGN-70 mAb, which is in early clinical development,13 blocked disease progression in SCID-hu mice with established WM. The mechanism(s) by which SGN-70 blocked disease progression in the SCID-hu WM mouse may be multifactorial (ie, immunologic and biological), and further studies to characterize its in vivo activity model are needed. ADCC or CDC activity, or some other immune effector function (ie, phagocytosis), may be possible, as suggested by our previous studies using rituximab in this mouse model,22 as is also blockade of sCD27-CD70 interactions. We propose in future experiments to use anti–SGN-70 Fab fragments in SCID-hu mice to further clarify this point.
In summary, the results of these studies suggest a functional role for sCD27 in WM pathogenesis, along with its utility as a surrogate marker of disease and a potential target for the development of therapeutics for WM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Peter and Helen Bing Fund for Waldenstrom macroglobulinemia, the Edward and Linda Nelson Fund for Translational Studies in WM, the Bailey Family Fund at the Dana-Farber Cancer Institute, and a National Institutes of Health Career Development Award (K23CA087977-03) to S.P.T.
National Institutes of Health
Authorship
A.W.H., E.H., A.R.B., Z.R.H., X.L., O.T., L.X., and K.O. performed basic science experiments, including ELISAs, flow cytometry, and RT-PCR; A.W.H., D.D.S., and M.C. performed animal studies; C.J.P., J.S., and R.J.M. provided clinical correlative data; J.A.M., C.L.L., and I.S.G. provided SGN-70 and supported therapeutic antibody studies; N.C.M. and S.P.T. oversaw research; and S.P.T. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: J.A.M., C.L.L., and I.S.G. are paid employees of Seattle Genetics Inc, manufacturer of SGN-70. All other authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström's Macroglobulinemia, Dana-Farber Cancer Institute, M548, 44 Binney Street, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu.