Abstract
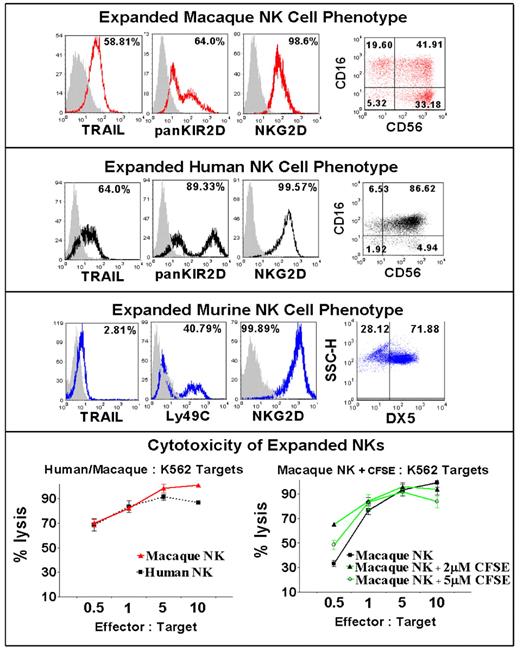
NK cell based immunotherapy represents a promising treatment approach for patients with cancer. Although preliminary clinical trials in humans suggest NK cell infusions can mediate anti-tumor effects, animal models are needed to provide insight into methods to enhance both the function and in vivo longevity of adoptively infused NK cells. Research conducted in our laboratory has shown that ex vivo expanded human NK cells are highly activated, up-regulating NKG2D, Granzyme B, TRAIL and Fas-ligand expression making them much more cytotoxic to tumor cells compared to freshly isolated NK cells. However, important questions remain regarding whether in vitro expansion alters the capacity of these cells to replicate, and traffic to tissues in vivo following their adoptive infusion into recipients. Differences in the genotype and phenotype of mouse NK cells compared to human NK cells limit the value of murine animal models to address these questions. In contrast to mice, Rhesus macaques have orthologues to most of the human MHC class I and II genes and possess NK cells expressing KIRs that are phenotypically and functionally similar to human NK cells, thus providing an excellent model system for evaluating questions related to adoptive NK cell therapy. We developed an in vitro method to expand macaque NK cells to characterize their in vivo longevity and tissue trafficking following adoptive infusion. Macaque NK cells were enriched from peripheral blood mononuclear cells by depleting CD3+ cells using immunomagnetic beads and were then expanded in vitro with autologous plasma and a human EBV-LCL feeder cell line using culture conditions identical to those used to expand NK cells from humans. NK cell cultures expanded 50- to 100-fold over 7 to 20 days, were greater than 99% CD3 negative, and had a similar phenotype to human NK cells including a large proportion of CD16/CD56 double positive cells, and ubiquitous expression of NKG2D, KIR2D, LFA-1, granzyme B, and CXCR3. In contrast to mice but analogous to human NK cells, macaque expanded NK cells upregulated surface expression of TRAIL and were highly cytotoxic to K562 cells and other human tumor lines (Figure). CFSE labelling of expanded NK cells did not alter their phenotype or tumor cytotoxic function. Data characterizing the longevity, proliferative capacity, and tissue trafficking patterns in the blood, bone marrow and lymph node of in vitro expanded and adoptively infused CFSE labeled NK cells (up to 1 × 108 NK Cells/kg i.v.) in macaque recipients will be presented from this analysis.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal