Abstract
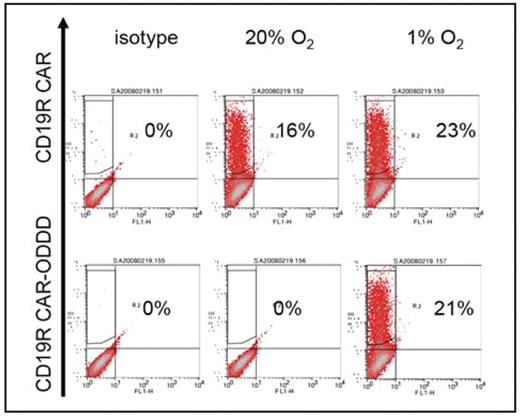
Solid tumors are characterized by cell clusters experiencing chronic and intermittent hypoxia due to haphazard growth. Importantly, highly migratory tumorigenic stem cells are localized within hypoxic niches, shielded by hypoxia-induced metabolites that inhibit tumor-infiltrating T cells, restricting their survival, cytotoxicity, and beneficial immunomodulatory cytokine responses. To eliminate bulky solid tumors including stem cells, T cells must overcome physiological changes distinctive for the tumor microenvironment, such as hypoxia, depleted nutrient levels, and low extracellular pH. To exploit the hypoxic tumor microenivornment, we introduce a new approach that exploits hypoxia as a condition for T-cell activation. We demonstrate a chimeric antigen receptor (CAR), which is specific for CD19 and capable of activating T cells through chimeric CD28 and/or CD3-zeta signaling endodomain, that can be conditionally expresed in a strictly oxygen-sensitive manner. Using the Sleeping Beauty transposon/transposase system, we can achieve stable, persistent CAR transgene expression without the expense and complexity of retroviral production. Cell surface expression of our CAR is high at 1% O and not detectable at 20% O (Figure). Anticipating the circulatory nature of T cells in vivo, our oxygen-sensitive CAR is designed to deactivate upon exiting from hypoxic tumor microenvironment to minimize deleterious off-target effects. When supplemented with one or more survival factors and/or homing receptors, this approach to CAR expression has the advantage of
transforming hypoxia from an adverse factor to a triggering mechanism,
efficiency, and
high levels of transgene expression.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal