Abstract
Factor VIII (FVIII) circulates bound to von Willebrand factor, and upon proteolytic activation it dissociates and attaches to activated membranes, e.g. at the platelet surface, that expose negatively-charged phosphatidylserine. This membrane association is mediated entirely by the FVIIIa light chain (A3-C1-C2), and the C2 domain is known to be a primary contributor to the membrane affinity. We previously demonstrated that the C1 domain, which is homologous to C2, also contributes to the affinity for activated platelets, (
Hsu et al., Blood 111, 200–208, 2008
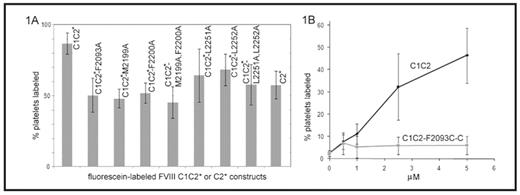
). Our earlier work showed that the platelet affinity of recombinant C1C2, as well as the total number of binding sites per platelet, were significantly higher than those measured for recombinant C2. Thus C1 either interacts with the platelet surface directly, or it stabilizes a conformation of C2 that promotes membrane binding. In this study, the affinities for activated platelets of a series of mutant C1C2 proteins were evaluated to determine residues involved in FVIII binding to platelets. C1C2 and C2 proteins were generated with a free cysteine residue substituted for the wild-type serine at position 2296 in C2 (S2296C does not disrupt the protein structure or affect membrane binding), to which a sulfyhydryl-linked fluorescein probe was attached covalently (C1C2* and C2*). Single or double alanine substitutions were introduced at the two beta-hairpin turns in C2 that mediate membrane binding (M2199A, F2200A, L2251A, L2252A), and at C1 residue F2093, which aligns with C2 residue L2252. Washed platelets were activated with SFLLRN-amide, incubated for 1 hr with the wild-type or mutant proteins, and analyzed by flow cytometry on a FACSCaliber. The relative binding affinities were estimated by using multiple protein concentrations and comparing the fluorescent signals to the values for saturation binding of C1C2* and C2*. Alanine substitutions at all of these positions resulted in decreased binding of C1C2*, comparable to the difference in affinity of C2* versus C1C2* (figure 1A). C1C2-F2093C was then generated, and the introduced sulfhydryl was blocked by adding free cysteine, thus introducing the bulky, charged cystine residue at this putative membrane-binding site (C1C2-F2093C-C). Binding assays utilizing detection by monoclonal antibody ESH8, which does not interfere with membrane attachment, followed by a PE-labeled secondary antibody showed that C1C2-F2093C-C had markedly reduced binding to activated platelets compared to C1C2 (figure 1B). These results are consistent with the hypothesis that the C1 domain contacts the membrane directly when FVIIIa becomes attached to the activated platelet surface and indicate that F2093 contributes significantly to this interaction.Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author
2008, The American Society of Hematology
2008

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal