Abstract
Data from several investigators suggest that the α2β1 integrin, a receptor for collagens, laminins, decorin, E-cadherin, matrix metalloproteinase-1, endorepellin, and several viruses, is required for innate immunity and regulation of autoimmune/allergic disorders. We demonstrated that the innate immune response to Listeria monocytogenes required α2β1 integrin expression by peritoneal mast cells (PMCs). Ligation of the α2β1 integrin by C1q contained in immune complexes comprised of Listeria and antibody was required for PMC activation in vitro and in vivo. However, ligation of the α2β1 integrin alone was insufficient to activate cytokine secretion, suggesting that one or more additional signals emanating from a coreceptor were required for PMC activation. Here, we demonstrate that C1q, but neither other complement proteins nor FcRγ, is required for early innate immune response to Listeria. The binding of Listeria's Internalin B (InlB) to hepatocyte growth factor receptor (HGF-R)/c-met provides the costimulatory function required for PMC activation. Either HGF or Listeria InlB bound to c-met and either C1q or type I collagen bound to α2β1 integrin stimulates PMC activation. These findings suggest that crosstalk between c-met and the α2β1 integrin may contribute to mast-cell activation in autoimmune and inflammatory disorders.
Introduction
The role of the α2β1 integrin in the innate and acquired immune response has been an area of active investigation. We initially reported that the α2β1 integrin-deficient mice exhibited markedly diminished inflammatory responses to Listeria monocytogenes because of a requirement for α2β1 integrin expression on peritoneal mast cells (PMCs) for mast-cell activation and cytokine release in vivo.1 Although the α2β1 integrin serves as a receptor for several matrix and nonmatrix ligands, the ligand for the integrin during the PMC response to infection was unknown.2,3 We demonstrated that C1q complement protein and collectin family members, including mannose binding lectin and surfactant protein A, all served as ligands for the integrin.4 In addition, the α2β1 integrin was required for mast-cell activation in vitro in response to Listeria. However, ligation of the α2β1 integrin alone was insufficient to activate cytokine secretion because mast-cell adhesion to collagen or C1q alone failed to support cytokine secretion.4
We hypothesized that one or more additional signals emanating from an additional receptor was required to activate mast-cell cytokine secretion in response to immune complexes. At that time, we presented 2 alternative models by which α2β1 integrin-ligand interactions may stimulate mast-cell activation and cytokine secretion. Our first model proposed that ligation of the α2β1 integrin simultaneously with a second, costimulatory receptor elicited mast-cell activation, in a manner reminiscent of the role proposed for the α2β1 integrin and the glycoprotein VI/Fc receptor common γ chain (FcRγ) during platelet adhesion to collagen.5,6 In our second model, binding of the α2β1 integrin to an immune complex containing C1q may directly activate the complement cascade, resulting in the deposition of C3b or iC3b and generation of complement byproducts, such as C3a or C5a, which would subsequently stimulate mast cell activation.7-9
We now report that neither the FcRγ nor components of the complement cascade are required in α2β1 integrin-dependent mast-cell activation. Instead, we describe a novel coreceptor required for mast-cell activation, hepatocyte growth factor (HGF-R)/c-met. The Listeria-specific molecule, Internalin B (InlB), binds to its host cell receptor, c-met to mediate internalization into epithelial and hepatic cells.10 We demonstrate that activation of mature PMCs by Listeria plus immune complex requires costimulatory signals from α2β1 integrin ligation to either type I collagen or C1q and c-met binding to either InlB or HGF. The synergistic signals from the 2 coreceptors result in mast-cell activation and the release of the proinflammatory cytokine interleukin-6 (IL-6) that induce the early innate immune responses to L monocytogenes.
Methods
Mice and Listeria
α2 integrin-subunit (α2−/−), FcRγ (FcRγ−/−), C1q (C1q−/−)-deficient mice, and WT (WT) littermate controls on a C57BL/6 × 129/Sv background were used at 6 to 20 weeks of age. FcRγ-deficient mice were obtained from Dr Paul Allen (Washington University School of Medicine). C1q-deficient mice, originally generated by Drs Walport and Botto, were obtained on a pure C57BL/6 background from Drs Michael Diamond (Washington University School of Medicine, St Louis, MO) and Gregory L. Stahl (Harvard University School of Medicine, Boston, MA).11 Mice were maintained under specific pathogen-free conditions in the Vanderbilt University School of Medicine (Nashville, TN) mouse facilities. Within individual experiments, mice were age and sex matched. Wild-type Listeria (EGD) and its isogenic mutants, ΔInlA and ΔInlB (provided by Dr E. Unanue from Washington University, St Louis, MO), were cultured in brain heart infusion broth (BD Biosciences, San Diego, CA) at 37°C.
Mast-cell preparation
PMCs were isolated from resident peritoneal exudates using Percoll gradient centrifugation (∼85% purity).1 Expression of c-kit, α2β1 integrin, or c-met was carried out by flow cytometric analysis using the following antibodies (all from BD Biosciences): fluorescein isothiocyanate-anti-CD117 (c-kit; 2B8), phycoerythrin–anti-CD49b (integrin subunit; HMα2).
In vitro adhesion and activation assays
Adhesion assays were performed as previously described.4 Static adhesion assays were performed in 96-well plates (Immulon 2HB; Thermo Electron, Waltham, MA).12,13 Wells were coated with bovine serum albumin (BSA) (5 μg/mL; Sigma-Aldrich, St Louis, MO), type 1 collagen (25 μg/mL rat tail; BD Biosciences), human C1q (25 μg/mL; Calbiochem, San Diego, CA), a matrix of L monocytogenes, anti-Listeria antibody, and serum, or a matrix of BSA, anti-BSA, and serum. The Listeria or BSA matrix was formed by allowing Listeria (strain EGD, 108 organisms/mL in 0.1 M carbonate buffer, pH 8.5) or BSA (5 μg/mL in phosphate-buffered saline [PBS]) to adhere to wells of a 96-well plate overnight. Unattached Listeria or BSA was removed and polyclonal anti-Listeria antibody (1:200 dilution in PBS; Difco, Detroit, MI) or anti-BSA antibody (1:1000 dilution in PBS; Invitrogen Life Technologies, Carlsbad, CA) was added and incubated at 37°C for 1 hour. Fresh mouse serum from WT, C1q−/−, C3−/−, C4−/−, C5−/− or factor B−/− mice (sera from C3−/−, C4−/−, C5−/−, and factor B−/− kindly provided by Michael Diamond, Washington University, St Louis, MO, 50%) was added for 1 hour at 37°C. PMCs (2000 cells/well) were allowed to adhere for 1 hour at 37°C in the presence of 2 mM MgCl2 or 2 mM EDTA. Nonadherent cells were removed and adherent cells were quantitated as previously described.13
For in vitro mast-cell activation by Listeria, purified PMCs (5 × 104 cells/well) were incubated for 1 hour at 37°C with a washed suspension of Listeria (107 organisms), incubated with rabbit anti-Listeria antibody, and 50% serum from either WT, C1q−/−, C3−/−, C4−/−, C5−/−, or factor B−/− mice. For in vitro mast-cell activation by BSA immune complexes, purified PMCs (5 × 104 cells/well) were incubated with a washed suspension of latex beads (Polysciences, Warrington, PA) coated with BSA (3 mg/mL), anti-BSA antibody, and serum (50%) alone or in the presence of lipopolysaccharide (LPS, 100 ng/mL, Sigma-Aldrich), Pam3Cys-Ser-(Lys)4 × 3HCl (Pam3Cys, 100 ng/mL, EMC Microcollections, Tuebingen, Germany), Listeria (108), heat-killed L monocytogenes (108 heated for 30 minutes at 60°C), or HGF (2 mg/mL, R&D Systems Minneapolis, MN). Anti–c-met antibody was obtained from R&D Systems. Supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) for IL-6 (BD Biosciences).
In vivo model of peritonitis
Listeria strain EGD and its isogenic mutants were stored at mid-log growth as glycerol stocks at −80°C and diluted in pyrogen-free saline for injection into mice. Bacteria were injected at a dose of 5 × 104Listeria/mouse intraperitoneally in 500 μL. At indicated time points after injection, mice were killed and peritoneal exudates were collected by lavage in 10 mL RPMI. Cell-free supernatants were stored at −20°C and later used for the determination of IL-6, tumor necrosis factor-α (TNF-α), and IL-1β by ELISA (BD Biosciences). Total cell number was determined for each mouse, and cells were cytospun onto slides and stained with the Hem 3 staining kit (Fisher Scientific, Pittsburgh, PA). The percentage of polymorphonuclear leukocytes (PMNs) was determined by differential cell counting.
Results
α2β1 integrin-dependent mast-cell activation results in IL-1β secretion
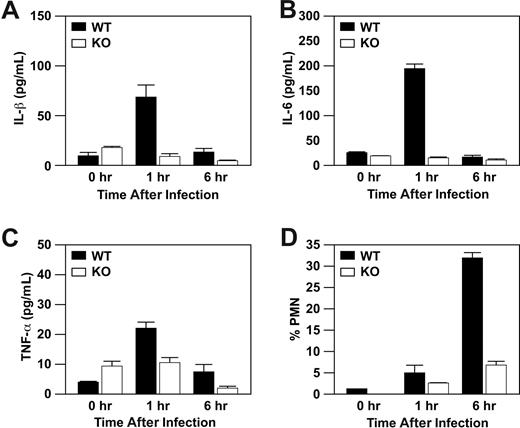
Previously, we reported that α2β1 integrin expression on mast cells is required for IL-6 production at 1 hour and neutrophil recruitment at 6 hours after infection with Listeria. However, IL-6 is not a chemoattractant. To define chemoattractants responsible for neutrophil influx after Listeria infection, we measured the levels of IL-1β, leukotriene B4 (LTB4), and TNF-α in the peritoneal fluid of WT and α2-null mice. Wild-type mice, but not α2-null mice, demonstrated a marked increase of IL-1β and IL-6 levels at 1 hour after infection with Listeria that was diminished by 6 hours after infection (Figure 1A,B).14 In addition, there was a small increase in the level of TNF-α in the WT, but not α2-null, mice at 1 and 6 hours (Figure 1C). Wild-type mice, but not α2-null mice, manifested a robust influx of PMNs into the peritoneal cavity that peaked at 6 hours after infection, as previously reported (Figure 1D). The level of IL-1β that we observe is consistent with several reports demonstrating the neutrophil chemotactic properties of IL-1β. Although LTB4 is secreted by mast cells and is a potent stimulator of neutrophil migration, we did not observe increased LTB4 above baseline at the time points tested (data not shown). Therefore, in addition to IL-6, several chemoattractants are secreted in an α2β1 integrin-dependent manner that may contribute to the acute inflammatory response to L monocytogenes.
Wild-type mice, but not α2β1 integrin-deficient mice, display increased levels of IL-1β, IL-6, and TNF-α in response to Listeria infection. Wild-type and α2β1 integrin-deficient mice were infected with 5 × 104Listeria intraperitoneally. At the indicated time points after infection, the concentration of IL-1β (A), IL-6 (B), TNF-α (C), and the percentage of neutrophils (D) were determined. Shown is a combination of 3 experiments (mean ± SEM) with each time point representing 3 mice.
Wild-type mice, but not α2β1 integrin-deficient mice, display increased levels of IL-1β, IL-6, and TNF-α in response to Listeria infection. Wild-type and α2β1 integrin-deficient mice were infected with 5 × 104Listeria intraperitoneally. At the indicated time points after infection, the concentration of IL-1β (A), IL-6 (B), TNF-α (C), and the percentage of neutrophils (D) were determined. Shown is a combination of 3 experiments (mean ± SEM) with each time point representing 3 mice.
FcRγ is not required for mast-cell activation
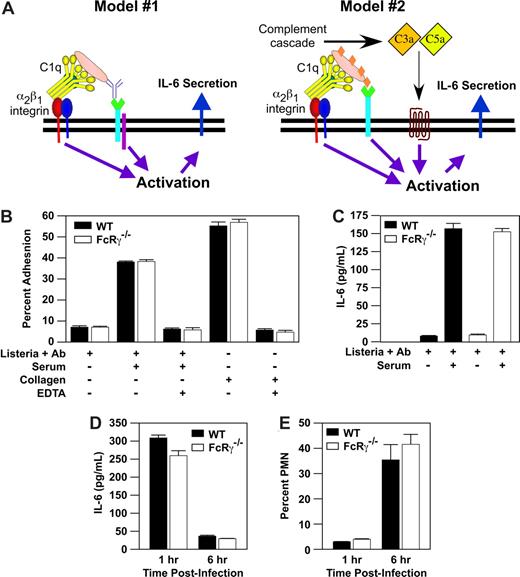
We demonstrated that α2β1 integrin-C1q-immune complex interaction was required, but not sufficient, for mast-cell activation in response to L monocytogenes. These data suggested that one or more additional signals emanating from an additional receptor, such as the FcRγ chain, was required to activate cytokine secretion (Figure 2A).1 To determine whether the FcRγ chain was required, we compared the ability of purified PMCs from WT or FcRγ−/− mice to bind to a matrix of either Listeria-containing immune complexes or type I collagen. PMCs from both WT and FcRγ−/− mice adhered to collagen and Listeria containing immune complexes in an α2β1 integrin-mediated, divalent cation-dependent manner (Figure 2B). These data demonstrate that PMC adhesion to immune complex via the α2β1 integrin does not require FcRγ. To determine whether α2β1 integrin-dependent PMC activation requires signaling downstream of the FcRγ, we measured the release of IL-6 from WT and FcRγ−/− PMCs after stimulation with a complex of Listeria plus anti-Listeria IgG antibody or an immune complex of Listeria plus anti-Listeria IgG antibody plus serum. Neither WT nor FcRγ−/− PMCs were activated in the presence of Listeria and antibody alone. In contrast, both, WT and FcRγ−/− mice released similarly high levels of IL-6 in response to Listeria plus antibody plus serum (Figure 2C). These results indicate that FcRγ is not required in vitro for the α2β1 integrin-dependent activation of PMC by Listeria plus immune complex. In addition, these data suggest that the FcRγ does not serve as a coreceptor for the α2β1 integrin in immune complex–stimulated mast-cell activation.
Mast-cell activation by Listeria immune complex does not require FcRγ. (A) A proposed model is a 2-site, 2-receptor model in which concurrent activation of the α2β1 integrin and secondary receptor (complement receptor, FcRγ, Listeria receptor) stimulates mast-cell activation. Adapted from Edelson et al1 with permission. (B) Purified PMCs (2 × 104) from either WT (WT) mice or from mice lacking FcRγ (FcRγ−/−) were assayed for adhesion to a matrix consisting of (1) Listeria plus anti-Listeria antibody alone, (2) Listeria, anti-Listeria antibody and 50% murine serum, or (3) type I collagen in the presence or absence of 1 mM EDTA. (C) Purified PMCs (5 × 104) from WT and FcRγ−/− mice were incubated for 1 hour with a washed suspension of Listeria, anti-Listeria antibody, and 50% murine serum. Supernatants were collected and analyzed for IL-6 production by ELISA. All adhesion and activation experiments were carried out in the presence of 2 mM MgCl2. Results are mean plus or minus SEM from triplicate wells of a single experiment and represent 1 of at least 3 experiments demonstrating similar results. (D,E) WT and FcRγ−/− mice were infected for 1 or 6 hours with 5 × 104Listeria intraperitoneally. At the indicated time points, the percentage of PMNs and concentration of IL-6 in the peritoneal fluid were determined. Shown is a representative example of at least 3 experiments (mean ± SEM), all carried out in triplicate.
Mast-cell activation by Listeria immune complex does not require FcRγ. (A) A proposed model is a 2-site, 2-receptor model in which concurrent activation of the α2β1 integrin and secondary receptor (complement receptor, FcRγ, Listeria receptor) stimulates mast-cell activation. Adapted from Edelson et al1 with permission. (B) Purified PMCs (2 × 104) from either WT (WT) mice or from mice lacking FcRγ (FcRγ−/−) were assayed for adhesion to a matrix consisting of (1) Listeria plus anti-Listeria antibody alone, (2) Listeria, anti-Listeria antibody and 50% murine serum, or (3) type I collagen in the presence or absence of 1 mM EDTA. (C) Purified PMCs (5 × 104) from WT and FcRγ−/− mice were incubated for 1 hour with a washed suspension of Listeria, anti-Listeria antibody, and 50% murine serum. Supernatants were collected and analyzed for IL-6 production by ELISA. All adhesion and activation experiments were carried out in the presence of 2 mM MgCl2. Results are mean plus or minus SEM from triplicate wells of a single experiment and represent 1 of at least 3 experiments demonstrating similar results. (D,E) WT and FcRγ−/− mice were infected for 1 or 6 hours with 5 × 104Listeria intraperitoneally. At the indicated time points, the percentage of PMNs and concentration of IL-6 in the peritoneal fluid were determined. Shown is a representative example of at least 3 experiments (mean ± SEM), all carried out in triplicate.
In light of the importance of the FcRγ in many immune responses to Ig complexes, we wanted to determine whether the FcRγ was required for the early neutrophil recruitment in response to Listeria. To determine the role of the FcRγ in vivo in response to Listeria, PMN influx and IL-6 secretion into the peritoneal cavity were evaluated in WT and FcRγ−/− mice. Both WT and mice deficient in the FcRγ exhibited maximal IL-6 secretion at 1 hour and a robust neutrophil response at 6 hours (Figure 2D,E). These data demonstrate that signals downstream of the FcRγ are not required for the early innate immune response to Listeria.
Complement activation is not required for mast-cell activation
We therefore hypothesized that mast-cell binding via the α2β1 integrin to an immune complex containing C1q may directly activate the complement cascade, resulting in the deposition of C3b or iC3b and the generation of complement byproducts such as C3a or C5a. In turn, binding of complement components to their cognate receptors on mast cells (complement receptor 1 [CR1], CR3, CR4, C3aR, or C5aR) would then stimulate mast-cell activation, as has previously been shown.7-9
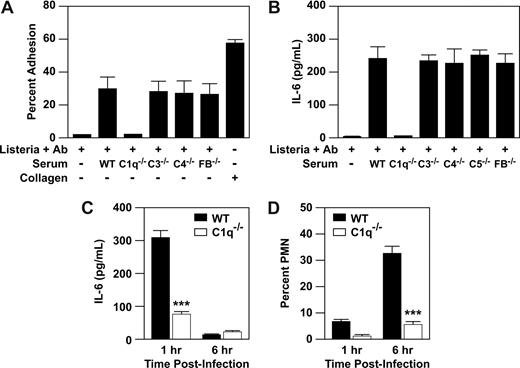
To determine whether complement activation provided the costimulatory signal for IL-6 release, we evaluated adhesion and activation of WT PMCs in response to immune complex formed from Listeria plus anti-Listeria antibody and murine serum from WT mice or mice deficient in classic complement cascade components, C1q, C3, C4, or C5, or the alternate cascade component, factor B. Listeria plus anti-Listeria antibody plus serum from WT mice and mice deficient in C3, C4, C5, and factor B resulted in formation of an adhesive substrate (Figure 3A). As previously demonstrated, C1q-deficient serum failed to form an adhesive substrate for the α2β1 integrin.4 To evaluate PMC activation, we measured secretion of IL-6 by PMCs after stimulation with Listeria plus anti-Listeria antibody plus WT serum or serum deficient in C1q, C3, C4, C5, or factor B. Wild-type PMCs secreted high levels of IL-6 in response to immune complexes formed with WT serum, as well as with serum deficient in C3, C4, C5, or factor B. As expected from our earlier report,4 immune complexes from C1q-deficient serum failed to activate WT PMCs (Figure 3B). Therefore, C1q, but not other complement components, is required for mast-cell activation. These results indicate that neither FcRγ nor complement components other than C1q act as costimulatory signals for PMC activation by Listeria immune complexes.
C1q, but not other complement components, supplies stimulatory signal for mast-cell activation by Listeria-immune complex. (A) Purified PMCs (2 × 104 cells/well) isolated from WT (WT) mice were assayed for adhesion to a matrix consisting of Listeria plus anti-Listeria antibody, Listeria, anti-Listeria antibody alone, and 50% murine serum obtained from either WT mice or mice deficient in the complement components, C1q, C3, C4, C5, or factor B (C1q−/−, C3−/−, C4−/−, C5−/−, FB−/−) or type I collagen. (B) Purified PMCs (5 × 104) from WT and mice were incubated for 1 hour with a washed suspension of Listeria, anti-Listeria antibody, and 50% murine serum from either WT or mice deficient in the complement components C1q, C3, C4, C5, and factor B. Supernatants were collected and analyzed for IL-6 production by ELISA. All adhesion and activation experiments were carried out in the presence of 2 mM MgCl2. Results are mean plus or minus SEM from triplicate wells of a single experiment and represent 1 of at least 3 experiments demonstrating similar results. (C,D) WT and C1q−/− mice were infected for 1 or 6 hours with 5 × 104Listeria intraperitoneally. At the indicated time points, the percentage PMN and IL-6 in the peritoneal fluid were determined. Shown is representative of at least 3 experiments (mean ± SEM), carried out in triplicate. Statistics were performed using unpaired Student t test (***P < .001).
C1q, but not other complement components, supplies stimulatory signal for mast-cell activation by Listeria-immune complex. (A) Purified PMCs (2 × 104 cells/well) isolated from WT (WT) mice were assayed for adhesion to a matrix consisting of Listeria plus anti-Listeria antibody, Listeria, anti-Listeria antibody alone, and 50% murine serum obtained from either WT mice or mice deficient in the complement components, C1q, C3, C4, C5, or factor B (C1q−/−, C3−/−, C4−/−, C5−/−, FB−/−) or type I collagen. (B) Purified PMCs (5 × 104) from WT and mice were incubated for 1 hour with a washed suspension of Listeria, anti-Listeria antibody, and 50% murine serum from either WT or mice deficient in the complement components C1q, C3, C4, C5, and factor B. Supernatants were collected and analyzed for IL-6 production by ELISA. All adhesion and activation experiments were carried out in the presence of 2 mM MgCl2. Results are mean plus or minus SEM from triplicate wells of a single experiment and represent 1 of at least 3 experiments demonstrating similar results. (C,D) WT and C1q−/− mice were infected for 1 or 6 hours with 5 × 104Listeria intraperitoneally. At the indicated time points, the percentage PMN and IL-6 in the peritoneal fluid were determined. Shown is representative of at least 3 experiments (mean ± SEM), carried out in triplicate. Statistics were performed using unpaired Student t test (***P < .001).
These studies demonstrated a role for C1q in in response to Listeria in vitro. To demonstrate a requirement for C1q in vivo, we infected WT and C1q−/− mice with Listeria. In WT mice, IL-6 was released into the peritoneal cavity at 1 hour after infection. In contrast, C1q−/− mice failed to respond with IL-6 in response to Listeria (Figure 3C). In addition, there was a significant decrease in the number and percentage of PMNs that infiltrated the peritoneal cavity of C1q−/− mice at 6 hours compared with the WT mice (Figure 3D). These results support our hypothesis that C1q is important in the mast cell–dependent response to peritoneal Listeria infection. Furthermore, C1q binding to α2β1 integrin provided a signal that could not be duplicated by peritoneal matrix components, such as collagen.
Listeria surface molecules stimulate PMC activation
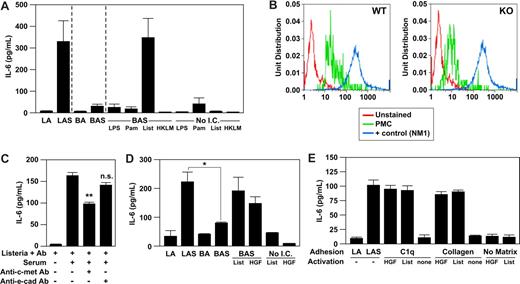
We had previously shown that WT PMCs adhere to plate-bound immune complexes formed between BSA, anti-BSA antibody, and serum in an α2β1 integrin-dependent manner.4 To determine whether binding of WT PMCs to an immune complex alone, without Listeria, was sufficient to mediate cytokine secretion, we compared IL-6 secretion by PMCs after 1-hour stimulation with immune complexes consisting of BSA-coated latex beads plus anti-BSA antibody and serum or immune complexes consisting of Listeria plus anti-Listeria antibody plus serum. Wild-type PMCs secreted abundant IL-6 in response to Listeria plus immune complex, as previously shown, but failed to secrete IL-6 in response to BSA plus immune complex. Therefore, the immune complex alone was not sufficient to stimulate PMC activation (Figure 4A). The addition of Listeria alone to the BSA-immune complex restored IL-6 secretion to levels similar to activation with Listeria containing immune complex (Figure 4A). This result suggested that interactions of the PMC with Listeria were providing the additional activation signal required after α2β1 integrin-ligation to immune complex.s
HGF-R/c-met is the receptor providing the costimulatory signal for mast-cell activation. (A) Purified PMCs (5 × 104) isolated from WT mice were incubated for 1 hour with a washed suspension of Listeria and anti-Listeria antibody alone (LA), Listeria, anti-Listeria antibody plus 50% serum from WT mice (LAS), latex beads coated with bovine serum albumin (BSA) plus anti-BSA antibody alone (BA), BSA plus anti-BSA antibody and 50% WT murine serum (BAS), BSA plus anti-BSA antibody and 50% WT murine serum (BAS) with either LPS (100 ng/mL), Pam3Cys (100 μg/mL), Listeria (108), heat-killed Listeria (108 organisms), or LPS (100 ng/mL), Pam3Cys (100 μg/mL), Listeria (108), heat-killed Listeria (108 organisms) alone. Supernatants were collected and analyzed for IL-6 production by ELISA. (B) Representative flow cytometric histograms of PMCs stained with c-met PMCs from WT (panel A) and α2β1 integrin-deficient (KO, panel B) were stained with phycoerythrin–anti–c-kit and APC–anti–c-met and assessed by flow cytometry. Mast cells were identified as c-kithigh–staining cells and represented 1% to 3% of resident peritoneal cells in both WT and KO mice. (C) Purified PMCs isolated from WT mice were pretreated with inhibitory antibodies toward E-cadherin, c-met, or irrelevant control antibody for 1 hour before stimulation with a washed suspension of Listeria, anti-Listeria antibody alone, or Listeria, anti-Listeria antibody, and 50% WT murine serum. Supernatants were collected and analyzed for IL-6 production by ELISA. (D) Purified PMCs (5 × 104) isolated from WT mice were incubated with Listeria and anti-Listeria antibody alone (LA), Listeria, and anti-Listeria antibody plus 50% serum from WT mice (LAS), latex beads coated with BSA, plus anti-BSA antibody alone (BA), or latex beads coated with BSA, anti-BSA antibody, plus 50% serum (BAS) plus or minus the addition of Listeria (108) or HGF (2 mg/mL), or Listeria (108), or HGF (2 mg/mL) alone. Supernatants were collected and analyzed for the concentration of IL-6 by ELISA. (E) Purified PMCs (5 × 104) isolated from WT mice were allowed to adhere to a matrix of Listeria and anti-Listeria antibody (LA), Listeria, anti-Listeria antibody plus serum (LAS), type I collagen (25 mg/mL), C1q (25 mg/mL), or tissue culture plastic (No Matrix) with or without either Listeria (108) or HGF (2 mg/mL). Supernatants were collected and analyzed for IL-6 production by ELISA. All experiments were carried out in the presence of 2 mM MgCl2. Results are presented as means plus or minus SEM from triplicate wells of a single experiment and represent 1 of at least 3 experiments demonstrating similar results. The P values were determined by unpaired Student t test (*P < .05, **P < .01).
HGF-R/c-met is the receptor providing the costimulatory signal for mast-cell activation. (A) Purified PMCs (5 × 104) isolated from WT mice were incubated for 1 hour with a washed suspension of Listeria and anti-Listeria antibody alone (LA), Listeria, anti-Listeria antibody plus 50% serum from WT mice (LAS), latex beads coated with bovine serum albumin (BSA) plus anti-BSA antibody alone (BA), BSA plus anti-BSA antibody and 50% WT murine serum (BAS), BSA plus anti-BSA antibody and 50% WT murine serum (BAS) with either LPS (100 ng/mL), Pam3Cys (100 μg/mL), Listeria (108), heat-killed Listeria (108 organisms), or LPS (100 ng/mL), Pam3Cys (100 μg/mL), Listeria (108), heat-killed Listeria (108 organisms) alone. Supernatants were collected and analyzed for IL-6 production by ELISA. (B) Representative flow cytometric histograms of PMCs stained with c-met PMCs from WT (panel A) and α2β1 integrin-deficient (KO, panel B) were stained with phycoerythrin–anti–c-kit and APC–anti–c-met and assessed by flow cytometry. Mast cells were identified as c-kithigh–staining cells and represented 1% to 3% of resident peritoneal cells in both WT and KO mice. (C) Purified PMCs isolated from WT mice were pretreated with inhibitory antibodies toward E-cadherin, c-met, or irrelevant control antibody for 1 hour before stimulation with a washed suspension of Listeria, anti-Listeria antibody alone, or Listeria, anti-Listeria antibody, and 50% WT murine serum. Supernatants were collected and analyzed for IL-6 production by ELISA. (D) Purified PMCs (5 × 104) isolated from WT mice were incubated with Listeria and anti-Listeria antibody alone (LA), Listeria, and anti-Listeria antibody plus 50% serum from WT mice (LAS), latex beads coated with BSA, plus anti-BSA antibody alone (BA), or latex beads coated with BSA, anti-BSA antibody, plus 50% serum (BAS) plus or minus the addition of Listeria (108) or HGF (2 mg/mL), or Listeria (108), or HGF (2 mg/mL) alone. Supernatants were collected and analyzed for the concentration of IL-6 by ELISA. (E) Purified PMCs (5 × 104) isolated from WT mice were allowed to adhere to a matrix of Listeria and anti-Listeria antibody (LA), Listeria, anti-Listeria antibody plus serum (LAS), type I collagen (25 mg/mL), C1q (25 mg/mL), or tissue culture plastic (No Matrix) with or without either Listeria (108) or HGF (2 mg/mL). Supernatants were collected and analyzed for IL-6 production by ELISA. All experiments were carried out in the presence of 2 mM MgCl2. Results are presented as means plus or minus SEM from triplicate wells of a single experiment and represent 1 of at least 3 experiments demonstrating similar results. The P values were determined by unpaired Student t test (*P < .05, **P < .01).
Toll-like receptors have been demonstrated to induce mast-cell activation both in vivo and in vitro. Because L monocytogenes is a gram-positive bacteria, we hypothesized that toll-like receptor (TLR) 2 may serve as the necessary coreceptor required to mediate the α2β1 integrin-dependent mast-cell activation. To examine the role of Listeria and TLRs in immune complex–mediated mast-cell activation, we compared IL-6 secretion from PMCs after stimulation with BSA-immune complexes plus either live Listeria, heat-killed L monocytogenes, the TLR2 agonists Pam3Cys, or the TLR4 agonist, LPS. The addition of heat-killed Listeria, TLR2, or TLR4 agonist to the BSA immune complexes failed to result in activation of PMCs (Figure 4A). These results suggest that a heat-labile protein serves as the coreceptor for mast-cell activation and that TLR2 and TLR4 do not provide costimulatory activity.
c-met acts as a coreceptor for mast-cell IL-6 release
Listeria is an intracellular pathogen that infects humans using 2 bacterial surface receptors for bacterial internalization, InlA and InlB, that bind to E-cadherin and c-met, respectively, on the host cell.10,15,16 Mouse E-cadherin does not bind to InlA because of a single point mutation in its binding site on mouse E-cadherin.17 We evaluated the expression of c-met on mature peritoneal mast cells by flow cytometric analysis. As shown in Figure 4B, WT and α2-null PMCs express high levels of c-met.
To determine whether Listeria–c-met interaction was required, inhibitory anti–c-met or anti–E-cadherin antibodies were used to block PMC-Listeria interactions. Preincubation of WT PMCs with anti–c-met antibody before stimulation with Listeria-containing immune complexes significantly diminished the IL-6 release by 40% (P = .03), suggesting that c-met may serve as a coreceptor. As expected, the inhibitory anti–E-cadherin did not significantly alter IL-6 release (Figure 4C).
The best defined ligand for c-met is HGF. We hypothesized that Listeria-stimulated PMC activation would be mimicked by HGF binding to c-met. To determine whether HGF binding alone was sufficient to activate WT PMCs when the α2β1 integrin was ligated, we compared secretion of IL-6 by WT PMCs after stimulation by BSA-immune complex plus Listeria, BSA-immune complex plus HGF, or Listeria-immune complex. The level of IL-6 produced by WT PMCs stimulated by Listeria-immune complex, BSA-immune complex plus Listeria, and BSA-immune complex plus HGF was equivalent (Figure 4D). HGF failed to stimulate IL-6 secretion when incubated with PMCs in the absence of BSA-immune complex (Figure 4D). These data suggest that stimulation of c-met by either Listeria or HGF mediates WT PMC activation in the presence of ligated α2β1 integrin.
To determine whether coactivation of the α2β1 integrin and c-met is sufficient for PMC activation, we stimulated WT PMCs adherent via the α2β1 integrin to either C1q or type I collagen with either Listeria or HGF. α2β1 integrin-dependent adhesion of WT PMCs to either collagen or C1q alone failed to result in IL-6 release, as previously shown.4 c-met activation of PMCs by Listeria or HGF alone failed to result in IL-6 secretion. However, ligation of both the α2β1 integrin and c-met resulted in PMC activation and IL-6 release (Figure 4E). These data indicate that costimulation of c-met and the α2β1 integrin is sufficient to induce mast-cell activation in the absence of additional stimuli such as immune complexes.
c-met–InlB interactions are required for the innate immune response to Listeria
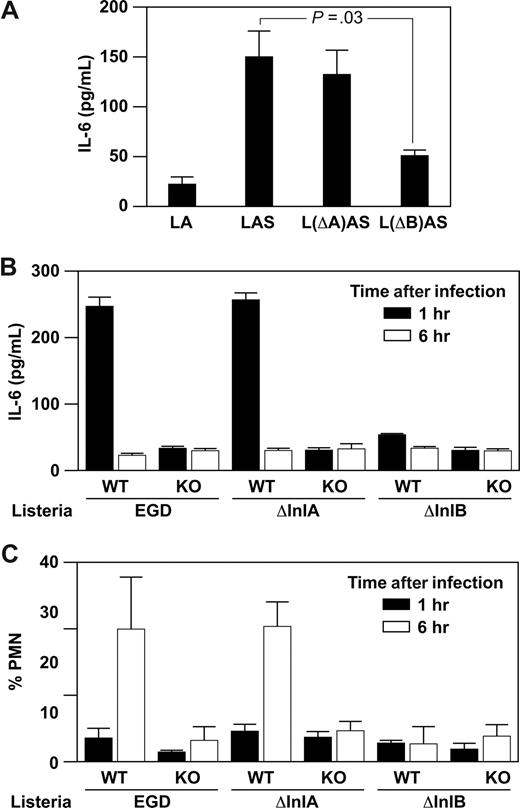
InlB and InlA promote Listeria internalization into host cells. The c-met receptor on the host cell binds to InlB on Listeria; E-cadherin binds to InlA. To determine the role of InlB–c-met interaction in PMC activation, we quantitated IL-6 secretion by WT PMCs after stimulation with immune complexes formed by WT Listeria or mutant Listeria containing mutations in either InlA (ΔInlA) or InlB (ΔInlB). Activation of WT PMCs with ΔInlA Listeria-immune complex resulted in secretion of IL-6 at similar levels to that secreted by PMCs stimulated by WT Listeria-immune complex. In contrast, activation of WT PMCs with ΔInIB Listeria-immune complex resulted in significantly reduced secretion of IL-6 to baseline levels (Figure 5A).
Internalin B is required for mast cell–mediated innate and adaptive immune responses. (A) Purified PMCs (5 × 104) from WT and α2-null mice were incubated for 1 hour with a washed suspension of WT Listeria EGD, Listeria ΔInlA (L(ΔA)), or Listeria (L(ΔB)), and anti-Listeria antibody plus or minus 50% murine serum. Supernatants were collected and assayed for IL-6 by ELISA. (B,C) WT and α2-null mice were infected for 1 or 6 hours with 5 × 104Listeria (EGD), Listeria (ΔInlA), or Listeria (ΔInlB) intraperitoneally. At the indicated time points, the percentage PMN and IL-6 in the peritoneal fluid were determined. Shown is representative of 2 experiments (mean ± SEM), with each point representing 5 or 6 mice.
Internalin B is required for mast cell–mediated innate and adaptive immune responses. (A) Purified PMCs (5 × 104) from WT and α2-null mice were incubated for 1 hour with a washed suspension of WT Listeria EGD, Listeria ΔInlA (L(ΔA)), or Listeria (L(ΔB)), and anti-Listeria antibody plus or minus 50% murine serum. Supernatants were collected and assayed for IL-6 by ELISA. (B,C) WT and α2-null mice were infected for 1 or 6 hours with 5 × 104Listeria (EGD), Listeria (ΔInlA), or Listeria (ΔInlB) intraperitoneally. At the indicated time points, the percentage PMN and IL-6 in the peritoneal fluid were determined. Shown is representative of 2 experiments (mean ± SEM), with each point representing 5 or 6 mice.
Because the early α2β1-dependent innate immune responses to Listeria mediated by PMC secretion of IL-6 resulted in neutrophil recruitment at 6 hours,1 we hypothesized that the c-met–InlB interaction was required not only in vitro but also for α2β1 integrin-dependent response in vivo. Wild-type and α2-null mice were infected intraperitoneally with WT, ΔInlA, or ΔInlB Listeria. As reported, WT mice, but not α2-null mice, when infected with WT Listeria, demonstrate high levels of IL-6 in the peritoneal cavity 1 hour after infection and robust neutrophil recruitment at 6 hours after infection. Wild-type, but not α2-null, mice responded with a rapid cytokine secretion and a similar robust neutrophil response to ΔInlA Listeria. In contrast, ΔInlB Listeria failed to elicit IL-6 secretion or neutrophil recruitment in either WT or α2-null mice (Figure 5A,B). These data demonstrate that binding of c-met to InlB cooperates with α2β1 integrin binding to C1q or collagen in the early mast cell–dependent innate immune response to Listeria both in vivo and in vitro.
Discussion
We have identified c-met as a novel coreceptor required for α2β1 integrin-dependent mast-cell activation. The synergistic contributions of α2β1 integrin and c-met receptor in immune modulation were entirely unexpected. We initially reported that the α2β1 integrin-deficient mouse demonstrated a profound and surprising defect in the innate immune response to L monocytogenes.1 α2β1 integrin expression on the PMCs was required for activation and cytokine secretion in vivo.1 Here, we demonstrate that the α2β1 integrin-dependent immune response is characterized by IL-6, IL-β, and low level TNF-α release at 1 hour after infection, followed by neutrophil recruitment at 6 hours. IL-6, IL-1β, and TNF-α have been described to have roles in response to Listeria infection.18-23
In addition, our previous studies demonstrated a requirement for α2β1 integrin ligation for PMC activation.4 However, α2β1 integrin-ligand interactions alone were insufficient for PMC activation because α2β1 integrin-dependent adhesion to collagen or C1q alone failed to support cytokine secretion.4 We hypothesized that one or more additional signals emanating from an additional receptor, similar to that downstream of glycoprotein VI/FcRγ on platelets, was required for PMC activation.5,6 Alternatively, α2β1 integrin binding to C1q may result in activation of the complement cascade. We now demonstrate that neither complement receptors nor FcRγ provides the necessary costimulatory signal for α2β1 integrin-dependent IL-6 release in vivo or in vitro. Indeed, the FcRγ is not required for the early innate immune response to Listeria either in vitro or in vivo.
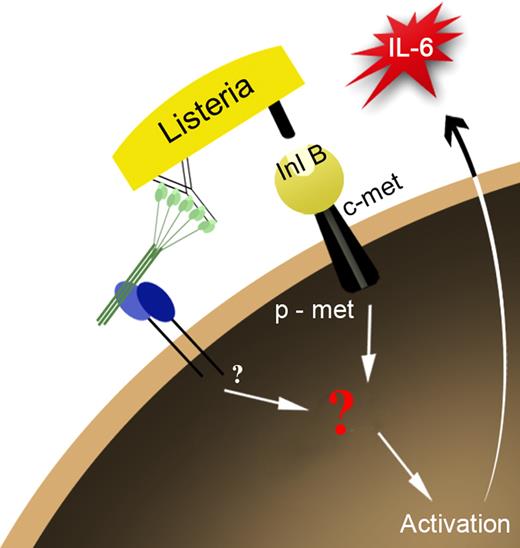
Instead, we identified c-met as a novel α2β1 integrin coreceptor that is essential for activation of the innate immune response to Listeria. In addition, we demonstrated cooperation between the α2β1 integrin and HGF-R/c-met in immune modulation (Figure 6). In the innate immune response to Listeria, the surface receptor InlB, a ligand for the c-met, provides the costimulatory signal. In our initial in vitro observations, mast-cell activation required Listeria-generated immune complexes. Because α2β1 integrin-dependent adhesion to either type I collagen or C1q alone was required but not sufficient for mast-cell activation, we suggested that perhaps the correct orientation of the immune complex was required for recognition.4 We have demonstrated that the orientation of the immune complex is not required for activation. Simple coligation of the integrin with either type I collagen or C1q and c-met with either Listeria organisms or c-met's natural ligand HGF is sufficient to induce activation. InlB binding to host receptor c-met is essential for infectivity and internalization into epithelial cells and hepatocytes. However, because stimulation with HGF and an α2β1 integrin ligand is sufficient for activation, we propose that internalization is not required for mast-cell activation.15 Our data now support a role for the α2β1 integrin–c-met interaction for the innate inflammatory response to Listeria.
Predicted model of mast-cell activation through c-met and the α2β1 integrin. Mast cell stimulation through c-met and the α2β1 integrin results in cross talk between the 2 receptors, resulting in the activation of the mast cell leading to release of the pro-inflammatory cytokine, IL-6.
Predicted model of mast-cell activation through c-met and the α2β1 integrin. Mast cell stimulation through c-met and the α2β1 integrin results in cross talk between the 2 receptors, resulting in the activation of the mast cell leading to release of the pro-inflammatory cytokine, IL-6.
HGF was originally identified as a mitogen for hepatocytes and as a scatter factor for several cell types.24 Although the role of the HGF/c-met interaction in tumor progression and tissue fibrosis has been extensively studied, the role of HGF and c-met in immunity is less well defined. Data from several groups suggest that HGF promotes B- and T-cell migration,25-27 counters the immunosuppressive effects of TGF-β,28-30 suppresses dendritic cell function,31 and reduces acute and chronic rejection.32 In our model, synergistic stimulation of mast cells by HGF or InlB and the α2β1 integrin results in activation of the innate immune response and the recruitment of neutrophils to the site of Listeria infection. Mast cells also secrete chymases that hydrolyze HGF to generate an HGF antagonist.33 This may serve as a negative feedback loop to inhibit subsequent immune activation.
Several different cell-surface receptors for C1q and other collectin family members have been reported, including the C1q receptor for phagocytosis enhancement (C1qRp), CR1, calreticulin (CRT), and binding protein for the globular head of C1q (gC1qbp).34-47 The precise role of each receptor remains an area of active investigation. Although the ability of InlB to interact with the cellular glycoprotein gC1q-R/p3248 raised questions concerning whether gC1q-R was involved in the α2β1 and c-met–dependent PMC response, our studies suggest that gC1q-R cannot replace the α2β1 integrin in initiating the innate immune response. Using isolated components in an in vitro system, we demonstrated that PMC activation required α2β1 integrin binding to either C1q or type I collagen plus the additional interaction of c-met with either Listeria or HGF. Although C1q and Listeria both interact with gC1q-R through either the globular head of C1q or InlB of Listeria, neither type I collagen nor HGF interacts with gC1q-R. IL-6 secretion is similar regardless of whether the α2β1 integrin interacts with C1q or type I collagen and whether c-met is bound by Listeria or HGF. These data suggest that, although gC1q-R may play a role in Listeria internalization, there is no role for gC1q-R in α2β1 integrin/c-met–induced mast-cell activation. Although HGF and InlB are both ligands for c-met, they lack sequence and structural similarity.49,50 In addition, the data illustrate that these 2 ligands bind separate sites on c-met.10,51,52 Despite their differences, both HGF and InlB activate the intrinsic phosphorylation activity of Met and induce tyrosine phosphorylation at Y134910 as well as cellular changes, such as scattering and DNA synthesis.52
We now describe an example of cooperation between the α2β1 integrin and a receptor for either a critical growth factor, HGF, or for a Listeria protein required for internalization. This cross-talk then leads to activation of the mast cell, a cell critical to the innate immune system. The crosstalk between integrins and c-met in endothelial and epithelial cells regulates cell migration and invasion downstream of signaling pathways that activate FAK, which transduces signals to adaptor molecules, such as Src family kinases, PI3K, phospholipase C, and Grb2.53 After HGF or InlB binding, c-met is phosphorylated on Y1349 and 1356, which serve as docking sites for multiple signal transducers, such as Grb2-associated binder1 (Gab1) and multiple Src homology 2 domains. Given the fact that isolated ligands of α2β1 integrin and c-met can stimulate mast-cell activation in the absence of immune complex, we propose that the α2β1 integrin–c-met crosstalk occurs via downstream signaling pathways rather than by direct interaction at the cell membrane. Studies to determine the mechanism of convergence of these downstream pathways are currently under way.54-56
The role for mast cells in immunity has traditionally been limited to their role in IgE-mediated allergic responses and immunity to parasites. Mast cells are known to play an important role in the early response of the immune system and can be stimulated through a range of receptors important in the early immune response, such as TLRs 1, 3, 4, 6, and 9, complement receptors (CR2, 4, 5, C3aR, C5aR), and cytokine/chemokine receptors (IL-1R, IL-10R, IL-12R, and IFN-gR).57 We have demonstrated a novel α2β1 integrin-dependent and c-met–dependent pathway for mast-cell activation that results in the rapid release of IL-6 and occurs independently of signals downstream of FcRγ. IgE cross-linking mediates signals through FcRγ and results in rapid degranulation and release of histamine and β-hexosamidase.58 The secretion of IL-6 has not been described after IgE-mediated degranulation. In addition to IgE cross-linking, TLR agonists, such as LPS (TLR4) and PGN (TLR2), are able to induce degranulation of mast cells in some circumstances, although we were unable to detect IL-6 release (Figure 3A).59,60 We are actively investigating the differential degranulation of mast cells to different stimuli.
We describe an innate immune response mediated by the α2β1 integrin binding C1q, a soluble, nonmatrix factor. We previously demonstrated that the α2β1 integrin is a cellular receptor for C1q, the first component in the complement cascade and mediator of innate immune response.4 In vitro interaction between α2β1 and C1q on mast cells resulted in activation and cytokine secretion. Here, using in vivo models, we show that the C1q/α2β1 integrin interaction is, indeed, required for innate immune response to Listeria. C1q-deficient mice do not release IL-6 or recruit PMNs into the peritoneum after Listeria infections. Although collagen and other ECM components are abundant in the peritoneum, collagen binding either does not occur or is not sufficient to mediate mast- cell activation after Listeria infection. Because the homology between C1q and the integrin binding site of collagen is high, the importance of the integrin and C1q interaction is probably significant given the low amount of genetic drift between these 2 sequences over time.
The α2β1 integrin in expressed on numerous cells, including cells of the innate immune system, such as mast cells and natural killer cells as well as cells of the adaptive immune system, such as a subset of activated T cells.61-63 The α2β1 integrin in these cells has been thought to act primarily through its ability to bind collagen in the extracellular matrix, thereby affecting cellular localization. Our work is the first to show that α2β1 integrin can bind C1q and transduce a signal that is absolutely necessary, but not sufficient, to initiate a proinflammatory cytokine response. C1q is known to play an important role in immune complex diseases, such as systemic lupus erythematosus, Arthus reaction, autoantibody-induced arthritis, glomerulonephritis, and experimental autoimmune encephalitis.11,64-66 In addition, mast cells have been associated with these diseases.36,67-70 Our findings provide a molecular mechanism linking C1q and activation of these inflammatory cells. Mast cells act as sentinels for the immune system at the body's boundaries. C1q binding of the α2β1 integrin is an initiating event of mast-cell degranulation and, hence, an initial trigger for the subsequent inflammatory response. Therefore, inhibition ofthe C1q/α2β1 interaction would probably be an effective target for therapeutic intervention.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laura Ford and Ping Zhao for expert animal care, Jean McClure for secretarial assistance, and Drs Emil Unanue, Claudio Mosse, and Brian Edelson for critical review of the manuscript.
This work was supported by National Institutes of Health grants RO1 CA098027 and RO1 CA115984.
National Institutes of Health
Authorship
Contribution: K.D.M.-C. designed and performed the research, analyzed the data, and wrote the paper; Z.L. participated in performing experiments; M.M.Z. was responsible for the overall study, participated in designing and analyzing the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary M. Zutter, Department of Pathology, C-3321A, 1161 21st Avenue South, Vanderbilt University School of Medicine, Nashville, TN 37232-2561; e-mail: mary.zutter@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal