Much of the efficacy of allogeneic hematopoietic stem cell transplantation (alloSCT) in curing hematologic malignancies is due to a graft-versus-leukemia (GVL) effect mediated by donor T cells that recognize recipient alloantigens on leukemic cells. Donor T cells are also important for reconstituting immunity in the recipient. Unfortunately, donor T cells can attack nonmalignant host tissues and cause graft-versus-host disease (GVHD). We previously reported that donor CD4+ effector memory T cells (TEMs) do not cause GVHD but transfer functional T-cell memory. In the present work, we demonstrate in an MHC-mismatched model that CD4+ TEMs (unprimed to recipient antigens) mediate GVL against clinically relevant mouse models of chronic phase and blast crisis chronic myelogenous leukemia, without causing GVHD. By creating gene-deficient leukemias and using perforin-deficient T cells, we demonstrate that direct cytolytic function is essential for TEM-mediated GVL, but that GVL is retained when killing via FasL, TNF-α, TRAIL, and perforin is individually impaired. However, TEM-mediated GVL was diminished when both FasL and perforin pathways were blocked. Taken together, our studies identify TEMs as a clinically applicable cell therapy for promoting GVL and immune reconstitution, particularly in MHC-mismatched haploidentical alloSCTs in which T cell–depleted allografts are commonly used to minimize GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) is a potentially curative therapy for hematologic malignancies, including acute and chronic leukemias and lymphomas. In an alloSCT, donor T cells in the allograft are critical for reconstituting T-cell immunity in the host1 and mediate an antitumor effect called graft-versus-leukemia (GVL).2,,–5 Unfortunately, donor T cells also broadly attack recipient tissues, including the skin, bowel, and liver, in a process called graft-versus-host disease (GVHD). GVHD is a major cause of morbidity and mortality in alloSCT, which greatly limits the efficacy and applicability of this life-saving therapy. GVHD is particularly severe when the donor and recipient are not fully major histocompatibility complex (MHC)-matched. This is because the precursor frequency of T cells that recognize non–self-MHC has been estimated to be at least 1000- to 10 000-fold higher than that of T cells that recognize minor histocompatibility antigen peptides presented by self-MHC. Therefore, many centers that perform transplantations using HLA-haploidentical donors rigorously deplete allograft T cells.6,7 While this T-cell depletion is highly effective in minimizing GVHD, it essentially abrogates donor T cell–mediated immune reconstitution and GVL. Preserving the positive effects of donor T cells—GVL and immune reconstitution—without GVHD remains the central challenge in the alloSCT field
We and others have previously reported that effector memory T cells (TEMs) do not induce GVHD in MHC-matched and MHC-mismatched models and can transfer functional T-cell memory.8,,–11 This is consistent with the prior demonstration that antipathogen T-cell memory is transferred from donor to recipient in human alloSCT.12,,,–16 These data suggest a strategy wherein donor TEMs would be selectively infused along with a CD34-selected allograft to improve immune reconstitution with a reduced risk of GVHD. Such an approach would be particularly attractive when patients do not have an HLA-matched donor and stem cells from an HLA-mismatched haploidentical donor are used. However, this would not decrease leukemia relapse unless TEMs also mediate GVL. Here we demonstrate using an MHC-mismatched model that CD4+ TEMs, unprimed to recipient antigens, mediate GVL against murine models of chronic phase and blast crisis chronic myelogenous leukemia,17,18 without causing GVHD. Effective GVL by both TEMs and naive T cells (TNs) required cognate interactions with leukemia targets, which contrasts with how GVHD is mediated in the same MHCII-disparate strain pairing used in our studies.19 TN or TEM killing via perforin, FasL, TNF-α, and TRAIL could individually be blocked without compromising GVL. However, TEM- but not TN-mediated GVL was significantly reduced when both perforin and FasL killing were simultaneously blocked. Our studies identify purified TEMs as a clinically applicable cell therapy for promoting GVL and immune reconstitution with a reduced risk for GVHD.
Methods
Mice
Allogeneic bone marrow transplant (alloBMT) recipients and mice used to generate murine model of chronic phase chronic myelogenous leukemia (mCP-CML) were 7 to 10 weeks of age. Mice used as T-cell and bone marrow (BM) donors were between 8 to 16 weeks of age. B6 mice were obtained from the NCI (Frederick, MD). B6bm12, Faslpr, and perforin-deficient mice (Prf1−/−) mice were obtained from Jackson Labs (Bar Harbor, ME) and then bred in our animal colony. IAb beta chain–deficient (IAbβ−/−) mice were obtained from Taconic (Germantown, NY). We made B6bm12Prf1−/− mice by crossing B6bm12 mice with B6 Prf1−/− mice. For polymerase chain reaction (PCR) screening of B6bm12 and Prf1 alleles, the following primers were used: forward for IAbβ chain of both B6 and B6bm12: 5′-atgggcgagtgctacttcac-3′; B6 reverse: 5′-cgcgttcgctccaggatct-3′; and bm12 reverse: 5′-ccgcttttgctccaggaact-3′; forward for perforin of both wt and knockout: 5′-taccaccaaatgggccaa-3′, wt reverse: 5′-gctatcaggacatagcgttgg-3′, and knockout reverse: 5′-ggaggctctgagacaggcta-3′. B6 mice deficient in both TNFR1 and TNFR2 (TNFR1/R2−/−) were created by us via crossing TNFR1−/− and TNFR2−/− mice (C57BL/6-Tnfrsf1atm1Imx and B6.129S2-Tnfrsf1btm1Mwm; Jackson Labs). These mice were screened via PCR for both the wild-type and knockout alleles as previously described.18 TRAILR−/− mice20 were kindly provided by Dr Astar Winoto (University of California Berkeley, Berkeley, CA) and were bred in our colony. For PCR screening of TRAILR−/− mice, we used the following primers: for the wt allele: 5′-tccttcagtaccatctcagggatg-3′ and 5′-ttatacagagcaaccattgcctcc-3′; for the knockout allele: 5′-ggaagtgtgtctccaaaacggc-3′ and 5′-gcagatggcacagacctgtaatg-3′.
Cell purifications
CD4+ cells were enriched from total spleen cells using BioMag (Qiagen, Hilden, Germany) cell separation as follows. Cells were incubated with the following antibody supernatants (all laboratory grown): anti-CD8α (TIB105), anti-B220 (RA3–6B2), anti-CD11b (M1/70), and anti-FcR (24G.2) for 30 minutes on ice, followed by 2 washes in MACS buffer (PBS, 2 mM EDTA, 0.5% BSA). Cells were then incubated with prepared BioMag goat antirat magnetic beads for 30 minutes on ice in a T75 or T125 flask that was then placed next to a strong magnet. Cells not bound to magnetic particles were collected and were typically 70% to 80% CD4+. For further separations of naive and memory cells, enriched CD4+ cells were incubated with MoAbs anti–CD4-PECy7 (GK1.5; eBioscience, San Diego, CA), anti–CD62L-FITC (Mel-14, laboratory prepared), anti–CD44-APC (Pgp-1; BD Pharmingen, Torrey Pines, CA), and biotin anti-CD25 (7D4; BD Pharmingen) for 20 minutes on ice, washed once in MACS buffer, and incubated with streptavidin-PE (BD Biosciences, San Jose, CA) for 15 minutes on ice, washed again, and resuspended in PBS with 0.5% FBS. Cells were sorted into CD4+CD62L+CD44−CD25− naive T cells (TNs) and CD4+CD62L−CD44+CD25− effector memory cells (TEMs) using a FACS Aria (BD Biosciences). Bone marrow was depleted of T cells using Miltenyi anti-Thy1.2 microbeads and the AutoMACs cell separator (Miltenyi Biotec, Auburn, CA).
GVHD transplantation protocol
All transplantations were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee. B6 hosts received 800 cGy irradiation, followed by tail vein injection of 7 × 106 T cell–depleted (TCD) B6bm12 BM, with no T cells, CD4+ TNs, or CD4+ TEMs. In GVHD experiments, mice were weighed approximately every 3 days. If a death occurred, the last recorded weight was maintained in mean weight loss calculations for the remainder of the experiment.
Histologic analysis
Mice were killed 43 days after transplantation. Tissues were fixed in 10% phosphate-buffered formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Slides were read by pathologists expert in skin (J.M.), liver, and gastrointestinal disease (D.J.) without knowledge as to experimental group. Scoring was as described previously.8,21,22
Retrovirus production
MSCV2.2 expressing the human bcr-abl p210 cDNA and a nonsignaling truncated form of the human low-affinity RJM receptor driven by an internal ribosome entry site (Mp210/NGFR) was a gift of Warren Pear. MSCV2.2 expressing the NUP98/HOXA9 fusion cDNA with EGFP expressed by an internal ribosome entry site (MNUP98/HOXA9-EGFP) was a gift of D. G. Gilliland.17 Retroviral supernatants were generated by transfection of the Plat-E retrovirus packing cell line23 as described18,24,25 and titered on 3T3 cells.
Progenitor infections
p210-infected progenitors were generated as previously described.18,22,24 Briefly, B6 mice were injected on day −6 with 5 mg 5-fluorouracil (5FU; Pharmacia & Upjohn, Kalamazoo, MI). On day −2, BM cells were harvested and cultured in prestimulation media (DMEM, 15% FBS, IL-3 [6 ng/mL], IL-6 [10 ng/mL], and SCF [10 ng/mL]; all cytokines from Peprotech, Rocky Hill, NJ). On days −1 and 0, cells underwent “spin infection” with Mp210/NGFR retrovirus as described.18 Murine blast crisis CML cells (mBC-CMLs) were created by infecting B6 progenitors with both Mp210/NGFR and MNUP98/HOXA9-EGFP retrovirus as described.17 Splenic mBC-CML cells from primary recipients of cells infected with both retroviruses were passaged in secondary mice and then in limiting numbers in tertiary mice. mBC-CML cells from spleens of tertiary mice were clonal based on analysis of retroviral integration sites and were predominantly CD34+ without expression of lineage markers (W.S. and C.M., manuscript in preparation).
mCP-CML GVL transplantation protocol
On day 0, B6 mice received 800 cGy and were reconstituted (intravenously) with 5 × 106 TCD donor B6bm12 BM with 7 × 105 BM cells that underwent spin infection, with or without B6bm12 CD4+ TNs or TEMs. Mice were bled weekly beginning the second week after transplantation, and peripheral blood was analyzed for the number of NGFR+ cells by flow cytometry. In mice that spontaneously died, cause of death was deemed to be from mCP-CML if the mouse had a rising number of NGFR+ cells in peripheral blood and had splenomegaly at necropsy. In mice that were killed, spleen, peripheral blood, and BM were analyzed for the presence of NGFR+ cells by flow cytometry and spleen weights were measured. All mice were weighed and scored for clinical GVHD approximately 3 times per week as we have described.22
mBC-CML transplantation protocol
On day 0, recipient B6 received 800 cGy and were reconstituted (intravenously) with 5 × 106 TCD donor B6bm12 BM with 104 mBC-CML cells with or without 5 × 105 CD4+ TNs or 106 TEMs from B6bm12 donors. Cause of death was determined by serial analysis of peripheral blood for NGFR+EGFP+ cells and by spleen weight.
Antibodies and flow cytometry
Antibodies used to characterize mCP-CML were as follows: Gr-1-FITC, TER119-PE (both from BD Pharmingen) and Alexa647-conjugated anti-NGFR (clone 20.4; laboratory prepared). Whole blood was stained with appropriate antibodies, followed by red blood cell (RBC) lysis with ACK Lysing Buffer (Cambrex Bio Science, Walkersville, MD). Propidium iodide was added to exclude dead cells. Cells were analyzed on a FACS Calibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) and results analyzed with FlowJo (TreeStar, Ashland, OR).
Intracellular cytokine staining was performed as we have described.26 Briefly, splenocytes were harvested and cultured with phorbol myristic acid (PMA) and ionomycin for 5 hours; monensin was added for the final 2 hours. Prior to permeabilization, cells were incubated with ethidium monoazide (EMA) to allow exclusion of dead cells. Cells were stained with antibodies against CD45.1 (biotin; followed by streptavidin-PerCP; clone A20) and CD4 (Alexa488; clone GK1.5), permeabilized, and then stained with antibodies against IFN-γ (PE; clone XMG1.2) and IL-2 (APC; clone JES6–5H4) or isotype controls (for the anticytokine antibodies). EMA+ dead cells and CD45.1+ cells (all in FL-3) were excluded, and donor-derived CD45.1−CD4+ cells were identified.
Analysis of serum cytokines
Serum cytokines were measured using the LINCOpleX kit (LINCO Research, St Charles, MI) and a Luminex 100 system (Luminex, Austin, TX).
Statistical methods
Significance for differences in weight loss, donor T-cell numbers, percentages of cytokine+ cells, and serum cytokine levels was calculated by an unpaired t test. P values for survival curves were calculated by log-rank test. P values for histology, leukemia cell number, and spleen weight comparisons were calculated by Mann-Whitney.
Results
CD4+ TEMs do not cause GVHD in the B6bm12→B6 MHCII-disparate strain pairing
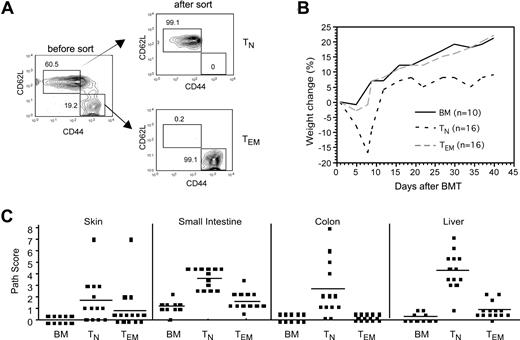
We chose to examine GVL in the B6bm12→ B6 major histocompatibility complex II antigen (MHCII)–disparate strain pairing to parallel the dominant mechanism of T-cell alloreactivity in MHC-haplotype–mismatched transplantations—direct recognition of MHC—that necessitates rigorous T-cell depletion to prevent severe GVHD.6,7 We first determined whether CD4+ TEMs induce GVHD in this strain pairing. We performed these experiments under experimental conditions that were generally nonlethal such that we could evaluate histologic GVHD. Irradiated B6 recipients were reconstituted with T cell–depleted (TCD) B6bm12 bone marrow (BM) with no T cells, CD4+CD62L+CD44−CD25− naive T cells (TNs), or CD4+CD62L−CD44+CD25− TEMs (Figure 1A) and were followed for the development of GVHD. B6bm12 CD4+ TEMs did not cause clinical GVHD, whereas CD4+ TNs induced severe disease manifested by weight change (Figure 1B), ruffled fur, and diarrhea. CD4+ TNs induced significant histologic GVHD of the liver, small intestine, and colon, whereas CD4+ TEMs did not cause significant GVHD in any tissue examined (Figure 1C). Thus, as has been reported in other models,8,,–11 CD4+ TEMs are less potent inducers of GVHD than are TNs in the B6bm12→B6 strain pairing.
CD4+ TEMs do not induce GVHD in the B6bm12→B6 strain pairing. B6 mice were irradiated and reconstituted with T-cell–depleted B6bm12 BM, with no T cells, or with 5 × 105 TN or 106 TEM B6bm12 CD4 cells. Data were combined from 2 independent experiments. (A) Sorting of TN and TEM CD4 cells from splenocytes. The first panel is gated on CD4+CD25− cells, which were sorted into TNs and TEMs based on the expression of CD62L and CD44. Numbers on plots are percentages of total cells within the rectangles. (B) Weight change (P < .007 at days 5, 7, and 9; P < .02 at days 34, 37, and 40 comparing recipients of TNs versus TEMs). P values are not significant at any time point comparing BM versus TEMs. (C) Pathology scores from day 43 after transplantation. P values comparing TN and TEM recipients: P < .001 for liver, small intestine, and colon; P = .055 for skin. P values are not significant comparing scores for recipients of BM alone versus CD4+ TEMs. Horizontal lines represent mean values.
CD4+ TEMs do not induce GVHD in the B6bm12→B6 strain pairing. B6 mice were irradiated and reconstituted with T-cell–depleted B6bm12 BM, with no T cells, or with 5 × 105 TN or 106 TEM B6bm12 CD4 cells. Data were combined from 2 independent experiments. (A) Sorting of TN and TEM CD4 cells from splenocytes. The first panel is gated on CD4+CD25− cells, which were sorted into TNs and TEMs based on the expression of CD62L and CD44. Numbers on plots are percentages of total cells within the rectangles. (B) Weight change (P < .007 at days 5, 7, and 9; P < .02 at days 34, 37, and 40 comparing recipients of TNs versus TEMs). P values are not significant at any time point comparing BM versus TEMs. (C) Pathology scores from day 43 after transplantation. P values comparing TN and TEM recipients: P < .001 for liver, small intestine, and colon; P = .055 for skin. P values are not significant comparing scores for recipients of BM alone versus CD4+ TEMs. Horizontal lines represent mean values.
CD4+ TEMs mediate GVL against murine chronic phase and blast crisis CML
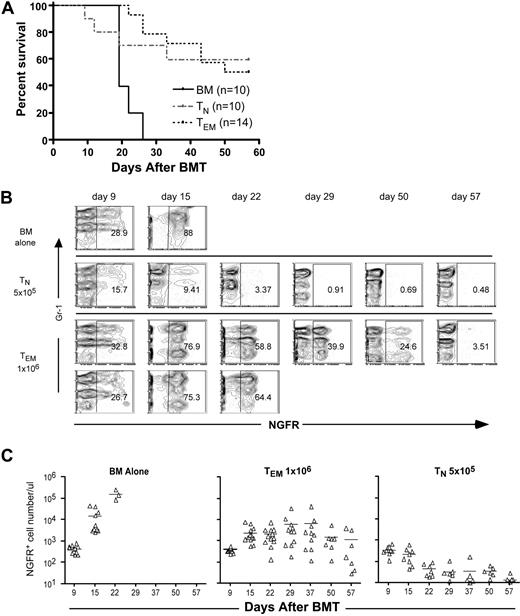
We next determined whether B6bm12 CD4+ TEMs mediate GVL against a murine model of chronic phase chronic myelogenous leukemia (mCP-CML) generated via retroviral insertion into murine hematopoietic progenitors of the human bcr-abl (p210) fusion cDNA, the defining genetic abnormality in human chronic phase CML.24,27,–29 An advantage of mCP-CML is that it can be induced in BM from any mouse, including strains genetically deficient in molecules important for leukemia immunogenicity.18,22 Irradiated mice that receive p210-transduced BM cells develop a myeloproliferative disease manifest by a high white blood cell count and splenomegaly, with hematopoiesis dominated by maturing myeloid cells.24,27,28 The retrovirus also expresses a nonsignaling form of the low-affinity nerve growth factor receptor (NGFR) linked to the p210 cDNA by an internal ribosome entry site, which allows detection of infected cells by flow cytometry. NGFR and p210 are not target antigens in this model as no GVL effect occurs in syngeneic B6→B6 transplantations, and alloantigen expression by mCP-CML cells is absolutely required.18,30 B6 mice were irradiated and reconstituted with p210-transduced B6 BM, TCD B6bm12 BM with no T cells, 5 × 105 CD4+ TNs, or 106 CD4+ TEMs. Mice that did not receive donor B6bm12 T cells died from leukemia between days 18 and 23 (Figure 2A). As expected, recipients of CD4+ TNs cleared mCP-CML cells, but 40% of them died from GVHD. Strikingly, CD4+ TEMs mediated GVL that resulted in significantly improved survival relative to mice that received no T cells (Figure 2A) and survival of TEM recipients was similar to recipients of TNs, though the causes of death were different. TEM recipients died from mCP-CML, whereas TN recipients died from GVHD. This GVL effect was also manifested by a reduced number of NGFR+ cells in peripheral blood (Figure 2B,C) and some long-term survivors cleared all NGFR+ cells in blood, BM, and spleen. Of mice that received TEMs and mCP-CML cells, 38 of 154 total (of which 60 survived) completely cleared NGFR+ cells. Importantly, we observed no clinical GVHD in CD4+ TEM recipients.
CD4+ TEMs mediate GVL against mCP-CML without causing GVHD. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, with B6 mCP-CML with no T cells, or with 5 × 105 TN or 106 TEM B6bm12 CD4 cells. (A) Shown is survival data combined from 2 independent experiments. P < .001 for recipients of TEMs versus only T cell–depleted BM. (B) Representative serial flow cytometry of peripheral blood. Each row represents serial bleeds of individual mice. Data from 2 TEM recipients are shown to illustrate the types of responses we observed. Numbers on plots are percentages of total cells within the rectangles. (C) Numbers of NGFR+ cells in the peripheral blood of mice that underwent transplantation taken at different time points. Each symbol represents an individual animal; solid lines are mean values. P < .002 comparing TNs versus TEMs from day 15 onward.
CD4+ TEMs mediate GVL against mCP-CML without causing GVHD. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, with B6 mCP-CML with no T cells, or with 5 × 105 TN or 106 TEM B6bm12 CD4 cells. (A) Shown is survival data combined from 2 independent experiments. P < .001 for recipients of TEMs versus only T cell–depleted BM. (B) Representative serial flow cytometry of peripheral blood. Each row represents serial bleeds of individual mice. Data from 2 TEM recipients are shown to illustrate the types of responses we observed. Numbers on plots are percentages of total cells within the rectangles. (C) Numbers of NGFR+ cells in the peripheral blood of mice that underwent transplantation taken at different time points. Each symbol represents an individual animal; solid lines are mean values. P < .002 comparing TNs versus TEMs from day 15 onward.
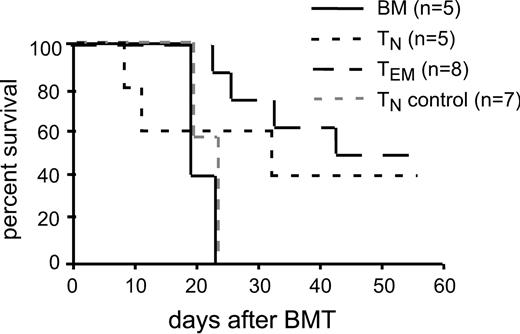
Sorted TEMs had between 0.1% and 0.2% contaminating CD4+ TNs. To exclude the possibility that GVL mediated by sorted TEMs was from these contaminating TNs (which are more potent mediators of GVL than are TEMs), we repeated the GVL experiment with an additional group of mice that received 2000 CD4+ TNs, the number of TNs in the sorted CD4+ TEMs. As in the prior experiment, 106 CD4+ TEMs and 5 × 105 CD4+ TNs mediated GVL, whereas recipients of only 2000 CD4+ TNs died from mCP-CML with the same kinetics as did recipients of no T cells (Figure 3). Thus the GVL mediated by CD4+ TEMs was not due to the activity of contaminating CD4+ TNs.
GVL mediated by TEMs was not due to contaminating CD4+ TNs. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, B6 mCP-CML with no T cells, 5 × 105 TNs, 106 TEMs, or 2 × 103 TNs (TN control indicates the number of TNs contaminating the TEM sorted cells) B6bm12 CD4 cells. Shown are the survival data.
GVL mediated by TEMs was not due to contaminating CD4+ TNs. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, B6 mCP-CML with no T cells, 5 × 105 TNs, 106 TEMs, or 2 × 103 TNs (TN control indicates the number of TNs contaminating the TEM sorted cells) B6bm12 CD4 cells. Shown are the survival data.
CD4+ TEMs also mediated GVL against a murine model of blast crisis CML (mBC-CML) induced by the retroviral introduction of both bcr-abl (p210) and a fusion cDNA from the NUP98/HOXA9 translocation found in both acute myeloid leukemia and blast crisis CML.17 In contrast to mCP-CML, mBC-CML is dominated by CD34+ myeloblasts17 (and data not shown). The retroviral construct that drives the expression of the NUP98/HOXA9 cDNA also expresses EGFP, and thus mBC-CML cells are NGFR+EGFP+ and disease can be monitored by flow cytometry. As is the case with mCP-CML, no GVL is observed against mBC-CML in syngeneic transplantations (our unpublished data and Shlomchik et al31 ). B6 mice were irradiated and reconstituted with TCD B6bm12 BM, 10 000 mBC-CML cells, with or without donor 5 × 105 CD4+ TNs or 1 to 2 × 106 CD4+ TEMs. Data combined from 2 independent experiments are shown in Figure 4. CD4+ TEMs clearly improve survival over recipients of only TCD BM (P = .026), and the majority of surviving mice cleared mBC-CML from bone marrow, spleen, and peripheral blood (data not shown). All deaths in the BM alone and CD4+ TEMs were from mBC-CML (as confirmed by flow cytometry of peripheral blood and postmortem spleen weight; data not shown), whereas only 2 of 5 deaths in TN recipients were attributable to mBC-CML.
CD4+ TEMs mediate GVL against mBC-CML. Lethally irradiated B6 mice were reconstituted with T cell–depleted B6bm12 BM, 104 B6 mBC-CML cells, with no T cells, or with 106 CD4+ TEMs or 5 × 105 CD4+ TNs. Shown are results combined from 2 similar independent experiments. P = .024 comparing BM alone versus TEM recipients.
CD4+ TEMs mediate GVL against mBC-CML. Lethally irradiated B6 mice were reconstituted with T cell–depleted B6bm12 BM, 104 B6 mBC-CML cells, with no T cells, or with 106 CD4+ TEMs or 5 × 105 CD4+ TNs. Shown are results combined from 2 similar independent experiments. P = .024 comparing BM alone versus TEM recipients.
Mechanisms by which CD4+ TEMs mediate GVL
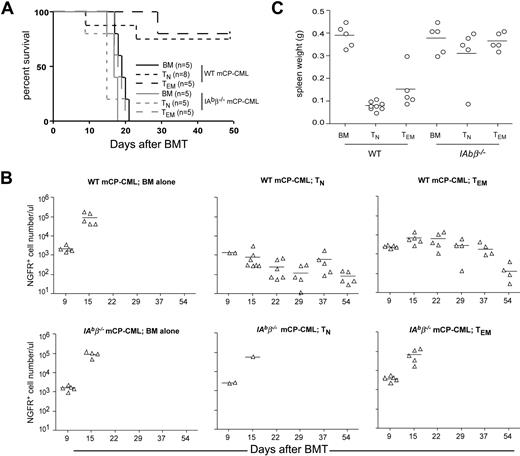
To better understand how CD4+ TEMs mediate GVL without causing GVHD, we investigated the mechanisms by which CD4+ TEMs kill leukemic targets in vivo. A fundamental question is whether CD4+ TEMs require cognate interactions between their T-cell receptors and MHCII molecules expressed by mCP-CML cells. To address this, we determined whether B6bm12 CD4+ TEMs mediate GVL against MHCII-deficient mCP-CML generated by p210 transduction of BM taken from IAb beta chain–deficient (IAbβ−/−) B6 mice.32 As in prior experiments, CD4+ TNs and TEMs mediated GVL against wild-type (wt) mCP-CML. However, neither CD4+ TNs nor TEMs mediated GVL against IAbβ−/− MHCII-deficient mCP-CML (Figure 5A). That death was from mCP-CML was confirmed by the number of NGFR+ cells in peripheral blood (Figure 5B) and the presence of massive splenomegaly at necropsy (Figure 5C). Thus, GVL mediated by either TNs or TEMs requires cognate interactions between alloreactive CD4+ T cells and leukemia targets.
CD4+ TNs and TEMs require cognate interactions with MHCII+ mCP-CML cells to mediate GVL. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, wt B6, or IAbβ−/− mCP-CML, with no T cells or with 5 × 105 TN or 106 TEM B6bm12 CD4 cells. (A) Survival data from 1 of 2 similar experiments; P = .002 for recipients of IAbβ−/− mCP-CML and TEMs versus wt B6 mCP-CML and TEMs; P = .004 comparing recipients of IAbβ−/− mCP-CML and TNs versus wt B6 mCP-CML and TNs. (B) Numbers of NGFR+ cells in the peripheral blood of mice that underwent transplantation taken at different time points. At day 9, peripheral blood was harvested from only 2 of 8 recipients of wt mCP-CML and TNs and 2 of 4 recipients of IAbβ−/− mCP-CML and TNs, as we were concerned that the remainder might not tolerate the procedure due to the severity of GVHD. Comparing recipients of wt mCP-CML and TNs versus TEMs, P < .04 on days 15, 22, and 39 and P > .3 on days 37 and 54. (C) Spleen weight of each recipient after death or killing at day + 50. P > .4 comparing spleen weights of recipients of IAbβ−/− mCP-CML and either TNs or TEMs versus no T cells; P < .008 comparing spleen weights of recipients of wt mCP-CML and either TNs or TEMs versus no T cells. Horizontal bars represent mean values.
CD4+ TNs and TEMs require cognate interactions with MHCII+ mCP-CML cells to mediate GVL. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, wt B6, or IAbβ−/− mCP-CML, with no T cells or with 5 × 105 TN or 106 TEM B6bm12 CD4 cells. (A) Survival data from 1 of 2 similar experiments; P = .002 for recipients of IAbβ−/− mCP-CML and TEMs versus wt B6 mCP-CML and TEMs; P = .004 comparing recipients of IAbβ−/− mCP-CML and TNs versus wt B6 mCP-CML and TNs. (B) Numbers of NGFR+ cells in the peripheral blood of mice that underwent transplantation taken at different time points. At day 9, peripheral blood was harvested from only 2 of 8 recipients of wt mCP-CML and TNs and 2 of 4 recipients of IAbβ−/− mCP-CML and TNs, as we were concerned that the remainder might not tolerate the procedure due to the severity of GVHD. Comparing recipients of wt mCP-CML and TNs versus TEMs, P < .04 on days 15, 22, and 39 and P > .3 on days 37 and 54. (C) Spleen weight of each recipient after death or killing at day + 50. P > .4 comparing spleen weights of recipients of IAbβ−/− mCP-CML and either TNs or TEMs versus no T cells; P < .008 comparing spleen weights of recipients of wt mCP-CML and either TNs or TEMs versus no T cells. Horizontal bars represent mean values.
That MHCII− mCP-CML cells are resistant to CD4-mediated GVL indicates that T-cell cytolytic function is essential. Direct T-cell cytotoxicity is mediated by 4 main mechanisms: perforin/granzyme, FasL, TNF-α, and TRAIL. Using the same B6bm12→B6 strain pairing, we assessed the roles of FasL-, TNF-α–, and TRAIL-mediated killing by inducing mCP-CML in BM from mice genetically lacking functional Fas (Faslpr), both TNF receptors 1 and 2 (TNFR1/R2−/−) or the TRAIL receptor (TRAILR−/−).20 The roles of these pathways in GVL have been interrogated mostly with gene-deficient donor T cells and reagent-based blockade.33 Both of these approaches could affect the generation and function of alloreactive T cells,34,35 and reagent-based blockade lacks specificity as it can act on multiple cell types. In contrast, by comparing GVL against leukemias deficient in death receptors, any observed differences would be due to only a block in the final cytolytic event.
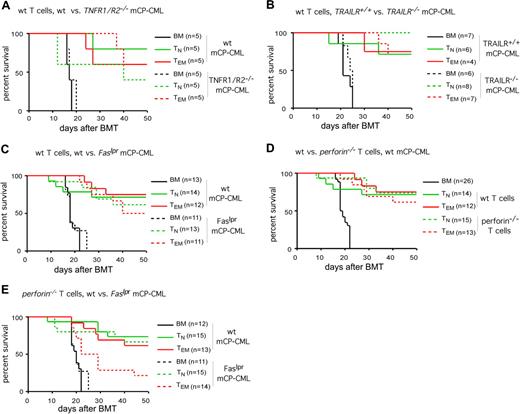
Both CD4+ TNs and TEMs mediated equivalent GVL against wt, Faslpr, TNFR1/R2−/−, and TRAILR−/− mCP-CML (Figure 6A-C). Importantly, recipients of gene-deficient and wt mCP-CML and no GVL-inducing T cells died with similar kinetics indicating that the absence of these death receptors did not change the fundamental behavior of the leukemias. As in prior experiments, TN recipients died from GVHD while TEM recipients died from mCP-CML (“Methods”). Thus, individually impaired, GVL is unaffected when killing via FasL, TNF-α, or TRAIL is prevented.
GVL mediated by CD4+ TEMs is intact when killing by FasL, TNF, TRAIL, and perforin is individually blocked, but is reduced when killing by both FasL and perforin is prevented. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, mCP-CML derived from wt, Faslpr, or TNFR1R2−/− B6 mice (A,C,D) or TRAILR−/− or TRAILR+/+ littermates (B; data from 1 of 2 similar experiments), with no T cells, CD4+ TNs, or CD4+ TEMs from wt or perforin−/− B6bm12 mice. Survival curves are plotted (A-E). The strains from which donor T cells and mCP-CML were derived are noted above each graph. When individually blocked, killing via TNFR1/R2 (A; P > .21 for recipients of wt TEMs or TNs and TNFR1R2−/− mCP-CML versus wt mCP-CML), TRAILR (B; P > .12 for recipients of wt TEMs or TNs and TRAILR−/− mCP-CML versus TRAILR+/+ mCP-CML), Fas (C; P > .63 for recipients of wt TEMs or TNs and Faslpr mCP-CML versus wt mCP-CML), or perforin (D; P > .47 for recipients of wt mCP-CML and Prf1−/− versus wt TNs or TEMs) is not required for GVL by either CD4+ TNs or TEMs. However, when killing via both perforin and Fas was prevented (E), GVL by TEMs but not TNs was diminished (P = .03 comparing recipients of CD4+Prf1−/− TEMs and wt mCP-CML versus CD4+Prf1−/− TEMs and Faslpr mCP-CML; P = .01 for recipients of Faslpr mCP-CML and no T cells versus Faslpr mCP-CML and CD4+Prf1−/− TEMs; P = .71 for recipients of CD4+Prf1−/− TNs and wt mCP-CML versus CD4+Prf1−/− TNs and Faslpr mCP-CML). Survival plots in panels C-E are from data combined from 2 independent experiments.
GVL mediated by CD4+ TEMs is intact when killing by FasL, TNF, TRAIL, and perforin is individually blocked, but is reduced when killing by both FasL and perforin is prevented. B6 mice were irradiated and reconstituted with T cell–depleted B6bm12 BM, mCP-CML derived from wt, Faslpr, or TNFR1R2−/− B6 mice (A,C,D) or TRAILR−/− or TRAILR+/+ littermates (B; data from 1 of 2 similar experiments), with no T cells, CD4+ TNs, or CD4+ TEMs from wt or perforin−/− B6bm12 mice. Survival curves are plotted (A-E). The strains from which donor T cells and mCP-CML were derived are noted above each graph. When individually blocked, killing via TNFR1/R2 (A; P > .21 for recipients of wt TEMs or TNs and TNFR1R2−/− mCP-CML versus wt mCP-CML), TRAILR (B; P > .12 for recipients of wt TEMs or TNs and TRAILR−/− mCP-CML versus TRAILR+/+ mCP-CML), Fas (C; P > .63 for recipients of wt TEMs or TNs and Faslpr mCP-CML versus wt mCP-CML), or perforin (D; P > .47 for recipients of wt mCP-CML and Prf1−/− versus wt TNs or TEMs) is not required for GVL by either CD4+ TNs or TEMs. However, when killing via both perforin and Fas was prevented (E), GVL by TEMs but not TNs was diminished (P = .03 comparing recipients of CD4+Prf1−/− TEMs and wt mCP-CML versus CD4+Prf1−/− TEMs and Faslpr mCP-CML; P = .01 for recipients of Faslpr mCP-CML and no T cells versus Faslpr mCP-CML and CD4+Prf1−/− TEMs; P = .71 for recipients of CD4+Prf1−/− TNs and wt mCP-CML versus CD4+Prf1−/− TNs and Faslpr mCP-CML). Survival plots in panels C-E are from data combined from 2 independent experiments.
Because there are not mice with a gene deficiency that renders their cells specifically resistant to killing by perforin/granzyme, we used B6bm12 perforin knockout (Prf1−/−) CD4+ T cells to investigate the role of perforin in TEM GVL activity. Perforin-mediated killing is not required as both CD4+Prf1−/− TNs and TEMs mediated GVL against B6 mCP-CML that was equivalent to that mediated by wt CD4+ TNs and TEMs (Figure 6D). To block killing by both perforin and FasL, we performed GVL experiments with Prf1−/− donor CD4+ T cells and Faslpr mCP-CML. GVL mediated by CD4+ TNs was intact even when both perforin- and FasL-mediated killing were prevented. In contrast, GVL mediated by TEMs was significantly reduced, but not eliminated, when both pathways were blocked (Figure 6E).
Analysis of cytokines and cytokine-producing cells in recipients of TEMs and TNs
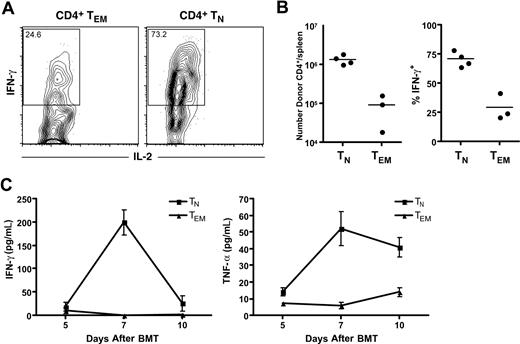
Because GVHD in the B6bm12→B6 strain pairing is diminished by treatment with reagents that block TNF-α and IL-1,19 we hypothesized that B6bm12 TEMs would not induce high levels of cytokines in B6 recipients, whereas TNs would. To test this idea, we reconstituted irradiated B6 mice with TCD B6bm12 BM and B6bm12 TEMs or TNs. We harvested serum for quantitation of cytokine concentration (by Luminex) on days +5, +7, and +10 after BM transplantation. Cohorts of mice were killed on day +7 and splenocytes were analyzed for donor CD4+ cells and their ability to produce IFN-γ, IL-2, TNF-α, and IL-4 by intracellular cytokine staining. B6 hosts were CD45.1+ congenic which, allowed the identification of donor-derived CD45.1− T cells. At day +7, the total number of donor CD4 cells in allogeneic TN recipients was approximately 15-fold greater than in allogeneic TEM recipients (Figure 7B; P = .002). A greater percentage also produced IFN-γ (Figure 7B; representative flow cytometry, Figure 7A; P = .001), but few cells produced IL-2 (Figure 7), IL-4, or TNF-α (not shown). Recipients of TNs (relative to TEMs) also had higher serum levels of IFN-γ (day +7; P < .001) and TNF-α (days +7 and +10; P = .002 and P = .015, respectively). The discordance between serum levels of TNF-α and the presence of few if any TNF-α–producing CD4 cells suggests that donor TNs induce TNF-α production by other cells, perhaps by activating recipient macrophages or dendritic cells.
TEMs are defective in their ability to produce cytokines and induce lower levels of TNF-α and IFN-γ in recipients. B6 CD45.1 mice were irradiated and reconstituted with TCD B6bm12 BM with either 106 B6bm12 CD4+ TEMs or 5 × 105 CD4+ TNs. (A) Representative staining for intracellular IFN-γ and IL-2 in donor-derived CD45.1−CD4+ cells in spleens harvested day +7 after BM transplantation. Numbers on plots are the percentages of total cells in the rectangles. (B) Total number of donor CD4+Thy1.2+ cells in spleen on day +7 (left panel; P = .002) and the percentage of these that stain for intracellular IFN-γ (right panel; P = .001). Each symbol represents data from an individual mouse; the horizontal line is the mean. (C) Mice were bled on days +5, +7, and +10, and serum levels of IFN-γ and TNF-α were measured by Luminex technology. Recipients of TNs (relative to TEMs) had higher serum levels of IFN-γ (day +7; P < .001) and TNF-α (days +7 and +10; P = .002 and P = .015, respectively). Error bars represent SD.
TEMs are defective in their ability to produce cytokines and induce lower levels of TNF-α and IFN-γ in recipients. B6 CD45.1 mice were irradiated and reconstituted with TCD B6bm12 BM with either 106 B6bm12 CD4+ TEMs or 5 × 105 CD4+ TNs. (A) Representative staining for intracellular IFN-γ and IL-2 in donor-derived CD45.1−CD4+ cells in spleens harvested day +7 after BM transplantation. Numbers on plots are the percentages of total cells in the rectangles. (B) Total number of donor CD4+Thy1.2+ cells in spleen on day +7 (left panel; P = .002) and the percentage of these that stain for intracellular IFN-γ (right panel; P = .001). Each symbol represents data from an individual mouse; the horizontal line is the mean. (C) Mice were bled on days +5, +7, and +10, and serum levels of IFN-γ and TNF-α were measured by Luminex technology. Recipients of TNs (relative to TEMs) had higher serum levels of IFN-γ (day +7; P < .001) and TNF-α (days +7 and +10; P = .002 and P = .015, respectively). Error bars represent SD.
Discussion
In the present work, we demonstrate that CD4+ TEMs can mediate meaningful GVL without causing GVHD. We did so using clinically relevant murine models of chronic phase and blast crisis CML induced by the genetic abnormalities responsible for their human equivalents. The mCP-CML dose used in our experiments was at least several fold greater than the minimal lethal dose, and mCP-CML cells are readily apparent in peripheral blood by 7 days after transplantation. This likely represents a greater leukemia challenge than is the case in human alloSCT where overt leukemia relapse is not usually manifest until months after transplantation. Nonetheless, a substantial fraction of our mice completely cleared leukemia cells in bone marrow, spleen, and peripheral blood.
Nearly 50% of patients who could benefit from an alloSCT do not undergo one because they lack an HLA-matched donor. In contrast, most patients have a HLA-haploidentical relative who could serve as a donor. However, because of the severity of GVHD with haploidentical allografts, most centers that perform such transplantations do so with rigorous depletion of allograft T cells, which completely abrogates donor T cell–mediated GVL and immune reconstitution.6,7 We performed the present studies in a MHCII-mismatched system to parallel the dominant form of T-cell recognition that drives the severity of GVHD in haploidentical transplantation. Our results indicate that CD4+ TEMs could be added to T cell–depleted MHC-mismatched allografts and that these TEMs will promote both GVL and immune reconstitution with far less GVHD than would be induced by unfractionated T cells. This approach could be safer than the infusion of small number of unfractionated donor T cells, which carries a high risk of significant GVHD. Moreover, transfer of memory T cells in large number would likely be more efficacious than a small number of TNs in promoting antipathogen T-cell immunity. Prior work has shown that memory T cells from donors vaccinated against fully allogeneic leukemia cells can mediate GVL without GVHD.9 However, vaccination of healthy donors with allogeneic tumor cells may not be practical due to regulatory barriers. Moreover, recent data indicate that CD4+ TEMs from donor mice vaccinated to recipient allogeneic MHC do mediate GVHD.36,37 In contrast, harvesting preexisting TEMs does not require vaccination, and the cell processing necessary to purify TEMs could be performed by any facility capable of selecting CD34+ cells from donor allografts.
A central question is why TEMs mediate GVL but do not induce GVHD. For TEMs to have this property, they must retain properties essential for GVL but lack features key for GVHD. GVHD may require a sustained and high-magnitude T-cell response that TNs, but not TEMs, can generate. Studies in a different MHC-mismatched GVHD model support this idea in that the progeny of transferred donor CD4+ TEMs did not accumulate after transplantation to the same extent as did the progeny of CD4+ TNs.11 Consistent with this study, at day 7 after transplantation, recipients of TNs had a 15-fold greater number of donor T cells than did recipients of TEMs (Figure 7B). It has yet to be determined why TEMs do not generate the type of response as do TNs,and this is an active area of research by several groups, including our own.36,,,–40 Nonetheless, we can speculate on why GVHD might require a higher threshold number of alloreactive effectors than does GVL. A consistent feature of GVHD in MHC-mismatched models is the generation of high systemic levels of cytokines produced by the rapidly expanding population of alloreactive T cells.41 This is particularly well demonstrated in a prior study using the B6bm12→B6 strain pairing used in our studies wherein cytokines were shown to cause GVHD and death, even if donor CD4 cells are unable to directly contact GVHD target tissues.19 This type of pathophysiology is likely to be important in human MHC-mismatched transplantations in which early hyperacute GVHD is a significant clinical problem. We observed that TEMs did not induce high systemic levels of TNF-α and IFN-γ in recipients, as did TNs. In addition, a greater fraction of TNs produced IFN-γ. Thus, combined with the work of Teshima et al19 , our results suggest that TNs but not TEMs can accumulate sufficient cytokine-producing cells so as to generate high levels of systemic cytokines.
The magnitude of the T-cell response may also be essential for T cell–infiltrative (as opposed to cytokine-mediated) GVHD. At the onset of GVHD, a threshold number of effectors may need to enter a given tissue to cause sufficient tissue damage to establish local changes (such as changes in vascular integrin expression and the generation of chemokine gradients) that promote more efficient recruitment of additional activated T cells.42,–44 The maintenance of a GVHD lesion also is likely to require an ongoing source of alloreactive T cells provided by ongoing alloreactive T-cell generation.22,26,45 The limitation on TEM expansion would retard both of these steps.
Our studies provide a detailed mechanistic understanding of how TEMs mediate GVL. Both CD4+ TNs and TEMs required cognate interactions with leukemia cells, which indicates that direct cytolytic activity is essential, and this is consistent with our prior results in an MHC-matched model.18 Because p210 overexpression alone does not block differentiation in human or murine CP-CML, mCP-CML cells are heterogeneous as is MHCII expression. We infer that a key target, perhaps a leukemia stem cell,46 is MHCII+, and this is currently being explored. We further investigated these cytolytic functions using gene-deficient leukemias and perforin-deficient T cells. When killing via FasL, TNF-α, TRAIL, and perforin was individually impaired, GVL by both CD4+ TNs and TEMs was intact. However, CD4+ TEMs were reduced in their ability to induce GVL when both perforin- and FasL-mediated killing were prevented. Nonetheless, survival in recipients of Faslpr mCP-CML and Prf1−/− CD4+ TEMs was significantly greater than in mice that received Faslpr mCP-CML and no T cells (P = .01). Thus both TNs and TEMs can mediate GVL via TRAIL or TNF-α, alone or in combination. Perforin has been shown to contribute to the down-regulation of T-cell responses.47,48 It is therefore possible that in the absence of perforin, alloreactive CD4 cell expansion was increased, thereby masking a more essential role for perforin-mediated killing, even by TNs.
GVL dependence on FasL or perforin has been model dependent with roles for both killing mechanisms described (reviewed in Molldrem et al33 and van den Brink and Burakoff49 ). We extend these prior studies by demonstrating that in a model in which CD4 cells must be directly cytolytic, they can mediate GVL independent of both FasL and perforin. In contrast to our experiments with TRAILR−/− mCP-CML, a prior study reported reduced GVL with reagent-based blockade of TRAIL and in transplantations with TRAIL-deficient donor T cells.50 In those experiments, killing of the model leukemia cell lines was CD8-dependent, and whether TRAIL is important for CD4-mediated GVL was not addressed. These cell lines may also be relatively resistant to FasL and perforin, and therefore GVL may be more reliant on TRAIL. It is also possible that TRAIL is important for the generation of GVL-inducing T cells and not in the final killing step, which was specifically inhibited in our approach. Nonetheless, the key point from our studies is that GVL-inducing CD4 cells, be they TEMs or TNs, have redundant killing mechanisms.
So then why can the relatively low magnitude response generated by TEMs induce GVL? The multiple and redundant killing mechanisms available to TEMs likely contribute to the efficiency of TEM-mediated GVL, though they are less potent than TNs. Threshold effects may also be less important for GVL than for GVHD as TEMs and their progeny have excellent access to leukemia cells, as leukemia is mostly a disease of blood, BM, and spleen, sites easily accessible to activated T cells without additional inflammatory signals that may be required to recruit T cells into other tissues.51,52 Taken together, efficient killing and ready access to leukemia targets may allow for effective GVL even when the magnitude of the alloresponse is below what is necessary for GVHD.
In summary, we demonstrate for the first time that CD4+ TEMs unprimed to recipient cells mediate GVL without causing GVHD. Our data further indicate that CD4+ TEMs induce GVL because they retain key cytolytic functions, but lack other features pivotal for initiating GVHD. These results, combined with the observation that TEMs can transfer functional T-cell memory,8 support a strategy of selective administration of memory T cells in clinical alloSCT. Reagents are already available to perform such purifications,53,54 and thus this is a feasible approach.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Yale Animal Resources Center for expert animal care.
This work was supported by National Institutes of Health (NIH) grants R01-HL66279 and R01-CA96943. W.D.S. is a recipient of a Clinical Scholar Award from the Leukemia and Lymphoma Society. H.Z. was a recipient of Christian Jacobsen Postdoctoral Fellowship Award from The Marrow Foundation and the National Marrow Donor Program. H.L. is supported by T32 HL07262-31.
National Institutes of Health
Authorship
Contribution: H.Z. designed and performed experiments, analyzed results, and wrote the paper; C.M.-M. developed key techniques, performed experiments, and analyzed data; B.E.A. provided important conceptual insight, contributed to experimental design, and established critical techniques; H.L. designed and performed the work analyzing cytokines produced and induced by TNs and TEMs; S.V. and H.S.T. performed experiments; D.J. and J.M. analyzed histopathology; and W.D.S. designed experiments, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Warren D. Shlomchik, Section of Medical Oncology, Yale Comprehensive Cancer Center, PO Box 208032, Yale University School of Medicine, New Haven, CT 06520-8032; e-mail: warren.shlomchik@yale.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal