Abstract
Patients undergoing hematopoietic cell transplantation (HCT) are at increased risk for infections with Streptococcus pneumoniaeand have long-lasting, impaired antibody responses to pneumococcal polysaccharide vaccines. We examined whether donor immunization with a heptavalent pneumococcal conjugate vaccine (PCV7) would elicit protective antibody responses to additional doses of vaccine administered early after transplantation. Ninety-six patients scheduled to receive an allogeneic hematopoietic cell transplant were randomized with their donors to receive either a dose of PCV7 vaccine or no vaccine before transplantation. All patients received PCV7 at 3 months, 6 months, and 12 months following transplantation, and serotype-specific antibody concentrations were determined after each dose. Following HCT, geometric mean antibody concentrations of patients in the immunized donor group were significantly higher for 5 of the 7 vaccine serotypes after one dose (P < .05) and for 4 of the 7 serotypes after 2 doses of vaccine (P < .03). Sixty-seven percent of patients in the immunized donor group had presumed protective IgG concentrations more than or equal to 0.50 μg/mL to all 7 serotypes following the first dose of vaccine compared to 36% in the unimmunized donor group (P = .05). After the third dose of vaccine, both groups had more than 60% of patients with concentrations at least 0.50 μg/mL to all vaccine serotypes. Donor immunization enhances early antibody responses of patients undergoing HCT to pneumococcal conjugate vaccine. A 3-dose schedule of PCV7 vaccine at 3, 6, and 12 months is immunogenic in these patients regardless of donor immunization.
Introduction
Patients undergoing hematopoietic cell transplantation (HCT) are at increased risk for infections due to defects in humoral and cell-mediated immunity that persist for several months to years after transplantation.1,2Streptococcus pneumoniaeinfections including pneumonia, sepsis, and meningitis occur in these patients at an estimated incidence of 2% to 36%.3-9 The median time of occurrence is 9 to 15 months, but infections occur as early as 3 months following transplantation.6,8-10 Current guidelines recommend immunizing patients with 23-valent pneumococcal polysaccharide vaccines at 12 and 24 months after HCT because antibody responses to these vaccines are impaired early after transplantation.11 Even when immunization is delayed until 12 months, antibody responses to capsular polysaccharide vaccines are poor and are unlikely to prevent disease.7,12 13
Polysaccharide conjugate vaccines have now been developed that link capsular polysaccharides to protein carriers and elicit enhanced antibody responses.13-16 One such vaccine, a 7-valent pneumococcal conjugate vaccine, has recently been shown to prevent invasive pneumococcal disease in healthy infants (PCV7; Prevnar, Wyeth Lederle Vaccines, Wyeth Lederle Laboratories, Pearl River, NY).17 In this study, we examined whether immunization with this 7-valent pneumococcal conjugate vaccine would be immunogenic in patients undergoing HCT and whether donor immunization would elicit early protective responses to vaccine doses administered within the first year of transplantation.
Patients, materials, and methods
Study design
Patients for this randomized, controlled study were recruited from the Dana-Farber Cancer Institute, Brigham and Women's Hospital, and Children's Hospital, all in Boston, MA. The study protocol was approved by institutional review boards of participating institutions, and informed consent was obtained from patients, donors, and their parents or guardians. Eligibility criteria, outcome variables, and the analysis plan were determined before study initiation and laboratory assays were performed with coded samples to blind personnel to patient group assignment.
Enrollment, randomization, and vaccine administration
Eligible patients were individuals scheduled to receive a non-T cell–depleted allogeneic transplant from a related donor for an underlying hematologic malignancy. Patients and their donors had to be at least 2 years of age and donors may not have received 23-valent pneumococcal polysaccharide vaccine (PPV23) within the 6 years prior to stem cell harvest. Patients were conditioned for transplantation according to diagnosis and current institutional protocols. Prophylaxis for graft-versus-host disease (GVHD) consisted of methotrexate or cyclosporine or both.
Randomization of patients with their donors was stratified by age (pediatric or adult) and performed in a permuted block design. Prelicensure lots of heptavalent pneumococcal conjugate vaccine (PCV7) containing serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F individually conjugated to a nontoxic variant of diphtheria toxin (CRM197) were used. Those patients and their donors randomized to the immunized donor group each received a dose of heptavalent pneumococcal conjugate vaccine (Wyeth Lederle Laboratories) intramuscularly at approximately 7 to 10 days before transplantation. Patients and their donors randomized to the unimmunized donor group received no vaccine before transplantation. Following transplantation, all study patients were immunized with PCV7 at 3 months, 6 months, and 12 months.
Blood samples and adverse event monitoring
Blood samples from patients undergoing HCT were obtained prior to each posttransplantation dose of vaccine at 3, 6, and 12 months and approximately 4 weeks following the third dose of vaccine (designated 13 months). Immunized donors had a blood sample collected before immunization and at the time of stem cell harvest. Unimmunized donors had a blood sample collected 0 to 10 days before harvest. Serum samples were aliquoted and stored at −80°C until assayed.
Immunized donors and all study patients completed a standardized vaccination report card at 24, 48, and 72 hours after each immunization. Fever was defined as an oral temperature of 99°F or higher. General symptoms categorized on the report card included headache, malaise, and joint pain with a section to specify other symptoms. Local site reactions were scored based on defined criteria of size of reaction for redness and swelling and restriction of arm movement for pain.
Antibody assays
IgG antibody to serotype-specific pneumococcal capsular polysaccharides was measured by an enzyme-linked immunosorbent assay (ELISA). To ensure comparability of study results to pneumococcal antibody concentrations measured in a vaccine efficacy trial in infants, the ELISA was standardized with controls and reagents qualified by Wyeth Lederle Vaccines and Pediatrics.17 IgG antibody concentrations were measured for the 7 vaccine serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) and for 2 control serotypes (1,5) not contained in the vaccine. The reference standard of the assay was US standard reference serum lot 89-SF from the Center for Biological Evaluation and Review (Food and Drug Administration, Bethesda, MD). The lower limit of assay quantitation was 10 ng/mL for each serotype.
Statistical analysis
The primary outcome of the study was comparison of serotype-specific antibody concentrations of patients in the immunized donor and unimmunized donor groups at 13 months after transplantation following receipt of 3 doses of PCV7. Comparisons of geometric mean antibody concentrations were also performed at 3, 6, and 12 months after HCT. Antibody concentrations below the limit of assay quantitation were assigned values of one half the lower limit. Comparisons of geometric mean antibody concentrations between immunization groups were performed by 2-tailed t test for parametric analyses and by Mann-Whitney rank sum test for nonparametric analyses. The proportion of patients in each immunization group with concentrations of antibody more than or equal to 0.5 μg/mL to all 7 vaccine serotypes was compared by Fisher exact test. Data analyses were performed by the PROPHET System, a national computer system sponsored by the Chemical/Biological Information Handling Program of the National Institutes of Health (Bethesda, MD).
Results
Patients and donor populations
A total of 96 patients scheduled for a non-T cell–depleted allogeneic HCT were enrolled with their donors between December 1, 1995 and December 31, 1998. Sixty-five patients (68%) were evaluable as defined by survival to at least 3 months after transplantation and without evidence of relapse. Of the 31 patients not evaluable, 5 patients were excluded prior to transplantation as their transplantation was either canceled or changed and 4 had been randomized to the immunized donor group and 1 to the unimmunized donor group. Three patients randomized to the immunized donor group had a relapse of their underlying disease before 3 months and 21 patients died in the peritransplantation period (10 in the immunized donor group and 11 in the unimmunized donor group). Two other patients were not evaluable due to protocol violations of missed pretransplantation immunization or misclassified type of transplantation.
Clinical characteristics of the 65 evaluable patients are shown in Table 1. The HCT patients in each group did not differ significantly in terms of recipient age, donor age, race, sex, diagnosis, receipt of total body irradiation, presence of GVHD, and receipt of intravenous immunoglobulin (IVIG) after transplantation.
Characteristics of evaluable allogeneic HCT study patients
| Characteristic . | Immunized donor group, n = 30 . | Unimmunized donor group, n = 35 . |
|---|---|---|
| Median age, y (range) | ||
| Recipient | 41 (2-59) | 39 (4-64) |
| Donor | 42 (4-63) | 39 (3-66) |
| White race/other | 28/2 | 33/2 |
| Male/female | 13/17 | 22/13 |
| Diagnosis | ||
| Myelodysplastic syndrome (%) | 3 (10) | 5 (14) |
| Leukemia (%) | 21 (70) | 28 (80) |
| Aplastic anemia (%) | 4 (13) | 2 (6) |
| Lymphoma (%) | 2 (7) | 0 (0) |
| Total body irradiation (%) | 25 (83) | 31 (89) |
| No. with GVHD/no. evaluable*(%) | ||
| 3 mo | 6/30 (20) | 5/35 (14) |
| 6 mo | 2/24 (8) | 6/29 (21) |
| 12 mo | 2/20 (10) | 5/26 (19) |
| No. who received IVIG†/no. evaluable (%) | ||
| 3 mo | 6/30 (20) | 9/35 (26) |
| 6 mo | 2/24 (8) | 2/29 (7) |
| 12 mo | 1/20 (5) | 1/26 (4) |
| Geometric mean concentration of serum antibody of donors at harvest, μg/mL | ||
| Pneumococcal serotype | ||
| 4 | 2.48 | 0.94‡ |
| 6B | 3.87 | 1.05‡ |
| 9V | 3.49 | 1.18‡ |
| 14 | 8.94 | 1.52‡ |
| 18C | 3.68 | 1.10‡ |
| 19F | 4.99 | 2.831-153 |
| 23F | 4.31 | 0.71‡ |
| Characteristic . | Immunized donor group, n = 30 . | Unimmunized donor group, n = 35 . |
|---|---|---|
| Median age, y (range) | ||
| Recipient | 41 (2-59) | 39 (4-64) |
| Donor | 42 (4-63) | 39 (3-66) |
| White race/other | 28/2 | 33/2 |
| Male/female | 13/17 | 22/13 |
| Diagnosis | ||
| Myelodysplastic syndrome (%) | 3 (10) | 5 (14) |
| Leukemia (%) | 21 (70) | 28 (80) |
| Aplastic anemia (%) | 4 (13) | 2 (6) |
| Lymphoma (%) | 2 (7) | 0 (0) |
| Total body irradiation (%) | 25 (83) | 31 (89) |
| No. with GVHD/no. evaluable*(%) | ||
| 3 mo | 6/30 (20) | 5/35 (14) |
| 6 mo | 2/24 (8) | 6/29 (21) |
| 12 mo | 2/20 (10) | 5/26 (19) |
| No. who received IVIG†/no. evaluable (%) | ||
| 3 mo | 6/30 (20) | 9/35 (26) |
| 6 mo | 2/24 (8) | 2/29 (7) |
| 12 mo | 1/20 (5) | 1/26 (4) |
| Geometric mean concentration of serum antibody of donors at harvest, μg/mL | ||
| Pneumococcal serotype | ||
| 4 | 2.48 | 0.94‡ |
| 6B | 3.87 | 1.05‡ |
| 9V | 3.49 | 1.18‡ |
| 14 | 8.94 | 1.52‡ |
| 18C | 3.68 | 1.10‡ |
| 19F | 4.99 | 2.831-153 |
| 23F | 4.31 | 0.71‡ |
Of the 30 patients in the immunized donor group, 6 dropped out of the study between 3 and 6 months (2 died, 2 relapsed, 1 missed immunization, and 1 had refractory thrombocytopenia). Four additional patients dropped out between 6 and 12 months (1 died, 2 relapsed, 1 received donor lymphocyte infusions). Of the 35 patients in the unimmunized donor group, 6 dropped out between 3 and 6 months (1 died, 3 relapsed, 1 had severe thrombocytopenia, 1 had persistent pancytopenia). Three more patients dropped out of the study between 6 and 12 months (1 died, 1 relapsed, 1 missed immunization).
Administered within 30 days of immunization.
P ≤ .002 by t test comparing immunized donors to unimmunized donors.
P = .048 by t test comparing immunized donors to unimmunized donors.
Donors of study patients immunized with a single dose of PCV7 had significantly higher geometric mean antibody concentrations to all 7 vaccine serotypes at the time of stem cell collection compared to those of unimmunized donors (Table 1). There were no significant differences in preimmunization antibody concentrations of donors who were immunized and those of the unimmunized donors (data not shown).
Effect of donor immunization on antibody concentrations after HCT
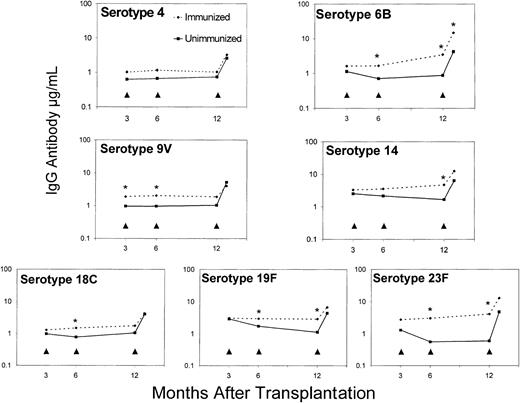
Transplantation patients in the immunized donor group had higher geometric mean antibody concentrations to all vaccine serotypes after 1 and 2 doses of vaccine compared to those patients whose donors were unimmunized (Figure 1). Differences in geometric mean antibody concentrations between the groups were significant at 6 months following the 3-month immunization for serotypes 6B (P = .036), 9V (P = .019), 18C (P = .039), 19F (P = .043), and 23F (P = .03) and at 12 months following the second immunization at 6 months for serotypes 6B (P = .013), 14 (P = .019), 19F (P = .023), and 23F (P = 0004). Prior to posttransplantation immunization with PCV7, geometric mean antibody concentration to serotype 9V was significantly higher at 3 months in the immunized donor group (P = .02). There were no significant differences in geometric mean antibody concentrations between the immunized donor group and unimmunized donor group for nonvaccine serotype 1 at 3 months (0.85 μg/mL versus 0.91 μg/mL), 6 months (0.66 μg/mL versus 0.58 μg/mL), and 12 months (0.50 μg/mL versus 0.44 μg/mL) and for nonvaccine serotype 5 at 3 months (1.55 μg/mL versus 1.56 μg/mL), 6 months (0.85 μg/mL versus 0.99 μg/mL), and 12 months (0.54 μg/mL versus 0.46 μg/mL) after HCT.
Effect of donor immunization on antibody responses to heptavalent pneumococcal conjugate vaccine (PCV7) following HCT.
Geometric mean IgG antibody concentrations to the 7 vaccine serotypes of patients whose donors were immunized with PCV7 compared to those patients whose donors were not immunized before transplantation. As indicated by the arrowheads, all patients received PCV7 at 3 months, 6 months, and 12 months after HCT. Geometric mean antibody concentrations marked with asterisks (*) were significantly higher for the immunized donor group compared to the unimmunized donor group for all vaccine serotypes (P ≤ .05) except for serotype 4.
Effect of donor immunization on antibody responses to heptavalent pneumococcal conjugate vaccine (PCV7) following HCT.
Geometric mean IgG antibody concentrations to the 7 vaccine serotypes of patients whose donors were immunized with PCV7 compared to those patients whose donors were not immunized before transplantation. As indicated by the arrowheads, all patients received PCV7 at 3 months, 6 months, and 12 months after HCT. Geometric mean antibody concentrations marked with asterisks (*) were significantly higher for the immunized donor group compared to the unimmunized donor group for all vaccine serotypes (P ≤ .05) except for serotype 4.
Following 3 doses of vaccine after HCT, geometric mean antibody concentrations at 13 months were similar for both groups, ranging from 2.55 μg/mL to 14.8 μg/mL, except for serotype 6B, which was significantly higher in the immunized donor group (14.8 μg/mL versus 4.22 μg/mL, P = .046). For control serotypes 1 and 5, geometric mean concentrations were low and similar between the immunized and unimmunized donor groups at 0.51 μg/mL versus 0.49 μg/mL for serotype 1 and 0.51 μg/mL versus 0.37 μg/mL for serotype 5.
The potential clinical impact of donor immunization with a pneumococcal conjugate vaccine was also examined by comparing the number of patients in each immunization group who achieved IgG antibody concentrations to all 7 vaccine serotypes considered to be protective. From the infant efficacy trial with PCV7, IgG concentrations within the range of 0.15 to 0.5 μg/mL are proposed to be associated with long-term protection against invasive disease.17 A threshold IgG concentration of more than or equal to 0.5 μg/mL was used in our study as an estimated serologic correlate of protection. A higher proportion of patients in the immunized donor group achieved this threshold concentration after 1 and 2 doses (Table2). The difference in the percent of patients with concentrations of 0.5 μg/mL or higher to all 7 serotypes was significant at 6 months after transplantation (67% versus 36%, P = .05). After 3 posttransplantation doses of vaccine, the proportion of patients who achieved this threshold concentration to all 7 vaccine serotypes was 75% in the immunized donor group and 64% in the unimmunized donor group.
Comparison of patients protected to all 7 vaccine serotypes following HCT
| Mo after HCT . | No. vaccine doses received . | Immunized donor group, % (n) . | Unimmunized donor group, % (n) . | P . |
|---|---|---|---|---|
| 3 | 0 | 57 (17/30) | 54 (19/35) | 1.00 |
| 6 | 1 | 67 (16/24) | 36 (10/28)* | .05 |
| 12 | 2 | 60 (12/20) | 35 (9/26) | .14 |
| 13 | 3 | 75 (15/20) | 64 (16/25) | .53 |
| Mo after HCT . | No. vaccine doses received . | Immunized donor group, % (n) . | Unimmunized donor group, % (n) . | P . |
|---|---|---|---|---|
| 3 | 0 | 57 (17/30) | 54 (19/35) | 1.00 |
| 6 | 1 | 67 (16/24) | 36 (10/28)* | .05 |
| 12 | 2 | 60 (12/20) | 35 (9/26) | .14 |
| 13 | 3 | 75 (15/20) | 64 (16/25) | .53 |
The presumed protective antibody concentration to all serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) was 0.5 μg/mL or higher.
Serum not available on one patient.
Antibody responses of patients who received PCV7 compared to patients who received PPV23
Current guidelines recommend that patients undergoing HCT receive a dose of 23-valent pneumococcal polysaccharide vaccine (PPV23) at 12 and 24 months after transplantation.11 To compare antibody responses elicited in this study with PCV7 to those achieved by immunization with standard 23-valent pneumococcal vaccine, we evaluated sera available from a previous study conducted in the same centers that included immunization of allogeneic HCT patients with PPV23 at 12 and 24 months following transplantation.18 Geometric mean antibody concentrations to the 7 serotypes common to both vaccines were compared between 22 HCT patients who received an initial dose of PPV23 at 12 months and the 45 HCT patients in this study who completed the 3-dose series of PCV7 at 3, 6, and 12 months.
Clinical characteristics of the 22 HCT patients who received PPV23 were similar to the PCV7 study patients with 17 (77%) having an underlying diagnosis of leukemia, 3 (14%) an underlying diagnosis of aplastic anemia, and 2 (9%) an underlying diagnosis of lymphoma. Twenty of the 22 had received total body irradiation as part of their conditioning regimen for HCT. Seven of the 22 patients had GVHD present within 30 days of immunization with PPV23 at 12 months. The median age of the PPV23 patients was significantly younger than the median age of the PCV7 group (29 years versus 40 years, P = .005) and the proportion of patients in the PPV23 group that received IVIG within 30 days of the 12 month immunization (23%) was significantly more than the 4% in the PCV7 group (P = .025). Blood samples for serum antibody measurements from the PPV23 patients were drawn a median of 7 weeks (range, 3-12 weeks) after the 12-month immunization and samples from the PCV7 group were obtained a median of 4 weeks (range, 3-18 weeks) after the 12 month (third) dose of pneumococcal conjugate vaccine.
Geometric mean antibody concentrations to the 7 serotypes included in both vaccines were significantly higher for patients who received a 3-dose series of PCV7 at 3, 6, and 12 months after HCT compared to those of patients who received an initial dose of PPV23 at 12 months. Geometric mean antibody concentrations of the PCV7 group compared to those of the patients who received PPV23 were 2.83 μg/mL versus 0.60 μg/mL for serotype 4 (P = .0001), 7.38 μg/ml versus 1.11 μg/mL for serotype 6B (P = .0001), 4.60 μg/mL versus 0.90 μg/mL for serotype 9V (P = .0001), 8.57 μg/mL versus 1.52 μg/mL for serotype 14 (P = .0001), 4.14 μg/mL versus 0.74 μg/mL for serotype 18C (P = .0001), 5.25 μg/mL versus 2.27 μg/mL for serotype 19F (P = .022), and 6.95 μg/mL versus 0.54 μg/mL for serotype 23F (P = .0001). The benefit of early immunization with PCV7 was also evaluated by comparing antibody concentrations of the 2 groups at 12 months. Geometric mean antibody concentrations were significantly higher for 6 of the 7 serotypes in PCV7 study patients at 12 months after 2 doses of pneumococcal conjugate vaccine compared to those of patients before immunization with PPV23 (data not shown).
Factors influencing IgG pneumococcal antibody concentrations following HCT
To determine whether other factors had an effect on pneumococcal antibody concentrations after HCT, multiple stepwise linear regression analyses were performed. The effect of donor immunization, recipient age, donor age, sex, race, diagnosis, total body irradiation, presence and treatment of GVHD within 30 days of immunization, and receipt of IVIG within 30 days of immunization on serotype-specific antibody concentrations measured at 3, 6, 12, and 13 months after HCT were determined. Only variables with P ≤ .05 were entered in the model.
Donor immunization was independently associated with higher serotype-specific antibody concentrations for 9V at 3 months (r = 0.269, P = .033); for 6B (r = 0.302,P = .01) 9V (r = 0.326, P = .018), 18C (r = 0.287, P = .039), 23F (r = 0.312,P = .024) at 6 months; for 6B (r = 0.417,P = .004), 14 (r = 0.420, P = .004), 19F (r = 0.365, P = .014), 23F (r = 0.453,P = .002) at 12 months; and for 6B at 13 months (r = 0.362, P = .016) after HCT. Prior to immunization with PCV7 after HCT, the use of IVIG was independently significantly associated with higher serotype-specific IgG antibodies for each of the 7 vaccine serotypes at 3 months, ranging from r = 0.277,P = .026 for serotype 23F to r = 0.482,P = .0001 for serotype 19F. An association of serotype-specific IgG pneumococcal antibody concentrations and receipt of IVIG was not significant at 6, 12, and 13 months after HCT. Younger donor age was independently associated with higher antibody concentrations to serotype 4 at 6 months (r = 0.306,P = .028) and 12 months (r = 0.301,P = .047) and to serotype 9 at 12 months (r = 0.347,P = .019). No other variable was consistently independently associated with multiple serotype-specific antibody concentrations.
Safety of pneumococcal conjugate vaccine in HCT patients and donors
Pneumococcal conjugate vaccine was well tolerated by immunized donors and patients as shown in Table 3. Fever was reported by a minority and 30% or less had systemic complaints after immunization. Pain was the most frequent local adverse reaction reported after each vaccine dose. Of note, one patient reported a temperature of 103°F at 10 hours after receiving the 12-month dose of vaccine. This patient also developed a local reaction with greater than an inch of redness, mild swelling, and moderate pain noted at 24 hours. Ibuprofen was taken on day 1 following immunization and her symptoms largely resolved over the next 6 days.
Side effects of pneumococcal conjugate vaccine in HCT patients and donors
| Reaction3-150 . | Total number reported (%) . | ||||
|---|---|---|---|---|---|
| Pre-HCT dose . | Post-HCT dose . | ||||
| Donors, n = 45 . | Patients, n = 41 . | 3 mo, n = 63 . | 6 mo, n = 54 . | 12 mo, n = 46 . | |
| Local3-151 | |||||
| Pain | 26 (58) | 19 (46) | 39 (62) | 29 (54) | 30 (65) |
| Redness | 7 (16) | 5 (12) | 11 (18) | 11 (20) | 10 (22) |
| Swelling | 9 (20) | 7 (17) | 14 (22) | 11 (20) | 12 (26) |
| Fever | |||||
| 99°F or higher | 3 (7) | 11 (27) | 11 (18) | 14 (26) | 4 (9) |
| 102°F or higher | 0 (0) | 2 (5) | 0 (0) | 0 (0) | 1 (2) |
| Systemic | |||||
| Headache | 2 (4) | 3 (7) | 7 (11) | 6 (11) | 4 (9) |
| Malaise | 7 (16) | 2 (5) | 7 (11) | 3 (6) | 8 (17) |
| Joint pain | 3 (7) | 1 (2) | 5 (8) | 9 (17) | 5 (11) |
| Other3-152 | 9 (20) | 5 (12) | 7 (11) | 3 (6) | 4 (9) |
| Reaction3-150 . | Total number reported (%) . | ||||
|---|---|---|---|---|---|
| Pre-HCT dose . | Post-HCT dose . | ||||
| Donors, n = 45 . | Patients, n = 41 . | 3 mo, n = 63 . | 6 mo, n = 54 . | 12 mo, n = 46 . | |
| Local3-151 | |||||
| Pain | 26 (58) | 19 (46) | 39 (62) | 29 (54) | 30 (65) |
| Redness | 7 (16) | 5 (12) | 11 (18) | 11 (20) | 10 (22) |
| Swelling | 9 (20) | 7 (17) | 14 (22) | 11 (20) | 12 (26) |
| Fever | |||||
| 99°F or higher | 3 (7) | 11 (27) | 11 (18) | 14 (26) | 4 (9) |
| 102°F or higher | 0 (0) | 2 (5) | 0 (0) | 0 (0) | 1 (2) |
| Systemic | |||||
| Headache | 2 (4) | 3 (7) | 7 (11) | 6 (11) | 4 (9) |
| Malaise | 7 (16) | 2 (5) | 7 (11) | 3 (6) | 8 (17) |
| Joint pain | 3 (7) | 1 (2) | 5 (8) | 9 (17) | 5 (11) |
| Other3-152 | 9 (20) | 5 (12) | 7 (11) | 3 (6) | 4 (9) |
Cumulative occurrence at 24, 48, and 72 hours after immunization.
Local site reaction of redness and swelling scored as: 0, none; 1, less than 1 inch; 2, 1 inch or greater. Pain at injection sited scored as: 0, none; 1, present, but no restriction of arm movement; 2, restricts use of immunized arm. Severe reactions defined as a score of 2 reported 24 to 72 hours after immunization. Zero to 4 individuals reported pain, redness, or swelling to be severe at each time point.
Report of nausea (14), vomiting (3), diarrhea (4), itchiness (2), light-headedness (1), throat pain (1), tender nipples (1), stiffness (1), insomnia (1), back pain (2), and ache in hips or legs (2).
Discussion
Infections due to polysaccharide-encapsulated bacteria such asS pneumoniae and Haemophilus influenzae type b (HIB) cause significant morbidity and mortality following HCT. Responses to polysaccharide antigens are T-cell–independent and are impaired in HCT patients as well as in healthy infants. Thus, prevention of pneumococcal infections with standard 23-valent pneumococcal vaccine has not been possible for either of these 2 populations. Recently a pneumococcal conjugate vaccine that elicits T cell–dependent responses has been shown to prevent invasive disease in infants and is now recommended as routine immunization for infants in the United States.19 We therefore examined whether heptavalent pneumococcal conjugate vaccine would elicit responses in patients undergoing allogeneic HCT and whether donor immunization resulted in earlier protection.
In this study, patients and their donors randomized to the immunized donor group each received a dose of pneumococcal conjugate vaccine before HCT, whereas patients and donors randomized to the unimmunized donor group received no vaccine before HCT. Following transplantation, patients in both immunization groups received 3 doses of pneumococcal conjugate vaccine. Patients in the immunized donor group had significantly higher IgG pneumococcal antibody concentrations to 6 of 7 vaccine serotypes after 1 and 2 doses of vaccine compared to those patients with unimmunized donors. Following the first posttransplantation dose of PCV7, geometric mean antibody concentrations at 6 months to the 7 vaccine serotypes in the immunized donor group ranged from 1.15 to 3.54 μg/mL compared to 0.56 to 2.16 μg/mL for the unimmunized donor group. At 12 months following the receipt of PCV7 at 3 and 6 months, geometric mean antibody concentrations ranged from 1.02 to 4.72 μg/mL in the immunized donor group compared to 0.59 to 1.67 μg/mL for patients in the unimmunized donor group. These data suggest that donor immunization has a positive effect on patients' ability to respond to immunization early after transplantation. In contrast, after the final dose in the series, both groups of patients achieved similar antibody concentrations with geometric mean antibody concentrations ranging from 2.55 to 14.8 μg/mL. These antibody concentrations at 13 months after transplantation are similar to those achieved in healthy infants after 3 doses of conjugate vaccine (1.21-5.04 μg/mL) and shown to prevent invasive pneumococcal disease.17 The immunogenicity of 3 doses of conjugate vaccine administered during the first year after HCT was also superior to standard 23-valent vaccine administered at 12 months after transplantation (0.54-2.27 μg/mL). It is likely that both the conjugate nature of the vaccine and the multiple dose schedule contributed to the higher antibody concentrations elicited in HCT patients immunized with pneumococcal conjugate vaccine. A previous study with HIB polysaccharide vaccines demonstrated that a protein-conjugated HIB capsular polysaccharide vaccine was more immunogenic than HIB capsular polysaccharide vaccine in patients undergoing allogenic HCT.16 Immunization studies in HCT patients have also shown that multiple doses of protein and polysaccharide conjugate vaccines elicit significantly higher antibody concentrations compared to single doses after transplantation, whereas multiple doses of unconjugated capsular polysaccharide vaccines do not prime for increased responses.13,16 20
To further assess the potential clinical impact of immunizing HCT patients with pneumococcal conjugate vaccine, we examined the proportion of patients protected after transplantation using a conservative estimate of serum antibody concentrations associated with long-term protection from invasive disease derived from analyses of the efficacy trial in infants.17 Using a threshold concentration of IgG antibody of 0.5 μg/mL or higher as an estimate of protection, 67% and 60% of patients whose donors were immunized had protective concentrations to all vaccine serotypes after 1 and 2 doses, respectively, in contrast to 36% and 35% for those whose donors were unimmunized. Following a third posttransplantation dose of vaccine, the majority of patients in both groups had serum concentrations of 0.5 μg/mL or higher to all 7 vaccine serotypes.
Previous investigators have shown that vaccine-specific antibody-secreting cells and T-cell clones after donor immunization are of donor origin in HCT patients.21-24 The findings in our present study suggest that either polysaccharide-specific B cells or carrier protein T cells (or both) were expanded in donors immunized with pneumococcal conjugate vaccine, which enhanced responses of patients to additional vaccine doses administered following transplantation. These results confirm our previous findings of donor immunization with HIB-conjugate vaccine in patients undergoing HCT.18
The strategy of donor immunization to elicit early immune recovery may be effective to prevent other infectious diseases. Infections with herpes viruses (varicella-zoster virus [VZV], cytomegalovirus [CMV], and Epstein-Barr virus [EBV]) cause significant morbidity in the first few months after transplantation. Adoptive immunotherapy with donor T cells specific for CMV and EBV has demonstrated limited efficacy in HCT patients when sufficient numbers of antigen-specific T cells are present.25-27 However, this is a prohibitively expensive approach and an effective immunization strategy is needed. A recent report of substantial protection from zoster in patients undergoing autologous HCT immunized before and after transplantation with an inactivated varicella vaccine supports the approach of using vaccines to elicit protective immunity.28 Our findings are also relevant for the use of donor immunization as an effective antitumor therapy that is being explored for a variety of hematologic and solid organ tumors.29-32
In summary, donor immunization with pneumococcal conjugate vaccine followed by patient immunization at 3, 6, and 12 months after HCT enhanced vaccine antibody responses within the first year of transplantation. In addition, 3 doses of conjugate vaccine administered after HCT regardless of donor immunization produced antibody concentrations considered protective by 13 months following transplantation. One limitation of this study is the reliance on serum antibody concentrations as a correlate of protection. However, this approach is necessary given the relatively low incidence of pneumococcal disease and sample size requirements to study efficacy outcomes in this patient population. In fact, recommendations for use of all vaccines in HCT patients have relied on antibody responses.11 Current guidelines recommend 2 doses of 23-valent pneumococcal polysaccharide vaccine at 12 and 24 months after HCT.11 Unfortunately, antibody responses of HCT patients to 23-valent pneumococcal vaccines are weak and unlikely to be protective during the time of greatest risk for disease.7,12 13 We therefore suggest that immunization with pneumococcal conjugate vaccine be considered for allogeneic HCT patients beginning at 3 months after transplantation. The utility of combining this approach with later doses of 23-valent pneumococcal polysaccharide vaccine to provide extended serotype coverage will need to be addressed. We also suggest that donor immunization with pneumococcal conjugate vaccine be used when possible to maximize early benefit for these patients. Whether the strategy of donor immunization or a multidose schedule of pneumococcal conjugate vaccine would have a similar impact in T cell–depleted allogeneic HCT or with autologous HCT is currently under investigation.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-03-0832.
Supported in part by Wyeth Lederle Vaccines, a fellowship award (D.C.M.) from Harvard Medical School 50th Anniversary Program for Scholars in Medicine, grant PO1 AI 41584 (to E.C.G.) from the National Institute of Allergy and Infectious Diseases and by the American Pediatric Society and The Society for Pediatric Research Summer Student Research Program (S.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Deborah C. Molrine, Massachusetts Biologic Laboratories, 305 South St, Jamaica Plain, MA 02130; e-mail:deborah.molrine@state.ma.us.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal