Adhesion of leukemic cells to vascular cells may confer resistance to chemotherapeutic agents. We hypothesized that disruption of leukemic cell cytoskeletal stability and interference with vascular cell interactions would promote leukemic cell death. We demonstrate that low and nontoxic doses of microtubule-destabilizing agent combretastatin-A4-phosphate (CA4P) inhibit leukemic cell proliferation in vitro and induce mitotic arrest and cell death. Treatment of acute myeloid leukemias (AMLs) with CA4P leads to disruption of mitochondrial membrane potential, release of proapoptotic mitochondrial membrane proteins, and DNA fragmentation, resulting in cell death in part through a caspase-dependent manner. Furthermore, CA4P increases intracellular reactive oxygen species (ROS), and antioxidant treatment imparts partial protection from cell death, suggesting that ROS accumulation contributes to CA4P-induced cytotoxicity in AML. In vivo, CA4P inhibited proliferation and circulation of leukemic cells and diminished the extent of perivascular leukemic infiltrates, prolonging survival of mice that underwent xenotransplantation without inducing hematologic toxicity. CA4P decreases the interaction of leukemic cells with neovessels by down-regulating the expression of the adhesion molecule VCAM-1 thereby augmenting leukemic cell death. These data suggest that CA4P targets both circulating and vascular-adherent leukemic cells through mitochondrial damage and down-regulation of VCAM-1 without incurring hematologic toxicities. As such, CA4P provides for an effective means to treat refractory organ-infiltrating leukemias.
Introduction
Although chemotherapy induces remission in most adult patients with acute myeloid leukemia (AML), only a small percentage are cured with conventional chemotherapy.1 Recurrence of leukemias is in part due to the persistence of minimal residual leukemias that remain viable within specialized niches, such as vascular niches. Hence, novel treatment strategies are urgently needed to block the interaction of leukemic cells with activated vasculature, interfering with the establishment of proleukemic niches in various organs, and to eradicate resistant disease.
Adhesion of leukemic cells to stromal cells has been shown to confer increased resistance to chemotherapeutic agents and diminish the rate of apoptosis of the leukemic cells. This process, named cell adhesion–mediated drug resistance (CAM-DR),2 depends on the interaction of integrins with their ligands. Adhesion of VLA4 (very-late antigen 4; α4β1) integrin–positive myeloid cells to VCAM-1+ stromal cells is an important mediator of CAM-DR. Indeed, expression of VLA4 by leukemic cells portends a poor prognosis and a decreased 5-year survival rate.3 Therefore, identification of novel antileukemic agents that inhibit interaction of leukemic cells with vascular cells provides novel strategies to target organ-infiltrating, angiogenesis-dependent leukemias.
Within the marrow or in circulation, leukemic cells are closely associated with endothelium,4,5 supporting establishment of neovessels by elaboration of angiogenic factors.4,,–7 In addition, leukemic cells may activate endothelial cells by releasing proinflammatory factors, including interleukin-1 (IL-1), facilitating invasion into tissues and formation of infiltrative organ disease or subcutaneous tumors, namely chloromas, thereby establishing chemotherapy-refractory leukemic minimal residual disease.
One approach to destabilize interactions of leukemic cells with endothelium is through disruption of the cytoskeletal organization of the leukemic cells.8,9 Indeed, disruption of cytoskeletal stability of leukemic cells may not only promote cell death directly, but also diminish the cellular interaction of the leukemic cells with vascular cells, thereby increasing sensitivity to chemotherapy. Therefore, in search of factors that may target leukemic microtubules, we investigated the mechanisms of action and treatment efficacy of combretastatin-A4 in AML. Combretastatin-A4, a novel tubulin-destabilizing agent, was isolated from the South African tree Combretum caffrum. Combretastatin-A4 binds to tubulin at the same site as colchicine does, but with even higher affinity.10,11 Its prodrug, combretastatin-A4-phosphate (CA4P), induces rapid microtubule depolymerization and vascular shutdown in subcutaneous solid tumors, causing tumor necrosis at concentrations well below the maximum tolerated dose.12,,–15 We have also shown that CA4P can induce apoptosis of the endothelial cells by disengaging VE-cadherin interaction. Henceforth, we hypothesized that CA4P may not only target rapidly proliferating leukemic cells directly, but may also diminish interaction of the leukemic cells with activated endothelial cells, thereby preventing establishment of a perivascular nidus for leukemic chloromas.
In this report, we show that CA4P at low, nontoxic doses induces rapid cell death of nonadherent leukemic cells through capase activation, mitochondria destabilization, and accumulation of reactive oxygen species (ROS), accompanied by the release of proapoptotic mitochondrial membrane proteins. We also demonstrate that single-agent CA4P treatment is effective in eradicating both circulating and vascular-adherent leukemic cells in subcutaneous and systemic mouse models of AML without affecting normal hematopoiesis. CA4P-treated mice had significantly prolonged survival and showed a drastic reduction of detectable leukemic cells in the marrow and peripheral circulation, and significantly decreased leukemic organ infiltration. In addition, CA4P decreases expression of VCAM-1 on endothelial cells both in vitro and in vivo, thereby decreasing leukemic cell adhesion to the vascular cells, reversing drug resistance. Therefore, CA4P delivered in combination with chemotherapeutic agents represents a promising novel therapeutic approach to eradicate vascular-dependent leukemic minimal residual disease.
Methods
Reagents
CA4P (OXiGENE, Waltham, MA) was dissolved in phosphate-buffered saline (PBS) to 1 mM and stored at 4°C.
Cell culture
All leukemia/lymphoma cells were cultured in Iscove modified Dulbecco medium (IMDM) with 10% fetal bovine serum (FBS) at 37°C. R81 AML cells were derived from a patient with primary AML as previously described.16 All remaining AML cell lines were obtained from the American Type Culture Collection (Manassas, VA): HL60, KG-1a, HEL, THP-1, K562, NB4, and U937.
Cell proliferation assay
Leukemic cells were seeded at 1 × 105 cells/mL in X-vivo medium (Lonza, Walkersville, MD) with 5% FBS and CA4P, or preincubated with the poly(ADP-ribose) polymerase (PARP) inhibitor DPQ or caspase inhibitors Z-VAD-fmk and Q-VD-OPh (R&D Systems, Minneapolis, MN). After incubation for 48 hours, cells were counted using trypan blue exclusion.
Annexin-V/PI apoptosis analysis
Leukemic cells were seeded at 105/mL in X-vivo supplemented with 5% FBS and then incubated with CA4P. After incubation for 48 hours, apoptotic cells were quantified by ApoAlert annexin-V–fluorescine isothiocyanate (FITC) propidium iodide (PI) Apoptosis Kit (BD Biosciences, San Jose, CA) using a Coulter Elite flow cytometer (Beckman Coulter, Fullerton, CA).
Western blot analysis
For detection of HMG1 protein, KG1a leukemic cells (106 in 2 mL X-vivo/5% FBS) were incubated with 100 μM staurosporine to induce apoptosis, freeze-thawed 3 times to induce necrosis, or treated with 20 mM CA4P for 24 hours. Cells were spun down and pelleted, and the supernatant was subjected to Western blot for HMG1.17
DNA damage assessment
DNA damage in CA4P-incubated leukemic cells was assessed by comet assay (R&D Systems). The concept behind this assay is based on the ability of denatured, cleaved DNA fragments to migrate out of the cell under the influence of an electric field, whereas undamaged DNA migrates more slowly and remains within the confines of the nucleus. Evaluation of the DNA “comet” tail shape and migration pattern allows for assessment of DNA damage. Results were expressed as the percentage of cells with a comet tail in 100 randomly selected, nonoverlapping cells visualized by standard light microscopy.
Activated caspase-3 staining
The percentage of activated caspase-3–positive cells was determined on cytospins by immunofluorescence using rabbit anti-human polyclonal cleaved caspase-3 antibody (1:50; Cell Signaling Technology, Danvers, MA) and AlexaFluor 488 anti-rabbit secondary antibody (1:200; Molecular Probes, Eugene, OR). Staurosporine was used as a positive control for caspase-induced apoptosis at 1 μM.
Intracellular calcium measurement and assessment of calpain activity
Leukemic cells were seeded at a concentration of 1 × 105/mL in X-vivo/5% FBS with CA4P. After 48 hours, cells were incubated with the Ca2+ indicator FLUO-3 (Molecular Probes) for 30 minutes or with cell-permeable calpain substrate Boc-Leu-Met-CMAC (10 μM; Molecular Probes) that fluoresces upon cleavage by calpain. Leukemic cells were also incubated with CA4P and the Ca2+ chelator BAPTA-AM at 10 μM (Calbiochem, San Diego, CA) or a specific inhibitor of calpain activity calpeptin at 10 μM (Calbiochem). The intracellular Ca2+ levels and calpain activity seen as fluorescent signal were then assessed by flow cytometry.
Measurement of mitochondrial membrane potential
To determine mitochondrial membrane potential, leukemic cells incubated with CA4P for 48 hours were harvested and then incubated for 10 minutes at 37°C in serum-free culture medium at a concentration of 2 × 105 cells/mL with 20 nM of DiOC6,(3) (Molecular Probes), a cell-permeant, green fluorescent, lipophilic dye that is selective for the mitochondria of viable cells. Cells were collected by centrifugation and analyzed by flow cytometry.
Fluorescence microscopy analysis of AIF, cytochrome c, and ARTS
The fluorescent microscopy analysis of apoptosis-inducing factor (AIF) was performed on leukemic cells after a 48-hour incubation with 10 nM CA4P, with or without pretreatment with DPQ. Cells were cytospun and fixed in 4% paraformaldehyde. After permeabilization with methanol (90% vol/vol) for 10 minutes at −20°C, slides were incubated with polyclonal antibody against AIF (1:50; Cell Signaling Technology) and analyzed by fluorescence microscopy. Cytochrome c and ARTS (apoptosis-related protein in the TGF-beta signaling pathway) were detected using mAb clone 6H2.B4 (1:100; BD Pharmingen, San Diego, CA) and polyclonal antibody A3720 (1:50; Sigma-Aldrich, St Louis, MO), both followed by AlexaFluor 488–conjugated secondary antibody (1:200; Molecular Probes), and analyzed by confocal microscopy.
Intracellular ROS detection
Intracellular ROS were detected as previously described.18 Briefly, leukemic cells were loaded with 2 μM H2DCFDA (Molecular Probes) in assay buffer (RPMI with 10 mM HEPES) for 30 minutes at 37°C, and mean fluorescent intensity was measured by flow cytometry.
Lentivirus production and generation of GFP+ HL60 and U937 cells
Lentiviruses were produced by transient transfection of 293T cells with the vectors pMDLgpRRE, pRSV.REV, pMD2.VSVG, and pCCLhPGK–green fluorescent protein (GFP). GFP+ HL60 and U937 cells were generated by transducing 105 HL60 cells with 2 to 4 infectious particles per cell. Transduced leukemic cells were more than 99% GFP+ as measured by flow cytometry. Leukemic/endothelial cell adhesion, VCAM-1 expression, and coculture assays.
Human umbilical vein endothelial cells (HUVECs)15 were activated with IL-1β (5 ng/mL) for 24 hours, with CA4P added at concentrations from 0 to 5 nM. VCAM-1 expression was determined by flow cytometry with phycoerythrin-conjugated anti-CD106 (VCAM-1) mAb. To assess leukemic cell adhesion, 105 GFP+ HL60 or U937 cells in X-vivo/5% FBS were added per well. The percentage of GFP+ adherent cells was quantified by fluorescent microscopy. To compare the resistance of vascular-adherent to nonadherent leukemic cells in coculture, leukemic cells were seeded on either IL-1β–activated or nonactivated HUVECs, with addition of CA4P. After 48 hours, GFP+ leukemic cells were removed from the wells by trypsinization and quantified by fluorescence microscopy and flow cytometry.
Subcutaneous in vivo leukemia model
Tumor model in mice.
HL60 (5 × 106 cells) were injected subcutaneously into the dorsa of 7-week-old nonobese diabetic–severe combined immunodeficiency (NOD-SCID) mice (The Jackson Laboratory, Bar Harbor, ME). When mice bore a tumor (ie, after 12 days), 4 experimental groups were randomized, each with 9 animals. Daily treatment was initiated at this time: the CA4P groups were subjected to intraperitoneal injection of CA4P at 10, 25, and 50 mg/kg body weight, and the control group received PBS. After a 3-day treatment, animals were killed and tumors were removed and then subjected to immunohistochemical analysis. Tumors were embedded in paraffin, serially sectioned, and stained with hematoxylin and eosin for histologic analysis. In a second experiment, mice were treated with CA4P at 25 mg/kg per day every other day, and tumors were harvested after 3 weeks and snap-frozen for cryosections. The Investigational Review Board of Weill Medical College of Cornell University approved all animal procedures.
TUNEL staining.
Cell death within paraffin-embedded tumor sections was detected by TUNEL reaction (Roche Diagnostics, Indianapolis, IN). The detection of cell death in this assay relies on the detection of free 3′OH DNA ends. Positive signal was revealed by fast red, and tumor sections were analyzed by light microscopy after hematoxylin counterstain.
Immunohistochemistry/immunofluorescence
Immunohistochemistry was used to assess the effect of CA4P on intratumoral vascularization and AIF localization in tumor cells. Antibodies were applied to paraffin-embedded tumors following heat antigen retrieval (Dako North America, Carpinteria, CA) and endogenous peroxidase quenching using 3% aqueous H2O2. For vessel staining, sections were incubated overnight at 4°C with a rat anti-mouse panendothelial cell antigen mAb (MECA-32; 1:50; BD Pharmingen), followed by incubation with a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), and then detection with Elite ABC Reagent and VIP (Vector Laboratories). The microvessel density was evaluated by microscopic counting of 5× fields at 10× magnification, and presented as mean number of microvessels per microscopic field. For AIF staining, slides were incubated with a rabbit anti–human AIF polyclonal antibody (1:50; Cell Signaling Technology), followed by secondary Texas Red–conjugated antibodies (1:1000). For fluorescence microscopy, sections were mounted in Vectashield (Vector Laboratories) containing DAPI. For VCAM-1 staining, rat anti–mouse CD106 (VCAM-1) monoclonal antibody (10 μg/mL; BD Pharmingen) was used. Images of tumor sections were taken with a Retigo EX digital camera (QImaging, Surry, BC) mounted on an Olympus BX51 microscope (Olympus, Tokyo, Japan). UPLFLN 10×/0.25, 20×/0.50, and 40×/0.75 objective lenses were used. Images were recorded using Mac QCapture version 2.68.6 acquisition software (QImaging) and were processed using Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
Systemic in vivo leukemia model
Xenotransplantation model.
NOD-SCID mice were intravenously inoculated with 107 GFP+ HL60 cells. At 3 days after inoculation, mice were divided into 2 groups of 5 mice. One group was treated every other day with PBS (control), and the second group received 25 mg/kg CA4P every other day. Each experiment was done 3 times. At day 30 after the start of the experiment, 2 mice from each group were killed, and their organs (spleen, liver, and lung), peripheral blood, and marrow of surgically removed femurs were collected and analyzed for the presence of human leukemic cells by flow cytometry. Single-cell suspension was stained using phycoerythrin-labeled anti-human CD45 mAb (BD Pharmingen), and the percentage of double-positive human CD45-PE and GFP cells was determined flow cytometer. The extent of GFP+ HL60 cell infiltration was assessed by fluorescence microscopy. In a second set of experiments, GFP+ U937 cells were used instead of HL60 cells, and the animals were killed after 30 days.
Hematopoietic toxicity.
Sex-matched 8-week-old CD1 mice (The Jackson Laboratory) were treated with CA4P at 25 mg/kg subcutaneously every other day for 4 weeks. Serial complete blood counts were monitored using a Bayer ADVIA 120 hematology analyzer (Emeryville, CA). Cord blood CD34+ cells were isolated by magnetic procedure and cultured with recombinant human stem cell factor (SCF; 50 ng/mL; Peprotech, Rocky Hill, NJ) and CA4P for 48 hours. Annexin-V/PI staining was then performed. CD34+ cells were also cultured in methylcellulose supplemented with cytokines and CA4P (Stem Cell Technologies, Vancouver, BC) for 14 days. Colonies were scored.
Statistical analysis
All in vitro and in vivo results were statistically analyzed using a 2-tailed nonparametric Mann-Whitney test. The results are expressed as mean value plus or minus standard error of the mean (SEM). A P value less than .05 was considered significant.
Results
CA4P inhibits leukemic cell proliferation
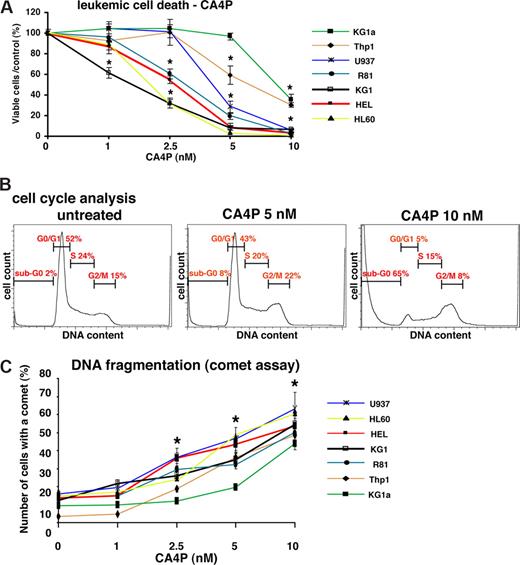
High doses of CA4P (5 to 100 nM) are required to induce cell death in anchorage-dependent solid tumors, mesenchymal stromal cells, and endothelial cells. Here, we show that CA4P in concentrations as low as 1 nM initiated cell death of nonadherent, anchorage-independent AML cells in vitro, with the IC50 ranging from 2.5 to 5 nM (Figure 1A). The majority of the AML cell lines tested were sensitive to CA4P at a concentration of 2.5 nM or less. All leukemic cell lines, as well as a recently established primary leukemic cell line (R81),16 were sensitive at low doses of CA4P (less than 10 nM).
CA4P blocks leukemic cell growth, and induces G2/M arrest, cell death with morphologic evidence of mitotic catastrophe and DNA fragmentation. (A) CA4P inhibits leukemic cell proliferation and causes cell death. A panel of leukemic cells was incubated with CA4P at the different concentrations indicated, and viable cells were counted after 48 hours using trypan blue exclusion. Results of 4 experiments in duplicate are expressed as the ratio of the percentage of viable cells/control plus or minus SEM (*P < .05 compared with CA4P-untreated cells; n = 4). (B) CA4P induces G2/M arrest and cell death (increase in sub-G0/G1 peak). KG1a leukemic cells were treated with CA4P at 0-, 5-, and 10-nM concentrations for 48 hours, and cell-cycle analysis was performed by PI staining and flow cytometry. (C) CA4P induces DNA fragmentation. CA4P-induced DNA damage was assessed by the comet assay. Results of 3 experiments in duplicate are expressed as the mean of the number of cells with a comet tail (percentage) plus or minus SEM (*P < .05 compared with CA4P-untreated cells; n = 3).
CA4P blocks leukemic cell growth, and induces G2/M arrest, cell death with morphologic evidence of mitotic catastrophe and DNA fragmentation. (A) CA4P inhibits leukemic cell proliferation and causes cell death. A panel of leukemic cells was incubated with CA4P at the different concentrations indicated, and viable cells were counted after 48 hours using trypan blue exclusion. Results of 4 experiments in duplicate are expressed as the ratio of the percentage of viable cells/control plus or minus SEM (*P < .05 compared with CA4P-untreated cells; n = 4). (B) CA4P induces G2/M arrest and cell death (increase in sub-G0/G1 peak). KG1a leukemic cells were treated with CA4P at 0-, 5-, and 10-nM concentrations for 48 hours, and cell-cycle analysis was performed by PI staining and flow cytometry. (C) CA4P induces DNA fragmentation. CA4P-induced DNA damage was assessed by the comet assay. Results of 3 experiments in duplicate are expressed as the mean of the number of cells with a comet tail (percentage) plus or minus SEM (*P < .05 compared with CA4P-untreated cells; n = 3).
CA4P causes cell-cycle arrest in G2/M phase, DNA fragmentation, and morphologic evidence of mitotic catastrophe
To investigate the mechanism by which CA4P induces leukemic cell death, the effect of CA4P on cell cycle was evaluated. In CA4P-treated cells, cell-cycle analysis with PI showed G2/M arrest and evidence of DNA fragmentation (sub-G0 phase) at 48 hours (Figure 1B). The effect of CA4P on DNA integrity was further analyzed using the comet assay. Quantification of the number of leukemic cells displaying a comet tail strongly increased after CA4P treatment (Figure 1C), consistent with CA4P-induced DNA damage.
CA4P induces cell death through apoptosis without evidence of necrosis
The mechanism by which CA4P induces death of endothelial or hematopoietic cells is unknown.14,–16,19,20 CA4P has been shown to promote cell death through recruitment of both apoptotic and non–caspase-dependent pathways. To elucidate the mechanism by which CA4P exerts its antileukemic effects, CA4P-treated AML cells were subjected to annexin-V/PI staining and quantification by flow cytometry. In contrast to endothelial cells, CA4P induced phosphatidylserine externalization in all leukemic cell lines tested, suggesting that CA4P promotes leukemic cells death through apoptosis (Figure 2A). In the majority of AML cell lines tested, CA4P at a concentration of 5 nM induced phosphatidylserine externalization (annexin-V+) in more than 50% of the leukemic cells. Only a small number of PI+, annexin-V− cells were detected, suggesting that CA4P-mediated cell death is apoptotic rather than necrotic.
Combretastatin A4 phosphate (CA4P) induces caspase-independent apoptosis in leukemic cells. (A) CA4P induces apoptosis of leukemic cells. Leukemic cells were treated with or without CA4P for 48 hours, and the percentage of apoptotic leukemic cells was determined by annexin-V and PI staining using flow cytometry. Results are representative of 3 independent experiments. Numbers in the quadrants are log fluorescent intensity. (B) CA4P induces cell death without evidence of necrosis. Western blot shows release of pelleted (“P”) nuclear HMG1 protein into the supernatant (“S”) in necrotic (freeze-thaw), but not apoptotic (staurosporine-treated) or CA4P-treated KG1a leukemic cells. Identical results were obtained in 3 independent experiments. (C) CA4P-mediated apoptosis of leukemic cells is partially reversed by the caspase inhibitor Q-VD. Leukemic cells were treated with or without CA4P for 48 hours in presence of caspase inhibitor Q-VD, and the percentage of apoptotic leukemic cells was determined by annexin-V and PI staining using flow cytometry. Percentage of live annexin-V−/PI− cells was plotted. Results are average of 3 independent experiments (*P = .008 as compared with CA4P-treated cells).
Combretastatin A4 phosphate (CA4P) induces caspase-independent apoptosis in leukemic cells. (A) CA4P induces apoptosis of leukemic cells. Leukemic cells were treated with or without CA4P for 48 hours, and the percentage of apoptotic leukemic cells was determined by annexin-V and PI staining using flow cytometry. Results are representative of 3 independent experiments. Numbers in the quadrants are log fluorescent intensity. (B) CA4P induces cell death without evidence of necrosis. Western blot shows release of pelleted (“P”) nuclear HMG1 protein into the supernatant (“S”) in necrotic (freeze-thaw), but not apoptotic (staurosporine-treated) or CA4P-treated KG1a leukemic cells. Identical results were obtained in 3 independent experiments. (C) CA4P-mediated apoptosis of leukemic cells is partially reversed by the caspase inhibitor Q-VD. Leukemic cells were treated with or without CA4P for 48 hours in presence of caspase inhibitor Q-VD, and the percentage of apoptotic leukemic cells was determined by annexin-V and PI staining using flow cytometry. Percentage of live annexin-V−/PI− cells was plotted. Results are average of 3 independent experiments (*P = .008 as compared with CA4P-treated cells).
Nonetheless, to rule out the possibility that CA4P may trigger necrotic cell death in subsets of the leukemic cells,21 we quantified the release of nuclear HMG1 protein into the culture supernatant, which has been described as a sensitive and highly specific marker for necrotic cell death.22 As shown in Figure 2B, treatment of leukemic cells with CA4P did not lead to release of HMG1 into the supernatant, indicating that necrosis is not involved in CA4P-induced cell death.
CA4P-induced cell death is partially caspase dependent
As microtubule targeting agents, such as paclitaxel, induce leukemic cell death through a caspase-dependent mechanism,23,24 we evaluated the role of caspase-mediated pathways in CA4P-induced cell death using caspase inhibitors. Both Z-VAD-fmk and Q-VD, 2 potent general caspase inhibitors, blocked CA4P-induced caspase-3 activation (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), but only partially affected CA4P-induced leukemic cell death (Figure 2C). Quantification of apoptosis by annexin-V/PI staining showed that only 33% of the CA4P-induced apoptosis was blocked by Q-VD in HL60 cells (Figure 2C). Z-VAD-fmk had no effect (data not shown). These data suggest that CA4P induces apoptosis of leukemic cells through caspase-dependent and non–caspase-dependent pathways.
CA4P decreases mitochondrial transmembrane potential, leading to cytochrome c and ARTS release from mitochondria
In order to elucidate the mechanism by which CA4P induces non–caspase-dependent cell death, the effect of CA4P on mitochondrial transmembrane potential (MTP) was analyzed. MTP was monitored by quantifying the fluorescence of the cationic lipophilic dye DiOC6(3), a potential-sensitive dye. The pattern of DiOC6(3) fluorescence taken up by control leukemic cells showed cell populations with bright fluorescence, representing cells with intact high MTP (Figure 3). In contrast, the amount of DiOC6(3) dye taken up in CA4P-treated leukemic cells was strongly decreased. The percentage of cells with fluorescence below control ranged from 12% to 89% of total cells, and for each cell line tested, the results correlated well with the extent of annexin-V positivity (Figure 2A). These data indicated that CA4P-induced leukemic cell death is possibly mediated through alteration of mitochondrial permeability. Mitochondrial damage may result in the release of proapoptotic mitochondrial membrane proteins (MMPs) such as cytochrome c, SMAC/diablo, and ARTS. ARTS is a proapoptotic protein derived by differential splicing from the human septin (sept4) gene.25 By immunofluorescence, we observed the release of cytochrome c and ARTS from mitochondria in leukemic cells after CA4P exposure (Figure S2A,B), consistent with mitochondrial destabilization.
CA4P decreases mitochondrial transmembrane potential of leukemic cells. Leukemic cells were incubated with CA4P at different concentrations for 48 hours and mitochondrial membrane potential was determined by incorporation of the potential-sensitive fluorescent dye DiOC6(3) using flow cytometry. CA4P-untreated cells have a strong uptake of the dye, whereas CA4P decreases the amount of dye uptake. Results are representative of 3 independent experiments. Numbers in the quadrants are log fluorescent intensity.
CA4P decreases mitochondrial transmembrane potential of leukemic cells. Leukemic cells were incubated with CA4P at different concentrations for 48 hours and mitochondrial membrane potential was determined by incorporation of the potential-sensitive fluorescent dye DiOC6(3) using flow cytometry. CA4P-untreated cells have a strong uptake of the dye, whereas CA4P decreases the amount of dye uptake. Results are representative of 3 independent experiments. Numbers in the quadrants are log fluorescent intensity.
To examine potential CA4P targets upstream of mitochondria, the effect of CA4P was assessed on the upstream regulators of mitochondria function, in particular on the intracellular level of Ca2+ and on calpain activity.26 Remarkably, CA4P increased cytosolic levels of Ca2+, thereby enhancing calpain activity (Figure S3A,B). However, using Ca2+ chelator (10 μM BAPTA-AM; data not shown) or a specific inhibitor of calpain activity (10 μM calpeptin) did not abrogate CA4P-induced cell death (data not shown). These data further support the notion that CA4P may target mitochondria directly via tubulin disruption and led us to explore other downstream proapoptotic pathways, possibly through translocation of apoptosis inducing factor into the nucleus.
AIF translocation from mitochondria to the nucleus has been shown to result in DNA fragmentation and cell death.27 We therefore hypothesized that AIF may be involved in CA4P-induced cell death. CA4P induced translocation of AIF from the cytoplasm to the nuclei in all the leukemic lines tested (Figure S4). To evaluate the functional role of AIF in CA4P-induced cell death, we blocked AIF translocation through inhibition of PARP, since PARP activation is required for AIF translocation into the nucleus.28 However, coincubation with the PARP inhibitor DPQ, which blocked AIF translocation to the nucleus, failed to block CA4P-induced cell death (data not shown). Thus, AIF translocation is not a significant contributor to CA4P-induced cell death.
CA4P causes rapid accumulation of ROS, and antioxidant treatment reduces CA4P-induced cell death
Another mechanism through which tubulin destabilization by CA4P may induce leukemic cell death is through disruption of the mitochondrial respiratory chain, leading to ROS accumulation and cellular damage. Indeed, coincubation of leukemic cells with the antioxidant ROS scavenger, ascorbic acid (AA), led to a partial decrease in CA4P-mediated cell death (Figure 4A). In addition, we observed rapid accumulation of superoxide anions after CA4P exposure, as evidenced by an increase in intracellular fluorescence after H2DCFDA loading and reversal of ROS accumulation by AA (Figure 4B). Another ROS scavenger, deferoxamine (DFX), also slightly inhibited CA4P-induced cell death and had an additive effect with caspase inhibitor Q-VD in HL60 cells, inhibiting up to 71% of CA4P-induced cell death (Figure 4C). Taken together, these data indicate that CA4P may induce cell death in part through a caspase-dependent as well as in part through a non–caspase-dependent cell pathway, by accumulation of ROS as a result of tubulin destabilization and disruption of the mitochondrial respiratory chain.
CA4P induces cell death through ROS accumulation. (A) CA4P-induced cell death can be partially prevented by coincubation with ascorbic acid, an ROS scavenger, in a concentration-dependent manner. Leukemic cells were treated with 5 nM (HL60, R81, or U937) or 10 nM (KG1a or THP-1) for 48 hours with the ascorbic acid concentrations indicated and viable cells determined by trypan blue exclusion. Results of 3 experiments in triplicate are expressed as percentage of viable cells compared with control plus or minus SEM (*P < .05 compared with CA4P-untreated cells; n = 3). (B) Early ROS accumulation during CA4P treatment. ROS levels as measured by H2DCFDA fluorescence. Numbers reflect relative mean fluorescence intensity ratio between KG1a AML cells treated with CA4P and control cells (medium alone). Results are shown from triplicate measurements (± SEM) and are representative of 3 independent experiments (*P < .05 compared with untreated cells; n = 3). (C) CA4P-mediated apoptosis of leukemic cells is reversed by inhibiting ROS and caspase pathways. HL-60 cells were treated with or without CA4P for 48 hours in presence of ROS scavenger deferoxamine (DFX) and caspase inhibitor Q-VD and the percentage of apoptotic leukemic cells was determined by annexin-V and PI staining using flow cytometry. Results are average of 3 independent experiments (*P = .01 compared with CA4P-treated cells; **P = .04 compared with CA4P+ Q-VD–treated cells).
CA4P induces cell death through ROS accumulation. (A) CA4P-induced cell death can be partially prevented by coincubation with ascorbic acid, an ROS scavenger, in a concentration-dependent manner. Leukemic cells were treated with 5 nM (HL60, R81, or U937) or 10 nM (KG1a or THP-1) for 48 hours with the ascorbic acid concentrations indicated and viable cells determined by trypan blue exclusion. Results of 3 experiments in triplicate are expressed as percentage of viable cells compared with control plus or minus SEM (*P < .05 compared with CA4P-untreated cells; n = 3). (B) Early ROS accumulation during CA4P treatment. ROS levels as measured by H2DCFDA fluorescence. Numbers reflect relative mean fluorescence intensity ratio between KG1a AML cells treated with CA4P and control cells (medium alone). Results are shown from triplicate measurements (± SEM) and are representative of 3 independent experiments (*P < .05 compared with untreated cells; n = 3). (C) CA4P-mediated apoptosis of leukemic cells is reversed by inhibiting ROS and caspase pathways. HL-60 cells were treated with or without CA4P for 48 hours in presence of ROS scavenger deferoxamine (DFX) and caspase inhibitor Q-VD and the percentage of apoptotic leukemic cells was determined by annexin-V and PI staining using flow cytometry. Results are average of 3 independent experiments (*P = .01 compared with CA4P-treated cells; **P = .04 compared with CA4P+ Q-VD–treated cells).
CA4P induces leukemic cell death in vivo (subcutaneous leukemia model)
To investigate the effect of CA4P on leukemia tumor growth in vivo, escalating doses of CA4P (10, 25, and 50 mg/kg body weight) were injected intraperitoneally daily for 3 days into mice bearing pre-established, subcutaneously xenotransplanted HL60 leukemia tumors (ie, chloroma-like tumors). Tumors from mice treated with CA4P for 3 days were softer and hemorrhagic (particularly when treated with 50 mg/kg) than those observed in the control group (Figure 5A). Tumor sections of the control untreated mice showed large areas of viable HL60 cells without significant necrosis or fibrosis. In contrast, all tumors in CA4P-treated mice were largely necrotic (Figure 5A).
CA4P induces leukemic cell death in vivo. NOD-SCID mice bearing established xenotransplanted HL60 tumors were treated every day for 3 days with intraperitoneal injection of CA4P (10, 25, or 50 mg/kg), and compared with the control group, which received PBS. (A) CA4P induces leukemic cell death and targets neovessels. HL60 tumors were removed and photographed, and tumor sections were stained with hematoxylin and eosin for histologic analysis. Cell death was assessed by TUNEL assay. Red staining represents positive signal within the tumors (blue cells are negative [ie, viable] cells). Tumor neovessels (yellow arrows) were identified by MECA-32 staining. All photomicrographs: original magnification, 10×; scale bar equals 50 μm. (B) Quantification of the microvessel density in HL60 tumor sections. The microvessel density was evaluated by microscopic counting of 5 fields at 10× magnification, and presented as mean number of microvessels per microscopic field plus or minus SEM (*P < .05 compared with the PBS control group; n = 5).
CA4P induces leukemic cell death in vivo. NOD-SCID mice bearing established xenotransplanted HL60 tumors were treated every day for 3 days with intraperitoneal injection of CA4P (10, 25, or 50 mg/kg), and compared with the control group, which received PBS. (A) CA4P induces leukemic cell death and targets neovessels. HL60 tumors were removed and photographed, and tumor sections were stained with hematoxylin and eosin for histologic analysis. Cell death was assessed by TUNEL assay. Red staining represents positive signal within the tumors (blue cells are negative [ie, viable] cells). Tumor neovessels (yellow arrows) were identified by MECA-32 staining. All photomicrographs: original magnification, 10×; scale bar equals 50 μm. (B) Quantification of the microvessel density in HL60 tumor sections. The microvessel density was evaluated by microscopic counting of 5 fields at 10× magnification, and presented as mean number of microvessels per microscopic field plus or minus SEM (*P < .05 compared with the PBS control group; n = 5).
To gain insight into the mechanisms by which CA4P induces leukemic cell death in vivo, we investigated the effect of CA4P on tumor cell viability by TUNEL assay. The control tumor sections were negative for TUNEL reaction (Figure 5A). In sharp contrast, most of the leukemic cells were nonviable following treatment with low to high doses of CA4P (Figure 5A). The extent of intratumor vascularization within the different groups was assessed by immunostaining for the endothelial-specific antigen MECA-32. Control tumors exhibited abundant number of vessels, whereas CA4P-treated tumors showed only a very small number of neovessels (Figure 5A,B; P < .05, n = 5). The decrease in intratumor vascularization induced by CA4P was dose dependent (54% ± 13%, 68% ± 6%, and 90% ± 3% decrease for 10, 25, and 50 mg/kg, respectively). These data suggest that targeting leukemic xenografts is associated with tumor regression and inhibition of neoangiogenesis.
CA4P blocks the growth of organ-specific and circulating leukemic cells in vivo (systemic leukemia model)
Endothelial cells may not only support leukemic cell growth by providing vascular conduits for oxygen delivery, but also facilitate perivascular adhesion and infiltration, thereby providing a niche for CAM-DR, possibly setting the stage for the establishment of chemoresistant “minimal residual disease.” To evaluate the efficacy of CA4P in diminishing organ-specific, perivascular leukemic infiltrates in vivo, HL60 cells were labeled with GFP by a lentiviral construct, and inoculated systemically through tail vein injection. In the untreated control group, the mice survived for 32 to 40 days only and succumbed to systemic spread of leukemia. In contrast, CA4P treatment significantly increased survival of the mice who received intravenous xenotransplants (Figure 6A). This increase in survival was accompanied by a decrease in circulating leukemic cells observed 30 days after leukemic cell injection, as assessed by flow cytometry determination of double-positive cells for human CD45 and GFP (0.9% for control vs 0.2% for CA4P treatment; P < .05, n = 5; Figure 6B). Remarkably, engraftment of leukemic cells in the bone marrow was completely eradicated in the CA4P-treated mice (Figure 6B). In addition, there was no evidence of infiltration of GFP+ HL60 cells in the spleen and the lung of CA4P-treated mice, whereas a small population (0.1%) of human CD45+ and GFP+ cells was detected in the liver (Figure 6B). In contrast, control mice had considerable leukemic infiltrates in the spleen (5.2%), liver (2.6%), and lung (3.5%; Figure 6B). Concurrent histologic analysis showed the presence of leukemic infiltrates in spleen, liver, and lung sections of control mice, but only minimal foci of leukemic cells in the liver of CA4P-treated mice (Figure 6C), confirming a drastic decrease in the amount of residual disease.
CA4P improves survival of mice that underwent xenotransplantation with human leukemia cells. NOD-SCID mice were intravenously inoculated with GFP+ HL60 cells and 3 days after injection, mice were treated every other day with intraperitoneal injection of CA4P (25 mg/kg) or PBS (control). (A) CA4P prolongs survival of xenotransplanted mice. Survival of mice treated with CA4P was enhanced compared with the control mice (P < .05; n = 5). (B) CA4P decreases leukemic cell circulation in the peripheral blood and engraftment in the bone marrow, spleen, liver, and lung. At 30 days after xenotransplantation of GFP+ HL60 cell injection, the presence of GFP+ HL60 cells in the peripheral blood and the different tissues of the mice was assessed by quantification of double positive GFP and human CD45 cells by flow cytometry. (C) CA4P decreases organ-specific leukemic cell infiltration. On day 30 after inoculation of GFP+ HL60 cells, CA4P-treated mice showed evidence of minor foci of leukemic infiltrates in the liver, but had no evidence of invading leukemic cells in the lung and the spleen. In contrast, control mice had considerable tumor infiltrates in the spleen, liver, and lung. Original magnification, 40×. Scale bar equals 50 μm; percentages in graphs are percent positive cells.
CA4P improves survival of mice that underwent xenotransplantation with human leukemia cells. NOD-SCID mice were intravenously inoculated with GFP+ HL60 cells and 3 days after injection, mice were treated every other day with intraperitoneal injection of CA4P (25 mg/kg) or PBS (control). (A) CA4P prolongs survival of xenotransplanted mice. Survival of mice treated with CA4P was enhanced compared with the control mice (P < .05; n = 5). (B) CA4P decreases leukemic cell circulation in the peripheral blood and engraftment in the bone marrow, spleen, liver, and lung. At 30 days after xenotransplantation of GFP+ HL60 cell injection, the presence of GFP+ HL60 cells in the peripheral blood and the different tissues of the mice was assessed by quantification of double positive GFP and human CD45 cells by flow cytometry. (C) CA4P decreases organ-specific leukemic cell infiltration. On day 30 after inoculation of GFP+ HL60 cells, CA4P-treated mice showed evidence of minor foci of leukemic infiltrates in the liver, but had no evidence of invading leukemic cells in the lung and the spleen. In contrast, control mice had considerable tumor infiltrates in the spleen, liver, and lung. Original magnification, 40×. Scale bar equals 50 μm; percentages in graphs are percent positive cells.
To confirm these results with a different leukemic cell line, we repeated the experiments using GFP-labeled U937 cells and harvested organs after 30 days. The results obtained were similar to the prior experiment using HL60 cells: in the treatment group, less than 0.05% GFP+ U937 leukemic cells were detectable in the liver and bone marrow, whereas the control animals had significant leukemic organ infiltration present in the spleen, liver, and lungs (Figure S5). These data show that CA4P can efficiently block systemic leukemic cell growth in vivo and inhibit organ-specific spread of leukemic cells, apparently through disruption of leukemic cell growth and migration, and possibly by interfering with the activation of vascular stromal cells.
CA4P decreases attachment of AML cells to endothelial cells and adhesion-mediated resistance to CA4P
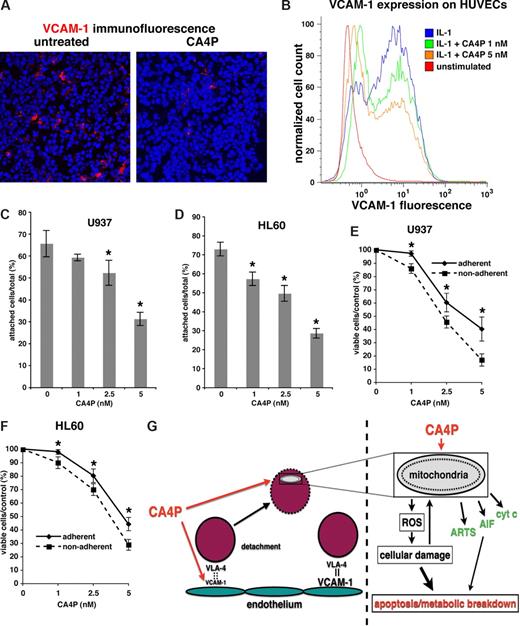
Adhesion of leukemic cells attached to vascular stromal cells may confer resistance to chemotherapy. Therefore, we hypothesized CA4P may modulate leukemia-vascular interactions, disrupting chemo-protected niches for leukemic cells. Indeed, we found significantly reduced expression of VCAM-1, a VLA4 ligand and key molecule in leukemia-stroma adhesion, in CA4P-treated leukemia xenografts (Figure 7A). Furthermore, treatment of HUVECs with low, nontoxic (1–5 nM range) doses of CA4P significantly reduced expression of VCAM-1 (Figure 7B), without inducing apoptosis. Furthermore, treatment with CA4P led to a decreased attachment of HL60 and U937 AML cells to HUVECs in vitro (Figure 7C,D). In turn, decreased adhesion to HUVECs rendered the leukemic cells more sensitive to subsequent CA4P treatment (Figure 7E,F). Taken together, these findings suggest that CA4P reduces expression of VCAM-1 on vascular cells, thereby increasing the chemosensitivity of leukemic cells. Most notably, low CA4P concentrations reduced the expression of VCAM-1 without inducing endothelial cell death, suggesting that CA4P exerts an antileukemic effect by modulating adhesive function of the endothelial cells prior to its antiangiogenic effect.
CA4P decreases expression of VCAM-1 in vivo and on HUVECs, and reduces AML adhesion and survival. (A) CA4P decreases VCAM-1 expression in vivo. NOD-SCID mice with subcutaneous HL60 AML tumors were treated with CA4P or PBS (untreated control). Immunofluorescence for VCAM-1 (red staining) shows significantly decreased VCAM-1 expression in CA4P-treated tumors. (B) CA4P prevents up-regulation of VCAM-1 in HUVECs. VCAM-1 expression in IL-1–activated HUVECs, with or without addition of CA4P, was measured by flow cytometry. (C,D) CA4P reduces leukemic cell adhesion to HUVECs. Adhesion of GFP+ U937 (C) and HL60 (D) leukemic cells to IL-1–activated HUVECs treated with various concentrations of CA4P was measured. The number of adherent cells is expressed as a percentage of total leukemic cells and representative of 3 independent experiments performed in triplicate plus or minus SEM (*P < .05 compared with CA4P-untreated control). (E,F) Leukemic cells adherent to HUVECs are more resistant to CA4P. Survival of GFP+ U937 (E) and HL60 (F) leukemic cells cocultured with HUVECs is expressed as a percentage of total cells. Compared are adherent (ie, attached to IL-1β–activated HUVECs) versus nonadherent leukemic cells (ie, cultured with nonactivated HUVECs). Results are representative of 3 independent experiments performed in triplicate plus or minus SEM (*P < .05). (G) Proposed mechanisms for antileukemic activity of CA4P. Right panel: CA4P modulates leukemia-vascular stroma interactions. Leukemic cells adhere to vascular stromal cells expressing VCAM-1, creating a chemoprotected niche. CA4P down-regulates VCAM-1 on vascular stromal cells, leading to decreased adhesion of leukemic cells and increased chemosensitivity. Left panel: CA4P causes leukemic cell death by targeting mitochondria. CA4P causes cell death by disrupting mitochondrial function, leading to accumulation of ROS, which in turn enhance mitochondrial damage and cause cellular damage via free radicals. Proapoptotic mitochondrial membrane proteins are released from the damaged mitochondria, contributing to cell death.
CA4P decreases expression of VCAM-1 in vivo and on HUVECs, and reduces AML adhesion and survival. (A) CA4P decreases VCAM-1 expression in vivo. NOD-SCID mice with subcutaneous HL60 AML tumors were treated with CA4P or PBS (untreated control). Immunofluorescence for VCAM-1 (red staining) shows significantly decreased VCAM-1 expression in CA4P-treated tumors. (B) CA4P prevents up-regulation of VCAM-1 in HUVECs. VCAM-1 expression in IL-1–activated HUVECs, with or without addition of CA4P, was measured by flow cytometry. (C,D) CA4P reduces leukemic cell adhesion to HUVECs. Adhesion of GFP+ U937 (C) and HL60 (D) leukemic cells to IL-1–activated HUVECs treated with various concentrations of CA4P was measured. The number of adherent cells is expressed as a percentage of total leukemic cells and representative of 3 independent experiments performed in triplicate plus or minus SEM (*P < .05 compared with CA4P-untreated control). (E,F) Leukemic cells adherent to HUVECs are more resistant to CA4P. Survival of GFP+ U937 (E) and HL60 (F) leukemic cells cocultured with HUVECs is expressed as a percentage of total cells. Compared are adherent (ie, attached to IL-1β–activated HUVECs) versus nonadherent leukemic cells (ie, cultured with nonactivated HUVECs). Results are representative of 3 independent experiments performed in triplicate plus or minus SEM (*P < .05). (G) Proposed mechanisms for antileukemic activity of CA4P. Right panel: CA4P modulates leukemia-vascular stroma interactions. Leukemic cells adhere to vascular stromal cells expressing VCAM-1, creating a chemoprotected niche. CA4P down-regulates VCAM-1 on vascular stromal cells, leading to decreased adhesion of leukemic cells and increased chemosensitivity. Left panel: CA4P causes leukemic cell death by targeting mitochondria. CA4P causes cell death by disrupting mitochondrial function, leading to accumulation of ROS, which in turn enhance mitochondrial damage and cause cellular damage via free radicals. Proapoptotic mitochondrial membrane proteins are released from the damaged mitochondria, contributing to cell death.
CA4P has minimal bone marrow toxicity in therapeutic doses in vivo
Ultimately, the therapeutic efficacy of CA4P is dependent on its selective action on leukemic cells without exerting major toxicity on normal hematopoietic cells. We demonstrate here that 8-week-old age- and sex-matched mice treated with high-dose CA4P at 25 mg/kg subcutaneously every other day for 4 weeks displayed a slight decrease in white blood cell (WBC) and absolute neutrophil counts only (Figure S6A-D), suggesting minimal marrow suppression. In addition, we cultured human umbilical cord blood (CB)–derived CD34+ cells in vitro in presence of kit-ligand (SCF) and CA4P for 48 hours and observed only minimal effect on cell viability as assayed by annexin-V/PI staining (Figure S6E). Moreover, CA4P did not impaired colony-forming potential of CD34+ cells, demonstrating that CA4P at the concentrations used to target leukemic cells has no major toxic effect on normal stem or progenitor cell function (Figure S6F). As such, CA4P used as a single agent can selectively target circulating and or tissue-resident leukemic cells without incurring significant hematologic toxicity.
Discussion
Leukemic cells differ from solid tumors in being capable of circulating and having access to various organs through interaction with activated vascular cells. Indeed, subsets of leukemic cells may adhere to vascular cells, establishing perivascular infiltrates, and as such may be endowed with a unique mechanism of resistance to chemotherapy. Both circulating and vascular-adherent leukemic cells require cytoskeletal stability to maintain mitochondrial and cellular function and avoid cell death. Here, we show that low and nontoxic doses of CA4P selectively induce apoptosis of circulating and vascular-bound leukemic cells by a caspase-dependent as well as ROS-mediated mitochondrial damage, leading to cell death. CA4P is effective in targeting leukemic cells in vitro and in vivo, eradicating circulating and marrow- and organ-resident vascular-adherent leukemic cells.
The molecular mechanisms by which CA4P causes cell death are complex and are most likely mediated through recruitment of caspase- and non–caspase-dependent pathways. In endothelial cells, high concentrations of CA4P have been reported to result in caspase activation and apoptotic cell death.29 CA4P induces arrest of the cells in G2/M phase of the cell cycle and subsequent cell death in endothelial cells through a nonapoptotic mechanism, since DNA fragmentation was not observed.19,30 Here, we show that CA4P impairs leukemic cell survival by a caspase activation and in part through disruption of mitochondrial transmembrane potential, leading to ROS accumulation and release of proapoptotic mitochondrial membrane proteins cytochrome c and ARTS. These findings suggest that microtubule destabilization by CA4P has direct effects on mitochondrial membrane integrity, a notion that is supported by the known close interactions between the cytoskeleton and mitochondria.31
Mitochondrial membrane potential disruption and G2/M arrest has previously been described in endothelial cells upon treatment with paclitaxel, another microtubule-active drug.320 However, paclitaxel-induced cell death is primarily caspase dependent.24,33 These data suggest that CA4P induces leukemic cell death by an apoptotic pathway that is distinct from conventional tubulin-targeting agents. As such, combination of CA4P with paclitaxel may exert a synergistic effect in mediating leukemic cell death.
We also examined whether CA4P-induced mitochondrial damage can promote the translocation of AIF. AIF is an ubiquitously expressed flavoprotein, which may play a role in caspase-independent apoptosis.27,34 Similar to cytochrome c, AIF is normally localized to the mitochondrial intermembrane space and released in response to apoptotic stimuli.35 We show that in AML cells, AIF is released from mitochondria and translocates to the nucleus. However, when AIF translocation was blocked through inhibition of PARP,27 cell death was not affected after CA4P treatment. Thus, AIF translocation and subsequent DNA cleavage is not the predominant mechanism in CA4P-induced cell death.
As increased ROS accumulation and DNA damage have been identified as mediators or initiators of necrosis in certain conditions,21 we looked for specific evidence of necrosis in CA4P-treated AML cells. Assessment of HMG1 release into the supernatant, a highly specific marker for necrotic cell death,22,36 showed no evidence of necrosis in CA4P-treated AML cells. These data indicate that CA4P induced cell death in AML is mediated via disruption of mitochondrial membrane potential, leading to intracellular ROS accumulation. We further show that these events lead to cell death via non–caspase-dependent apoptosis with DNA fragmentation, but without evidence of necrosis. Although there is concomitant release of proapoptotic mitochondrial membrane proteins, such as AIF, cytochrome c, and ARTS, our data indicates that they do not represent major contributors to CA4P-induced cell death.
To assess the antileukemic and antiangiogenic properties of CA4P on AML cells in vivo, we also assessed the effect of CA4P on immunodeficient (NOD-SCID) mice bearing subcutaneous HL60 xenografts. Histologic examination of tumors showed dramatic differences between CA4P and control groups. Animals receiving CA4P treatment showed enhanced tumor necrosis and decreased neovessel density. Examination of tumor sections revealed that AIF localized to the nuclei of the leukemic cells in vivo, validating the in vitro finding. Furthermore, we noted a significant down-regulation of VCAM-1 expression in CA4P-treated xenografts, suggesting that CA4P disrupts attachment of leukemic cells to vascular stroma. Indeed, we found that pretreatment of endothelial cells with CA4P led to a decreased attachment of AML cells and reduced adhesion-mediated CA4P resistance. In line with these observations, CA4P prevented the up-regulation of VCAM-1 in activated endothelium. These data indicate that CA4P induces regression of AML through direct targeting of the leukemic cells and disruption of leukemia-associated vasculature, thereby increasing their sensitivity to chemotherapeutic agents (Figure 7G; schema).
Next, we assessed the therapeutic effect of CA4P in a systemic leukemia xenotransplantation model in NOD-SCID mice. Intravenous injection of GFP+ HL60 cells resulted in the localization of leukemic cells to the marrow, spleen, liver, lung, and peripheral circulation. Untreated NOD-SCID mice that underwent xenotransplantation succumbed to disease within 4 to 6 weeks after inoculation. However, CA4P treatment inhibited progression and organ spread of HL60 cells in NOD-SCID mice and prolonged their survival. CA4P reduced the percentage of GFP+ HL60 cells within the marrow below the detection limit and decreased the number of circulating GFP+ HL60 cells. Moreover, CA4P significantly reduced the perivascular invasion of leukemic cells to all of the organs studied. These findings were confirmed in a second experiment using GFP+ U937 leukemic cells. Collectively, these data demonstrate the efficacy of CA4P as a single-agent therapy for the treatment of AML and a novel approach to target vascular-dependent “minimal residual disease.” Remarkably, CA4P at the concentrations used to induce cell death in the leukemic cells has no toxic effect on normal hematopoietic cells. This suggests that CA4P may be used clinically to treat leukemias without conferring major hematopoietic suppression.
In summary, our findings provide mechanistic insight into the combined antileukemic and antivascular effects of CA4P in vitro and in vivo. Our data support further evaluation of CA4P as a promising agent for novel therapeutic interventions in combination with other chemotherapeutic agents for the treatment of acute leukemias, which have predilection for perivascular chloroma formation and establishment of minimal residual disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute, Ansary Center for Stem Cell Therapeutics and National Heart, Lung and Blood Institute grants HL075234, HL059312, and HL084936 (S.R.)
National Institutes of Health
Authorship
Contribution: I.P., M.A.K., L.V., L.Y., J.B., and A.T.H. designed research and contributed to analysis and writing the paper; K.J. assisted with execution of experiments; H.S. provided expertise on the experiments related to ARTS-mediated apoptosis; D.J.C. provided expertise for the use of CA4P; E.F. provided leukemic cells and expertise; and S.R. wrote the paper, designed experiments, executed in vitro and in vivo experiments, and analyzed and complied data for the results section of the paper.
Conflict-of-interest disclosure: D.J.C., who provided the agent Combretastatin-4-P (CA4P), is the chief scientific officer of the biotech company OXiGENE. OXiGENE is currently testing the efficacy of CA4P for the treatment of solid tumors.
Correspondence: Shahin Rafii, Howard Hughes Medical Institute, Ansary Center for Stem Cell Therapeutics, Weill Cornell Medical College, Department of Genetic Medicine, 1300 York Ave, Rm A-869, New York, NY 10021; e-mail: srafii@med.cornell.edu.




![Figure 5. CA4P induces leukemic cell death in vivo. NOD-SCID mice bearing established xenotransplanted HL60 tumors were treated every day for 3 days with intraperitoneal injection of CA4P (10, 25, or 50 mg/kg), and compared with the control group, which received PBS. (A) CA4P induces leukemic cell death and targets neovessels. HL60 tumors were removed and photographed, and tumor sections were stained with hematoxylin and eosin for histologic analysis. Cell death was assessed by TUNEL assay. Red staining represents positive signal within the tumors (blue cells are negative [ie, viable] cells). Tumor neovessels (yellow arrows) were identified by MECA-32 staining. All photomicrographs: original magnification, 10×; scale bar equals 50 μm. (B) Quantification of the microvessel density in HL60 tumor sections. The microvessel density was evaluated by microscopic counting of 5 fields at 10× magnification, and presented as mean number of microvessels per microscopic field plus or minus SEM (*P < .05 compared with the PBS control group; n = 5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-05-089219/3/m_zh80030814190005.jpeg?Expires=1765887500&Signature=L6mFx7yagOX-2clX0jjAn~wmV60syk45qxuccILnxNrzatbkCVMjvEizmmeocr55gR4xohBFEFGZhSwahQt4fOwigvGnQDQAD2-FLDJ4rnw88FwHOqhnNDSrywoEZZYTaXEMbT~iGRbCi4YKf8voUm4POw-p2kUmmsV8PDfESZnR8vRH6COunnypiDh7BwBGyCQR0F0CwrFqiFpwiQb-yLJAowsYZUxTog9pJQHt-QeocH6CFbbZJsdQbeNLduyIk8CbIrF6pn92xC7tU1pQEFSOz4RlI-aoMEPe3FZlJb1Kp1Lr7CUt7lZ2wv4pOA4JzPHWWp3qwQtmmz0DR82Bew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal