Children with Down syndrome (DS) display macrocytosis, thrombocytosis, and a 500-fold increased risk of developing megakaryocytic leukemia; however, the specific effects of trisomy 21 on hematopoiesis remain poorly defined. To study this question, we analyzed blood cell development in the Ts65Dn mouse model of DS. Ts65Dn mice are trisomic for 104 orthologs of Hsa21 genes and are the most widely used mouse model for DS. We discovered that Ts65Dn mice display persistent macrocytosis and develop a myeloproliferative disease (MPD) characterized by profound thrombocytosis, megakaryocyte hyperplasia, dysplastic megakaryocyte morphology, and myelofibrosis. In addition, these animals bear distorted hematopoietic stem and myeloid progenitor cell compartments compared with euploid control littermates. Of the 104 trisomic genes in Ts65Dn mice, Aml1/Runx1 attracts considerable attention as a candidate oncogene in DS–acute megakaryoblastic leukemia (DS-AMKL). To determine whether trisomy for Aml1/Runx1 is essential for MPD, we restored disomy at the Aml1/Runx1 locus in the Ts65Dn strain. Surprisingly, trisomy for Aml1/Runx1 is not required for megakaryocyte hyperplasia and myelofibrosis, suggesting that trisomy for one or more of the remaining genes can promote this disease. Our studies demonstrate the potential of DS mouse models to improve our understanding of chromosome 21 gene dosage effects in human hematologic malignancies.
Introduction
Trisomy 21 is a relatively common acquired aneuploidy observed in myeloid leukemia blasts. Furthermore, trisomy 21 predisposes individuals with Down syndrome (DS) to multiple hematologic abnormalities, including increased red cell volume, thrombocytosis in infancy, transient myeloproliferative disorder (TMD), and leukemia.1,–3 While TMD and acute megakaryoblastic leukemia (AMKL) are associated with somatic GATA1 mutations,4,,,–8 little is known about how trisomy 21 impacts hematopoiesis, interacts with GATA1 mutations, or functions in leukemia
Children with DS face a 10- to 20-fold greater risk of developing leukemia than other children, with acute lymphoblastic leukemia (ALL) and AMKL comprising the majority of cases. Relatively rare in the non-Down's population, AMKL occurs approximately 500 times more commonly in children with DS,9 underscoring a strong association between trisomy 21, the genetic determinant of DS, and megakaryocytic malignancy. AMKL affects DS children before their fifth year and often follows a unique, spontaneously resolving TMD. TMD and AMKL both involve a clonal proliferation of megakaryoblasts, which are virtually indistinguishable between the 2 diseases; however, TMD characteristically affects fetuses and neonates with DS and most often resolves spontaneously, while AMKL presents months to years later, requires chemotherapy, and is life threatening. The ontogeny of DS-AMKL supports a model in which AMKL derives from an original TMD clone after additional mutations are acquired.
If trisomy 21 is considered the first genetic event in DS-AMKL leukemogenesis, the second is likely to be mutation of the gene for GATA1, an X-linked, blood-specific transcription factor essential for development of the erythroid and megakaryocytic lineages. Both TMD and AMKL blasts harbor somatic GATA1 mutations that result in the exclusive generation of GATA1s,4,,,–8 a shorter GATA1 isoform that retains both DNA-binding zinc fingers, but lacks the N-terminal transcriptional activation domain.10 Normal human hematopoietic cells produce GATA1s in addition to the full-length protein, but its functions in normal and malignant hematopoiesis remain poorly understood. Interestingly, wild-type mice do not express detectable levels of Gata1s11 (and our unpublished data, Andrew G. Muntean and J.D.C., June 2003); however, mice have been engineered to express the shorter protein in place of full-length GATA1. These latter animals are affected by hyperproliferation of a yolk sac/fetal liver progenitor, which resolves and gives way to apparently normal hematopoiesis in adult mice.12 In contrast, humans with germ-line mutations that cause the exclusive expression of GATA1s suffer more severe consequences throughout their lifetimes, including anemia, trilineage dysplasia, and hyperproliferation of dysplastic megakaryocytes.13 Importantly, exclusive GATA1s production alone does not cause leukemia in either humans or mice.12,13 These key findings illustrate a requisite role for additional genetic events in DS-AMKL and refocus attention on the potential cooperating function of trisomy 21 in DS leukemogenesis.
Presumably overexpression of one or more genes on chromosome 21 makes hematopoietic cells vulnerable to transformation; however, because the number of trisomic genes is large in most cases of DS—chromosome 21 encodes 364 known genes—the developmental and malignant contributions of specific genes in the hematopoietic system are difficult to tease apart. Several studies have attempted to correlate DS traits with regions of chromosome 21 by examining the DS phenotypes affecting individuals with various partial chromosome 21 trisomies. These reports have helped shape a Down syndrome critical region (DSCR) hypothesis, which predicts that most of the common DS phenotypes can be assigned to a small region of chromosome 21. This region extends about 5 Mb from DS21S17 to MX1 and has been implicated in multiple DS phenotypes, including mental retardation, short stature, craniofacial anomalies, and hypotonia. Recently, this region has been shown to be necessary but not sufficient for the craniofacial characteristics observed in mouse models of DS, underscoring the complexity of gene interactions in DS.14 No such “critical region” has been reported for the increased incidence of leukemia in DS. A small increase in disomic homozygosity at 21q11.1-2 has been reported in DS-TMD and DS-AMKL patients, but the significance of this finding is not yet clear.
Of the characterized genes on chromosome 21, there are several with known functions in hematopoiesis and leukemia that are compelling potential leukemia oncogenes in DS leukemogenesis. One such candidate is the gene for AML1/RUNX1, a transcription factor required for megakaryopoiesis and hematopoietic stem cell maintenance.15 Translocations involving this gene account for up to 10% of adult AML,16,17 and loss-of-function mutations cause familial platelet disorder (FPD) with a predisposition to AML.18 Other proposed candidates include ETS2 and ERG, 2 ets family transcription factors expressed in megakaryocytes that are targets of translocation or gene amplification in leukemia.19
To investigate the effects of trisomy 21 on blood cell development, and a potential role in malignancy, we studied the hematopoietic system in the Ts65Dn mouse model of DS. Ts65Dn mice are the most widely used model for DS and display many of the features common to human DS, including craniofacial characteristics, learning deficits, and heart malformations.20,,,–24 Ts65Dn mice harbor a segmental trisomy for the distal end of chromosome 16q fused to a gene-poor region of 17p. The 16q breakpoint is distal to Ncam2 and proximal to Gapba, and the 15.6-Mb trisomic region encodes 104 conserved genes, including an estimated 94 of the highly conserved 170 orthologs from the Down syndrome critical region (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Hematopoiesis has not been extensively studied in the Ts65Dn strain, although one report has detailed decreased proliferation of CD34+ cells in vitro.25 Notably, leukemia has never been reported in these animals. Here we show that Ts65Dn mice develop a highly penetrant, progressive myeloproliferative disease featuring thrombocytosis, mild anemia, extramedullary hematopoiesis, bone marrow fibrosis, and distorted stem and myeloid progenitor compartments. Our findings suggest that trisomy for human chromosome 21 orthologs upsets the normal regulation of hematopoiesis, and particularly megakaryopoiesis, in mice. By extension, trisomy 21 may have a similar effect in humans, generating a context that cooperates with GATA1 mutations and facilitates the development of leukemia. Unexpectedly, the Ts65Dn mouse phenotype also resembles chronic idiopathic myelofibrosis (CIMF) in humans.26 Therefore, this study may also help to define a role for chromosome 21 genes in the development of myeloproliferative disease.
Methods
Mice
Ts65Dn mice bear a segmental trisomy for the distal end of mouse chromosome 16, which contains 104 orthologs of human chromosome 21 genes.23 Ts65Dn female mice were obtained from Jackson Laboratories (C3H/Bl6 hybrid, stock number 001924; Bar Harbor, ME) and bred to C57Bl/6 × C3H F1 hybrid males to generate Ts65Dn offspring and euploid controls. To determine whether mice were Ts65Dn or euploid, a mouse chromosome 16 bacterial artificial chromosome (BAC) clone was hybridized to peripheral blood cells (cytospin preparations) using fluorescence in situ hybridization (FISH) as described previously.26 A biotin-labeled BAC probe from mouse chromosome 16 containing the Aml1/Runx1 gene (RP23-36l12; Roswell Park Cancer Institute, Buffalo, NY) was prepared by nick-translation using Bio-16-dUTP (Enzo Diagnostics, New York, NY) and detected with fluorescein-conjugated avidin (Vector Laboratories, Burlingame, CA). Cells were stained with 4,6-diamidino-2-phenylindole-dihydrochloride (DAPI), and 100 interphase cells were scored for each sample. Aml1/Runx1 heterozygous knockout mice were a kind gift of Dr James Downing and maintained on a C57Bl/6 background before crossing to Ts65Dn. All animal studies were approved by the University of Chicago and Northwestern University animal care and use committees.
Complete blood counts
Blood (50 μL) was collected from the tail vein in EDTA-coated tubes and analyzed on a Hemavet 850 complete blood counter (Oxford, CT). Statistical analysis was performed using Student t test; error bars represent standard error.
Histology
Tissues were fixed in 10% buffered formalin and processed by the University of Chicago Histological Resources Core Facility. Bone marrow samples were decalcified prior to processing and sections were stained with H&E or for reticulin according to standard protocols. Slides were photographed at 200 × original magnification on a DM 4000B microscope with a DFC320 camera and captured with DFC Twain software version 6.6.0 (Leica Microsystems, Heerbrugg, Switzerland). Slides were reviewed by a board certified pathologist (S.G.).
Colony assays
Single-cell suspensions were obtained from bone marrow or spleen in cold PBS plus 1% BSA. Red cells were lysed in hypotonic KCl and nucleated cells were washed and plated in M3234 methylcellulose for erythroid burst-forming units (BFU-Es) and granulocyte-macrophage colony-forming units (CFU-GMs), or MegaCult-C for megakaryocyte colony-forming units (CFU-MKs) (Stem Cell Technologies, Vancouver, BC), supplemented with Epo, IL3, IL6, IL11, SCF, Tpo, and/or granulocyte-macrophage colony-stimulating factor (GMCSF). Cells (15 000/mL) were plated for BFU-Es and CFU-GMs, and 30 000 cells/mL for CFU-MKs. Cells were cultured for 3 to 8 days at 37°C and 5% CO2 and colonies were enumerated. CFU-MK cultures were fixed and stained with acetylcholiniodide according to the manufacturer's specifications, and acetylcholinesterase positive colonies were counted.
Flow cytometry
Single-cell suspensions from bone marrow or spleen were treated with hypotonic KCL to lyse red cells. Cells were washed and stained in PBS plus 1% BSA plus 5% NGS on ice for 1 hour with anti–CD41-FITC, anti–TER119-PE, anti–c-kit-APC, anti–CD3-PE, and/or anti–CD19-FITC (BD Pharmingen, San Diego, CA), washed, and analyzed on a BD FacsCanto or LSRII (BD Biosciences, San Jose, CA). For ploidy analysis, cells were stained with CD41, fixed in 0.5% paraformaldehyde on ice for 45 minutes, washed, and resuspended in PBS containing 2 mM MgCl2, 0.05% saponin, 0.01 mg/mL propidium iodide, and 10 U/mL RNase A overnight, washed, and analyzed on a BD FacsCanto.
Lineage−, c-kit+, Sca1+ and myeloid progenitor analysis
Bone marrow was lineage depleted with the Easy Sep Progenitor Enrichment Kit (Stem Cell Technologies), with added anti–IL7R-biotin (BD Pharmingen). Cells were incubated with streptavidin-PerCP-Cy5.5 (eBioscience, San Diego, CA), or streptavidin-PE-TexasRed (Caltag, Burlingame, CA), anti–Sca1-PE-Cy7 (eBioscience), anti–c-kit-APC, anti–Fcγ-PE, and anti–CD34-FITC (BD Pharmingen) on ice for 1 hour, washed, and analyzed on a MoFlo flow cytometer (Cytomation, Fort Collins, CO). For pyronin Y/Hoechst stain, lineage-depleted cells were incubated with antibodies against c-kit, Sca1, and biotin, washed, and incubated for 1 hour in Hanks balanced salt solution, 20 mM HEPES, 1g/L glucose, 10% FCS, 50 μg/mL verapamil, and 1.7 μM Hoechst 33342 at 37°C. Pyronin Y was added to a final concentration of 1 μg/mL for an additional 20 minutes at 37°C. Cells were analyzed using a MoFlo flow cytometer or LSRII and FlowJo software (Treestar, Ashland, OR).
Transplantation assays
Eight- to 10-week-old lethally irradiated (6 + 4 Gy) recipient mice (C57Bl/6 Ly 5.1, stock number 002014; Jackson Laboratories) received 106 nucleated bone marrow cells from 8- to 10-week-old Ts65Dn mice (C3H/Bl6 background Ly5.2) through the retroorbital sinus (n = 5 Ts65Dn recipients; n = 5 control recipients). Mice were maintained on trimethoprim sulfamethoxazole water from 1 week prior to irradiation and for the duration of the experiment.
Results
Ts65Dn mice develop multiple peripheral blood abnormalities
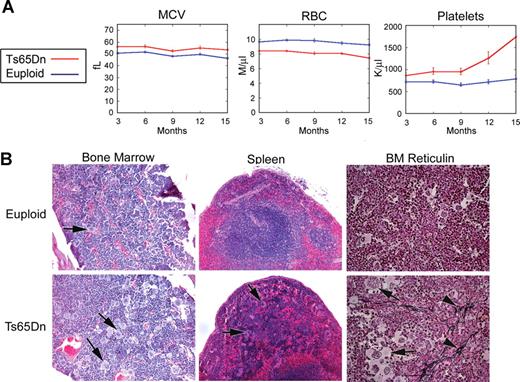
To establish baseline hematopoietic parameters for the Ts65Dn strain, monthly peripheral complete blood counts (CBCs) were performed for 14 Ts65Dn animals and 22 same-sex euploid littermate controls for 15 months. CBC data reveal the development of progressive thrombocytosis in Ts65Dn mice, with platelet numbers double to triple those of controls by 12 months of age (Figure 1A; Table S2). Platelet counts were significantly different between the groups at every time point examined. In addition, Ts65Dn mice present with significantly diminished erythrocyte numbers and elevated mean corpuscular volume (MCV) values, indicating larger red blood cell size, and lower hemoglobin concentrations (Figure 1A; Table S2). As humans with DS also display such erythroid macrocytosis,1 these data suggest that Ts65Dn mice share some features of hematopoiesis with human DS. To investigate whether a folate deficiency was the cause of macrocytosis, we measured folate levels in control and Ts65Dn peripheral blood erythrocytes and plasma, but did not detect significant differences (Table S3). Mice also displayed mildly increased numbers of white blood cells, monocytes, lymphocytes, and neutrophils for the first 12 months (Table S2).
Ts65Dn mice develop megakaryocytic myeloproliferative disease. (A) Complete blood counts (CBCs) are altered in Ts65Dn peripheral blood in comparison with euploid littermate controls. MCV indicates mean corpuscular volume; RBC, red blood cells. n = 14 Ts65Dn; n = 22 euploid same sex littermates. P < .05 for all parameters at all time points. Complete actual values and statistics are provided in Table S2. Error bars represent SE. (B) Hematoxylin and eosin staining of bone marrow and spleen sections (left and middle panels, respectively) highlight megakaryocyte hyperplasia in Ts65Dn animals, while reticulin staining reveals bone marrow fibrosis (right panels).  indicate megakaryocytes;
indicate megakaryocytes;  indicate reticulin fibrosis. All images were captured at 200× original magnification.
indicate reticulin fibrosis. All images were captured at 200× original magnification.
Ts65Dn mice develop megakaryocytic myeloproliferative disease. (A) Complete blood counts (CBCs) are altered in Ts65Dn peripheral blood in comparison with euploid littermate controls. MCV indicates mean corpuscular volume; RBC, red blood cells. n = 14 Ts65Dn; n = 22 euploid same sex littermates. P < .05 for all parameters at all time points. Complete actual values and statistics are provided in Table S2. Error bars represent SE. (B) Hematoxylin and eosin staining of bone marrow and spleen sections (left and middle panels, respectively) highlight megakaryocyte hyperplasia in Ts65Dn animals, while reticulin staining reveals bone marrow fibrosis (right panels).  indicate megakaryocytes;
indicate megakaryocytes;  indicate reticulin fibrosis. All images were captured at 200× original magnification.
indicate reticulin fibrosis. All images were captured at 200× original magnification.
Bone marrow and spleen from Ts65Dn mice show several abnormalities reminiscent of human chronic idiopathic myelofibrosis
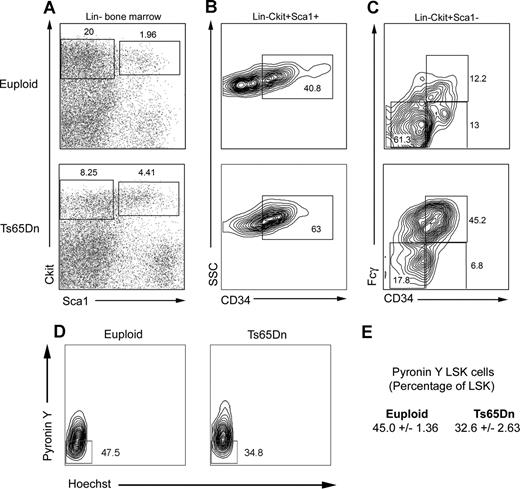
To investigate the basis for the peripheral blood phenotype, mice were euthanized for further study at 14 to 15 months of age. Histologic examination of bone marrow revealed an abundance of large megakaryocytes with deeply lobulated nuclei, often present in loose clusters (Figure 1B). Granulocyte hyperplasia was also present and there was dilation of bone marrow sinuses and a modest reticulin staining, indicating marrow fibrosis (Figure 1B). While the actual spleen size was not significantly different between the wild-type littermate control mice and the Ts65Dn mice, microscopic examination of splenic sections revealed extensive extramedullary hematopoiesis. This trilineage hematopoiesis infiltrated into the spleen, replacing both the red and white pulp (Figure 1B). While granulocytic as well as erythroid precursors could be identified, the megakaryocytes appeared to be particularly prominent. Flow cytometry data confirmed an increase in CD41+ megakaryocytes in the bone marrow and spleen, accompanied by a relative decrease in TER119-positive erythroid cells in the bone marrow (Figure 2A left panels). Furthermore, the ploidy profile of CD41+ cells was shifted toward 2N DNA content and away from the higher ploidy classes (Figure 2A right panels). In addition, flow cytometry revealed that Ts65Dn bone marrow contains more c-kit+ cells, suggesting an expansion of immature progenitors (Figure 2B), and apparently owing to, in part, left-shifted granulocytic hyperplasia. Finally, the lymphoid compartment displayed abnormalities in Ts65Dn, with a relative increase in CD3+ T cells in the spleen and a relative decrease in CD19+ B cells (Figure 2C).
Representative flow cytometric analysis of bone marrow and spleen cells. (A) Ts65Dn mice harbor increased CD41+ cells in the bone marrow and spleen (left panels). CD41+ megakaryocytes from Ts65Dn animals show an increase in the lower ploidy classes in both organs (right panels). (B) Ts65Dn mice display increased c-kit expression in the bone marrow. (C) The ratio of CD19+ to CD3+ lymphocytes is diminished in Ts65Dn mice. Numbers on plots and on histogram peaks represent the percentage of the total population of cells in the rectangle.
Representative flow cytometric analysis of bone marrow and spleen cells. (A) Ts65Dn mice harbor increased CD41+ cells in the bone marrow and spleen (left panels). CD41+ megakaryocytes from Ts65Dn animals show an increase in the lower ploidy classes in both organs (right panels). (B) Ts65Dn mice display increased c-kit expression in the bone marrow. (C) The ratio of CD19+ to CD3+ lymphocytes is diminished in Ts65Dn mice. Numbers on plots and on histogram peaks represent the percentage of the total population of cells in the rectangle.
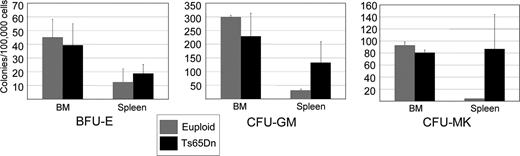
In vitro colony-forming assays were next used to assess the number of hematopoietic progenitors in Ts65Dn bone marrow and spleen. Ts65Dn mice displayed a trend toward increased numbers of erythroid, granulocyte-macrophage, and megakaryocytic progenitors in the spleen, consistent with extramedullary hematopoiesis (Figure 3). These data are not significant, likely owing to the disparate severity of disease and large variability of colony number between Ts65Dn animals. The colony-forming assays from the bone marrow yielded results that were more variable than those from the spleen and most likely a consequence of variation in disease severity and degree of bone marrow fibrosis. Importantly, we did not detect mutations in Gata1, Jak2, or Jak3 analogous to those seen in people with DS-AMKL or MPD (data not shown). Our data demonstrate that Ts65Dn mice develop a phenotype that can be classified by the Bethesda criteria as a myeloproliferative disease, manifest as megakaryocyte and granulocytic hyperplasia with bone marrow fibrosis and extramedullary hematopoiesis.28
Ts65Dn spleens harbor increased hematopoietic progenitors. Colony-forming assays were performed using nucleated bone marrow cells or splenocytes isolated from Ts65Dn mice and euploid littermate controls.  indicate euploid controls;
indicate euploid controls;  indicate Ts65Dn. BFU-E indicates erythroid burst-forming unit; CFU-GM, granulocyte-macrophage colony-forming unit; and CFU-MK, megakaryocyte colony-forming unit. n = 3 Ts65Dn; n = 3 euploid littermates. Differences in spleen colony number are P = .1 for CFU-MKs, P = .24 for CFU-GMs, and P = .6 for BFU-Es. Error bars represent SE.
indicate Ts65Dn. BFU-E indicates erythroid burst-forming unit; CFU-GM, granulocyte-macrophage colony-forming unit; and CFU-MK, megakaryocyte colony-forming unit. n = 3 Ts65Dn; n = 3 euploid littermates. Differences in spleen colony number are P = .1 for CFU-MKs, P = .24 for CFU-GMs, and P = .6 for BFU-Es. Error bars represent SE.
Ts65Dn spleens harbor increased hematopoietic progenitors. Colony-forming assays were performed using nucleated bone marrow cells or splenocytes isolated from Ts65Dn mice and euploid littermate controls.  indicate euploid controls;
indicate euploid controls;  indicate Ts65Dn. BFU-E indicates erythroid burst-forming unit; CFU-GM, granulocyte-macrophage colony-forming unit; and CFU-MK, megakaryocyte colony-forming unit. n = 3 Ts65Dn; n = 3 euploid littermates. Differences in spleen colony number are P = .1 for CFU-MKs, P = .24 for CFU-GMs, and P = .6 for BFU-Es. Error bars represent SE.
indicate Ts65Dn. BFU-E indicates erythroid burst-forming unit; CFU-GM, granulocyte-macrophage colony-forming unit; and CFU-MK, megakaryocyte colony-forming unit. n = 3 Ts65Dn; n = 3 euploid littermates. Differences in spleen colony number are P = .1 for CFU-MKs, P = .24 for CFU-GMs, and P = .6 for BFU-Es. Error bars represent SE.
Hematopoietic stem and progenitor compartment profiles are distorted in Ts65Dn mice
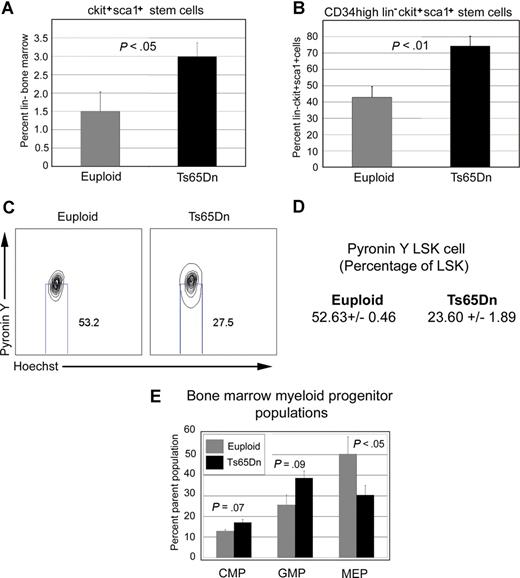
The myeloproliferative diseases are clonal disorders of the hematopoietic stem and progenitor compartments. To determine whether the myeloproliferative phenotype observed in Ts65Dn mice might also reflect changes in stem or progenitor populations, we examined lineage−, c-kit+, Sca1+ (LSK) and myeloid progenitor cell profiles in 14- to 15-month-old Ts65Dn bone marrow. We consistently noted an expansion of the LSK compartment, possibly indicating deregulation of the hematopoietic stem cell pool (Figure 4A). In addition, LSK cells displayed higher cell surface expression of CD34 (Figure 4B). This may be indicative of an increase in the number of more short-term repopulating stem cells. We next examined the proportion of quiescent cells in the LSK compartment with pyronin Y and Hoechst (to stain RNA and DNA, respectively), with quiescent cells defined as those with lower transcriptional activity (indicated by low pyronin Y staining) within the G0/G1 fraction (Figure 4D). We observed a significant decrease in the proportion of pyronin Y–low LSK cells in Ts65Dn mice compared with controls (32.6% ± 2.63% and 45.0% ± 1.36% of LSK cells, respectively, P = .01), indicating fewer quiescent LSK cells. The myeloid progenitor compartment (lineage−, c-kit+, Sca1−) in 14- to 15-month-old Ts65Dn mice was diminished in comparison with euploid controls, and Ts65Dn myeloid progenitors were predominantly granulocyte-monocyte progenitors (GMPs) with fewer megakaryocyte-erythrocyte progenitors (MEPs; Figure 4C).
LSK and myeloid progenitor cell profiles are distorted in Ts65Dn bone marrow. (A) LSK (lineage−, Sca1+, c-kit+) and myeloid progenitor (lineage−, Sca1−, c-kit+) compartments were analyzed in lineage-depleted bone marrow by flow cytometry. Ts65Dn mice displayed an increased percentage of LSK cells. (B) LSK cells stain more brightly for CD34 in Ts65Dn mice. (C) Analysis of myeloid progenitors demonstrates a bias toward the GMP compartment (granulocyte-monocyte progenitor; lineage−, c-kit+, Sca1−, CD34+, FcγR+) and away from the MEP compartment (megakaryocyte-erythrocyte progenitor; lineage−, c-kit+, Sca1−, CD34−, FcγR−). (D) Representative analysis of quiescent LSK cells in Ts65Dn and control bone marrow by pyronin Y/Hoechst staining. Pyronin Y–low cells represent the quiescent LSK fraction. (E) Average values and statistical analysis of quiescent LSK cells in bone marrow. n = 3 Ts65Dn; n = 3 controls. P = .01. Numbers on plots represent the percentage of the total population of cells in the rectangle.
LSK and myeloid progenitor cell profiles are distorted in Ts65Dn bone marrow. (A) LSK (lineage−, Sca1+, c-kit+) and myeloid progenitor (lineage−, Sca1−, c-kit+) compartments were analyzed in lineage-depleted bone marrow by flow cytometry. Ts65Dn mice displayed an increased percentage of LSK cells. (B) LSK cells stain more brightly for CD34 in Ts65Dn mice. (C) Analysis of myeloid progenitors demonstrates a bias toward the GMP compartment (granulocyte-monocyte progenitor; lineage−, c-kit+, Sca1−, CD34+, FcγR+) and away from the MEP compartment (megakaryocyte-erythrocyte progenitor; lineage−, c-kit+, Sca1−, CD34−, FcγR−). (D) Representative analysis of quiescent LSK cells in Ts65Dn and control bone marrow by pyronin Y/Hoechst staining. Pyronin Y–low cells represent the quiescent LSK fraction. (E) Average values and statistical analysis of quiescent LSK cells in bone marrow. n = 3 Ts65Dn; n = 3 controls. P = .01. Numbers on plots represent the percentage of the total population of cells in the rectangle.
Myeloproliferative disease is evident in Ts65Dn mice by 4 months of age
In humans, myeloproliferative disease may exist in a chronic phase for some time before the culmination of acute disease. For Ts65Dn mice, the complete penetrance and progressive nature of disease, the LSK cell changes, and the CBC changes we observed by 14 to 15 months led us to question whether bone marrow changes are present in younger animals, before thrombocytosis becomes profound. To better define the etiology of disease, we studied hematopoiesis in 3- to 4-month-old Ts65Dn mice and euploid controls. Similar to the older animals, young Ts65Dn mice harbor increased megakaryocytes and extramedullary hematopoiesis in the spleen, with an elevated number of c-kit+ bone marrow cells, and a diminished CD19+/CD3+ cell ratio (data not shown). Histologic analysis is consistent with increased extramedullary hematopoiesis (data not shown), and flow cytometry demonstrates a similar distortion in the stem and myeloid progenitor cell compartments: young Ts65Dn mice displayed a 2-fold increase in bone marrow LSK cells that express high levels of CD34 (Figure 5A,B). We next examined the proportion of quiescent cells in the LSK compartment with pyronin Y and Hoechst and observed a decreased proportion of quiescent LSK cells in young Ts65Dn mice (Figure 5C,D). In addition, a shift in the progenitor compartment was evident, with a relative increase in the GMP fraction and a decrease in the MEP compartment (Figure 5E). These data demonstrate that the development of myeloproliferative disease in Ts65Dn is progressive and initiates in relatively young mice, with obvious evidence of disease by 4 months of age.
Ts65Dn bone marrow displays signs of disease by 3 months of age. (A) Three- to 4-month-old Ts65Dn mice harbor a larger LSK cell compartment compared with euploid controls. n = 9 Ts65Dn; n = 9 euploid controls. (B) Bone marrow LSK cells express more CD34 in young Ts65Dn mice. (C) Representative pyronin Y/Hoechst staining of Ts65Dn LSK cells shows that they are more transcriptionally active than controls. Numbers on plots represent the percentage of the total population of cells in the rectangle. (D) Average values and statistical analysis of quiescent LSK cells in bone marrow. n = 3 Ts65Dn; n = 3 controls. P = .001. (E) Young Ts65Dn mice display a trend toward increased megakaryocyte progenitors in the spleen.  represent euploid controls;
represent euploid controls;  represent Ts65Dn. n = 9 Ts65Dn; n = 9 controls. Error bars represent SE.
represent Ts65Dn. n = 9 Ts65Dn; n = 9 controls. Error bars represent SE.
Ts65Dn bone marrow displays signs of disease by 3 months of age. (A) Three- to 4-month-old Ts65Dn mice harbor a larger LSK cell compartment compared with euploid controls. n = 9 Ts65Dn; n = 9 euploid controls. (B) Bone marrow LSK cells express more CD34 in young Ts65Dn mice. (C) Representative pyronin Y/Hoechst staining of Ts65Dn LSK cells shows that they are more transcriptionally active than controls. Numbers on plots represent the percentage of the total population of cells in the rectangle. (D) Average values and statistical analysis of quiescent LSK cells in bone marrow. n = 3 Ts65Dn; n = 3 controls. P = .001. (E) Young Ts65Dn mice display a trend toward increased megakaryocyte progenitors in the spleen.  represent euploid controls;
represent euploid controls;  represent Ts65Dn. n = 9 Ts65Dn; n = 9 controls. Error bars represent SE.
represent Ts65Dn. n = 9 Ts65Dn; n = 9 controls. Error bars represent SE.
Transplantability
The ability to give rise to transplantable tumors can be useful in distinguishing neoplasms from a reactive condition. Although transplantability often correlates with the degree of malignancy, not all malignancies are transplantable.28 To assess whether defects observed in Ts65Dn are cell autonomous, we reconstituted 8- to 10-week-old lethally irradiated C57Bl/6 recipients with 106 nucleated bone marrow cells from Ts65Dn animals. All recipients analyzed demonstrated substantial engraftment by expression of the CD45.2 marker at 3 months after transplantation; however, only 2 of 5 Ts65Dn bone marrow recipients survived to 6 months after transplantation, in contrast to 5 of 5 control recipients, indicating weaker long-term repopulating ability in Ts65Dn bone marrow (Figure S1). There was no evidence of thrombocytosis by CBC for Ts65Dn recipients by 6 months after transplantation, although at this time point, the sample size was small. Interestingly, Ts65Dn transplant recipients did display macrocytosis, indicating that this defect may occur by a cell-autonomous mechanism (Table S4).
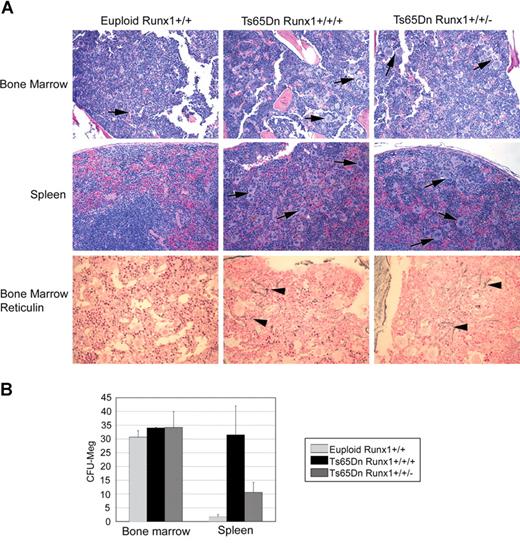
Increased dosage of Aml1/Runx1 is not required for Ts65Dn myelofibrosis and megakaryocytic hyperplasia
The trisomic region in Ts65Dn mice encodes 104 known genes. One of these, Aml1/Runx1, is an attractive candidate oncogene in DS leukemogenesis as it has an established function in hematopoietic stem cell development, megakaryopoiesis, and leukemia.15,,–18 To determine whether increased dosage of Aml1/Runx1 mediates the myeloproliferation seen in the Ts65Dn strain, we bred a null allele of Aml1/Runx1 onto the Ts65Dn background, generating mice that are trisomic for 103 of the 104 genes represented in Ts65Dn, but disomic at the Aml1/Runx1 locus. Based on examination of bone marrow and spleen histology at 15 months, Ts65Dn mice with only 2 functional alleles of Runx1 (hereafter Ts65Dn/Runx1+/+/−) developed hyperproliferation of megakaryocytes in the bone marrow and spleen that could not be distinguished histologically from that of Ts65Dn littermate controls (Ts65Dn/Runx1+/+/+) (Figure 6A). Bone marrow from Ts65Dn/Runx1+/+/− mice contained increased numbers of large megakaryocytes, present in loose clusters, with granulocytic hyperplasia and myelofibrosis in the most severe cases, which was never observed in euploid control mice. Spleens of Ts65Dn/Runx1+/+/− mice contained dramatically increased numbers of megakaryocytes, as well as erythroid precursors and granulocytic cells. Colony-forming assays showed that Ts65Dn/Runx1+/+/− spleens harbored a 5-fold increase in megakaryocyte progenitors over euploid controls (Figure 6B). Of note, colony numbers in Ts65Dn/Runx1+/+/− mice were not as high as those for Ts65Dn/Runx1+/+/+. Due to the small number Ts65Dn/Runx1+/+/+ littermate controls and the variability of phenotype severity observed among animals, it is difficult to assess whether Aml1/Runx1 gene dosage may accentuate extramedullary hematopoiesis in the Ts65Dn strain. Nevertheless, we conclude that increased dosage of Aml1/Runx1 is not essential for development of megakaryocytic hyperproliferation, extramedullary hematopoiesis, and reticulin fibrosis in Ts65Dn mice.
Three alleles of Aml1/Runx1 are not essential for the development of MPD. (A) Hematoxylin and eosin staining of bone marrow and spleen (top 2 panels) and bone marrow reticulin staining (bottom panels) demonstrate that Ts65Dn mice with 3 or 2 alleles of Runx1 (Ts65Dn/Runx1+/+/+ and Ts65Dn/Runx1+/+/−, respectively) develop a similar phenotype. (B) In vitro colony-forming assays demonstrate that restoring disomy at the Runx1 locus does not abrogate extramedullary hematopoiesis in Ts65Dn. Runx1 trisomy may contribute to the extent of extramedullary megakaryopoiesis. Error bars represent SE.
Three alleles of Aml1/Runx1 are not essential for the development of MPD. (A) Hematoxylin and eosin staining of bone marrow and spleen (top 2 panels) and bone marrow reticulin staining (bottom panels) demonstrate that Ts65Dn mice with 3 or 2 alleles of Runx1 (Ts65Dn/Runx1+/+/+ and Ts65Dn/Runx1+/+/−, respectively) develop a similar phenotype. (B) In vitro colony-forming assays demonstrate that restoring disomy at the Runx1 locus does not abrogate extramedullary hematopoiesis in Ts65Dn. Runx1 trisomy may contribute to the extent of extramedullary megakaryopoiesis. Error bars represent SE.
Discussion
In this study, we report the effects of trisomy for 104 orthologs of human chromosome 21 genes on hematopoiesis in the Ts65Dn mouse strain. Ts65Dn mice are widely used to model trisomy 21 and present craniofacial, cognitive, and heart defects20,–22,24 similar to those observed in DS.
Our results demonstrate that overexpression of chromosome 21 gene orthologs disrupts normal hematopoiesis and leads to abnormal development of multiple blood lineages. Most notably, megakaryocytes in Ts65Dn are morphologically altered and accumulate in the bone marrow and spleen. Profound thrombocytosis, often accompanied by myelofibrosis, develops by 1 year in virtually all animals. While thrombocytosis develops in more mature animals, bone marrow changes are present in younger mice, including an increase in CD41+ megakaryocytic cells. These findings confirm that trisomy for chromosome 21 gene orthologs dramatically impacts proliferation and development of the megakaryocyte lineage, resulting in a progressive myeloproliferative disease. Interestingly, infants with DS display profound thrombocytosis throughout the first year of life.2 It is unclear whether mechanisms leading to megakaryocyte proliferation in Ts65Dn mice are relevant to the thrombocytosis observed in DS infants or to leukemia; however, it is tempting to speculate that a similar process in human megakaryopoiesis could be compounded by GATA1 mutations and confer a profound growth advantage. Further study will be necessary to define the mechanism that causes myeloproliferative disease in Ts65Dn mice and evaluate a possible correlation with hematopoiesis in DS.
Unexpectedly, the phenotype observed in Ts65Dn mice resembles human myeloproliferative disease (MPD). In humans, the MPDs are caused by clonal hematopoietic stem and progenitor cell proliferation leading to hyperproliferation of one or more mature blood lineages. Two MPDs, essential thrombocythemia (ET) and chronic idiopathic myelofibrosis (CIMF), directly affect the megakaryocyte lineage, can be accompanied by thrombocytosis and myelofibrosis, and confer a tendency of evolution to acute leukemia.25 Interestingly, Ts65Dn mice display increased c-kit+cells in the bone marrow, consistent with an increased pool of immature myeloid cells.
An intriguing aspect of hematopoiesis in the Ts65Dn strain is the disruption of the LSK and myeloid progenitor compartments, with an increased proportion of LSK cells expressing high levels of CD34. CD34 is expressed at high levels on the long-term repopulating hematopoietic stem cells (LT-HSCs) of embryonic and young mice, but its expression decreases on adult LT-HSCs, and, in fact, most long-term repopulating activity in the adult animal lies in the CD34− HSC fraction. Increased CD34 expression on Ts65Dn LSK cells may then reflect a relative increase in the proportion of more short-term repopulating LSK cells; alternatively, it cannot be ruled out that CD34+ embryonic HSCs might persist in Ts65Dn. The decreased fraction of quiescent LSK cells and lower long-term repopulating ability of Ts65Dn bone marrow support either possibility. Likewise, others have shown that CD34+ bone marrow cells from Ts65Dn mice proliferate to a lesser extent in vitro than controls, supporting the hypothesis that these mice have fewer long-term repopulating cells in the bone marrow.28
The profile of myeloid progenitor cells is also skewed in Ts65Dn bone marrow, with an increased proportion of GMPs, and dramatically reduced MEP component. It may be of interest that GMPs are distinguished from MEPs in part by increased expression of CD34, although it is unclear whether this relates to increased CD34 expression on Ts65Dn LSK cells. A similar expansion in GMPs has also been documented in the blast crisis phase of human MPD.29,30 Our data suggest that Ts65Dn mice develop a disease with significant similarities to human MPD and roots in the hematopoietic stem and myeloid progenitor compartments.
Two of the most well-understood molecular causes of human myeloproliferative disease are the V617F-activating mutation in the tyrosine kinase JAK2 and the Philadelphia chromosome. The V617F JAK2 mutation, present in 90% of polycythemia vera (PV) patients and 30% to 50% of ET and CIMF patients, leads to constitutive activation of multiple signaling pathways including Stat, PI3K, and MAPK.31,,,–35 In addition, the homologous factor JAK3 is mutated in a subset of DS-AMKL.36 The BCR-Abl fusion protein generated by the Philadelphia chromosome constitutively activates the CRKL adaptor and multiple downstream pathways.36 Notably, we did not detect analogous Jak2 or Jak3 mutations in Ts65Dn bone marrow and no cytogenetic abnormalities were detected (data not shown). Therefore, our data may provide insight into genetic factors capable of causing Ph-myeloproliferative disease in patients who lack JAK2 mutations. Furthermore, because JAK2 mutations likely require cooperating events in MPD, these animals may provide insights into these unknown secondary factors.
The Ts65Dn trisomic region encodes 104 known orthologs of human chromosome 21 genes. Of these, Aml1/Runx1 has been proposed as a candidate leukemogenic factor in DS due to its known developmental function in hematopoietic stem cells, megakaryocytes, and leukemia.15,,–18 Our results demonstrate that 3 alleles of the Aml1/Runx1 gene are not necessary to cause megakaryocyte hyperplasia, thrombocytosis, and reticulin fibrosis. These results are consistent with the observed reduced expression of AML1/RUNX1 in DS-AMKL compared with non–DS-AMKL.37 Further, these studies indicate that increased dosage of one or more of the other genes in the Ts65Dn translocation must promote this phenotype. However, it is not possible to exclude the possibility that Aml1/Runx1 does participate in the myeloproliferative disease in Ts65Dn. Interestingly, increased dosage of Aml1/Runx1 may contribute to the maturation or survival of megakaryocyte progenitors in Ts65Dn, as in vitro colony-forming ability is somewhat attenuated in mice that are disomic for Aml1/Runx1. This finding illustrates the complexity behind Down syndrome gene-phenotype relationships, which may involve multiple complementary factors, rather than a single gene for each trait.
Our results demonstrate that Ts65Dn mice effectively model multiple features of DS hematopoiesis. Ts65Dn mice recapitulate the erythroid macrocytosis that is a hallmark of hematopoiesis in DS. According to one study, 66% of children with DS have MCV values greater than the 97th percentile of age-matched, non-DS controls, and while the average MCV in non-DS children was 80.6 fL, the average in DS children was 86.9 fL.1 Our study suggests that increased dosage of the 104 genes represented in Ts65Dn is sufficient to produce a significantly elevated MCV. Furthermore, this is a cell-autonomous defect, as transplant recipients of Ts65Dn bone marrow also developed macrocytosis, and is not caused by plasma or erythrocyte folate deficiency. Our findings may help identify potential causes of macrocytosis, improve our understanding of red blood cell development in DS, and shed light on a subset of red blood cell deficiencies. With some exceptions, most studies of lymphopoiesis in DS observe lymphopenia with a severe reduction of B cells.38 The decreased ratio of CD19+ to CD3+ cells in Ts65Dn spleens may indicate a similar lymphoid profile to that observed in DS. Thus, these animals may also be useful in studies of the B-cell lineage. In summary, Ts65Dn mice effectively model some, but not all, aspects of hematopoiesis in DS.
Finally, Ts65Dn mice did not develop leukemia in our study, possible owing to the absence of additional mutations, to physiological differences between mice and humans, or to the exclusion of HSA21 genes that are not trisomic in Ts65Dn. While we did not detect Gata1 mutations in Ts65Dn bone marrow, studies by others suggest that Gata1 mutations are insufficient to promote TMD or leukemia in Ts65Dn mice.40 This may indicate that additional cooperating mutations are required even for the development of TMD. In fact, GATA1 mutations have been detected in DS fetal liver samples when there is no histologic evidence of TMD, lending support to the idea that additional mutations are necessary.41 Alternatively, as mice are less sensitive than humans to the differential effects of GATA1s,11,12 Gata1 mutations in trisomic mice might not lead to the same clonal expansion that develops in human DS patients. Mice expressing GATA1s exclusively do in fact develop transient proliferation of a yolk sac progenitor, but the hematologic manifestations are much less severe than sole expression of GATA1s in humans.12
Overall, our data demonstrate that Ts65Dn mice are a useful model for hematopoiesis in Down syndrome and reveal the potential of chromosome 21 genes to promote megakaryocyte hyperproliferation and myeloproliferative disease. Further studies to better define the pathology of this phenotype and determine the specific genes that promote it will improve understanding of human myeloproliferative disease and hematologic abnormalities in DS.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr James Downing for Aml1/Runx1 heterozygous knockout mice, Roger Reeves and Cecilia Schmidt for advice and assistance with the Ts65Dn strain, Eileen Dolan for use of the Hemavet 850, and the University of Chicago Cancer Research Center Flow Cytometry facility for assistance with data collection. We also thank members of the Crispino lab and John Joslin for helpful discussions.
This work was supported by the Elsa U. Pardee Foundation, NCI (ROI CA101774), LLSA, and The University of Chicago MCB training grant. J.D.C. is a scholar of the Leukemia and Lymphoma Society.
Authorship
Contribution: G.K. contributed to experimental design, performed the research, analyzed the data, and wrote the paper; S.J., H.L., and E.D. contributed to performing the research; S.G. and M.M.L. contributed to analyzing the data and writing the paper; J.D.C. designed the research, analyzed and interpreted data, and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Crispino, Northwestern University, Lurie Research Bldg, 303 E Superior St, Rm 5-113, Chicago, IL 60611; e-mail: j-crispino@northwestern.edu.