Whereas regulatory T (Treg) cells play an important role in the prevention of autoimmunity, increasing evidence suggests that their down-regulatory properties negatively affect immune responses directed against tumors. Treg cells selectively express chemokine receptors CCR4 and CCR8, and specific migration occurs following the release of various chemokines. Neoplastic meningitis (NM) resulting from leptomeningeal spread of systemic non-Hodgkin lymphoma (NHL) or carcinoma has a poor prognosis. We hypothesized that Treg-cell accumulation within the subarachnoid space as a result of interfering with tumor immunity may be relevant for survival of neoplastic cells. We collected cerebrospinal fluid (CSF) from 101 patients diagnosed with lymphomatous/carcinomatous NM and various inflammatory diseases (IDs) and noninflammatory neurologic disorders (NIDs). CSF Treg- cell counts were determined by flow cytometry, Treg cell–specific chemokines by enzyme-linked immunsorbent assay (ELISA), and Treg-cell trafficking by chemotaxis assay. Both frequencies of Treg-cell and Treg cell–specific chemotactic activities were significantly elevated in CSF samples of patients with NM. Local Treg-cell accumulation occurred without concomitant rise of conventional T (Tconv) cells, coincided with elevated concentrations of Treg cell–attracting chemokines CCL17 and CCL22 and correlated with numbers of atypical CSF cells. We conclude that Treg cells are specifically recruited into the CSF of patients with NM, suggesting that the presence of Treg cells within the subarachnoid space generates a microenvironment that may favor survival and growth of malignant cells.
Introduction
Dissemination of malignant non-Hodgkin lymphoma (NHL) or systemic carcinoma to the leptomeninx carries a poor prognosis and is rapidly fatal in the most patients. The predictors of metastatic tumor spread to the meninges are elusive, and little is known as to what extent the microenvironment of the subarachnoid space affects local accumulation and expansion of neoplastic cells. One way in which tumors escape physiologic defense mechanisms is through counteracting antitumor immunity.1,,,–5 Recent findings demonstrate that naturally suppressive T cells of CD4+CD25+FOXP3+ phenotype (regulatory T [Treg] cells) are critically involved in this process and, in addition to their functional significance in the prevention of murine and human autoimmunity, may promote poor antitumor immunity by down-regulating the functional activity of tumor-infiltrating CD4+ T cells. Thus, experimental depletion of Treg cells in tumor-bearing mice enhances both immune-mediated tumor clearance and responses to immune-based therapies.6,–8 Likewise, tumor Treg cells are detectable in both solid human tumors and human B-cell NHL,9,,,,,–15 and in patients with ovarian cancer, intratumoral Treg cell accumulation correlates with an unfavorable clinical outcome.16 In contrast with these observations, a recent study in Hodgkin lymphoma reported that increased frequencies of infiltrating cytotoxic T cells in parallel with low numbers of local Treg cells negatively predict disease-free survival.17
Human Treg cells selectively express chemokine receptors CCR4 and CCR8, and specific migration of Treg cells occurs in response to release of CCR4 and CCR8 ligands, including the chemokines CCL17 (thymus and activation-regulated chemokine [TARC]), CCL22 (macrophage-derived chemokine [MDC]), CCL1 (I-309), and vMIP-I (viral macrophage inflammatory protein I).18 Secretion of Treg cell–attracting chemokines is not restricted to immune cells, but may also occur in carcinoma cells and neoplastic B-lymphocytes.19,–21 Curiel et al demonstrated that Treg cell migration to ovarian tumors is mediated by CCL22 released by cancer cells and tumor-associated macrophages.16 Secretion of CCR4 and CCR8 ligands was further demonstrated for activated and neoplastic B lymphocytes.22,,–25
In this study, we used cerebrospinal fluid (CSF) specimens from patients with lymphomatous or carcinomatous meningitis to assay Treg-cell migration into the CSF compartment. We comparatively tested the chemotactic activity toward Treg cells prompted by lymphoma/tumor infiltration of the leptomeninx versus Treg-cell trafficking to the CSF occurring in association with various inflammatory diseases (IDs) and noninflammatory neurologic disorders (NIDs). We also determined CSF Treg-cell prevalences and chemokine profiles in these conditions.
Methods
Human samples
We collected CSF and serum samples from 101 patients who underwent diagnostic lumbar puncture. Patients were diagnosed with lymphomatous meningitis (LM; n = 24) resulting from B-NHL; carcinomatous meningitis (CM; n = 18) due to breast (n = 9), lung (n = 5), gastric (n = 1), testicular (n = 1), or renal (n = 1) cancer, and cancer of unknown primary origin (n = 1); IDs of the nervous system (infections, n = 26); multiple sclerosis (MS; n = 16); and various NIDs (n = 17).
Mean CSF cell counts in samples obtained from patients with LM and CM were 62.9/μL (range, 1-375/μL) and 42.3 μL (range, 1-426/μL), respectively. The presence of NM was established by unequivocal cytologic evidence of atypical cells in routine CSF cytospins. Mean percentages of neoplastic cells were 30.2% (LM; range, 1%-100%) and 48.1% (CM; range, 1%-100%), respectively. The blood-CSF barrier (BCB) function was determined by nephelometric assessment of serum and CSF albumin concentrations and calculation of the CSF-to-serum albumin ratio (Qalb = albumin CSF [mg/L] / albumin serum [mg/L] × 10−3; normal = 2.0-8.0).26 Mean Qalb was 19.5 (range, 4.8-45.1) in LM and 22.6 (range, 4.7-60.0) in CM samples and revealed BCB dysfunction in 6 of 12 patients with LM and 13 of 16 patients with CM. Lack of serum samples excluded Qalb calculation in 12 patients with LM and 2 patients with CM. Infections were of viral (n = 12) or bacterial (n = 14) origin, with a mean cell count of 301.1/μL (range, 1-3584/μL). Mean Qalb in patients with infections was 22.1 (range, 4.0-161.9), with BCB dysfunction in 19 of 24 patients. Within the MS and NID cohorts, BCB dysfunction was present in 2 of 16 and 2 of 14 patients, respectively; mean Qalb was 4.9 (range, 3.1-9.2) for patients with MS and 6.9 (range, 2.9-15.3) for patients with NID. None of the patients with NM underwent intrathecal chemotherapy or radiation of the neuraxis prior to lumbar puncture. Likewise, treatment with anti-infectious agents or corticosteroids did not precede CSF analysis in patients with infections and MS.
The protocol was approved by the University of Heidelberg ethics committee, and written informed consent was obtained from all individuals.
Flow cytometry
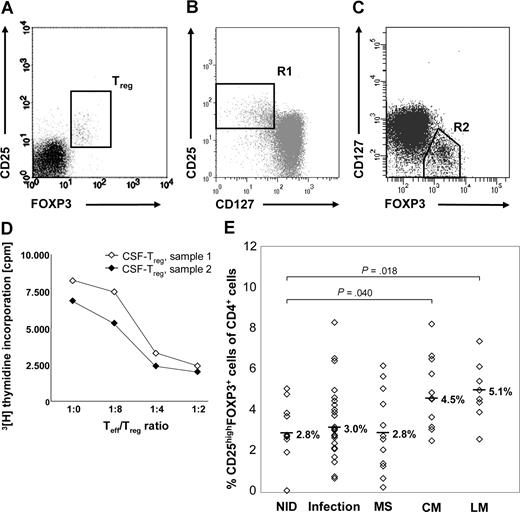
For flow cytometric analysis, freshly isolated peripheral blood mononuclear cells (PBMCs) or CSF cells were stained with monoclonal antibodies (mAbs) specific for human CD4 and CD25 (purchased from BD Pharmingen, Heidelberg, Germany) and FOXP3. FOXP3 staining was performed with a FOXP3 staining kit from eBioscience (San Diego, CA) according to the manufacturer's protocol. A minimum of 5000 CSF cells per staining was required, with at least 1000 events measured. Fluorescence-activated cell sorter (FACS) acquisition was performed immediately with a FACS Canto cytometer and analyzed with FACS Diva software (BD Biosciences, Palo Alto, CA). CSF cells were first gated according to forward and side light-scattering properties and CD4 expression and then analyzed for expression of CD25 and FOXP3, as only CD4+ T cells expressing the FOXP3+ gene product scurfin exhibit suppressive function.27 Treg-cell numbers were defined as the proportion of CD25highFOXP3+ cells among total CD4+ T cells (Figure 1A). As Treg cells, unlike activated conventional T (Tconv) cells, constitutively express CD127 at low levels,28,29 random CSF samples were further costained for CD127. CSF-derived CD4+CD25+FOXP3+ cells were CD127low and thus had the expected Treg-cell phenotype (Figure 1B,C).
Treg cells accumulate in the CSF of patients with NM. (A) Typical FACS staining of CSF cells (sample obtained from 1 patient with LM) for surface CD25 and intracellular FOXP3. For quantification of Treg cells, stained CSF cells were first gated according to forward and side light-scattering properties and CD4 expression, and then coexpression of FOXP3 and CD25 was assessed. (B) After staining for CD4, CD25, CD127, and FOXP3, cells were gated for CD4+ lymphocytes. (B) R1 shows CD4+CD25highCD127low cells. (C) R2 shows CD4+FOXP3+CD127low cells. (D) In vitro proliferation assay with 104 control-derived Teff cells and increasing numbers of CD4+CD25high Treg cells isolated from CSF samples obtained from 2 patients with viral infections. CSF-derived Treg cells suppress proliferation of activated Teff cells in a dose-dependent manner. (E) Percentages of CD25highFOXP3+ Treg cells among CD4+ CSF cells in patients with NM and control diseases. Symbols indicate percentages of individual patients. Medians are indicated.
Treg cells accumulate in the CSF of patients with NM. (A) Typical FACS staining of CSF cells (sample obtained from 1 patient with LM) for surface CD25 and intracellular FOXP3. For quantification of Treg cells, stained CSF cells were first gated according to forward and side light-scattering properties and CD4 expression, and then coexpression of FOXP3 and CD25 was assessed. (B) After staining for CD4, CD25, CD127, and FOXP3, cells were gated for CD4+ lymphocytes. (B) R1 shows CD4+CD25highCD127low cells. (C) R2 shows CD4+FOXP3+CD127low cells. (D) In vitro proliferation assay with 104 control-derived Teff cells and increasing numbers of CD4+CD25high Treg cells isolated from CSF samples obtained from 2 patients with viral infections. CSF-derived Treg cells suppress proliferation of activated Teff cells in a dose-dependent manner. (E) Percentages of CD25highFOXP3+ Treg cells among CD4+ CSF cells in patients with NM and control diseases. Symbols indicate percentages of individual patients. Medians are indicated.
Cell separation
CD4+CD25high cells were isolated from CSF samples with high cell counts obtained from 2 patients with viral infections of the central nervous system (CNS; 2.6 × 106 and 3.8 × 106 white CSF cells) as described previously.30,31 In short, CSF cells were sedimented for 10 minutes at 4° and 400g, and CD25high cells were then immunomagnetically separated using a Treg cell isolation kit (Dynal, Hamburg, Germany). Immune magnetic separation yielded 2.8 × 104 and 5.3 × 104 highly pure Treg cells (87.5% and 90.2% CD4+CD25high cells). For isolation of effector T (Teff) cells, CD4+ cells were enriched from the peripheral blood of 1 healthy donor using a negative CD4+ T-cell isolation kit (Dynal). CD4+CD25low/CD4+CD25int Teff cells were obtained by depleting CD25high cells with the Treg cell isolation kit.
Proliferation assay
For functional characterization, CD4+CD25high cells isolated from CSF were tested in subsequent in vitro proliferation assays as described previously,30,31 with the following modifications. A total of 104 freshly isolated Teff cells were cultured alone or together with Treg cells (Treg/Teff ratios: 1:2, 1:4, and 1:8) and stimulated with soluble anti-CD3 (1 μg/mL) and anti-CD28 mAbs (1 μg/mL). After 4 days at 37°C in 5% CO2, 0.0185 MBq (0.5 μCi) 3[H] thymidine per well was added for additional 16 hours, and proliferation was measured using a scintillation counter.
Migration assay
Treg-cell trafficking was assessed by using a transwell assay to measure the specific chemotactic activity in CSF. A total of 106 freshly isolated PBMCs obtained from 1 healthy standard donor were suspended in 200 μL RPMI and transferred into the upper chambers of 6.5-mm diameter, 5.0-μm pore-size polycarbonate membrane filter transwell plates (Costar Corning, Cambridge, MA). RPMI (600 μL), CCL22 (100 ng/mL; R&D Systems, Minneapolis, MN), or CSF supernatants with or without blocking mAbs were added to the lower chamber. After 4 hours at 37°C, migrated cells were collected in the lower chamber, stained with anti-CD4 and anti-CD25 mAbs, and analyzed by flow cytometry analysis. The chemotactic index for Treg cells (CI-Treg) was defined as the ratio of percentages of Treg cells in CD4+ T cells of migrated cells and percentages of Treg cells in CD4+ T cells of original PBMCs. Specific mAbs for inhibition of CCL17 (500 ng/mL) and CCL22 (500 ng/mL) were purchased from R&D Systems.
ELISA
Enzyme-linked immunosorbent assays (ELISAs) of CSF supernatants and serum samples were performed using Quantikine Immunoassay kits specific for human CCL17 (TARC) and CCL22 (MDC), according to the manufacturer's instructions (all from R&D Systems).
Statistical analysis
To determine whether differences and correlations in CSF cell counts, migration indices, and chemokine levels were statistically significant, data were analyzed with the Sigma STAT software (SPSS, Chicago, IL). Mann-Whitney U tests and Pearson correlation analyses were used for statistical assessment. P values less than .05 were considered significant.
Results
Enhanced Treg-cell frequencies within the CSF of patients with NM
Treg cell frequencies in CSF samples were flow-cytometrically defined as proportions of CD25highFOXP3+ cells among CD4+ T cells (Figure 1A). CSF-derived Treg cells were CD127low (Figure 1B,C) and functionally suppressive (Figure 1D), and thus represent Treg cells.
The CSF of patients with NID contained a median of 2.8% Treg cells of all CD4+ cells (range, 0.1%-5.1% of CD4+ T cells; Figure 1E). Treg-cell levels were significantly elevated in CSF specimens from patients with LM (median, 5.1%; range, 2.6%-7.4%; P = .018) and were also enhanced in CM (median, 4.5%; range, 2.5%-8.2%; P = .040). In contrast, Treg-cell frequencies obtained from both individuals with infections and MS did not differ compared with the NID cohort (infections: median, 3.0%; range, 0.7%-8.3%; P = .467; MS: median, 2.8%; range, 0.3%-6.3%; P = .939).
There was a strong correlation between the amount of atypical cells and the prevalence of Treg cells in CSF of patients with LM (r2 = 0.96) and also with CM (r2 = 0.67).
Proportions of CD4+ cells were equivalent between NID (median, 61.2%; range, 33.6%-77.2%), CM (median, 60.3%, range, 34.6%-85.1%; P = .571), and LM (median, 53.3%; range, 28.6%-76.5%; P = .324), but were increased in CSF of patients with infections (median 70.9%, range 13.4%-100.0%, P = .046; not depicted).
Increased Treg cell–specific chemotactic activity in CSF supernatants from patients with NM
Treg cell–specific chemotactic activities in CSF supernatants were tested by in vitro cell migration assays. Compared with individuals with NID (CI-Treg, 1.2 ± 0.4), the mean CI-Treg was moderately elevated in patients with infections (1.8 ± 0.4; P = .020; Figure 2), whereas differences were not statistically significant in patients with MS (1.3 ± 0.5; P = .743). In contrast, a pronounced increase of CI-Treg was detectable in both individuals with LM (3.0 ± 1.2; P = .008) and CM (2.5 ± 1.1; P = .015) versus NID.
Treg cells migrate in response to CSF supernatants obtained from patients with NM. CIs of Treg cells from a healthy donor toward CSF supernatants of patients with NM and control diseases as determined by transwell migration assays. Original input PBMCs and migrated cells were stained with CD4/CD25 mAbs and gated on CD4+ cells. CI was calculated by dividing percentages of Treg cells in CD4+ T cells of migrated cells by percentages of Treg cells in CD4+ T cells in original input PBMCs. Symbols indicate CI-Treg of individual patients. Means are indicated.
Treg cells migrate in response to CSF supernatants obtained from patients with NM. CIs of Treg cells from a healthy donor toward CSF supernatants of patients with NM and control diseases as determined by transwell migration assays. Original input PBMCs and migrated cells were stained with CD4/CD25 mAbs and gated on CD4+ cells. CI was calculated by dividing percentages of Treg cells in CD4+ T cells of migrated cells by percentages of Treg cells in CD4+ T cells in original input PBMCs. Symbols indicate CI-Treg of individual patients. Means are indicated.
Specific migration of all CD4+ cells was similar between NID (1.1 ± 0.4), LM (1.2 ± 0.4; P = .882), and CM (1.3 ± 0.3; P = 0.384), and significantly enhanced in CSF samples of patients with infections (1.8 ± 0.3; P = .001; not depicted).
Spontaneous migration of PBMCs toward RPMI medium always resulted in Treg cell– and CD4+-specific indices between 0.9 and 1.1 (data not shown).
Treg cell–specific trafficking toward CSF supernatants from patients with NM depends on CCL17 and CCL22
As several studies implicate an important role of CCL17 and CCL22 in migration of human Treg cells, we tested the effect of anti-CCL17 and anti-CCL22 mAbs on Treg cell–specific trafficking toward CSF supernatants from 9 patients with NM. In all patients studied, Treg cell–specific trafficking was significantly reduced when both blocking Abs were added to CSF supernatants prior to chemotaxis assays. The mean CI-Treg dropped from 3.7 ± 0.9 to 1.5 ± 1.0 (P = .006) in patients with LM (n = 6) and from 6.0 ± 1.9 to 1.6 ± 1.2 (P = .029; Figure 3) in patients with CM (n = 3). The use of isotypic control antibodies had no effect (data not shown).
Inhibition of Treg cell–specific trafficking toward CSF supernatants with the use of anti-CCL17 and anti-CCL22 mAbs. CI-Treg of CSF supernatants obtained from patients with LM (n = 6) and CM (n = 3). After preincubation for 30 minutes at 4°C with anti-CCL17 and anti-CCL22 mAbs (1 μg/mL), Treg cell migration was tested by transwell migration assays. Symbols indicate CI-Treg of individual patients. Medians are indicated.
Inhibition of Treg cell–specific trafficking toward CSF supernatants with the use of anti-CCL17 and anti-CCL22 mAbs. CI-Treg of CSF supernatants obtained from patients with LM (n = 6) and CM (n = 3). After preincubation for 30 minutes at 4°C with anti-CCL17 and anti-CCL22 mAbs (1 μg/mL), Treg cell migration was tested by transwell migration assays. Symbols indicate CI-Treg of individual patients. Medians are indicated.
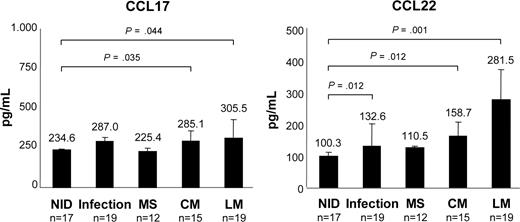
Elevated concentrations of Treg cell–specific chemokines in CSF of patients with NM
Concentrations of Treg cell–specific chemokines CCL17 and CCL22 in CSF samples were determined by ELISA. Compared with the NID cohort (CCL17, 234.6 ± 12.1 pg/mL; CCL22, 100.3 ± 3.8 pg/mL), CCL17 and CCL22 levels were increased in the CSF of patients with LM (CCL17: 305.5 ± 105.4 pg/mL; P = .044; CCL22: 281.5 ± 124.9 pg/mL; P = .001) and CM (CCL17: 285.1 ± 44.7 pg/mL; P = .035; CCL22: 158.7 ± 57.5 pg/mL; P = .012; Figure 4). Elevations were also detectable in infections (CCL17: 287.0 ± 79.4 pg/mL; P = .080; CCL22: 132.8 ± 26.4 pg/mL; P = .002), but not in MS (CCL17: 225.4 ± 4.9 pg/mL; P = .470; CCL22: 110.5 ± 18.8 pg/mL, P = .100).
Increased concentrations of CCL17 and CCL22 in the CSF of patients with NM. Amounts of CCL17 and CCL22 in CSF samples of patients with NIDs, infections, MS, LM, and CM as determined by ELISA. Bars represent mean chemokine concentrations; error bars represent standard deviation.
Increased concentrations of CCL17 and CCL22 in the CSF of patients with NM. Amounts of CCL17 and CCL22 in CSF samples of patients with NIDs, infections, MS, LM, and CM as determined by ELISA. Bars represent mean chemokine concentrations; error bars represent standard deviation.
High concentrations of Treg cell–specific chemokines CCL17 and CCL22 correlated well with both CSF Treg-cell frequencies (CCL17: LM r2 = 0.64, CM r2 = 0.50; CCL22: LM r2 = 0.51, CM r2 = 0.55) and numbers of atypical cells (CCL17: LM r2 = 0.55, CM r2 = 0.40; CCL22: LM r2 = 0.53, CM r2 = 0.48). Moreover, there was a clear correlation between chemokine concentrations and Treg cell–specific trafficking toward NM CSF samples (LM: CCL17 r2 = 0.42, CCL22 r2 = 0.65; CM: CCL17 r2 = 0.59, CCL22 r2 = 0.31).
Chemokine concentrations were also measured in 38 serum samples. Levels of CCL17 and CCL22 did not differ between patients with NM and the NID cohort (CCL17: NID, 196.3 ± 62.7 pg/mL; CM, 281.4 ± 150.3 pg/mL; P = .290; LM, 223.6 ± 88.8 pg/mL; P = .624; CCL22: NID, 601.7 ± 77.2 pg/mL; CM, 585.7 ± 304.5 pg/mL; P = .919; LM, 509.7 ± 77.4 pg/mL; P = .206; not depicted). Moreover, there was no correlation between chemokine levels in CSF and Qalb, indicating that the elevated Treg cell–specific chemokine concentrations observed in CSF specimens from patients with NM originate predominantly from synthesis within the CNS. Concentrations of CCL17 and CCL22 were also not elevated in serum samples from patients with MS (CCL17: 271.5 ± 149.3 pg/mL; CCL22: 591.0 ± 91.0 pg/mL) and from patients with infections of the CNS (CCL17: 249.1 ± 97.5 pg/mL; CCL22: 580.1 ± 82.9 pg/mL).
Discussion
Recent studies emphasize the unfavorable effect of Treg cells on tumor immunity, as local Treg cell accumulation driven by the presence of Treg cell–specific chemokines in the tumor environment promotes immune tolerance toward tumor antigens.
In line with these observations, we show here that CD25highFOXP3+ T cells are overrepresented in CD4+ T-cell populations within CSF samples from patients with both lymphomatous and carcinomatous NM versus control specimens. These cells are CD127low and, when isolated from the CSF are functionally Treg cells, exhibiting fully suppressive properties toward activated Tconv cells in vitro. These findings reinforce previous reports implicating enhanced Treg-cell numbers in ovarian, breast, lung, gastric, esophageal, and pancreatic cancers as well as in Hodgkin lymphoma and B-NHL.10,12,,,,–17,32,33
Irrespective of the underlying malignancy, the presence of neoplastic cells in the leptomeninges and CSF triggers trafficking of Treg cells from the systemic circulation to the subarachnoid space, as CSF supernatants from patients with LM and CM universally exhibit a marked chemotactic activity toward Treg cells that is significantly more pronounced compared with specimens from patients with NID and ID. Consequently, prevalences of Treg cells increase without a concomitant rise in CD4+ T-cell numbers. This is in sharp contrast with findings in CSF specimens from patients with infections, where CD4+ T cells increase, but Treg-cell proportions do not compared with the NID cohort. We also did not detect redistribution of Treg cells into the CSF in patients with MS, confirming our earlier observations.30 Both NM and infections coincided with a pathological Qalb of variable extent in most patients, whereas enhanced Treg-cell frequencies were detectable in NM only. Collectively, there was no correlation between Qalb and prevalences of Treg cells within CSF, indicating that the disturbed permeability of the BCB cannot fully explain the increase of Treg cells in the CSF of patients with NM. Instead, Treg-cell frequencies correlate with the amount of malignant cells in the CSF, suggesting that Treg cells are selectively recruited into the CSF in response to leptomeningeal carcinomatosis or lymphomatosis.
In concordance with this assumption, Treg cell–specific chemokines CCL17 and CCL22 are enriched in CSF specimens from patients with NM, and both their concentrations and the CI-Treg are determined by the magnitude of malignant cell populations detectable in individual CSF samples. To exclude passive diffusion of chemokines across the BCB, we tested CCL17 and CCL22 in paired serum and CSF samples. There was no correlation between chemokine levels in CSF and Qalb, and systemic chemokine concentrations were not elevated in the NM group, arguing for an intrathecal production of CCL17 and CCL22 in patients with LM and CM. Although we did not assess subsets of CSF cells independently for their respective chemokine profiles, the correlation between numbers of atypical cells and chemokine levels suggests that chemokine secretion from tumor cells contributes to the local synthesis of CCL17 and CCL22.
This conclusion is in concordance with a recent study demonstrating chemotaxis and migration of Treg cells in B-NHL tissues resulting from secretion of CCL22 by lymphoma cells.33 CCL22 production mediating intratumoral Treg-cell infiltration has also been detected in ovarian cancer cells.16 Moreover, CCL22 and/or CCL17 were found to be involved in trafficking of CCR4+ Treg cells in diffuse large B-cell lymphoma,34 Hodgkin lymphoma,35,,,–39 and B-cell chronic lymphocytic leukemia (B-CLL).35,39
The consequences of Treg-cell accumulation within neoplastic tissues are not yet conclusively defined. However, available evidence demonstrates that intratumoral Treg cells from patients with lymphoma as well as from patients with other malignancies are functionally capable of suppressing the proliferation and cytokine production of CD4+ T cells and to suppress tumor-specific T-cell immunity.6,30,33,40 Congruously, enhanced Treg-cell frequencies were correlated with poor prognosis of patients with solid tumors and with B-CLL.14,–16,39 Contradictory to these findings, high numbers of Treg cells are associated with improved survival in Hodgkin lymphoma and follicular lymphoma,17,41 possibly indicating a different role for Treg cells in the pathogenesis of these types of hematologic malignancies.
Collectively, this study shows that leptomeningeal metastasis from B-NHL and from various carcinomas prompts selective recruitment of Treg cells across the BCB to the subarachnoid space without concomitant accumulation of Tconv cells, and thereby might create a microenvironment which favors the survival and growth of malignant cells. Intriguingly, Treg cells might even more efficiently counteract local antitumor immune responses than outside the CNS, where the movement of tumor-infiltrating conventional human T cells is less restricted by barrier structures. Further studies are required to proof that Treg cells interfere with T-cell responses directed against the respective tumors. Proof of concept will provide a rationale for manipulating Treg cells to enhance immunotherapy for patients with lymphomatous or carcinomatous NM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all patients and healthy donors for participating in this study.
This work was supported by grants from the Deutsche José Carreras Leukaemie-Stiftung (1.319) and the Young Investigator Award from the Faculty of Medicine, University of Heidelberg, to A.S.
Authorship
Contribution: J.H. designed and performed research, analyzed data, and wrote the paper; L.S. performed research and analyzed data; B.S.-H. contributed clinical samples and assisted in writing the paper; B Fritzsching performed flow cytometry and analyzed data; C.J. contributed expertise in ELISA; L.M. performed research and analyzed data; B. Fritz performed research; A.S. assisted in writing the paper; E.S.-P. assisted in writing the paper; M.H. contributed clinical samples and assisted in writing the paper; and B.W. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brigitte Wildemann, Division of Molecular Neuroimmunology, Department of Neurology, University of Heidelberg, INF 350, D-69120 Heidelberg, Germany; e-mail: brigitte_wildemann@med.uni-heidelberg.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal