Abstract
Myeloablative conditioning results in thymic epithelial cell (TEC) injury, slow T-cell reconstitution, and a high risk of opportunistic infections. Keratinocyte growth factor (KGF) stimulates TEC proliferation and, when given preconditioning, reduces TEC injury. Thymocytes and TECs express androgen receptors, and exposure to androgen inhibits thymopoiesis. In this study, we have investigated whether TEC stimulation via preconditioning treatment with KGF and leuprolide acetate (Lupron), 2 clinically approved agents, given only before conditioning would circumvent the profound TEC and associated T-cell deficiency seen in allogeneic bone marrow transplant (BMT) recipients. Only combined treatment with KGF plus leuprolide acetate normalized TEC subset numbers and thymic architecture. Thymopoiesis and thymic output were supranormal, leading to the accelerated peripheral reconstitution of naive CD4 and CD8 T cells with a broad Vβ repertoire and decreased homeostatic T-cell proliferation. Combined therapy facilitated T:B cooperativity and enabled a B-cell humoral response to a CD4 T cell–dependent neoantigen challenge soon after BMT. In vivo antigen-specific CD8 T-cell responses and clearance of a live pathogen was superior with combined versus individual agent therapy. Thus, KGF combined with androgen blockade represents a novel approach to restore thymic function and facilitates the rapid recovery of peripheral T-cell function after allogeneic BMT.
Introduction
Allogeneic bone marrow transplantation (BMT) is a valuable treatment option for malignant and nonmalignant disorders.1,2 After myeloablative conditioning, a favorable outcome depends upon successful immune reconstitution, including the de novo generation of a polyclonal population of naive T cells in the thymus.2-6 Mature T-cell generation is substantially delayed after BMT, primarily because of thymic injury induced by pre-BMT chemoradiotherapy and graft-versus-host disease (GVHD).3,5 Fungal and viral infections normally controlled by T cells can occur at high frequency in BMT patients, resulting in significant morbidity and mortality.7 Thus, strategies are needed to speed thymopoiesis after BMT.
Normal thymopoiesis involves a program of thymocyte differentiation and maturation through sequential stages characterized by CD4 and CD8 expression—CD4−CD8− (“double negative”), CD4+CD8+ (“double positive”), and CD4+ or CD8+ (“single positive”)—culminating in the export of mature CD4+ and CD8+ T cells into the periphery.8 The thymic stroma is composed primarily of a 3-dimensional matrix of cortical and medullary thymic epithelial cells (TECs).9 TECs directly support thymocyte development and selection but are susceptible to BMT-conditioning-induced damage, impairing the ability of the thymus to produce T cells for prolonged periods of time after BMT.10-13
Several growth factors regulate the development, proliferation, and function of TECs throughout life, including fibroblast growth factor-7 (FGF-7), also known as keratinocyte growth factor (KGF).14-16 KGF is an epithelial growth factor mainly produced by mesenchymal cells in the thymus and binds exclusively to a specific member of the fibroblast growth factor receptor-2 family, FGFR2-IIIb (KGFR), which is expressed in the thymus by TEC.17 KGF can aid in the protection and/or repair of epithelial cells in murine models of radiation- and chemotherapy-induced injury and is FDA-approved for the prevention of oral mucositis associated with chemoradiotherapy and BMT.18-20 Murine studies have demonstrated that thymic injury and prolonged immune deficiency can be prevented by KGF pretreatment in models with and without GVHD.11,21,22 KGF also has been shown to facilitate engraftment and abrogate GVHD-induced lethality in murine BMT recipients.23
The thymic atrophy that occurs with advancing age has been partly linked to physiologic changes in sex steroid hormone production.24-26 Androgen receptors (ARs) are expressed on TECs, certain thymocyte subsets, and mature T cells, although the exact mechanisms by which androgens exert their effects on thymopoiesis and T-cell homeostasis/function are not fully understood.27-31 Physical castration of aged mice results in a complete restoration of thymic size and function to prepubertal levels, and mice castrated pre-BMT restore thymopoiesis and peripheral T-cell numbers more rapidly than sham-castrated recipients.32-36 Although physical castration has been proven effective in the murine model, methods of chemically induced castration are more directly translatable to the human BMT setting. Disrupting sex steroid production using a luteinizing hormone-releasing hormone agonist (LHRH-A) rapidly results in long-lasting changes in sex steroids similar to that of surgical castration.37 Leuprolide acetate (Lupron) is a potent LHRH-A that is currently used in the clinic to treat prostate cancer, and LHRH-A has been tested as a single agent in a pilot study of autologous and allogeneic hematopoietic stem cell transplant recipients and shown to increase levels of naive CD4+ T cells in the periphery in a cohort of patients.38
The receptor distribution of FGFR2-IIIb and ARs on TECs indicates the potential for additive effects from combined treatment with KGF and leuprolide acetate. We hypothesized that pre-BMT androgen blockade via chemical castration could act in an additive fashion with KGF to enhance thymic recovery and T-cell reconstitution in allogeneic BMT recipients. This study focuses on 2 currently FDA-approved agents (recombinant human KGF [Kepivance] and leuprolide acetate). We report that combined pre-BMT treatment resulted in a restoration of thymic architecture, number, and subset distribution of TECs. These changes led not only to additive effects on restoring thymopoiesis, thymic output, and recovery of peripheral naive CD4 and CD8 T-cell numbers but also in vivo responses to neoantigen and challenges with a live pathogen. These findings suggest a novel, clinically translatable approach to accelerate the recovery of functional immune system recovery after BMT.
Methods
Animals
C57BL/6 (H-2b; termed B6) and [C57BL/6xBALB/c]F1 (H-2d/b; termed CB6F1) male mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at 8 weeks of age as BMT recipients or control animals (non-BMT control mice). Donor female BALB/c (H-2d) or C57BL/6.Ly5.1 mice of the same age were purchased from the National Cancer Institute (Frederick, MD). Mice were housed in specific pathogen-free facilities. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
KGF administration and chemical castration
Recombinant human KGF (kindly provided by Amgen, Thousand Oaks, CA) was administered subcutaneously for 3 consecutive days (5 mg/kg per day) before radiation treatment as previously reported.11 Leuprolide acetate (Lupron; TAP Pharmaceuticals, Lake Forest, IL), an LHRH agonist that ablates sex steroid hormone production, was injected as a 3-month deposition into the hind leg of B6 recipients 13 days before total body irradiation (TBI) at a dose of 0.8 mg/mouse based upon dose-response studies in the same model demonstrating equivalency in day 28 after BMT thymopoiesis in doses of 0.4, 0.8, and 1.2 mg/mouse34 (and data not shown).
BM transplantation
Single-cell suspensions of BM cells obtained from femurs and tibiae of BALB/c (allogeneic) or B6.Ly5.1 (congenic) donors were CD4/8-depleted as described previously39 and 107 (allogeneic) or 5 × 106 (congenic) CD4/8-depleted BM cells were intravenously administered to recipients that had received 11-Gy TBI from a cesium source 24 h before BMT.
Lymphocyte flow cytometry
Thymocytes, splenocytes, and lymph nodes were suspended in 2% fetal calf serum/phosphate-buffered saline (PBS), and 106 cells were incubated with appropriate fluorochrome-conjugated monoclonal antibodies (BD Pharmingen, San Jose, CA) for 30 minutes at 4°C. A total of 104-105 live events were acquired on a FACScalibur flow cytometer (BD Pharmingen) and analyzed with FlowJo software (TreeStar, San Jose, CA).
TEC analysis by FACS
TECs were isolated and analyzed as described previously.40,41 Individual thymi were removed and small capsule incisions were made, followed by gentle disruption in ice-cold RPMI 1640 medium to deplete majority of thymocytes. Thymi were then incubated twice for 15 minutes in 0.1% collagenase-D plus 0.125% DNase-I (Roche, Indianapolis, IN), followed by incubation for 30 minutes in 0.1% collagenase/dispase plus 0.125% DNase-I (Roche). Supernatants from the final 2 enzymatic digestions were pooled and analyzed with the following antibodies: CD45-PerCp, Ly51/CDR1-fluorescein isothiocyanate, major histocompatibility complex-II-phycoerythrin (MHC-II-PE; BD Pharmingen), and biotinylated-Ulex-europaeus-agglutinin-1 (UEA-1; Vector Labs, Burlingame, CA) plus streptavidin-conjugated-Cy5 (Invitrogen, Carlsbad, CA). Anti-AIRE antibody was a generous gift of H. Scott (Monash University, Victoria, Australia) and was detected with mouse anti-rat IgG2c-Cy5 (BD Pharmingen).
BrdU incorporation assays
Mice were injected intraperitoneally with 200 μL of a 5 mg/mL solution of 5-bromo-2-deoxyuridine (BrdU)/PBS on days 3, 10, or 22 after BMT and subsequently given BrdU in their drinking water for 5 days (0.8 mg/mL). Recipients were killed after 5 days, and TECs or T cells were assessed for BrdU incorporation using the BrdU Flow Kit (BD Pharmingen) and the Alexa Fluor 647-conjugated monoclonal anti-BrdU antibody (clone PRB-1; Invitrogen). At least 5 × 104 CD45− (for TEC analyses) or total live events (for lymphocyte analyses) were acquired by fluorescence-activated cell sorting (FACS).
Detection of recent thymic emigrants
Anesthetized mice were injected in one thymic lobe with 10 μL of a 5 mg/mL solution of sulfosuccinimidyl-6-(biotinamido) hexanoate (sulfo-NHS-LC biotin) in PBS (Pierce, Rockford IL). After 24 hours, thymus and spleen were stained with streptavidin-conjugated-Cy5 and other markers and analyzed by flow cytometry as described previously.42
Immunofluorescence microscopy
Intact thymi embedded in optimum cutting temperature (OCT) compound (Sakura, Tokyo, Japan) were snap-frozen in liquid nitrogen and stored at −80°C. Cryosections (7 μm) were fixed by air-drying overnight, blocking with 10% normal horse serum/PBS (Jackson Immunoresearch Laboratories, West Grove, PA), and staining with rabbit-anti-mouse cytokeratin-5 (K5) antibody (Covance, Berkeley, CA) plus a cychrome-5-conjugated goat-anti-rabbit antibody (Invitrogen) and biotinylated mouse-anti-mouse K18 (Progen Biotechnik, Heidelberg, Germany) plus Alexa Fluor 555-conjugated streptavidin (Invitrogen). Sections were mounted under a cover slip with 4,6-diamidino-2-phenylindole anti-fade solution (Invitrogen) and imaged on the following day at room temperature using an Olympus FluoView 500 Confocal Scanning Laser Microscope (Olympus, Center Valley, PA). Identification of TEC subsets according to marker expression was done as reported previously.22
Immunization with KLH and quantification of serum immunoglobulin
Mice were injected intraperitoneally with 50 μg keyhole-limpet hemocyanin (KLH; CalBiochem, La Jolla, CA) in Complete Freund adjuvant (Sigma-Aldrich, St Louis, MO) followed 3 weeks later with 50 μg KLH in Incomplete Freund adjuvant (Sigma-Aldrich). After 7 days, peripheral blood was collected by retro-orbital bleeds and analyzed with the use of enzyme-linked immunosorbent assay (ELISA) for total and KLH-specific IgG1 levels.43
Listeria monocytogenes infection
The recombinant L monocytogenes strains Lm-OVA and ΔactA-Lm-OVA44 (attenuated) expressing full-length chicken ovalbumin were kindly provided by Dr S. S. Way (University of Minnesota). Mice were inoculated with early logarithmic phase (OD600 of 0.1) bacteria grown in brain heart infusion broth at 37°C. Mice were injected intravenously with 106 colony-forming units (CFU) of ΔactA-Lm-OVA (primary) or 105 CFU of Lm-OVA (secondary) diluted in 200 μL PBS.
Quantification of Lm-OVA-specific CD8 T cells
MHC-I-DimerX:mouse-Ig-PE was purchased from BD Biosciences, and purified OVA257-64 (SIINFEKL) peptide was purchased from Anaspec (San Jose, CA). MHC-I-DimerX:mouse-Ig:OVA257-64 conjugates were prepared according to manufacturer's instructions (BD Biosciences). Peripheral blood was incubated with DimerX:mouse-Ig:OVA257-64-PE plus antibodies for other markers (all from BD Biosciences), and 5 × 103 donor CD8 T cells were collected and analyzed by flow cytometry.
Determination of L monocytogenes CFU
Mice were immunized intravenously with 105 CFU and rechallenged with 2 × 106 CFU of Lm strain 2C45 diluted in 200 μL PBS. Four days after secondary infection, livers were removed and homogenized in 0.05% Triton X-100/PBS (Sigma-Aldrich). Serial dilutions were plated onto brain-heart infusion plates, and Lm colonies were enumerated after 24 to 48 hours at 37°C.
Statistical analysis
Differences between treated and untreated BMT groups were analyzed by a 2-tailed, paired Student t test with unequal distribution.
Results
Combined pretreatment with KGF and leuprolide acetate additively restored thymopoiesis soon after allogeneic BMT
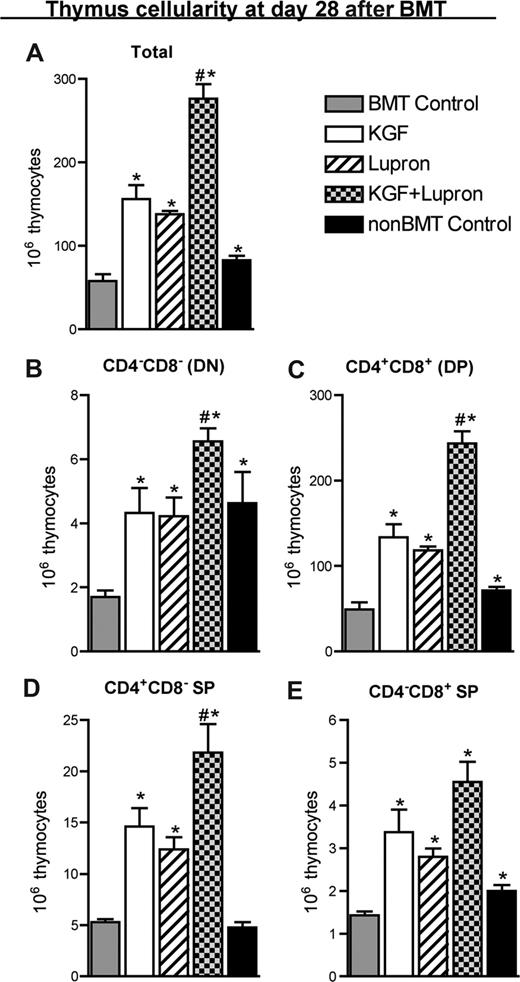
KGF administered before TBI and BMT enhanced thymopoiesis and peripheral T-cell reconstitution after BMT; maximal benefit was observed in the periphery 2 months after BMT.11 To determine whether KGF and androgen blockade before BMT could act in an additive fashion to more rapidly restore thymopoiesis, allogeneic murine BMT recipients of rigorously T cell–depleted BM cells were either left untreated (BMT control) or were pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate before transplant. On day 28 after BMT, total thymocyte cellularity was reduced by approximately 50% in BMT control mice compared with age/sex-matched, unmanipulated B6 control animals (non-BMT Control; Figure 1A). Mice treated with KGF or leuprolide acetate abrogated the reduction in thymocyte cellularity seen in BMT control mice. Combined treatment with KGF + leuprolide acetate showed an additive increase in thymic cellularity to numbers that were significantly greater than untreated or KGF-/leuprolide acetate-treated BMT recipients and non-BMT control mice (Figure 1A). The relative distribution of thymocyte subsets was not affected by any treatment with KGF and/or leuprolide acetate; therefore, concomitant increases in the DN, DP, CD4+ SP, and CD8+ SP subsets were observed (Figure 1A-E). Additive effects of KGF + leuprolide acetate were maintained through at least day 56 after BMT (Figure S1A-E, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Pretreatment with KGF combined with androgen blockade additively restored thymopoiesis early after allogeneic BMT. Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and analyzed for thymocyte cellularity at day 28 after BMT alongside age-/sex-matched, unmanipulated B6 control animals (non-BMT Control). Data shown are mean absolute numbers (± SEM) of (A) total thymocytes and of the thymocyte subsets: (B) CD8−CD4− double-negative, (C) CD8+CD4+ double-positive, (D) CD4+CD8− single-positive, and (E) CD4−CD8+ single-positive. The data are representative of 4 independent experiments with 4 to 5 mice per group. *P < .05 compared with untreated BMT recipients; #P < .05 compared with KGF-treated or leuprolide acetate-treated BMT recipients.
Pretreatment with KGF combined with androgen blockade additively restored thymopoiesis early after allogeneic BMT. Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and analyzed for thymocyte cellularity at day 28 after BMT alongside age-/sex-matched, unmanipulated B6 control animals (non-BMT Control). Data shown are mean absolute numbers (± SEM) of (A) total thymocytes and of the thymocyte subsets: (B) CD8−CD4− double-negative, (C) CD8+CD4+ double-positive, (D) CD4+CD8− single-positive, and (E) CD4−CD8+ single-positive. The data are representative of 4 independent experiments with 4 to 5 mice per group. *P < .05 compared with untreated BMT recipients; #P < .05 compared with KGF-treated or leuprolide acetate-treated BMT recipients.
Because both donor-derived and residual host-derived thymocytes might contribute to thymic cellularity early after BMT, we assessed thymocyte chimerism. On day 28 after BMT, more than 95% of thymocytes were of donor origin, increasing to more than 99% by day 56 (data not shown). Therefore, increased thymic cellularity was due to increased numbers of donor-derived thymocytes and not to preservation of host-derived thymocytes.
Combined pretreatment with KGF and leuprolide acetate maximally restored TECs after BMT
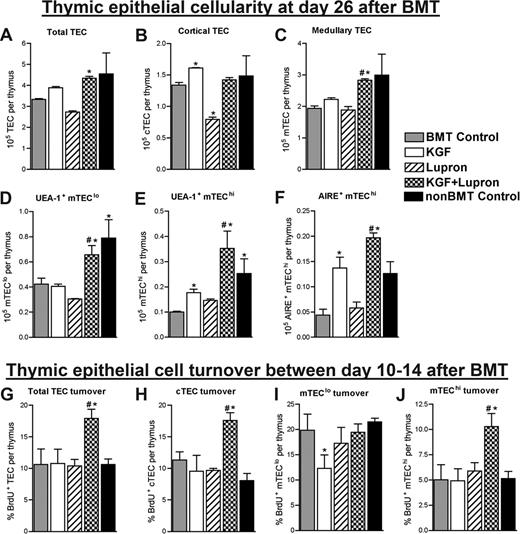
To determine whether protection/restoration of TECs was responsible for the enhancement of thymopoietic recovery observed on day 28 after BMT, absolute numbers of TECs, defined as CD45−MHC-II+ cells, were quantified in thymi of allogeneic BMT recipients left untreated or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and compared with non-BMT control mice. Regardless of pretreatment, TECs were severely depleted as early as day 7 after BMT (>75%), and TEC numbers remained significantly reduced through day 14 after BMT compared with non-BMT control mice (data not shown). On day 26 after BMT, all BMT groups still had reduced total TEC numbers compared with non-BMT control mice except for KGF + leuprolide acetate-treated BMT recipients that had restored TECs to levels above untreated BMT recipients and to similar numbers compared with non-BMT control mice (Figure 2A). With the exception of leuprolide acetate-treated BMT recipients, cortical TECs (cTEC; CD45−MHC-II+Ly51+) were restored to normal levels at day 26 (Figure 2B). Medullary TECs (mTECs; CD45−MHC-II+Ly51−) remained depleted in all groups except for the KGF + leuprolide acetate BMT recipients, which had similar mTEC numbers compared with non-BMT control mice (Figure 2C).
Combined pretreatment with KGF and androgen blockade maximally restores numbers of totalTECsand mTEC subsets by day 26afterBMT. (A-F) Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and analyzed for absolute numbers of TEC and TEC subsets at day 26 after BMT. Single-cell suspensions were prepared from enzymatic digests of individual thymi and used to determine total thymic epithelial cells (TEC; CD45−MHC-II+) (A), cortical TEC (cTEC; CD45−MHC-II+Ly51+) (B), medullary TEC (mTEC; CD45−MHC-II+Ly51−) (C), UEA-1+ mTEClo (CD45−MHC-IIlowLy51− UEA-1+) (D), UEA-1+ mTEChi (CD45−MHC-IIhighLy51− UEA-1+) (E), AIRE+ mTEChi (CD45−MHC-IIhighLy51− AIRE+) (F). (G-J) Total TECs and individual TEC subsets were assessed for proliferation by continuous administration of BrdU in the drinking water (0.8 mg/mL) between day 10 and 14 after BMT at which point Total TECs (G), cTEC (H), mTEClo (I), and mTEChi subsets (J) were analyzed for BrdU incorporation. Data shown are the mean numbers of TEC (± SEM) or mean percentages of BrdU+ TEC (± SEM) and are representative of one experiment of 4 mice per group. *P < .05 compared with BMT control mice; #P < .05 compared with KGF- or leuprolide acetate-treated BMT recipients.
Combined pretreatment with KGF and androgen blockade maximally restores numbers of totalTECsand mTEC subsets by day 26afterBMT. (A-F) Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and analyzed for absolute numbers of TEC and TEC subsets at day 26 after BMT. Single-cell suspensions were prepared from enzymatic digests of individual thymi and used to determine total thymic epithelial cells (TEC; CD45−MHC-II+) (A), cortical TEC (cTEC; CD45−MHC-II+Ly51+) (B), medullary TEC (mTEC; CD45−MHC-II+Ly51−) (C), UEA-1+ mTEClo (CD45−MHC-IIlowLy51− UEA-1+) (D), UEA-1+ mTEChi (CD45−MHC-IIhighLy51− UEA-1+) (E), AIRE+ mTEChi (CD45−MHC-IIhighLy51− AIRE+) (F). (G-J) Total TECs and individual TEC subsets were assessed for proliferation by continuous administration of BrdU in the drinking water (0.8 mg/mL) between day 10 and 14 after BMT at which point Total TECs (G), cTEC (H), mTEClo (I), and mTEChi subsets (J) were analyzed for BrdU incorporation. Data shown are the mean numbers of TEC (± SEM) or mean percentages of BrdU+ TEC (± SEM) and are representative of one experiment of 4 mice per group. *P < .05 compared with BMT control mice; #P < .05 compared with KGF- or leuprolide acetate-treated BMT recipients.
Within the medulla, 2 distinct mTEC subpopulations can be identified based upon MHC-II expression and binding to the lectin Ulex Europaeus Agglutinin-1 (UEA-1)–mTEClo (CD45−MHC-IIloLy51−UEA-1+) and mTEChi (CD45−MHC-IIhiLy51−UEA-1+).40,41 Both mTEC populations were reduced in number by 50% or more in untreated, KGF- or leuprolide acetate-treated BMT recipients compared with non-BMT control mice on day 26 after BMT (Figure 2D,E). However, KGF + leuprolide acetate treatment resulted in mTEClo and mTEChi numbers comparable with those of non-BMT control mice and 1.5-fold (mTEClo) and 4-fold (mTEChi) higher than untreated BMT control mice (Figure 2D,E). Numbers of AIRE+ mTEChi, known to be critical for the negative selection of thymocytes with autoreactive specificities,46 were significantly reduced (∼3-fold) in untreated and leuprolide acetate-treated BMT recipients compared with non-BMT control mice. In KGF- and KGF + leuprolide acetate-treated BMT recipients, AIRE+ mTEChi numbers were restored to levels similar to those of non-BMT control mice (Figure 2F). Medullary dendritic cells in the thymus, also involved in negative selection,46 were severely depleted after BMT and were significantly and maximally restored by day 26 after BMT in KGF + leuprolide acetate-treated BMT recipients (data not shown).
To determine whether the increased numbers of TECs observed on day 26 after BMT were due to enhanced TEC proliferation at an earlier time point, mice were injected between days 10 and 14 after BMT with the thymidine analog BrdU, which is incorporated into DNA of replicating cells. In agreement with the relative TEC numbers observed on day 26 after BMT, total TECs, cTECs, and mTEChi showed the greatest levels of proliferation in the thymi of BMT recipients treated with KGF + leuprolide acetate (Figure 2G-J).
In addition to the loss of TECs, immunofluorescence microscopy showed a loss of clear compartmentalization of cortex and medulla in the thymi of untreated BMT recipients on day 28 after BMT (Figure S2). In contrast, non-BMT control mice displayed well organized and densely clustered cortical and medullary regions with a distinct corticomedullary boundary, a finding largely recapitulated in KGF + leuprolide acetate-treated BMT recipients (Figure S2). All groups had restored thymic architecture by day 56 after BMT (Figure S2).
Pretreatment with KGF and leuprolide acetate resulted in enhanced T-cell reconstitution in the lymph nodes and spleen by day 35 after BMT
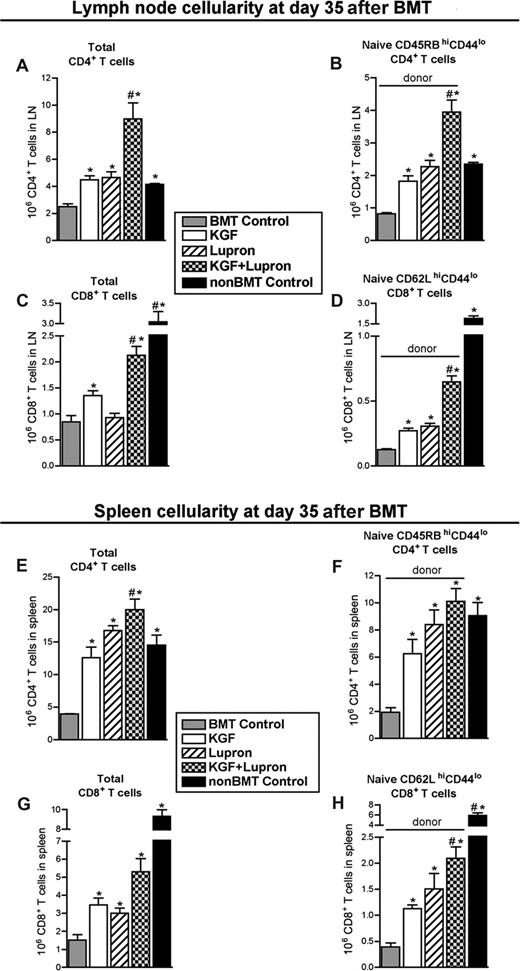
To determine whether improved thymopoiesis induced by KGF and leuprolide acetate resulted in higher numbers of peripheral T cells, lymph nodes of BMT recipients were analyzed for CD4+ and CD8+ T cells on day 35 after BMT. CD4+ and CD8+ T-cell numbers in untreated BMT recipients were reduced approximately 50% and approximately 75%, respectively, compared with non-BMT control mice and were partially restored in BMT recipients pretreated with KGF or leuprolide acetate alone (Figure 3A,C). Combined, these treatments provided an additive increase in CD4+ and CD8+ T cells that was significantly greater than either agent alone (Figure 3A,C), completely eliminating CD4+ and virtually eliminating CD8+ T-cell lymphopenia. Because small numbers of radioresistant host-derived peripheral T cells still remained at this time point, we specifically quantified naive, donor-derived, CD4+ and CD8+ T cells to assess the contribution of thymus-derived T cells to reconstitution of peripheral T cells. Additive increases of donor-derived, naive CD4+ (H2Kd+CD4+CD45RBhiCD44lo) and CD8+ (H2Kd+CD8+CD62LhiCD44lo) T cells were observed in BMT recipients treated with KGF + leuprolide acetate that were significantly greater than was observed in untreated, KGF-treated, and leuprolide acetate-treated BMT recipients, respectively (Figure 3B,D). By day 60 after BMT, CD4+ T-cell compartments in untreated BMT recipients were restored to their non-BMT control levels, whereas leuprolide acetate- and KGF + leuprolide acetate treated BMT recipients maintained comparatively higher numbers of total and naive CD4+ T cells (Figure S3A,B). Donor-derived naive CD8+ T cells remained significantly reduced through day 60 after BMT in all BMT groups (compared with non-BMT control mice), except those pretreated with KGF + leuprolide acetate (Figure S3C,D).
Combined pretreatment with KGF and androgen blockade significantly restore numbers of total and donor-derived naive CD4+ and CD8+ T cells in lymph node and spleen by day 35 after BMT. Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and analyzed for the presence of T cells in the lymph nodes and spleen at day 35 after BMT alongside unmanipulated age/sex-matched B6 control animals (non-BMT Control). Mean absolute num-bers (± SEM) of total CD4+ T cells (A,E), naive (CD45RBhighCD44low) CD4+ T cells (B,F), total CD8+ T cells (C,G), and naive (CD62LhighCD44low) CD8+ T cells (D,H) in the lymph nodes and spleen are shown. Lymph node data are represented here by pooled cells from 2 inguinal, 2 axillary, and mesenteric lymph nodes. Data are representative of 3 experiments, each with 4 mice per group; *P < .05 compared with BMT control mice; #P < .05 compared with KGF-treated BMT recipients.
Combined pretreatment with KGF and androgen blockade significantly restore numbers of total and donor-derived naive CD4+ and CD8+ T cells in lymph node and spleen by day 35 after BMT. Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and analyzed for the presence of T cells in the lymph nodes and spleen at day 35 after BMT alongside unmanipulated age/sex-matched B6 control animals (non-BMT Control). Mean absolute num-bers (± SEM) of total CD4+ T cells (A,E), naive (CD45RBhighCD44low) CD4+ T cells (B,F), total CD8+ T cells (C,G), and naive (CD62LhighCD44low) CD8+ T cells (D,H) in the lymph nodes and spleen are shown. Lymph node data are represented here by pooled cells from 2 inguinal, 2 axillary, and mesenteric lymph nodes. Data are representative of 3 experiments, each with 4 mice per group; *P < .05 compared with BMT control mice; #P < .05 compared with KGF-treated BMT recipients.
Similar to the lymph node findings, treatment with KGF + leuprolide acetate resulted in complete reconstitution of splenic T-cell numbers at day 35 after BMT. Whereas there were highly significant (>4-fold) reductions in total CD4+ and CD8+ T cells in the spleen of untreated BMT recipients compared with non-BMT control mice (Figure 3E,G), combined treatment completely prevented the state of T-cell lymphopenia, resulting in the highest increase in total CD4+ and CD8+ T cells, which appeared additive for total CD8+ T cells but not for total CD4+ T cells (Figure 3E,G). Compared with BMT control mice, naive, donor-derived CD4+ T cells were significantly increased by KGF (3-fold), leuprolide acetate (4-fold), and KGF + leuprolide acetate (5-fold), reaching levels that were no longer significantly lower than those in non-BMT control mice (Figure 3F). Significant increases in naive, donor-derived CD8+ T cells were observed with KGF or leuprolide acetate (3-fold) and KGF + leuprolide acetate (5-fold) compared with untreated BMT recipients (Figure 3H). Total and naive donor-derived CD8+ T-cell numbers remained deficient through day 60 after BMT; only the combined treatment resulted in a significant restoration of these cells compared with untreated control animals (Figure S3G,H). These data demonstrate that combined treatment with KGF + leuprolide acetate before BMT additively and durably enhanced recovery of donor-derived naive CD4+ and CD8+ T cells in both lymph nodes and spleen after BMT. Analysis of TCR Vβ repertoire by flow cytometric analysis representing 12 Vβ alleles confirmed that KGF and leuprolide acetate did not affect the diversity of TCR Vβ usage in donor-derived T cells compared with non-BMT control mice (Figure S4).
Combined pretreatment with KGF and leuprolide acetate resulted in enhanced thymic output and less homeostatic proliferation early after BMT
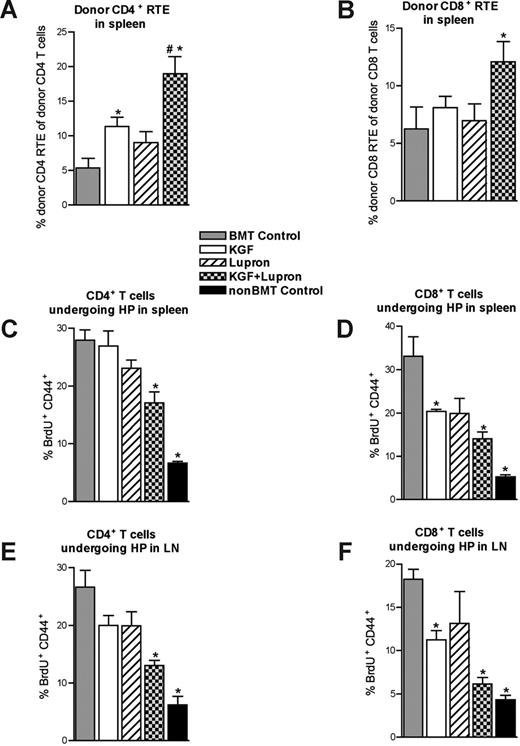
Because KGF and leuprolide acetate each may increase peripheral T-cell expansion, we sought to determine the relative contributions of thymic export and peripheral homeostatic expansion to the observed increased numbers of total and naive peripheral CD4+ and CD8+ T cells found in secondary lymphoid organs. Thymic export was quantified at day 28 after BMT by detection of intrathymically biotin-labeled donor-derived T cells that had recently emigrated to the spleen.42 Consistent with higher naive splenic CD4+ T-cell numbers, we observed a significant and almost significant increase in the export of donor-derived CD4+ T cells into the periphery in BMT recipients treated with KGF and leuprolide acetate, respectively, compared with the untreated BMT control mice (Figure 4A). Although thymic export of donor-derived CD8+ T cells was not significantly improved with KGF or leuprolide acetate alone, there was a significant and additive increase in the export of donor-derived CD4+ and CD8+ T cells into the periphery in KGF + leuprolide acetate-treated BMT recipients on day 28 after BMT, consistent with higher naive CD4+ and CD8+ T-cell numbers in this group (Figure 4A,B).
Enhanced thymocyte cellularity in KGF + leuprolide acetate-treated BMT recipients correlates with increased T-cell export into the periphery and diminished homeostatic proliferation early after BMT. (A,B) Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow that were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate were intrathymically injected (one thymic lobe) with biotin at 28 days after BMT. After 24 hours of in vivo labeling, the export of thymus-derived CD4 (A) and CD8 (B) T cells into the periphery was assessed by staining total splenocytes with fluorescently labeled streptavidin in combination with monoclonal antibodies specific for CD4 and CD8. Absolute numbers of recent thymic emigrants (RTE) were normalized to absolute numbers of donor-derived CD4+ or CD8+ T cells in panels A and B, respectively. (C-F) Homeostatic proliferation in the CD4+ and CD8+ T-cell compartment was also assessed between days 30 and 35 after BMT. Treated and untreated BMT recipients and non-BMT control mice were injected intraperitoneally with BrdU (1 mg) on day 30, followed by continuous administration of BrdU in the drinking water (0.8 mg/mL) through day 35 after BMT, at which point CD4+ and CD8+ T cells were analyzed for BrdU incorporation and concomitant up-regulation of CD44, thus indicating that proliferation had occurred within the 5-day labeling window. Data shown are the mean percentages of BrdU+CD44+ (± SEM) of total CD4+ T cells (C,E) and CD8+ T cells (D,F) isolated from the spleens and lymph nodes of these mice. These data are representative of one experiment with 4 mice per group; *P < .05 compared with BMT control mice; #P < .05 compared with KGF- or leuprolide acetate-treated BMT recipients.
Enhanced thymocyte cellularity in KGF + leuprolide acetate-treated BMT recipients correlates with increased T-cell export into the periphery and diminished homeostatic proliferation early after BMT. (A,B) Lethally irradiated B6 recipients of allogeneic (BALB/c) bone marrow that were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate were intrathymically injected (one thymic lobe) with biotin at 28 days after BMT. After 24 hours of in vivo labeling, the export of thymus-derived CD4 (A) and CD8 (B) T cells into the periphery was assessed by staining total splenocytes with fluorescently labeled streptavidin in combination with monoclonal antibodies specific for CD4 and CD8. Absolute numbers of recent thymic emigrants (RTE) were normalized to absolute numbers of donor-derived CD4+ or CD8+ T cells in panels A and B, respectively. (C-F) Homeostatic proliferation in the CD4+ and CD8+ T-cell compartment was also assessed between days 30 and 35 after BMT. Treated and untreated BMT recipients and non-BMT control mice were injected intraperitoneally with BrdU (1 mg) on day 30, followed by continuous administration of BrdU in the drinking water (0.8 mg/mL) through day 35 after BMT, at which point CD4+ and CD8+ T cells were analyzed for BrdU incorporation and concomitant up-regulation of CD44, thus indicating that proliferation had occurred within the 5-day labeling window. Data shown are the mean percentages of BrdU+CD44+ (± SEM) of total CD4+ T cells (C,E) and CD8+ T cells (D,F) isolated from the spleens and lymph nodes of these mice. These data are representative of one experiment with 4 mice per group; *P < .05 compared with BMT control mice; #P < .05 compared with KGF- or leuprolide acetate-treated BMT recipients.
To correlate thymic output with the degree of homeostatic proliferation (HP) occurring in peripheral lymphoid organs, BrdU was given continuously in the drinking water between days 30 and 35 after BMT. BrdU incorporation with up-regulation of CD44 in CD4+ and CD8+ T cells in the spleen and lymph nodes was measured. An inverse relationship was observed between the number of recently exported CD4+ and CD8+ T cells into the periphery around day 28 after BMT and the degree of HP detected in peripheral lymphoid organs between days 30 and 35 after BMT (Figure 4C-E). The higher donor-derived naive CD4+ and CD8+ T-cell numbers are probably due to increased thymic output rather than higher levels of HP.
Pretreatment with KGF and leuprolide acetate enhanced T cell–dependent antibody responses early after BMT
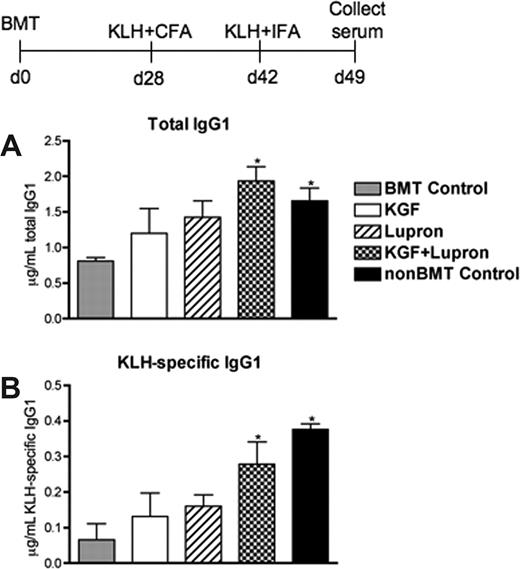
The prolonged CD4+ T-cell deficiency after BMT can preclude the generation of normal immune responses to T cell–dependent B-cell antigens.47 Because KGF + leuprolide acetate-treated BMT recipients had normalization of donor naive CD4+ T cells and donor B cells by day 35 after BMT (data not shown), we sought to determine whether this treatment could enhance a humoral immune response against a T cell–dependent neoantigen challenge given on day 28 after BMT. Mice were immunized with KLH plus adjuvant, rechallenged 2 weeks later with KLH, and serum immunoglobulin levels were measured after 7 days. Total and KLH-specific IgG1 levels were approximately 50% lower in untreated BMT recipients compared with non-BMT control mice, and pretreatment with KGF or leuprolide acetate only marginally improved T cell–dependent B-cellisotype switching as measured by anti-KLH specific IgG1 antibody levels (Figure 5A,B). BMT recipients treated with KGF + leuprolide acetate produced significantly greater amounts of total and KLH-specific IgG1 antibody, reaching levels comparable with non-BMT control mice (Figure 5A,B). The early increases of peripheral donor T cells and B cells in BMT recipients pretreated with KGF + leuprolide acetate permitted the normal generation of a CD4+ T cell–dependent B-cell immunoglobulin isotype-switched response to KLH even when given as early as day 28 after BMT.
KGF treatment and androgen blockadebeforeBMT enhance the secondary humoral immune response to KLH after allogeneic BMT. Lethally irradiated C57BL/6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and immunized at day 28 after BMT with 50 μg of KLH in Complete Freund's adjuvant (KLH/CFA) alongside unmanipulated age/sex-matched C57BL/6 control animals (non-BMT Control). Two weeks after primary immunization (equivalent to day 42 after BMT), mice were rechallenged with 50 μg of KLH in Incomplete Freund's adjuvant (KLH/IFA). Serum was then collected after 7 days and analyzed with the use of ELISA for total IgG1 (A) and KLH-specific IgG1 (B) antibody levels. Data shown are mean micrograms of IgG1 per milliliter of serum (± SEM) from one experiment with 4 mice per group; *P < .05 compared with untreated BMT control mice.
KGF treatment and androgen blockadebeforeBMT enhance the secondary humoral immune response to KLH after allogeneic BMT. Lethally irradiated C57BL/6 recipients of allogeneic (BALB/c) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and immunized at day 28 after BMT with 50 μg of KLH in Complete Freund's adjuvant (KLH/CFA) alongside unmanipulated age/sex-matched C57BL/6 control animals (non-BMT Control). Two weeks after primary immunization (equivalent to day 42 after BMT), mice were rechallenged with 50 μg of KLH in Incomplete Freund's adjuvant (KLH/IFA). Serum was then collected after 7 days and analyzed with the use of ELISA for total IgG1 (A) and KLH-specific IgG1 (B) antibody levels. Data shown are mean micrograms of IgG1 per milliliter of serum (± SEM) from one experiment with 4 mice per group; *P < .05 compared with untreated BMT control mice.
Pretreatment with KGF and leuprolide acetate enhances functional immune response against L monocytogenes after BMT
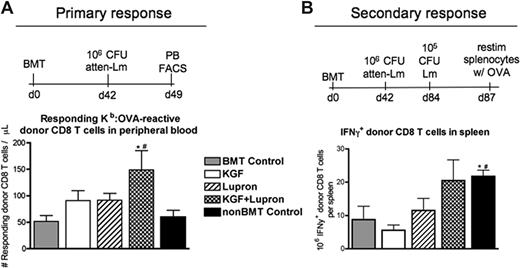
To determine whether improved immune reconstitution induced by pre-BMT treatment with KGF and/or leuprolide acetate would permit a functional immune response to challenge with a live intracellular pathogen after BMT, mice were immunized with 106 CFU of an attenuated strain of L monocytogenes. To monitor pathogen specific immune responses, a congenic BMT system was used with L monocytogenes (Lm) engineered to express chicken ovalbumin (ΔactA-Lm-OVA). Thus, activated Lm-specific CD8 T-cell responses were quantifiable in peripheral blood by MHC class-I:OVA tetramer binding to donor-derived CD44+ CD8 T cells (Ly5.1+CD8+CD44+Kb:OVA257-264+). Seven days after primary infection, KGF- or leuprolide acetate-treated BMT recipients contained modestly higher numbers of donor-derived, OVA-specific CD44+ CD8 T cells compared with untreated BMT recipients, and treatment with KGF + leuprolide acetate resulted in additively higher numbers that were significantly greater than in all other BMT groups (Figure 6A). Postmortem necroscopic analysis revealed that all BMT and non-BMT groups had effectively cleared this attenuated strain by 3 weeks after infection (data not shown).
Combined pretreatment with KGF and androgen blockade before BMT significantly improves CD8 T-cell responses against L monocytogenes after allogeneic BMT. Lethally irradiated C57BL/6 Ly5.2+ recipients of congenic (C57BL/6 Ly5.1+) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and immunized at day 42 after BMT alongside unmanipulated age/sex-matched B6 control animals (non-BMT Control). For primary immunization, 106 CFU of an attenuated strain of L monocytogenes that express recombinant full-length chicken ovalbumin (ΔactA-Lm-OVA) was intravenously injected. (A) Absolute numbers of donor-derived Ly5.1+CD44+CD8+ Kb-OVA257-64–specific T cells were quantified in peripheral blood of infected animals by FACS 7 days after primary infection. (B) Immunized mice were then rechallenged with 105 CFU of the virulent parent strain, Lm-OVA, 42 days after primary infection. After 3 days, isolated splenocytes of infected animals were restimulated ex vivo for 5 h with OVA257-64, and donor-derived CD8 T cells were analyzed by FACS for IFNγ production. *P < .05 compared with untreated BMT control mice; #P < .05 compared with KGF- and leuprolide acetate–treated BMT recipients.
Combined pretreatment with KGF and androgen blockade before BMT significantly improves CD8 T-cell responses against L monocytogenes after allogeneic BMT. Lethally irradiated C57BL/6 Ly5.2+ recipients of congenic (C57BL/6 Ly5.1+) bone marrow were left untreated (BMT Control) or pretreated with KGF, leuprolide acetate, or KGF + leuprolide acetate and immunized at day 42 after BMT alongside unmanipulated age/sex-matched B6 control animals (non-BMT Control). For primary immunization, 106 CFU of an attenuated strain of L monocytogenes that express recombinant full-length chicken ovalbumin (ΔactA-Lm-OVA) was intravenously injected. (A) Absolute numbers of donor-derived Ly5.1+CD44+CD8+ Kb-OVA257-64–specific T cells were quantified in peripheral blood of infected animals by FACS 7 days after primary infection. (B) Immunized mice were then rechallenged with 105 CFU of the virulent parent strain, Lm-OVA, 42 days after primary infection. After 3 days, isolated splenocytes of infected animals were restimulated ex vivo for 5 h with OVA257-64, and donor-derived CD8 T cells were analyzed by FACS for IFNγ production. *P < .05 compared with untreated BMT control mice; #P < .05 compared with KGF- and leuprolide acetate–treated BMT recipients.
To investigate the effects of KGF and leuprolide acetate on a secondary immune response against Lm-OVA, mice were rechallenged 42 days after primary infection with 105 CFU of the nonattenuated parent strain, Lm-OVA. Three days later, we restimulated splenocytes from infected animals ex vivo with soluble OVA257-264 and found that only KGF + leuprolide acetate-treated BMT recipients demonstrated a comparable number of interferon-γ (IFNγ)–positive CD8 T cells as non-BMT control mice, at least 2-fold higher than all other groups (Figure 6B).
In separate experiments, untreated, KGF-, leuprolide acetate- or KGF + leuprolide acetate-treated allogeneic BMT recipients were assessed for clearance of Lm as measured by CFU determination in livers of infected animals 4 days after secondary infection. Untreated BMT recipients contained significantly higher bacterial burdens than non-BMT control mice and KGF or leuprolide acetate administered as single agents before BMT resulted in a marginal benefit for decreasing liver CFU (Figure S5). It is noteworthy that KGF + leuprolide acetate-pretreated BMT recipients significantly reduced the CFU burden from the liver to levels that were comparable with those in non-BMT control mice (Figure S5).
Discussion
We report a novel therapy given entirely before the BMT conditioning regimen that combines the administration of KGF and the LHRH agonist (leuprolide acetate) to enhance thymopoiesis and peripheral T-cell recovery and function in BMT recipients. Compared with no treatment in BMT reccipients, KGF + leuprolide acetate treatment additively increased thymocyte and peripheral T-cell recovery. Thymic architecture and TECs were significantly restored in KGF + leuprolide acetate-treated recipients early after BMT, improving peripheral donor-derived T-cell reconstitution as a result of increased thymic output. KGF + leuprolide acetate-pretreated BMT recipients mounted a superior immune response to the neoantigen KLH and cleared a live infection with Lm more effectively than other treatment groups.
In rodents and nonhuman primate models of chemoradiotherapy and BMT, KGF pretreatment has been shown to augment thymopoiesis in an interleukin-7 (IL-7)–dependent fashion.11,21,48 Because TECs are KGFR+, the putative mechanism of action proposed was stimulation of TEC proliferation and/or repair from radiation-induced injury.11 In mice that did not receive transplants, KGF administration increased TEC numbers.14 Thus, KGF may induce the expansion of a TEC progenitor population that, in turn, may rapidly mature to restore TEC numbers after BMT conditioning. Androgen blockade by physical castration can reverse the thymic atrophy accompanying aging and can improve thymopoietic recovery in rodents undergoing allogeneic BMT.32-34,36 Although our experiments focused on male mice, gonadal hormone blockade should be effective in female subjects, because leuprolide acetate has been successfully used to treat hormone-related disorders in women.49 ARs are expressed on TECs, non-TEC thymic stromal cells, thymocytes, and peripheral T cells,36 although chimeric studies have indicated that the restorative effect of castration on the thymus required AR expression on TECs.27,28 Because castration-induced enhancement of thymopoietic recovery after BMT was KGF-independent,36 these data suggest that each agent acts through distinct mechanisms.
Thymic cellularity is tightly controlled by the availability of thymic stromal niches to support thymocyte maturation.9,50 Although studies have demonstrated TBI-induced TEC depletion,10-12 this is the first to quantitatively analyze TEC subset depletion and recovery by FACS after TBI and BMT. TBI-induced TEC depletion is not surprising in light of data illustrating that TEC, especially mTEC, are dynamically proliferating in the steady state.46 The equivalent loss of TEC observed in all BMT groups early after BMT (day 7, day 14) indicates that KGF + leuprolide acetate accelerates the restoration rather than the protection of TEC compartment from TBI-induced depletion. KGF and leuprolide acetate appeared to act in concert to restore more rapidly TEC numbers without disrupting thymic architecture and may have provided for a larger stromal scaffold to support thymopoiesis. These proliferation events may have occurred early after BMT based upon earlier studies demonstrating maximal TEC proliferation at 1-3 days after discontinuation of KGF.14 KGF has been shown to be mitogenic for both cTEC and mTEC and can act to restore the thymic disorganization associated with aging and BMT14 (G.A.H., unpublished data, August 2007). A large percentage of UEA-1+ mTEC express FGFR2-IIIb.14 Although expression patterns of ARs on TEC subsets have not been described, androgen removal is mitogenic for mTEC.51 It is thought that mTEChi encompass mature, postmitotic TECs that are derived from immature mTEClo.46 Our findings of a large number of BrdU+ mTEChi in KGF + leuprolide acetate-treated BMT recipients are consistent with the interpretation that these cells are the progeny of mTEClo recovering from depletion and maturing to reconstitute mTEChi cells. Thus, KGF + leuprolide acetate appears to maximally enhance mTEChi regeneration after BMT, including the AIRE+ subset, critical for the expression of tissue-restricted antigens needed for negative repertoire selection of autoreactive T-cell clones during thymopoiesis.52
The additive increase in thymic cellularity in recipients pretreated with KGF + leuprolide acetate correlated with increased thymic export of T cells into the periphery. Previous studies have shown that KGF treatment and androgen blockade (physical castration), when analyzed individually, could increase thymic cellularity and enhance thymic output.14,35,36 In mice that did not receive transplants, thymic export is a direct function of thymic cellularity.53 Our data suggest that the same relationship holds true after BMT, even though the thymus is damaged and exports T cells into a lymphopenic environment. In agreement with an enhanced thymic output in KGF + leuprolide acetate-treated mice, we observed by day 35 after BMT additive increases in donor-derived naive CD4 and CD8 T cells in both spleen and lymph node. Because T cell–depleted BM was used, all donor-derived T cells with a naive surface phenotype must have been generated in the thymus in this transplant model. The BMT models used in these studies do not result in GVHD clinically or histologically. Because androgen blockade by physical castration pre-BMT does not exacerbate GVHD in other models and KGF has been shown to ameliorate thymic damage and lethality as a result of GVHD,22,23,36 KGF + leuprolide acetate may represent a viable clinical approach to speeding immune reconstitution even in the setting of GVHD risk.
Higher export of naive T cells in KGF + leuprolide acetate-treated BMT recipients also correlated with a decrease in HP observed in secondary lymphoid organs of these mice. T cells derived from lymphopenia-induced peripheral expansion or expansion driven by exogenous cytokines generally result in expansion of a limited number of peripheral T-cell clones and can create “holes” in the TCR repertoire.5,54-56 In our studies, TCR Vβ diversity was not skewed by KGF and/or leuprolide acetate. Therefore, KGF + leuprolide acetate in the clinical BMT setting may speed reconstitution of a diverse naive peripheral T-cell compartment capable of responding to a wider array of pathogens after BMT.
Poor immune responsiveness among BMT recipients leads to suboptimal responses to immunization for extended periods of time.5,47 In myeloablated recipients of autologous hematopoietic grafts, KLH responses were decreased up to 16 months.47 Although all BMT groups mounted similar primary anti-KLH IgM responses (data not shown), indicating that B-cell function was sufficient, only KGF + leuprolide acetate provided maximal benefit for enhancing T:B cooperativity with higher total IgG1 and KLH-specific IgG1 antibody titers indicative of isotype switching mechanisms requiring CD4 T-cell help.57 The demonstration that pretreatment with KGF + leuprolide acetate improved the T cell–dependent antibody response suggests a potential for KGF and leuprolide acetate as adjunct to immunization in BMT recipients.
Low peripheral CD4 and CD8 T-cell numbers results in poor immune responsiveness and increased susceptibility to and severity of infection among BMT recipients.7,58 Likewise, we observed a severe defect in the ability of untreated murine BMT recipients to clear Lm from the liver, whereas KGF + leuprolide acetate-pretreated BMT recipients cleared Lm from these sites as efficiently as non-BMT control mice. Consistent with this defective clearance, untreated BMT recipients showed less than 50% of the total number of Lm-reactive CD8 T cells compared with non-BMT control mice and BMT recipients pretreated with KGF + leuprolide acetate after a secondary infection. This same trend was observed when analyzing the percentage of IFNγ+ cells within the Kb:OVA-reactive CD8 T-cell compartment (data not shown). Because adaptive immunity against many intracellular pathogens involve highly conserved T-cell responses,59-61 the enhanced Lm immunity would likely be extended to a wider spectrum of viral and intracellular bacterial pathogens that pose a serious threat to BMT recipients. In this regard, we have shown that KGF-treated nonhuman primate BMT recipients develop improved responses to a challenge with simian immunodeficiency virus compared with untreated BMT recipients.48 T-cell responsiveness to CD3 signals also has been shown to be drastically diminished after BMT compared with that in healthy control animals, and cytomegalovirus reactivation after BMT has been associated with dysfunctional antigen-specific CD8 T cells.58,62 In contrast, peripheral T cells from castrated mice are hyperresponsive to antigen and CD3/28 signaling early after castration, although this effect is lost by 7 weeks after castration.35 Although heightened sensitivity to antigenic stimulation of T cells after androgen blockade may have played a role in driving early peripheral T-cell reconstitution, it is unlikely to be a major contributor to improved CD8 T-cell responses to Lm late after BMT.
In summary, combined KGF and androgen blockade was highly effective in speeding thymic recovery and peripheral T-cell reconstitution after BMT. Treated BMT recipients were resistant to a pathogenic challenge and were capable, relatively early after engraftment, of T:B-cell cooperativity in generating antibody responses to a neoantigen. Because KGF and leuprolide acetate are being used as single agents in the context of human BMT, our data suggest that future clinical trials designed to determine the safety and efficacy of combining these agents for facilitating immune recovery after BMT.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Chris Contag (Stanford University, Stanford, CA) for kindly providing L monocytogenes strain 2C and Hamish Scott (Monash University, Victoria, Australia) for providing the anti-AIRE antibody. We thank Emily Goren and Luna Liu for excellent animal care, Ryan Fremming for performing the ELISA analysis of KLH immune serum, and Jason Gill, Daniel Gray, Jonathan Hardy, and SingSing Way for kindly providing ΔactALmOVA and LmOVA, helpful discussions, and experimental advice.
This work was supported by National Institutes of Health grants R01-HL073794, R01-HL55209, R01-A1057477-01, and R01-A1057477-01, by SNF 3100-68310.02, by the Children's Cancer Research Fund, and by a grant from the European Community 6th Framework Program Eurothymaid Integrated Project. We would like to acknowledge the use of the confocal microscope made available through a National Center for Research Resources Shared Instrumentation Grant (1-S10-RR16851).
National Institutes of Health
Authorship
Contribution: R.M.K. designed and performed research, analyzed and interpreted data, made the figures, and wrote the manuscript. S.L.H. performed research. A.P-M. provided advice and edited the manuscript. P.A.T. performed research. R.L.B. provided advice and edited the manuscript. G.A.H. advised on experimental design and edited the manuscript. B.R.B. designed research, advised on experimental design, and edited the manuscript.
Conflict-of-interest disclosure: R.L.B. is named as an inventor on patents pertaining to immune reconstitution after androgen blockade. The remaining authors declare no competing financial interests.
Correspondence: Dr Bruce R. Blazar, University of Minnesota, Room 460F, Cancer Center Research Building, 425 East River Road, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.