Abstract
Adenosine deaminase (ADA)–deficient severe combined immune deficiency (SCID) may be treated by allogeneic hematopoietic stem cell transplantation without prior cytoreductive conditioning, although the mechanism of immune reconstitution is unclear. We studied this process in a murine gene knockout model of ADA-deficient SCID. Newborn ADA-deficient pups received transplants of intravenous infusion of normal congenic bone marrow, without prior cytoreductive conditioning, which resulted in long-term survival, multisystem correction, and nearly normal lymphocyte numbers and mitogenic proliferative responses. Only 1% to 3% of lymphocytes and myeloid cells were of donor origin without a selective expansion of donor-derived lymphocytes; immune reconstitution was by endogenous, host-derived ADA-deficient lymphocytes. Preconditioning of neonates with 100 to 400 cGy of total body irradiation before normal donor marrow transplant increased the levels of engrafted donor cells in a radiation dose–dependent manner, but the chimerism levels were similar for lymphoid and myeloid cells. The absence of selective reconstitution by donor T lymphocytes in the ADA-deficient mice indicates that restoration of immune function occurred by rescue of endogenous ADA-deficient lymphocytes through cross-correction from the engrafted ADA-replete donor cells. Thus, ADA-deficient SCID is unique in its responses to nonmyeloablative bone marrow transplantation, which has implications for clinical bone marrow transplantation or gene therapy.
Introduction
Adenosine deaminase (ADA) deficiency causes 15% to 20% of human severe combined immunodeficiency (SCID), most notably resulting in a profound pan-lymphocytopenia.1 Without treatment, most SCID patients die within the first years of life as a result of viral or bacterial infections. The current standard of care for ADA-deficient SCID with a human leukocyte antigen (HLA)–matched sibling donor is bone marrow transplantation (BMT), without prior marrow cytoreductive conditioning.2,3 Allogeneic transplantation with unfractionated, whole marrow from an HLA-matched sibling into an unconditioned SCID recipient usually results in complete and enduring restoration of immunity. The exact mechanisms by which nonmyeloablative allogeneic transplantation leads to immune reconstitution in SCID are not fully understood. Typically, very low levels of donor cell engraftment in the bone marrow are present, but essentially normal levels of donor lymphocytes are found in the blood and central lymphoid sites.4-6 These observations have led to the concept of “selective engraftment/expansion” of genetically normal T lymphocytes or progenitors in SCID patients from the few donor stem or progenitor cells that engraft.
We used a murine model of ADA-deficient SCID to characterize the effects of the transplantation of congenic normal bone marrow. Knockout of the Ada gene in mice caused perinatal mortality from hepatocellular damage, but crossing-in an Ada transgene expressed exclusively in placental trophoblasts allowed survival through gestation.7-9 However, ADA is required for postnatal life as well, and the ADA-deficient pups die by the fourth week of life from severe pulmonary insufficiency.10,11 Treatment of ADA-deficient mice with enzyme replacement therapy (ERT) by chronic administration of a clinical preparation of pegylated bovine ADA (PEG-ADA) begun shortly after birth or by “in vivo gene therapy” using intravenous injection of a lentiviral vector expressing Ada into neonates will keep the mice alive for more than 6 months, with partial restoration of immunity.12,13 Mortellaro et al14 recently reported successful immune restoration in ADA-deficient mice by transplantation of bone marrow corrected by transduction with an ADA lentiviral vector, after fully cytoablative conditioning.
To model the transplantation of ADA-deficient SCID patients with HLA-matched sibling donors without the use of cytoreductive conditioning, we gave neonatal ADA-deficient mice transplants of intravenous infusion of normal donor bone marrow without cytoreductive treatment. These mice had prolonged survival, with significant immune restoration, but only low levels of donor cell engraftment. Unexpectedly, there was no selective expansion of donor lymphocytes, relative to myeloid engraftment, contrary to typical findings in SCID patients who received transplants without cytoreduction. These findings suggest that the mechanisms of immune restoration after nonmyeloablative transplantation in ADA-deficient SCID may be novel.
Methods
Mice
A murine model of ADA deficiency (background of 129/Sv and FVB/N) was generated and characterized by Blackburn et al.10 When ADA ERT was administered, weekly intramuscular injections of 300 U/kg of ADA-GEN (Enzon Pharmaceutical, Piscataway, NJ) were given. Mice were housed in accordance with Institutional Animal Care and Use Committee (Saban Research Institute at Childrens Hospital, Los Angeles, CA) and the National Institutes of Health guidelines. All animals were handled in laminar flow hoods and housed in micro-insulator cages in a pathogen-free colony.
Neonatal bone marrow transplantation
Neonatal BMT was described by Sands and Barker.15 Congenic normal (+/+) donor mice were killed with CO2 narcosis. Femurs and tibias were harvested and washed in sterile Hank balanced salt solution without phenol red. Under aseptic conditions, marrow was flushed using a 23-G needle and 1-mL syringe filled with Hank balanced salt solution and centrifuged for 10 minutes at 800g at 10°C and resuspended at 5.0 × 107 cells/mL in injectable 0.9% sodium chloride (Hospira, Lake Forest, IL). Neonates (1-3 days old) were injected via the superficial temporal vein with 100 μL (5.0 × 106 cells) using a 30-G needle. Pups were immediately returned to the dam. Genotyping was performed as described.7,13
Cytoablative conditioning
Neonatal mice received transplants either with or without prior cytoablative conditioning by total body irradiation (TBI) on the day of BMT (100, 200, or 400 cGy from a 137Cesium source or busulfan (Sigma-Aldrich, St Louis, MO) was administered to the pregnant dam (15 mg/kg) 18 days postcoitus.16
Chimerism
Chimerism of normal donor cells in the ADA-deficient mice was determined by quantitative real-time polymerase chain reaction (qPCR), measuring the uninterrupted Ada gene of donor cells against the background of the disrupted Ada gene in the knock-out mice. DNA was extracted from peripheral blood samples using PURGENE KIT (Gentra Systems, Minneapolis, MN) and resuspended in Tris-ethylenediaminetetraacetic acid (TE) buffer and from thymus, spleen, bone marrow, liver, and lung using proteinase K digestion followed by phenol/chloroform extraction and resuspended in TE.
A primer/probe set detecting the normal donor Ada allele spanned the Aat1 site in exon 5, the site of Ada gene disruption by insertion of the neomycin resistance gene: forward primer: tccctcttcctctctccacaca; reverse primer: cacagaatggaccggacctt; and the Tamra probe: FAM-tcacccctgatgacgttgtggatcttg-Tamra. Another primer/probe set was used to quantify the knock-out allele by measuring the neomycin resistance gene: forward primer: actgggcacaacagacaatcg; reverse primer: cctcgtcctgcagttcattca; and the Tamra probe: TET-aagaccgacctgtccggtgccc-Tamra. The primers were used at 400 nM and the probe at 50 nM, and all reactions were performed with 10 ng of template and Universal Master Mix (Applied Biosystems, Fullerton, CA) in the 7700 Sequence Detector under default conditions. All unknown samples were compared with a standard curve constructed from mixtures (100%, 70%, 50%, 30%, 10%, 3%, 1%, 0.3%, 0%) of homozygote normal (+/+) tail DNA diluted in homozygote ADA-deficient (−/−) tail DNA.
Analysis of recipients
Recipient mice were killed at 16 days, 60 days, and 240 days after neonatal BMT and perfused with 15 mL of phosphate-buffered solution. The thymus, spleen, liver, and lung were harvested, and cell suspensions were made by pressing tissue through a 70-μm sterile nylon cell strainer (BD Biosciences, San Jose, CA). Bone marrow was collected from femurs and tibias. Cell isolation and immunophenotype analysis were done as described.13 Proliferation was also assessed in response to stimulation with lipopolysacharride (LPS; 10 μg/mL; Sigma-Aldrich).17 B-cell function was also assessed in vivo, by vaccination with Pneumovax 23 (Merck, Whitehouse Station, NJ), 10 μ in 100 μL injectable 0.9% sodium chloride (Hospira).18 Preimmune blood samples were collected at 5 days before vaccination, and postimmune samples were harvested 8 days after vaccination. All blood samples were collected into heparinized tubes and centrifuged at 600g for 10 minutes. Sera were harvested and frozen at −20°C. Sera were analyzed by enzyme-linked immunosorbent assay (ELISA) to Pneumovax 23 as described.18
Neonatal bone marrow transplantation in γc-knockout mice
The γc (common cytokine receptor gamma) gene knockout mice (B6.129S4-Il2rgtm1Wjl/J) were purchased from The Jackson Laboratories (Bar Harbor, ME). Mice are on a C57/BL/6 strain background and are CD45.1 (Ly5.1) allotype. Normal marrow donors were C576/SJL (Ly5.2); γc-knock-out neonates were injected with 5 × 106 cells of unfractionated whole marrow, without cytoreductive conditioning. Donor chimerism was determined by flow cytometry using a phycoerythrin-conjugated anti–mouse CD45.1 monoclonal antibody (BD Biosciences PharMingen, San Diego, CA) to detect the CD45.1 donor cells.
ADA enzyme activity and substrate analysis
Mice were killed under anesthesia, and tissues were removed and frozen rapidly in liquid nitrogen. ADA enzyme activity assay was performed as described.12 Adenine nucleosides were extracted from frozen lungs using 0.4 N perchloric acid as described,19 and adenosine and deoxyadenosine were separated and quantified using reversed-phase high performance liquid chromatography according to established procedures.19 Adenosine levels were normalized to protein content, and values are given as nanomoles of adenosine or deoxyadenosine per milligram protein.
Statistical analysis
Survivorship analysis was performed using the Kaplan-Meier approach. Tests between groups were made using the log rank tests. Comparisons between treatment groups over time (or using different conditions) were made using a general linear model For the chimerism data, tissue types and treatments (normal, untreated ADA−/−, ADA−/− BMT, ADA−/− ERT, ADA+/+) were the independent variables along with all possible 2-way interactions. Initially, a main-effects stepwise procedure was used followed by inclusion of all additional 2-way interactions that reached statistical significance (P < .05).
Results
ADA-deficient (Ada−/−) mice were treated by intravenous infusion of ADA-replete bone marrow from congenic donors as a model of the clinical practice of nonablated transplantation from HLA-matched sibling donors for patients with SCID. Because of the early mortality in these Ada gene knockout mice without treatment, BMT was performed in the neonatal period to potentially rescue them from lethality without imposing other therapies (such as ERT) that could complicate analyses. Mice were treated with a single dose of unfractionated, freshly isolated bone marrow, injected intravenously (via the superficial temporal [facial] vein), between days 1 and 3 after birth, without cytoreduction. Whole litters, homozygous Ada−/− mice, and heterozygous Ada+/− mice (Ada−/− males × Ada+/− females), received transplants and were subsequently genotyped. Mice were analyzed at 16, 60, and 240 days after neonatal BMT. Mice that received transplants showed signs of multisystem correction. Survival for a cohort of mice that were maintained for at least 1 year after neonatal BMT without further intervention was more than 83% (n = 6).
Analysis of immune reconstitution
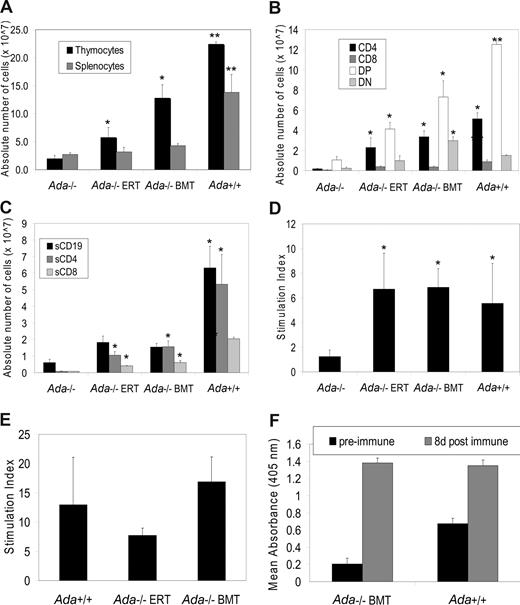
To characterize the effects of transplantation on immune reconstitution, mice were analyzed at 16 days after birth, before death of untreated ADA-deficient mice. The untreated ADA-deficient mice were severely lymphocyte-deficient in thymus and spleen at day 16, compared with the age-matched normal control mice (Figure 1A). In contrast, ADA-deficient mice that received transplants showed increased absolute numbers of thymocytes and splenocytes, with the number of thymocytes significantly higher after BMT than in the untreated Ada−/− mice (P < .001). Ada−/− mice treated with PEG-ADA ERT showed similar improvements in lymphocyte numbers.
Immunophenotype and lymphocyte function at 16 days after neonatal BMT. All mice were age-matched (16 days) in the experimental arms: (1) Ada−/− (n = 4), (2) Ada−/− ERT (n = 4), (3) Ada−/− BMT (n = 7), and (4) Ada+/+ (n = 5). *Significantly higher than untreated ADA-deficient mice (P < .001). **Significantly higher than untreated ADA-deficient mice (P < .007). Data are means plus or minus SEM. (A) Absolute numbers of thymocytes and splenocytes. (B) Absolute numbers in each thymocyte subpopulation (CD4+, CD8+, double-positive (DP): CD4+, CD8+, double-negative (DN): CD4−, CD8−) were calculated by multiplying the total numbers of cells collected from the thymus by the percentage of cells in each subpopulation. (C) Absolute numbers in each splenocyte subpopulation (CD4+, CD8+, CD19+) were calculated by multiplying the total numbers of cells collected from the spleen by the percentage of cells in each subpopulation. (D) Lymphocyte proliferative function was assessed by stimulating splenocytes with concanavalin A (conA) for 48 hours, pulsing with 3H-thymidine for 20 hours, and determining the stimulation index compared with cells not treated with ConA.
Immunophenotype and lymphocyte function at 16 days after neonatal BMT. All mice were age-matched (16 days) in the experimental arms: (1) Ada−/− (n = 4), (2) Ada−/− ERT (n = 4), (3) Ada−/− BMT (n = 7), and (4) Ada+/+ (n = 5). *Significantly higher than untreated ADA-deficient mice (P < .001). **Significantly higher than untreated ADA-deficient mice (P < .007). Data are means plus or minus SEM. (A) Absolute numbers of thymocytes and splenocytes. (B) Absolute numbers in each thymocyte subpopulation (CD4+, CD8+, double-positive (DP): CD4+, CD8+, double-negative (DN): CD4−, CD8−) were calculated by multiplying the total numbers of cells collected from the thymus by the percentage of cells in each subpopulation. (C) Absolute numbers in each splenocyte subpopulation (CD4+, CD8+, CD19+) were calculated by multiplying the total numbers of cells collected from the spleen by the percentage of cells in each subpopulation. (D) Lymphocyte proliferative function was assessed by stimulating splenocytes with concanavalin A (conA) for 48 hours, pulsing with 3H-thymidine for 20 hours, and determining the stimulation index compared with cells not treated with ConA.
Analysis of T lymphocyte subsets in the thymus from untreated ADA-deficient mice showed lower numbers of double-negatives (DN; CD4−/CD8−), double-positives (DP; CD4+/CD8+; P < .001), and single positives (CD4+, P < .007; and CD8+, P < .001) compared with healthy control mice (Figure 1B). The increase in the total thymocyte population in the BMT recipients was highlighted by significant increases in the numbers of single positive CD4+ (P < .007) and CD8+ (P < .007) cells and DP CD4+/CD8+ (P < .001) and DN CD4−/CD8− (P < .007) cells. Similar findings were seen in the ADA-deficient mice receiving ERT with PEG-ADA.
Analysis of lymphocytes in spleens showed the untreated ADA-deficient mice to have low numbers of B lymphocytes (CD19+) and T lymphocytes (CD4+ and CD8+) compared with normal mice (Figure 1C). Neonatal BMT led to a significant improvement in the number of splenic B lymphocytes (P < .001) but smaller increases in splenic T lymphocyte numbers. These responses were also seen in the mice treated with PEG-ADA ERT.
To assess immune function, the mitogenic responses of splenocytes were measured after stimulation with concanavalin A (conA) (Figure 1D). The ADA-deficient mice had very low proliferative responses to conA, and these were only slightly higher in 16-day-old mice treated with either neonatal BMT or PEG-ADA ERT.
For analysis at day 60, it was not possible to assess untreated Ada−/− mice because they uniformly died in the first month; therefore, the same data from the untreated ADA-deficient day 16 mice from Figure 1 are shown for comparison. At day 60, we observed that ADA-deficient mice treated by neonatal BMT had significantly higher levels of total thymocytes and splenocytes than untreated ADA-deficient mice historical controls (P < .001; Figure 2A), with thymocyte CD4+, CD4+, CD8+ (DP) and CD4−, CD8− (DN) populations significantly higher than untreated ADA-deficient mice (P < .001; Figure 2B). The thymocyte DN cell population after BMT was significantly higher than all treatment groups (P < .001), including Ada+/+ mice, and may suggest a partial block in maturation. By day 60, splenic B-cell numbers were not significantly improved in ADA-deficient mice after BMT, nor with ERT, in contrast to numbers observed at day 16. Splenic T lymphocytes (CD4+ and CD8+) were significantly higher (P < .001) than the untreated day 16 controls, but these were not at fully normal levels (Figure 2C). The mitogenic responses of splenic T cells to conA (Figure 2D) and splenic B cells to LPS (Figure 2E) from mice after BMT or PEG-ADA ERT did reach normal levels. Finally, in response to vaccination with Pneumovax 23, mice that received transplants produced similar amounts of immunoglobulin M (IgM) compared with ADA+/+ mice (Figure 2F). Thus, neonatal BMT without prior cytoreductive conditioning led to partial restoration of lymphocyte numbers and function at 2 months, similar to that achieved by chronic ERT.
Immunophenotype and lymphocyte function at 60 days after neonatal BMT. (A-D) The mice were age-matched (60 days) in the experimental arms: (1) Ada+/+ (n = 4), (2) Ada−/− BMT (n = 6), and (3) Ada−/− ERT (n = 7). Data from the Ada−/− with no treatment (at day 16) from Figure 1 are reproduced here as a historical control. *Significantly higher than untreated ADA-deficient mice (P < .001). **Significantly higher than untreated ADA-deficient mice or those treated with neonatal BMT or ERT (P < .001). Data are means plus or minus SEM. (A) Absolute numbers of thymocytes and splenocytes. (B) Absolute numbers in each thymocyte subpopulation (CD4+, CD8+, double-positive (DP): CD4+, CD8+, double-negative (DN): CD4−, CD8−). (C) Absolute numbers in each splenocyte subpopulation (CD4+, CD8+, CD19+) were calculated by multiplying the total numbers of cells collected from the organ by the percentage of cells in each subpopulation. (D) Lymphocyte proliferative function to conA was assessed as described in Figure 1. (E) Lymphocyte proliferative function was assessed by stimulating splenocytes with LPS for 48 hours, pulsing with 3H-thymidine for 20 hours, and determining the stimulation index compared with cells not treated with LPS: (1) Ada−/− BMT (n = 5), (2) Ada−/− ERT (n = 2), (3) Ada+/+ (n = 2). (F) IgM production in response to vaccination with Pneumovax 23 in vivo. Preimmune sera were collected before vaccination and compared with sera collected 8 days postvaccination. IgM production was assessed by ELISA. (1) Ada−/− BMT (n = 5), (2) Ada+/+ (n = 3).
Immunophenotype and lymphocyte function at 60 days after neonatal BMT. (A-D) The mice were age-matched (60 days) in the experimental arms: (1) Ada+/+ (n = 4), (2) Ada−/− BMT (n = 6), and (3) Ada−/− ERT (n = 7). Data from the Ada−/− with no treatment (at day 16) from Figure 1 are reproduced here as a historical control. *Significantly higher than untreated ADA-deficient mice (P < .001). **Significantly higher than untreated ADA-deficient mice or those treated with neonatal BMT or ERT (P < .001). Data are means plus or minus SEM. (A) Absolute numbers of thymocytes and splenocytes. (B) Absolute numbers in each thymocyte subpopulation (CD4+, CD8+, double-positive (DP): CD4+, CD8+, double-negative (DN): CD4−, CD8−). (C) Absolute numbers in each splenocyte subpopulation (CD4+, CD8+, CD19+) were calculated by multiplying the total numbers of cells collected from the organ by the percentage of cells in each subpopulation. (D) Lymphocyte proliferative function to conA was assessed as described in Figure 1. (E) Lymphocyte proliferative function was assessed by stimulating splenocytes with LPS for 48 hours, pulsing with 3H-thymidine for 20 hours, and determining the stimulation index compared with cells not treated with LPS: (1) Ada−/− BMT (n = 5), (2) Ada−/− ERT (n = 2), (3) Ada+/+ (n = 2). (F) IgM production in response to vaccination with Pneumovax 23 in vivo. Preimmune sera were collected before vaccination and compared with sera collected 8 days postvaccination. IgM production was assessed by ELISA. (1) Ada−/− BMT (n = 5), (2) Ada+/+ (n = 3).
Analysis of ADA enzyme activity and substrate concentration
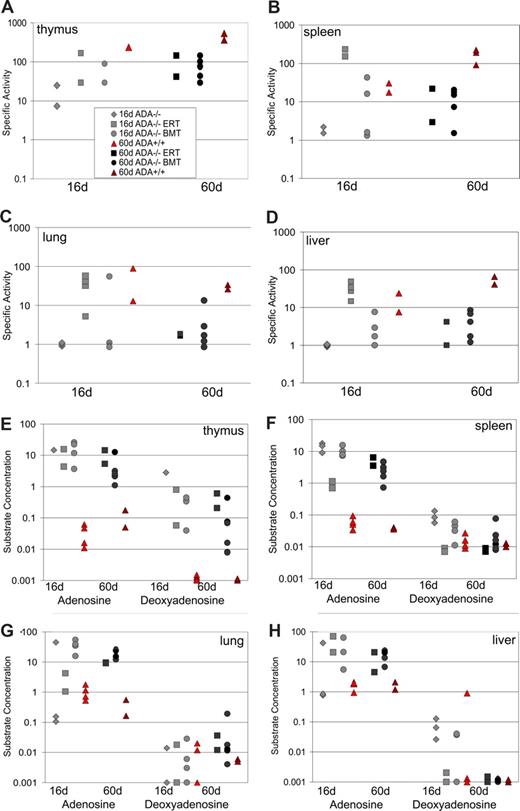
We measured the level of ADA enzyme activity in tissue samples from the mice at 16 and 60 days after birth (Figure 3A-D). In the normal control mice, the highest levels of ADA enzyme activity were seen in the thymus and the spleen (100-300 nmol/min per mg), with 10-fold lower levels in the liver and lung, and a further 10- to 30-fold lower level of activity (Figure 3A-D). ADA-deficient mice treated with neonatal BMT had 5- to 10-fold lower levels of ADA enzyme activity in all tissues analyzed compared with Ada+/+ mice at day 16 and day 60. By day 60, there was no difference in the levels achieved by both BMT and ERT in all tissues analyzed.
ADA specific activity and substrate levels after neonatal BMT. ADA enzyme specific activity (nmol of adenosine converted to inosine/min/mg protein) was determined in tissue lysates.12 Age-matched, Ada+/+ congenic controls were compared at day 16 (n = 4) and day 60 (n = 2) to untreated Ada−/− mice (n = 3) and Ada−/− treated with neonatal BMT at day 16 (n = 3) and day 60 (n = 6) and to Ada−/− mice receiving ERT with PEG-ADA at day 16 (n = 2) and at day 60 (n = 2). ADA specific activity in the thymus (A), spleen (B), lung (C), and liver (D). ADA substrate concentrations (nmol/mg protein) of adenosine and deoxyadenosine in thymus (E), spleen (F), lung (G), and liver (H).
ADA specific activity and substrate levels after neonatal BMT. ADA enzyme specific activity (nmol of adenosine converted to inosine/min/mg protein) was determined in tissue lysates.12 Age-matched, Ada+/+ congenic controls were compared at day 16 (n = 4) and day 60 (n = 2) to untreated Ada−/− mice (n = 3) and Ada−/− treated with neonatal BMT at day 16 (n = 3) and day 60 (n = 6) and to Ada−/− mice receiving ERT with PEG-ADA at day 16 (n = 2) and at day 60 (n = 2). ADA specific activity in the thymus (A), spleen (B), lung (C), and liver (D). ADA substrate concentrations (nmol/mg protein) of adenosine and deoxyadenosine in thymus (E), spleen (F), lung (G), and liver (H).
Concentrations for 2 ADA substrates, adenosine and deoxyadenosine, were also analyzed, and these levels were inversely related to the amount of ADA specific activity (Figure 3E,F). In general, however, concentrations of adenosine in the tissues analyzed from the Ada−/− mice after BMT were always between 100- and 1000-fold higher than the concentrations of adenosine measured in Ada+/+ mice with BMT or ERT with PEG-ADA. The concentrations of deoxyadenosine were greatly reduced in the spleen, liver, and lung; however, in the thymus, levels remained 100- to 1000-fold higher with either neonatal BMT or ERT compared with levels in Ada+/+ mice. Taken together, by day 60, BMT achieved levels of detoxification similar to those measured with ERT. However, despite the improvements in lymphocytes numbers seen, unexpectedly there was not a concordant increase in the level of ADA enzyme activity in the lymphoid organs.
Analysis of donor chimerism in ADA-deficient mice
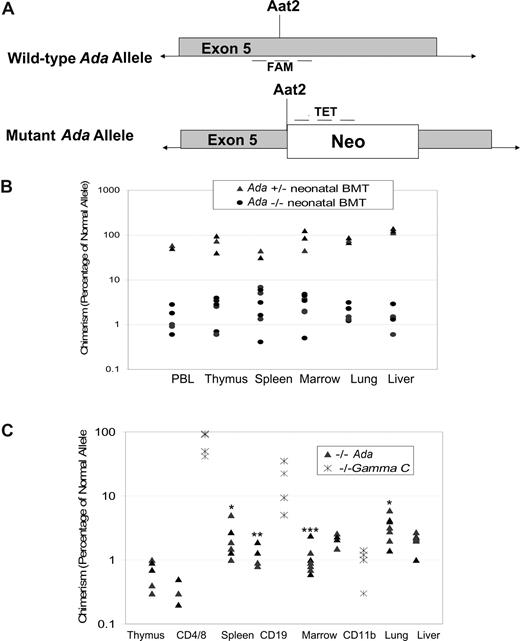
To assess the level of engraftment of normal donor cells in various organs, we developed a qPCR assay to measure the frequency of the normal, uninterrupted ADA gene in donor cells, but not the disrupted ADA gene in the knockout allele (Figure 4A). To validate this assay, DNA from some tissues was also analyzed for chimerism using qPCR for the neomycin resistance gene inserted into the knockout Ada allele, in addition to qPCR for the uninterrupted Ada allele. The amount of neomycin resistance gene was the reciprocal of the amount of uninterrupted Ada allele in all tissues tested (data not shown).
Determination of donor chimerism after neonatal BMT. (A) Schematic representation of qPCR approach for determining donor chimerism. A real-time quantitative PCR (qPCR) primer/probe set was designed to amplify the normal, wild-type Ada allele at the site of disruption in the mutant allele by insertion of the Neo gene at the unique Aat2 site in exon 5. A second set of primers/probe was designed to the neomycin resistance gene inserted at the Aat2 site in the mutant allele. (B) Chimerism at 16 days after neonatal BMT. Whole litters borne of heterozygous matings were injected with normal donor bone marrow within the first 1 to 3 days after birth; the individual genotypes were subsequently determined from tail DNA. Mice were killed at 16 days of age, and DNA from tissues was analyzed to measure donor chimerism. Both homozygous Ada−/− mice and heterozygous +/− mice were analyzed, with the heterozygote littermates (50% normal allele) serving as internal controls for the qPCR measurements. (C) Chimerism at 60 days after neonatal BMT. In ADA-deficient mice (n = 7), chimerism was determined at day 60 after neonatal BMT, analyzing DNA from tissue fragments as well as from the indicated cell subpopulations isolated with immunomagnetic beads from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells). γC gene knockout mice (Ly5.1) were treated by neonatal infusion of normal congenic bone marrow (Ly5.2, γC+/+) and killed after 60 days (n = 4). Cells from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells) were analyzed by flow cytometry to measure donor chimerism, based on the percentage of cells of the indicated cell subpopulations expression Ly5.2. Statistical analysis for Ada−/− only: *Significantly higher than thymus (P < .05), marrow (P < .05), and liver (P < .05). **Significantly higher than CD4 (P < .01). ***Significantly higher than CD19 (P < .006) and CD4 (P < .001).
Determination of donor chimerism after neonatal BMT. (A) Schematic representation of qPCR approach for determining donor chimerism. A real-time quantitative PCR (qPCR) primer/probe set was designed to amplify the normal, wild-type Ada allele at the site of disruption in the mutant allele by insertion of the Neo gene at the unique Aat2 site in exon 5. A second set of primers/probe was designed to the neomycin resistance gene inserted at the Aat2 site in the mutant allele. (B) Chimerism at 16 days after neonatal BMT. Whole litters borne of heterozygous matings were injected with normal donor bone marrow within the first 1 to 3 days after birth; the individual genotypes were subsequently determined from tail DNA. Mice were killed at 16 days of age, and DNA from tissues was analyzed to measure donor chimerism. Both homozygous Ada−/− mice and heterozygous +/− mice were analyzed, with the heterozygote littermates (50% normal allele) serving as internal controls for the qPCR measurements. (C) Chimerism at 60 days after neonatal BMT. In ADA-deficient mice (n = 7), chimerism was determined at day 60 after neonatal BMT, analyzing DNA from tissue fragments as well as from the indicated cell subpopulations isolated with immunomagnetic beads from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells). γC gene knockout mice (Ly5.1) were treated by neonatal infusion of normal congenic bone marrow (Ly5.2, γC+/+) and killed after 60 days (n = 4). Cells from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells) were analyzed by flow cytometry to measure donor chimerism, based on the percentage of cells of the indicated cell subpopulations expression Ly5.2. Statistical analysis for Ada−/− only: *Significantly higher than thymus (P < .05), marrow (P < .05), and liver (P < .05). **Significantly higher than CD4 (P < .01). ***Significantly higher than CD19 (P < .006) and CD4 (P < .001).
Engraftment of the donor marrow was evident by day 16 (Figure 4B). Levels of donor cell chimerism in peripheral blood lymphocytes and in thymus, spleen, liver, and lung were comparable among tissues and ranged between 0.5% and 9.0%. Heterozygote littermates were also analyzed as an internal control for the accuracy of the qPCR assay; these mice are expected to have at least 50% “donor chimerism” measured because of the presence of one normal Ada allele in their genomes and the small percentage of normal donors cells with 2 intact Ada alleles. The level of “donor chimerism” measured in the heterozygotes was 50% or more, with many mice having chimerism well above 50%, suggesting that there was some engraftment of donor-derived cells in the heterozygotes (Figure 4B).
Levels of donor chimerism at day 60 were highest in the spleen (P < .05) and lung (P < .002) compared with the thymus and marrow, ranging between 0.5% and 8.0% (Figure 4C). Cells sorted from the lymphoid tissues had similar levels of donor chimerism to those observed in the unsorted cell suspension from the whole organs (CD4+ and CD8+ thymocytes, 0.2%-0.5%; CD19+ B splenocytes, 0.8%-3.0%; CD11b+ myeloid bone marrow cells, 1.0%-3.0%). Most surprising, however, was the significantly higher level of donor chimerism seen in CD11b+ marrow cells (myeloid) compared with CD4+ thymocytes (P < .001) and CD19+ splenocytes (P < .006). The levels of donor chimerism in CD19+ cells and total splenocytes was also higher than that of the CD4+ thymocytes (P < .01).
Analysis of donor chimerism in γc gene knockout mice after neonatal bone marrow transplantation
The absence of selective donor engraftment or expansion of ADA-replete lymphocytes in the thymus and spleen, compared with myeloid cells, was a surprising finding. We expected to observe that the reconstitution of thymocytes and splenocytes after neonatal BMT would be the result of selective expansion of normal donor-derived lymphocytes expressing ADA enzyme, from a relatively low level of stem cell (and hence myeloid) engraftment in the absence of cytoreduction.
To determine whether this lack of selective expansion was specific to the ADA-deficient SCID model, we performed an analogous experiment in which γc gene knockout mice, a model of human X-linked SCID, received a neonatal transplant of unprocessed congenic bone marrow without cytoreduction. Because the γc gene knockout is on the C57/BL6j strain background (in contrast to the mixed FV1 × 129 background of the Ada−/− mice), we could inject γc gene knockout SCID neonates (Ly5.1) with congenic marrow harvested from normal C57/SJL (Ly5.2) mice, without prior cytoreduction and use fluorescence-activated cell sorting analysis to determine donor chimerism (Ly5.2).
In the γc gene knockout mice, at day 60 after neonatal BMT, thymocytes (CD4+ and CD8+) were mostly donor-derived (50%-97% Ly5.2+), splenic B lymphocytes showed moderate donor chimerism (4%-35%), and myeloid cells showed a low level of donor chimerism (0.3%-1.0%; Figure 4C). This pattern in the γc gene knockout mice of selective donor engraftment and expansion of thymocytes and, to a lesser extent, of splenic B lymphocytes compared with myeloid cells, is the pattern expected for SCID recipients of BMT without cytoreduction. Thus, the pattern observed in the Ada gene knockout mice without selective donor lymphoid expansion is unique to that model.
Effects of cytoreduction on survival
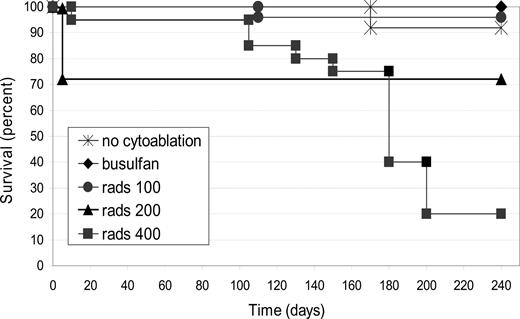
To examine the relationship between partial marrow cytoreduction and donor engraftment in the murine model of ADA-deficient SCID, submyeloablative dosages of TBI (100-400 cGy) were used to irradiate entire litters, 1 to 2 hours before neonatal BMT. As an alternative method for cytoreduction, busulfan (15 mg/kg) was administered to the pregnant dam (18 days postcoitus or ∼3 days before birth), an approach that has been reported to induce moderate cytoreduction in the neonates.16
The survival of mice receiving neonatal BMT without conditioning (0 cGy) or with radiation dosages of 100, 200, or 400 cGy TBI, as well as those mice receiving busulfan in utero, is shown (Figure 5). Survival was inversely related to the irradiation dose (P < .001). For those mice that received transplants but no TBI (0 cGy; n = 14), or 100 cGy TBI (n = 25), or in utero busulfan before BMT (n = 21), survival was more than 90%. Of mice receiving 200 cGy TBI (n = 22), 6 died within the first 2 weeks after TBI and BMT, but the remaining 16 survived until at least day 240 (73% survival), when they were killed. The group of mice receiving 400 cGy TBI (n = 20) had one death within the first 2 weeks but then had a continued progressive mortality over the next months for a final survival rate of 20% by day 240.
Survival after neonatal BMT with or without cytoreduction. Survival was recorded after ADA-deficient mice received neonatal BMT without cytoreduction (n = 14) or with cytoreduction using busulfan given to the pregnant dam (n = 21), or by total body irradiation (TBI) of 100 cGy (n = 25), 200 cGy (n = 22), or 400 cGy (n = 20). Survivorship was subjected to Kaplan-Meier analysis and shows a significant dose-response with increasing doses of TBI (P < .001).
Survival after neonatal BMT with or without cytoreduction. Survival was recorded after ADA-deficient mice received neonatal BMT without cytoreduction (n = 14) or with cytoreduction using busulfan given to the pregnant dam (n = 21), or by total body irradiation (TBI) of 100 cGy (n = 25), 200 cGy (n = 22), or 400 cGy (n = 20). Survivorship was subjected to Kaplan-Meier analysis and shows a significant dose-response with increasing doses of TBI (P < .001).
Chimerism at 240 days after neonatal bone marrow transplantation
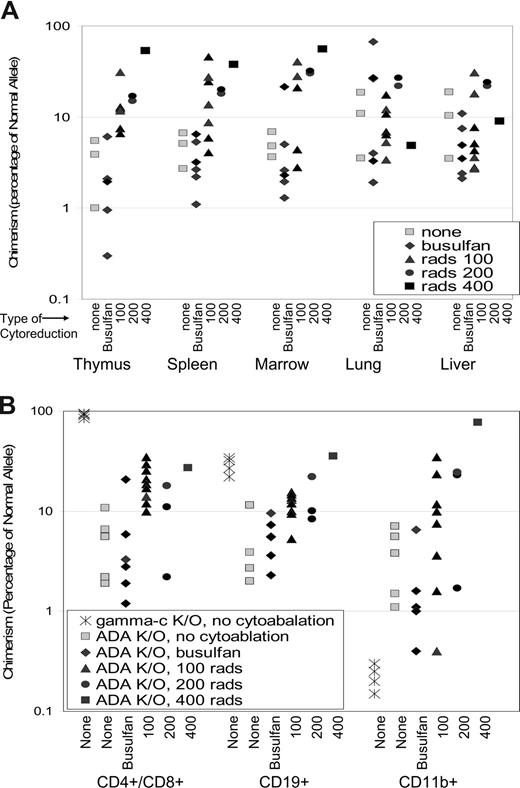
The levels of donor chimerism in mice receiving no cytoreduction or busulfan was between 1% and 15% in the thymus, spleen, bone marrow, lungs, and liver at 240 days, similar to those seen at 16 and 60 days after neonatal BMT (Figure 6A). However, the administration of 100, 200, or 400 cGy TBI did increase the level of donor chimerism in a dose-dependent manner in the thymus (P < .001), spleen (P < .03), and bone marrow (P < .001), but not in the lung or liver.
Donor chimerism after neonatal BMT and cytoreduction. (A) Chimerism was determined in tissues from ADA-deficient mice (no cytoreduction, n = 3; busulfan, n = 6; 100 cGy, n = 7; 200 cGy, n = 2; and 400 cGy, n = 1) after 240 days as described in Figure 5. Dose-response of chimerism with increasing TBI dosage is significant in thymus (P < .001), spleen (P < .05), and marrow (P < .001). (B) Chimerism was determined in cells from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells) isolated from ADA-deficient mice (no cytoreduction, n = 5; busulfan, n = 6; 100 cGy, n = 9; 200 cGy, n = 2; and 400 cGy, n = 1) as described in Figure 4B. γC gene knockout mice (Ly5.1) were treated by neonatal infusion of normal congenic bone marrow (Ly5.2, γC+/+) and killed after 200 days (n = 4). Cell populations were isolated and analyzed as described in Figure 4B. Statistical analysis for ADA−/− mice only: Dose-response is significant in CD4+ cells (P < .001), CD19+ cells (P < .001), and CD11b+ cells (P < .05).
Donor chimerism after neonatal BMT and cytoreduction. (A) Chimerism was determined in tissues from ADA-deficient mice (no cytoreduction, n = 3; busulfan, n = 6; 100 cGy, n = 7; 200 cGy, n = 2; and 400 cGy, n = 1) after 240 days as described in Figure 5. Dose-response of chimerism with increasing TBI dosage is significant in thymus (P < .001), spleen (P < .05), and marrow (P < .001). (B) Chimerism was determined in cells from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells) isolated from ADA-deficient mice (no cytoreduction, n = 5; busulfan, n = 6; 100 cGy, n = 9; 200 cGy, n = 2; and 400 cGy, n = 1) as described in Figure 4B. γC gene knockout mice (Ly5.1) were treated by neonatal infusion of normal congenic bone marrow (Ly5.2, γC+/+) and killed after 200 days (n = 4). Cell populations were isolated and analyzed as described in Figure 4B. Statistical analysis for ADA−/− mice only: Dose-response is significant in CD4+ cells (P < .001), CD19+ cells (P < .001), and CD11b+ cells (P < .05).
Donor chimerism was also measured in cells sorted from the thymus (CD4+ and CD8+ T lymphocytes), the spleen (CD19+ B lymphocytes), and the bone marrow (CD11b+ myeloid cells) (Figure 6B). In mice receiving no cytoreduction or in utero busulfan, donor chimerism in purified cell populations was similar to the level of donor chimerism seen in the bulk cell suspensions (0.6%-10%; Figure 6A). The administration of 100, 200, or 400 cGy did increase donor chimerism in a dose-dependent manner in T lymphocytes (P < .001), B lymphocytes (P < .001), and in myeloid cells (P < .05). As in the nonconditioned transplantations, the donor chimerism levels in lymphoid and myeloid cells were equivalent. The one surviving mouse that received 400 cGy TBI had further increases of donor chimerism seen in all analyzed cell lineages with between 30% and 80% donor chimerism. Thus, cytoreductive conditioning with TBI, but not with busulfan as used, led to increased levels of donor engraftment without a selective advantage in the lymphoid compartment. These findings are in marked contrast to donor chimerism measured in γc-gene knockout mice 200 days after neonatal BMT without cytoreduction. CD4+/CD8+ cells were almost 100% donor derived, and CD19+ cells were 30% to 55% donor derived, whereas the CD11b had less than 0.5% donor chimerism (Figure 4C).
Analysis of immune reconstitution in response to cytoreductive conditioning
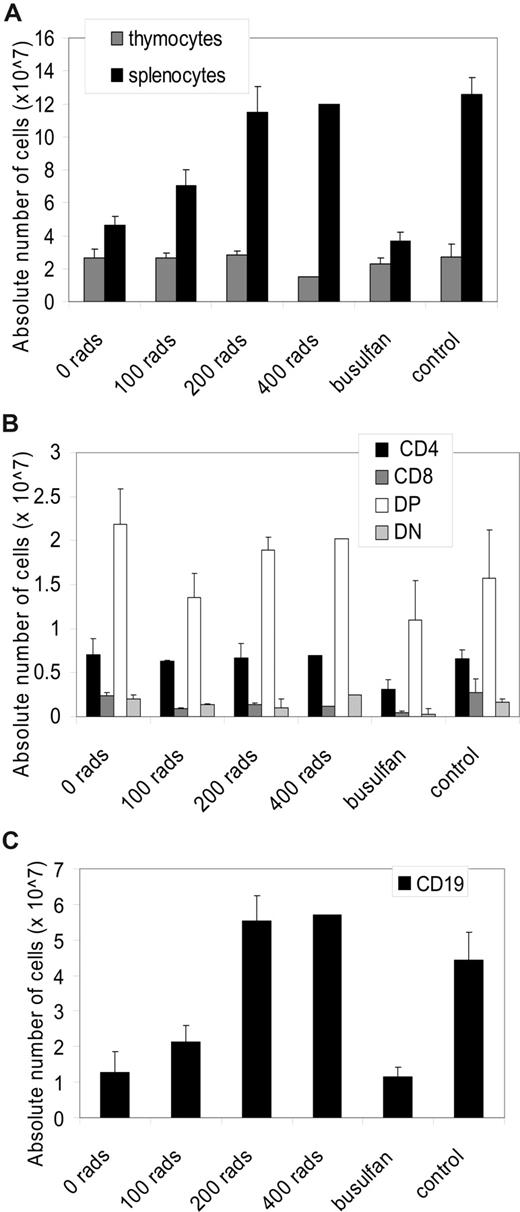
There was not a significant dose-response with increasing dose of TBI in the absolute number of T lymphocytes or T lymphocyte subsets (Figure 7A,B). The absolute numbers of splenocytes (P < .001), as well as the absolute number of CD19+ splenocytes (P < .001), did show a significant increase (Figure 7A,C). Furthermore, 200 cGy or more resulted in full restoration of the absolute numbers of splenocytes and CD19+ cells compared with Ada+/+ mice.
Immunophenotype after cytoreduction and neonatal BMT. All mice were age-matched (200-240 days) in the experimental arms: ADA-deficient mice (no cytoreduction, n = 3; 100 cGy, n = 7; 200 cGy, n = 2; and 400 cGy, n = 1; busulfan, n = 6) after 240 days. (A) Absolute numbers of thymocytes and splenocytes. Data are means plus or minus SEM. Significant dose-response in splenocytes (P < .001). (B) Absolute numbers in each thymocyte subpopulation (CD4+, CD8+, double-positive (DP): CD4+, CD8+, double-negative (DN): CD4−, CD8−) were calculated by multiplying the total numbers of cells collected from the thymus by the percentage of cells in each subpopulation. (C) Absolute numbers in each splenocyte subpopulation (CD4+, CD8+, CD19+) were calculated by multiplying the total numbers of cells collected from the spleen by the percentage of cells in each subpopulation. Significant dose-response in CD19+ (P < .001).
Immunophenotype after cytoreduction and neonatal BMT. All mice were age-matched (200-240 days) in the experimental arms: ADA-deficient mice (no cytoreduction, n = 3; 100 cGy, n = 7; 200 cGy, n = 2; and 400 cGy, n = 1; busulfan, n = 6) after 240 days. (A) Absolute numbers of thymocytes and splenocytes. Data are means plus or minus SEM. Significant dose-response in splenocytes (P < .001). (B) Absolute numbers in each thymocyte subpopulation (CD4+, CD8+, double-positive (DP): CD4+, CD8+, double-negative (DN): CD4−, CD8−) were calculated by multiplying the total numbers of cells collected from the thymus by the percentage of cells in each subpopulation. (C) Absolute numbers in each splenocyte subpopulation (CD4+, CD8+, CD19+) were calculated by multiplying the total numbers of cells collected from the spleen by the percentage of cells in each subpopulation. Significant dose-response in CD19+ (P < .001).
Discussion
Since 1968, allogeneic hematopoietic stem cell transplantation (HSCT) for infants with SCID from HLA-matched sibling donors, by infusing unprocessed bone marrow without prior cytoreductive conditioning, has been life-saving to prevent the lethality of SCID.2,3 These patients who received transplants generally develop protective immune function with a donor-derived immune system, despite low levels of donor engraftment, based on chimerism analyses of myeloid or progenitor cells.4-6 Using a murine model of ADA-deficient SCID, we report a similar profound improvement in survival by transplanting unprocessed congenic bone marrow into recipients without prior cytoreductive conditioning. The nonablative BMT also prevented the mouse-specific complication of pulmonary death in infancy. The mice that received transplants had significant immune reconstitution. Strikingly, we observed that the lymphoid compartment was not mostly reconstituted by donor-derived ADA-replete cells but by endogenous, host-derived ADA-deficient cells.
Immune reconstitution was evident at the earliest time analyzed after neonatal transplantation, (postnatal day 16), when direct comparisons could be made to age-matched untreated ADA-deficient controls. However, the degree of immune reconstitution that developed in mice was lower than in normal age-matched controls; the absolute numbers of thymocytes and splenocytes were reduced, and there was an absence of a proliferative response to ConA, indicating poor functional activity. The numbers of thymocytes and splenocytes remained subnormal when the neonatal BMT recipients were analyzed at 2 and 8 months of age. Despite their low number, splenocytes responded to stimulation with conA and LPS; furthermore, BMT recipients were capable of producing IgM in response to vaccination with Pneumovax 23. Thus, although multiple indices of immune reconstitution were significantly improved by the nonconditioned BMT, they remained subnormal for age. The protective benefits from this partial degree of immune reconstitution are unclear because the mice are fostered in a pathogen-free environment. Patients with ADA deficiency on PEG-ADA ERT appreciate immune reconstitution that is highly protective from infections, despite significantly subnormal numbers of blood T, B, and NK lymphocytes.20-22 The presence of the intact Ada gene in the donors and the Ada gene knockout allele in recipients allowed donor chimerism to be measured over a wide range and with high accuracy using real-time PCR to quantify the normal donor Ada allele. In addition, ADA enzyme activity and reduction of cytotoxic substrates, adenosine and deoxyadenosine, in tissues and cells also served as a measure of donor cell engraftment. The levels of engraftment of myeloid cells (and presumably hematopoietic stem cells) ranged from 0.3% to 3.0% in the marrow and spleen, similar to the levels of donor engraftment reported in human SCID patients receiving nonconditioned transplants.4-6 The neonatal mice (∼2 g) received 5 × 106 nucleated bone marrow cells, representing a cell dosage of 2.5 × 109 cells/kg. In clinical allogeneic HSCT for SCID, the typical bone marrow cell dosage may be 1 to 2 orders of magnitude lower (eg, 5 × 107 to 108 per kg). However, multiple factors may underlie the donor engraftment levels besides marrow cell dosage, including species-specific marrow stem cell content and the ages of the recipients (neonatal mice vs infant humans).
The finding that 0.3% to 3.0% of the T and B cells in the thymus, spleen, and blood were donor derived, based on measurements of ADA enzyme activity relatively uncorrected substrate levels (especially in the thymus) and low donor cell chimerism, indicates that more than 90% of the lymphocytes in the mice that received transplants are host-derived, ADA-deficient cells. In patients receiving PEG ADA ERT, the development and survival of ADA-deficient lymphocytes occur, although the mechanism is probably indirect, with the PEG protein primarily circulating in plasma, leading to systemic lowering of adenine nucleotide pools.12 The reconstitution with ADA-deficient cells in the mice that received transplants suggests that trans-rescue occurred; the total mass of ADA-replete donor cells must produce sufficient ADA enzymatic activity to allow survival of the ADA-deficient lymphoid cells, presumably by lowering the levels of toxic deoxyadenosine metabolites, either systemically or locally in lymphoid organs.
We reported a similar pattern of immune reconstitution with mostly endogenous ADA-deficient lymphocytes from direct in vivo Ada gene delivery using a single intravenous injection of a lentiviral vector in ADA-deficient neonatal mice.13 We found there was minimal transduction of hematopoietic stem cells or lymphocytes; ADA expression from the vector was highest in the liver and lungs, which provided a systemic source of ADA enzyme or functioned as a metabolic sink for adenine nucleotides.
In contrast to expectations from nonablated BMT for SCID patients, there was not a higher level of donor-derived lymphoid cells compared with myeloid cells. This surprising finding demonstrates that, in this murine model of ADA-deficient SCID, there is not a selective advantage for development and survival of normal lymphoid cells. As a comparison, similar nonconditioned neonatal transplantations of normal bone marrow were performed with a different murine model of SCID (X-linked SCID from γc gene knockout). These recipients showed the “classical” engraftment/reconstitution pattern of SCID with selective expansion of donor-derived lymphocytes. The genetic defect in intracellular signaling in response to cytokines in γc deficiency would be expected to be cell-intrinsic and therefore not amenable to trans-rescue; only genetically normal lymphoid progenitor cells expressing the normal γc gene would be expected to develop. In contrast, the donor-derived ADA replete hematopoietic and lymphoid cells may be acting, in effect as in vivo ERT, by provision of ADA enzyme activity that promotes the development of endogenous lymphoid progenitors, with no disadvantage relative to donor lymphocytes.
We compared the findings in ADA-deficient mice receiving neonatal BMT without any conditioning to those in mice receiving low dosages of TBI or systemic busulfan before HSCT. We observed that relatively low dosages of TBI increased donor chimerism approximately 10-fold over that in the nonconditioned recipients, reaching levels as high as 10% to 30%. The increases of donor chimerism were TBI-dosage dependent for the hematopoietic and lymphoid organs (bone marrow, spleen, and thymus), but not in the solid organs (liver and lung). The highest dosage of TBI given to neonatal mice (400 cGy) was highly toxic, with progressive death of recipients. The lowest dosage of TBI that was used (100 cGy) was well tolerated with survival equivalent to that achieved in nonconditioned mice, yet with significantly higher engraftment levels. It is not known whether the modest improvement in engraftment would outweigh potential late effects from the neonatal radiation. Busulfan, as administered, had no discernible effects, with all measured outcome values of engraftment and immune reconstitution being indistinguishable from those in nonconditioned mice. Differences between the strains of mice used may explain the lack of effect from busulfan we found, in contrast to those reported by Yoder et al.16
Recovery of B-cell activity with production of normal quantities and qualities of immunoglobulins remains a major clinical problem after allogeneic HSCT for SCID. As many as 50% of patients may not produce adequate levels of antibodies, despite adequate T-cell reconstitution, and require chronic intravenous immunoglobulin therapy.3 In one retrospective analysis, SCID patients who received cytoreduction before allogeneic HSCT had a higher rate of adequate B-cell recovery than nonconditioned patients.23 We observed that B-cell numbers were normal after HSCT after 100 to 200 cGy TBI, but not without TBI, although the mice were capable of producing IgM in response to Pneumovax 23 vaccination. Future studies will need to address B-cell reconstitution in more detail.
The degree to which there is selective donor lymphoid expansion compared with myeloid engraftment in ADA-deficient SCID patients after allogeneic HSCT has not been reported, with all publications on posttransplant chimerism describing patients with other genetic forms of SCID. A selective advantage exists in ADA-deficient SCID patients who have overall low amounts of ADA-expressing cells, based on rare case reports of ADA-deficient SCID patients who had a spontaneous reversion of the ADA mutation in a stem or progenitor cell with subsequent increase in ADA-expressing T cells, as well as observations from patients who underwent gene therapy targeting T cells or umbilical cord blood CD34+ cells followed by intermittent treatment and withdrawal of ADA ERT.24-28 In patients receiving gene therapy using autologous hematopoietic stem cells transduced by a retroviral vector with cytoreductive marrow conditioning using moderate dosages of busulfan, the level of gene-corrected T lymphocytes approached 75% to 100%, whereas myeloid cells with the inserted Ada gene were 10- to 100-fold lower, indicating the occurrence of a selective lymphoid expansion.29,30 Nevertheless, in mice, we observed that a moderate level of chimerism of normal donor cells produced sufficient ADA enzyme to lead to trans-rescue of ADA-deficient lymphocytes, in effect blunting any potential selective advantage of the ADA-replete lymphocytes. It remains to be determined whether our findings predict those that occur in nonconditioned ADA-deficient patients or if differences in purine metabolic pathways between mice and humans lead to different outcomes. The potential for trans-rescue, without comprehensive correction, may lead to novel clinical applications and a better understanding of the pathophysiology of ADA-deficient SCID.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yujin Zhang and Ling Song for their technical assistance in this study.
This work is in memory of Rocco L. Carbonaro, father of D.A.C., and Eugene L. Kohn, father of D.B.K.
This work was supported by National Institutes of Health grant HL073104-01 (D.B.K.), including the Cores for Animal Care, Research Vectors, and Cell & Tissue Analysis, and grant DK46207 (R.E.K.).
National Institutes of Health
Authorship
Contribution: D.A.C., X.J., D.C., X-J.Y., D.C.S., T.M., R.E.K., and M.R.B. performed experiments; F.D. did the statistical analysis; D.A.C. and D.B.K. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald B. Kohn, Division of Research immunology/Bone Marrow Transplantation, Saban Research Institute of Childrens Hospital of Los Angeles, MS 62, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: dkohn@chla.usc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal