The phosphatidylinositol 3-kinase (PI3K)/Akt and mTORC1 pathways are frequently activated, representing potential therapeutic targets in acute myeloid leukemia (AML). In 19 AML samples with constitutive PI3K/Akt activation, the rapamycin derivative inhibitor everolimus (RAD001) increased Akt phosphorylation. This mTOR C1-mediated Akt up-regulation was explained by an insulin-like growth factor-1 (IGF-1)/IGF-1 receptor autocrine loop: (1) blast cells expressed functional IGF-1 receptors, and IGF-1-induced Akt activation was increased by RAD001, (2) a neutralizing anti-IGF-1R α-IR3 monoclonal antibody reversed the RAD001-induced Akt phosphorylation, and (3) autocrine production of IGF-1 was detected in purified blast cells by quantitative reverse transcription-polymerase chain reaction and immunofluorescence. This RAD001-induced PI3K/Akt up-regulation was due to an up-regulated expression of the IRS2 adaptor. Finally, we observed that concomitant inhibition of mTORC1 and PI3K/Akt by RAD001 and IC87114 induced additive antiproliferative effects. Our results suggest that dual inhibition of the mTORC1 complex and the IGF-1/IGF-1R/PI3K/Akt pathway in AML may enhance the efficacy of mTOR inhibitors in treatment of this disease.
Introduction
Acute myeloid leukemia (AML) is associated with a low survival rate. Therefore, new therapeutic strategies may prove effective in addition to chemotherapy
The deregulation of several signal transduction pathways is a common feature in AML. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is activated in AML blast cells.1,,,–5 We showed previously that the p110 δ isoform of PI3K is a potential therapeutic target and that the p110 δ-selective inhibitor IC87114 blocks AML cell proliferation.6,7 The mammalian target of rapamycin (mTOR) is activated in response to stimuli activating the PI3K pathway,8 and mTORC1 inhibitors may have therapeutic value in the treatment of patients with AML9,10 ; however, rapamycin alone led to modest antileukemic activity.10 We investigated the effect of the mTORC1 inhibitor RAD001 (Everolimus; Novartis Pharmaceuticals, Basel, Switzerland) in 19 bone marrow samples from patients with newly diagnosed AML. We show that mTORC1 inhibition with RAD001 increased Akt activating phosphorylation, as a result of up-regulated expression of the IRS-2 protein adaptor that promoted insulin-like growth factor-1 (IGF-1) /IGF-1R signaling. Moreover, we show that the enhanced activation of Akt was dependent on the IGF-1 autocrine production by leukemic cells. Our results provide a rationale for combined inhibition of both mTORC1 and PI3K/Akt pathways in AML, and we observed an additive effect of both RAD001 and IC87114 on blast cell proliferation.
Methods
Bone marrow (BM) samples were obtained from 19 patients with newly diagnosed AML, all treated in the AML2001 chemotherapy trial, initiated by the French Multicenter Group, Groupe Ouest Est des Leucémies et Autres Maladies du Sang (GOELAMS). All biologic studies were approved by the GOELAMS Institutional Review Board, and signed informed consent was obtained in accordance with the Declaration of Helsinki. The clinical characteristics of patients are summarized in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). All patients presented a constitutive activation of Akt at diagnosis, as reported previously.5 Cells were incubated with the following inhibitors: RAD001 (Everolimus) kindly provided by Novartis, IC87114 provided by ICOS (Bothell, WA), LY294002 from Sigma (St Louis, MO), and αIR3 from Calbiochem (La Jolla, CA). IGF-1 was from Sigma. Expression of total and phosphorylated proteins was detected by Western blot (WB) analysis as reported previously.7 The references for the antibodies are summarized in Table S2. Immunofluorescence staining for IGF-1 expression and quantitative reverse transcription-polymerase chain reaction (RT-PCR) were performed on blast cells, sorted according to their CD45low expression and side scatter (see Document S1 for details). Blast cell proliferation was assessed by [3H]thymidine incorporation as reported previously.7
Results and discussion
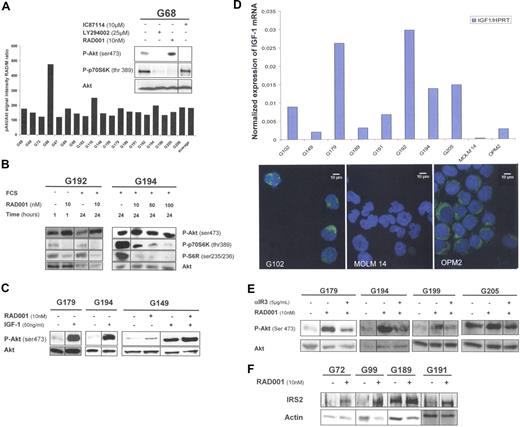
This study was conducted in an attempt to inhibit Akt and mTORC1 phosphorylation in AML samples. We compared the effect of 3 kinase inhibitors, (1) IC87114,7 (2) LY294002, and (3) the rapamycin derivative RAD001, in fresh BM blast cells from patients with AML presenting constitutive PI3K activation.4,7 We observed that IC87114 and LY294002 totally suppressed Akt phosphorylation, whereas, in contrast, RAD001 substantially increased Akt activation. The enhancement of Akt phosphorylation in the presence of RAD001 (mean 86%) was detected in all 19 AML samples (Figure 1A). Furthermore, increased Akt activation was maintained after a 24-hour incubation with 10 nmol/L RAD001 (Figure 1B, sample G192) and also with RAD001 at higher concentrations (Figure 1B, sample G194). Thus, RAD001 treatment led to an increased Akt activation in all AML samples presenting constitutive PI3K activation. Because mTORC1 has been reported to inhibit Insulin/IGF-1 signaling,11 we hypothesized that the IGF-1/IGF-1R pathway could play a role in the RAD001-induced Akt activation in AML cells. We found that exogenous IGF-1 stimulation increased Akt phosphorylation and that RAD001 increased IGF-1-stimulated Akt phosphorylation in AML samples (Figure 1C). Because BM cells were cultured in serum-free medium and harbored a functional IGF-1R, we analyzed the production of IGF-1 inside the leukemic cells. To that end, we purified the blast cell population by flow cytometry cell sorting as described previously.4 IGF-1 expression was detected at the mRNA level, and the IGF-1 protein was detected by immunofluorescence analysis in all samples tested (Figure 1D). From these data, we concluded that an autocrine production of IGF-1 was constantly observed in primary AML blast cells. We inhibited the interaction between IGF-1 and its receptor with a neutralizing monoclonal antibody αIR3, directed against the α-subunit of IGF-1 receptor.12 As shown in Figure 1E, blocking the IGF-1/IGF-1R interaction reversed the RAD001-induced increase of Akt phosphorylation. These data strongly suggest that the enhancement of Akt phosphorylation in response to RAD001 treatment involved the IGF-1R in leukemic cells.
mTORC1 inhibition with RAD001 induces Akt activation in primary AML samples by activation of the IGF-1/IGF1-R signaling pathway, dependent on an IGF-1 autocrine loop. (A) Bone marrow (BM) blast cells from 19 patients with AML were starved for 4 hours in cytokine and serum-free minimal essential medium (MEM), with or without the following kinase inhibitors: 25 μmol/L LY294002, 10 μmol/L IC87114, or 10 nmol/L RAD001, added during the last hour of starvation. Western blots (WBs) were performed with anti-phospho-Akt (ser 473), anti-phospho-p70S6K (thr 389), and anti-Akt antibodies. Quantification of phospho-Akt signal intensity was normalized to Akt signal intensity. Each histogram of the graph represents the phospho-Akt signal intensity in RAD001-treated blast cells, expressed as percentage of signal intensity in control cells (M, medium without inhibitors). (B) BM blast cells from patients G192 and G194 were collected after Ficoll-Hypaque density gradient separation, then washed once in PBS buffer. Blast cells (5 × 105/mL) from patient G192 were starved for 4 hours in cytokine and serum-free MEM, then incubated without or with 10 nmol/L RAD001 for 1 hour. In independent experiments, 5 × 105/mL blast cells from patients G192 and G194 were incubated without or with RAD001 for 24 hours, in α-MEM with 10% fetal calf serum (FCS). RAD was used at 10 nmol/L for sample G192, and at 10, 50, or 100 nmol/L for sample G194. WBs were performed with anti-phospho-Akt (ser 473), anti-phospho-p70S6 kinase (thr 389), anti-phospho-S6R (Ser 235/236), and anti-Akt antibodies. (C) RAD001 increases IGF-1-stimulated Akt phosphorylation in AML blast cells. BM blast cells from patients G179, G194, and G149 were starved for 4 hours in serum-free MEM, without or with 10 nmol/L RAD001, then stimulated or not with 50 ng/mL IGF-1 for 10 minutes. WBs were performed with anti-phospho-Akt (Ser 473) and anti-Akt antibodies. (D) AML blast cells express IGF-1 at the RNA and protein level. BM blast cells from 8 patients were highly purified by flow cytometry cell sorting according to CD45low expression and side scatter. The MOLM-14 AML cell line was used as negative control, and the OPM2 myeloma cell line was used as a positive control for IGF-1 expression. IGF-1 mRNA expression was quantified in the purified blast cells by quantitative RT-PCR, and their levels were expressed relative to HPRT (hypoxanthine phosphoribosyl transferase) mRNA levels. Similar results were obtained using another housekeeping gene, UBCV (C ubiquitin; data not shown). Immunofluorescence staining was performed on purified blasts for the same 8 patients mentioned above, and on MOLM-14 and OPM2 cell lines, using a mouse monoclonal anti-IGF-1 antibody and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Images obtained from the representative patient G102 and from MOLM-14 and OPM2 cell lines are presented. (E) The mTORC1-mediated positive feedback on PI3K/Akt activity involves the IGF-1 receptor. BM blast cells from patients G179, G194, G199, and G205 were starved for 4 hours in serum-free MEM. Cells were incubated in the following conditions: medium alone, 10 nmol/L RAD001 for 1 hour, 10 nmol/L RAD001 for 1 hour plus, and 5 μg/mL αIR3 (added 30 minutes before RAD001). WBs were performed with anti-phospho-Akt (Ser 473) and anti-Akt antibodies. αIR3 is a blocking mouse monoclonal antibody directed against the alpha subunit of the IGF-1 receptor and was obtained from Calbiochem. (F) mTORC1 inhibition by RAD001 increases the expression of the IRS2 adaptor. BM blast cells from patients G72, G99, G189, and G191 were starved for 4 hours in serum-free MEM then incubated with or without RAD001 (10 nmol/L) for 1 hour. WBs were performed with anti-IRS2 and anti-actin antibodies.
mTORC1 inhibition with RAD001 induces Akt activation in primary AML samples by activation of the IGF-1/IGF1-R signaling pathway, dependent on an IGF-1 autocrine loop. (A) Bone marrow (BM) blast cells from 19 patients with AML were starved for 4 hours in cytokine and serum-free minimal essential medium (MEM), with or without the following kinase inhibitors: 25 μmol/L LY294002, 10 μmol/L IC87114, or 10 nmol/L RAD001, added during the last hour of starvation. Western blots (WBs) were performed with anti-phospho-Akt (ser 473), anti-phospho-p70S6K (thr 389), and anti-Akt antibodies. Quantification of phospho-Akt signal intensity was normalized to Akt signal intensity. Each histogram of the graph represents the phospho-Akt signal intensity in RAD001-treated blast cells, expressed as percentage of signal intensity in control cells (M, medium without inhibitors). (B) BM blast cells from patients G192 and G194 were collected after Ficoll-Hypaque density gradient separation, then washed once in PBS buffer. Blast cells (5 × 105/mL) from patient G192 were starved for 4 hours in cytokine and serum-free MEM, then incubated without or with 10 nmol/L RAD001 for 1 hour. In independent experiments, 5 × 105/mL blast cells from patients G192 and G194 were incubated without or with RAD001 for 24 hours, in α-MEM with 10% fetal calf serum (FCS). RAD was used at 10 nmol/L for sample G192, and at 10, 50, or 100 nmol/L for sample G194. WBs were performed with anti-phospho-Akt (ser 473), anti-phospho-p70S6 kinase (thr 389), anti-phospho-S6R (Ser 235/236), and anti-Akt antibodies. (C) RAD001 increases IGF-1-stimulated Akt phosphorylation in AML blast cells. BM blast cells from patients G179, G194, and G149 were starved for 4 hours in serum-free MEM, without or with 10 nmol/L RAD001, then stimulated or not with 50 ng/mL IGF-1 for 10 minutes. WBs were performed with anti-phospho-Akt (Ser 473) and anti-Akt antibodies. (D) AML blast cells express IGF-1 at the RNA and protein level. BM blast cells from 8 patients were highly purified by flow cytometry cell sorting according to CD45low expression and side scatter. The MOLM-14 AML cell line was used as negative control, and the OPM2 myeloma cell line was used as a positive control for IGF-1 expression. IGF-1 mRNA expression was quantified in the purified blast cells by quantitative RT-PCR, and their levels were expressed relative to HPRT (hypoxanthine phosphoribosyl transferase) mRNA levels. Similar results were obtained using another housekeeping gene, UBCV (C ubiquitin; data not shown). Immunofluorescence staining was performed on purified blasts for the same 8 patients mentioned above, and on MOLM-14 and OPM2 cell lines, using a mouse monoclonal anti-IGF-1 antibody and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Images obtained from the representative patient G102 and from MOLM-14 and OPM2 cell lines are presented. (E) The mTORC1-mediated positive feedback on PI3K/Akt activity involves the IGF-1 receptor. BM blast cells from patients G179, G194, G199, and G205 were starved for 4 hours in serum-free MEM. Cells were incubated in the following conditions: medium alone, 10 nmol/L RAD001 for 1 hour, 10 nmol/L RAD001 for 1 hour plus, and 5 μg/mL αIR3 (added 30 minutes before RAD001). WBs were performed with anti-phospho-Akt (Ser 473) and anti-Akt antibodies. αIR3 is a blocking mouse monoclonal antibody directed against the alpha subunit of the IGF-1 receptor and was obtained from Calbiochem. (F) mTORC1 inhibition by RAD001 increases the expression of the IRS2 adaptor. BM blast cells from patients G72, G99, G189, and G191 were starved for 4 hours in serum-free MEM then incubated with or without RAD001 (10 nmol/L) for 1 hour. WBs were performed with anti-IRS2 and anti-actin antibodies.
mTORC1 activity down-regulates insulin/IGF-1 signaling through proteasome-mediated decrease of IRS adaptor proteins.13,–15 To confirm the involvement of the IGF-1R in the mechanism of Akt activation, a variation of IRS-2 expression after treatment with RAD001 was evaluated in AML samples. RAD001 treatment led to a significant increase of IRS2 protein expression, which in each case paralleled the level of RAD001-induced Akt phosphorylation (Figure 1F).
We have shown that IC87114, a PI3K p110δ-selective inhibitor, suppressed AML cell proliferation.7 On the other hand, we observed that the blockage of PI3K by IC87114 did not inhibit mTORC1, as assessed by the persistence of P70S6K phosphorylation (Figures 1A and 2A). Thus, these pathways seem to be independent in AML, thereby allowing us to assess the rationale of a treatment combining 2 inhibitors. We tested the effect of the association of RAD001 and IC87114 on blast cell proliferation. RAD001 and IC87114, when used alone, inhibited blast cell proliferation to the same level in our series of 19 patients. However, the concomitant inhibition of both pathways with RAD001 and IC87114 resulted in a significant additive antiproliferative effect, compared with the effect of each compound alone (P < .001) (Figure 2A). The additive effect of PI3K and mTOR inhibitors was confirmed in dose response curves for the 2 compounds, in 4 AML samples (Figure 2B).
Concomitant inhibition of mTORC1 and p110δ PI3K activity with RAD001 and IC87114, respectively, induces additive inhibition of blast cell proliferation. (A) Blast cells (105/mL) from the 19 patients were incubated 48 hours in duplicate in 5% FCS MEM under the following conditions: control (C), 10 nmol/L RAD001 (R), 10 μmol/L IC87114 (IC), 10 nmol/L RAD001 and 10 μmol/L IC87114 (R + IC), and pulsed for 6 hours with [3H]thymidine (1 μCi, [37 kBq]). A Student t test was performed to compare proliferation rates between the different conditions. Significance was: C/R, P <.001; C/IC, P <.001; R/R + IC, P <.001; IC/R + IC, P <.001. The WB analysis shows the effect of inhibitors on Akt (Ser 473) and P70S6K (Thr 389) phosphorylation. (B) BM blast cells (105/mL) from 4 AML samples were incubated 48 hours in triplicate in 5% FCS MEM, with or without inhibitors as described below. They were then pulsed 6 hours with [3H]thymidine 1 μCi [37 kBq]. The amount of radioactivity incorporated was determined by trichloracetic acid precipitation. In the top panel, increasing amounts (from 1.25 to 20 nmol/L) of RAD001 were used, without (top line) or with a constant concentration of IC87114 (10 μmol/L). In the bottom panel, increasing (from 1.25 to 20 μmol/L) amounts of IC87114 were used, without (top line) or with a constant concentration of RAD001 (10 nmol/L). Error bars indicate standard deviations.
Concomitant inhibition of mTORC1 and p110δ PI3K activity with RAD001 and IC87114, respectively, induces additive inhibition of blast cell proliferation. (A) Blast cells (105/mL) from the 19 patients were incubated 48 hours in duplicate in 5% FCS MEM under the following conditions: control (C), 10 nmol/L RAD001 (R), 10 μmol/L IC87114 (IC), 10 nmol/L RAD001 and 10 μmol/L IC87114 (R + IC), and pulsed for 6 hours with [3H]thymidine (1 μCi, [37 kBq]). A Student t test was performed to compare proliferation rates between the different conditions. Significance was: C/R, P <.001; C/IC, P <.001; R/R + IC, P <.001; IC/R + IC, P <.001. The WB analysis shows the effect of inhibitors on Akt (Ser 473) and P70S6K (Thr 389) phosphorylation. (B) BM blast cells (105/mL) from 4 AML samples were incubated 48 hours in triplicate in 5% FCS MEM, with or without inhibitors as described below. They were then pulsed 6 hours with [3H]thymidine 1 μCi [37 kBq]. The amount of radioactivity incorporated was determined by trichloracetic acid precipitation. In the top panel, increasing amounts (from 1.25 to 20 nmol/L) of RAD001 were used, without (top line) or with a constant concentration of IC87114 (10 μmol/L). In the bottom panel, increasing (from 1.25 to 20 μmol/L) amounts of IC87114 were used, without (top line) or with a constant concentration of RAD001 (10 nmol/L). Error bars indicate standard deviations.
In summary, our study shows that mTORC1 inhibition enhanced Akt activation in AML cells and may contribute to limit the efficacy of rapamycins when used in monotherapy, as described in other systems.16 It has been reported that a 24-hour exposure to rapamycin may impair mTORC2 assembly and, subsequently, down-regulate Akt phosphorylation.17 Similar observations were made by this group in primary AML samples treated with CCI-779 (temsirolimus; Wyeth-Ayerst Pharmaceuticals, Princeton, NJ).18 In our hands, Akt activation was unchanged after 24-hour incubation with RAD001, even at higher RAD001 concentrations (Figure 1B). The reasons for this discrepancy are unknown. It is possible that the culture conditions for primary blast cells were different; alternatively, CCI-779 could be more active on a prolonged incubation period than RAD001 or could have additional side inhibitory effects.
In conclusion, our results show that autocrine IGF-1 signaling is present in primary AML blast cells and is responsible, at least in part, for the effect of RAD001 on mTORC1-mediated PI3K/Akt up-regulation. Our results provide a molecular basis for understanding the increased Akt activation induced by mTORC1 inhibitors and suggest that a combined therapeutic strategy targeting both PI3K and mTORC1 pathways might be useful in patients with AML.
The online version of ths article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the Novartis Institutes for Medical Research Basel, Oncology for the supply of RAD001. We thank all participating investigators from the GOELAMS. We thank Dr David Fruman (University of California-Irvine) for helpful criticism of the manuscript.
This work was supported by grants from the Ligue Nationale Contre le Cancer (LNCC, laboratoire associé), the Association pour le Recherche contre le Cancer (ARC), the Institut National du Cancer (INCA), the Association Laurette Fugain, and the Fondation pour la Recherche Medicale (FRM).
Authorship
Contribution: J.T. performed research, analyzed data, and wrote the manuscript. N.C., P.S., V.B., S.P., and L.W. performed research and analyzed data. N.I. and F.D. contributed AML patient samples and analyzed clinical data. C.L. and P.M. analyzed data and wrote the manuscript. D.B. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Didier Bouscary, Département d'Hématologie, Institut Cochin, 27 rue du Faubourg Saint-Jacques, F-75014 Paris, France; e-mail: bouscary@cochin.inserm.fr.

![Figure 2. Concomitant inhibition of mTORC1 and p110δ PI3K activity with RAD001 and IC87114, respectively, induces additive inhibition of blast cell proliferation. (A) Blast cells (105/mL) from the 19 patients were incubated 48 hours in duplicate in 5% FCS MEM under the following conditions: control (C), 10 nmol/L RAD001 (R), 10 μmol/L IC87114 (IC), 10 nmol/L RAD001 and 10 μmol/L IC87114 (R + IC), and pulsed for 6 hours with [3H]thymidine (1 μCi, [37 kBq]). A Student t test was performed to compare proliferation rates between the different conditions. Significance was: C/R, P <.001; C/IC, P <.001; R/R + IC, P <.001; IC/R + IC, P <.001. The WB analysis shows the effect of inhibitors on Akt (Ser 473) and P70S6K (Thr 389) phosphorylation. (B) BM blast cells (105/mL) from 4 AML samples were incubated 48 hours in triplicate in 5% FCS MEM, with or without inhibitors as described below. They were then pulsed 6 hours with [3H]thymidine 1 μCi [37 kBq]. The amount of radioactivity incorporated was determined by trichloracetic acid precipitation. In the top panel, increasing amounts (from 1.25 to 20 nmol/L) of RAD001 were used, without (top line) or with a constant concentration of IC87114 (10 μmol/L). In the bottom panel, increasing (from 1.25 to 20 μmol/L) amounts of IC87114 were used, without (top line) or with a constant concentration of RAD001 (10 nmol/L). Error bars indicate standard deviations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/1/10.1182_blood-2007-03-080796/3/m_zh80020811320002.jpeg?Expires=1769139741&Signature=iF1Y5b73J9dwCdPP7b2SZSiUOw0okxq60JyXKoSWy7ojLWsYfOhWtNH2J3EM~nmL6Z31AxwyBwL5yZHL688ijrrF4L6kiGDMPqVWPJqqxTriXTT8IqlG4xhcgg0q72GJSRwPdwoaxvxi1~9uNeuw-vXtVONABN8neNj~Zue5a8-lhYTka-bpPWvcg-1zFca7IxAE~TjGhYLQTUSfzoxtIwo6OnaZNYXeKL4FY8KVchB1RRYXCfChhQpptZkbg5YB2ZjbCa2esP9pNdAJ7dq~nGptsgYNJsaqFdGRKvHS0YHlamB8xu0hGLdSLnCjvKDH16mcc~GEPaJkQ1381Y8znA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal