Coagulation activation by tissue factor (TF) is implicated in cancer progression, cancer-associated thrombosis and metastasis. The role of direct TF signaling pathways in cancer, however, remains incompletely understood. Here we address how TF contributes to primary tumor growth by using a unique pair of isotype-matched antibodies that inhibit either coagulation (monoclonal antibody [Mab]-5G9) or direct signaling (Mab-10H10). We demonstrate that the inhibitory antibody of direct TF-VIIa signaling not only blocks TF-VIIa mediated activation of PAR2, but also disrupts the interaction of TF with integrins. In epithelial and TF-expressing endothelial cells, association of TF with β1 integrins is regulated by TF extracellular ligand binding and independent of PAR2 signaling or proteolytic activity of VIIa. In contrast, α3β1 integrin association of TF is constitutive in breast cancer cells and blocked by Mab-10H10 but not by Mab-5G9. Mab-5G9 has antitumor activity in vivo, but we show here that Mab-10H10 is at least as effective in suppressing human xenograft tumors in 2 different models. Breast tumor growth was also attenuated by blocking PAR2 signaling. These results show that tumor cell TF-PAR2 signaling is crucial for tumor growth and suggest that anti-TF strategies can be applied in cancer therapy with minor impairment of TF-dependent hemostatic pathways.
Introduction
The tissue factor (TF)-initiated coagulation pathway plays important roles in normal hemostasis, cardiovascular disease and thrombosis. Coagulation activation in the vicinity of TF-expressing tumor cells and shedding of procoagulant activity into the circulation contribute significantly to cancer associated-thrombosis, thromboembolism, and Trousseau syndrome.1,2 TF expression is correlated with tumor progression in several cancers, and TF initiates thrombin-dependent tumor cell metastasis.3 Although the duration of anticoagulant therapy for recurrent thromboembolism is correlated with reduced cancer incidence,4 the role of TF in cancer development and primary tumor growth is incompletely understood.
The prometastatic pathway downstream of TF5 has been delineated in considerable detail and is dependent on several activities of thrombin that converge to promote tumor cell survival during the initial phase of homing to target organs (for reviews, see Ruf and Mueller3 and Nierodzik and Karpatkin6 ). The TF cytoplasmic domain also contributes to metastasis, but effects of cytoplasmic domain deletion vary between tumor models.7,–9 Whether the TF pathway plays a role in primary tumor growth remains controversial. Overexpression of TF in fibrosarcoma,10 pancreatic cancer cells,11 and melanoma cells12 enhances tumor growth and, conversely, knock-down of TF in colon cancer cells13 attenuates tumor expansion. To the contrary, TF-deficient cell lines that have been established from mouse embryos by oncogene transformation are not changed in their tumor growth behavior upon reexpression of TF, although metastatic activity increases.9 These data indicate that TF supports tumor growth and metastasis by independent pathways.
Tumor growth in transplanted models is reduced by treatment with the thrombin inhibitor hirudin, indicating the possibility that TF enhances tumor growth indirectly through proangiogenic thrombin signaling, fibrin deposition, or platelet activation.6 However, protease-activated receptor-1 (PAR1)-deficient animals show normal growth of transplanted tumors14 and metastases,15 suggesting that thrombin-dependent PAR1 signaling is dispensable in the host cell compartment with the possible exception of platelets that can provide local hemostasis in angiogenic vessels.16 These indirect coagulant effects of TF are difficult to separate from potential direct signaling functions of TF on tumor cells in the tumor microenvironment.
TF is linked to 2 major cellular signaling pathways. On the one hand, TF regulates integrins and suppresses cell migration specifically on laminin 5, a matrix for the integrin α3β1.17 Suppression of migration is mediated by TF cytoplasmic domain signaling and binding of some antibodies (ie, Mab-5G9 or the physiologic ligand VIIa) can reverse suppression.17 Active site-blocked VIIa also stimulates migration in certain tumor cells.18 In this and other systems,19 the TF cytoplasmic domain regulates p38 mitogen-activated kinase, extracellular signal-regulated kinase 1/2 (ERK1/2) and rac pathways. How ligand binding to TF per se stimulates cell migration is incompletely understood.
On the other hand, TF is linked to 7-transmembrane, G-protein-coupled receptor signaling by direct TF-VIIa-mediated cleavage of PAR2.20 In part, PAR2 signaling stimulates migration by a feedback pathway that phosphorylates the TF cytoplasmic domain and releases integrin suppression.17,21 TF-VIIa-PAR2 signaling also promotes breast cancer migration dependent on the chemokine interleukin 8 (IL-8) and regulation of the cofilin pathway.22,–24 In addition, TF-VIIa signaling has antiapoptotic effects and prevents cells from death in the absence of matrix (anoikis).25,26 TF-VIIa signaling may regulate angiogenesis or play immunomodulatory roles through the up-regulation of angiogenic regulators and cytokines.27,–29 Because thrombin up-regulates a similar repertoire of genes in either tumor or host cells,6,30 it is possible that direct TF signaling is redundant with other protease signaling pathways in the tumor microenvironment.
TF-VIIa typically binds and activates coagulation factor X to initiate the coagulation pathway. However, we have found recently that TF procoagulant activity can be switched off by protein disulfide isomerase-dependent pathways without impairing direct TF-VIIa signaling. We further identified 2 prototypic antibodies with relative specificity to block either direct TF-VIIa signaling (Mab-10H10) or coagulation (Mab-5G9) without displacing VIIa.31 Here, we use these tools to determine the contributions of TF signaling versus coagulation to tumor growth in vivo. We show that TF is constitutively associated with β1 integrins in highly aggressive breast cancer cells, whereas TF-integrin interaction is regulated in noncancerous keratinocytes or endothelial cells by extracellular ligand binding. Mab-10H10, but not Mab-5G9, specifically disrupts the association of TF with integrins and potently inhibits cancer cell TF-VIIa-PAR2 signaling, which is enhanced by ligation of β1 integrin. Blocking TF signaling with Mab-10H10 is sufficient to attenuate tumor growth of aggressive human breast cancer cells. Blockade of PAR2 cleavage further supports the conclusion that direct tumor cell TF signaling is crucial for tumor growth.
Methods
Materials
N-Hydroxysuccinimide (NHS)–biotin was from Pierce (Rockford, IL), and paramagnetic Dynabeads were from Invitrogen (Carlsbad, CA). We used anti-phosphorylated and total ERK1/2 antibodies (Cell Signaling Technologies, Danvers, MA), anti-integrin β1 AIIB2 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), anti-α3 P1B5, anti-α2 P1H5, anti–α6 GoH3, and anti-α5 KH72 that were purified from ascites and culture supernatant or purchased from Chemicon (Temecula, CA). Monoclonal antibodies to TF (Mab-10H10 and Mab-5G9), and the PAR2 blocking rabbit polyclonal antibody31 were purified under endotoxin- free conditions. Fab′2 fragments of the anti-PAR2 antibody were generated by digestion of IgG fractions with immobilized pepsin followed by purification using ion exchange chromatography to remove uncleaved residual IgG. Recombinant VIIa and active site-blocked VIIa (VIIai) was a generous gift from Dr L. C. Petersen (Novo Nordisk, Malov, Denmark). Human fibronectin (Collaborative Biomedical Products, Bedford, MA), collagen I (Cohesion, Palo Alto, CA), poly-L-lysine (Sigma-Aldrich, St Louis, MO), laminin 5-containing supernatant from 805G rat bladder carcinoma cells, and vitronectin purified from human plasma were used for adhesion assays.
Cell culture and adenoviral transductions
Human HaCaT immortalized keratinocytes were subcultured twice per week. Keratinocyte standard culture medium contained Dulbecco modified Eagle medium (DMEM), 10% FBS, and 2 mmol/L glutamine. The human breast cancer cell line MDA-MB-231 and the in vivo selected, highly aggressive MDA-MB-231mfp32 cell lines were typically cultured in L15 medium (Lonza, Walkersville, MD), 10% FBS, glutamine, and insulin. Cells were rendered quiescent for 4 hours in DMEM without FBS and stimulated as indicated in text and figures. The cells were then washed in ice-cold phosphate-buffered saline (PBS) and processed for cell surface biotinylation, cell lysis for immunoprecipitation, or mRNA extraction. Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial growth medium (Lonza) and transduced with TF and PAR2 adenoviral constructs as described previously.31,33 HUVEC were rendered quiescent overnight in Endothelial Basal Media (Lonza). Proliferation was determined by seeding cells on culture dishes coated with collagen I (5 μg/mL), laminin 5-containing supernatant from 805G cells, or vitronectin (2 μg/mL) followed by blocking of residual binding sites with 5% bovine serum albumin. After 48–72 hours, counts of antibody-treated cells (50 μg/mL) were quantified relative to control using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT)-based assay. Anchorage-independent survival in the presence or absence of antibody was quantified after 3-day suspension culture in 0.5% methylcellulose under serum-free conditions.
Cell surface biotinylation, immunoprecipitation, and Western blotting
For surface biotinylation, cells were incubated with 2 mmol/L NHS-biotin for 30 minutes in HEPES-buffered saline (HBS; 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], pH 7.4, 137 mmol/L NaCl, 5.3 mmol/L KCl, and 1.5 mmol/L CaCl2) on ice and washed twice in ice-cold HBS. Biotinylated or washed cells were lysed in Brij35 buffer (50 mmol/L Tris, pH 7.4, 1% Brij35, 150 mmol/L NaCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, and protease/phosphatase inhibitors) for 15 minutes on ice. Cells were scraped and centrifuged for 10 minutes at 800g to remove cellular debris (cleared cell lysate). Cleared cell lysates were then centrifuged for 30 minutes at 16 000g and the supernatant was immunoprecipitated for at least 2 hours at 4°C using anti-integrin antibodies that were directly coupled to paramagnetic beads. Immunoprecipitation of TF was similarly performed from cleared lysates with Mab-10H10 or Mab-5G9 directly coupled to paramagnetic beads. Beads were washed 3 times in lysis buffer and resuspended in sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. TF and β1 integrin in the immunoprecipitates were detected by polyclonal goat anti-TF or anti-β1 antibody. Western blots were digitized and intensities were quantified by densitometry using Scion Image (Scion, Frederick, MD).
FXa generation
To evaluate coagulant activity of TF in integrin immunoprecipitates, beads were washed in HBS and resuspended in the presence of 10 nmol/L VIIa and 100 nmol/L X. Reactions were quenched after 10 minutes, and generated Xa was determined with the chromogenic substrate Spectrozyme FXa. For cell surface procoagulant activity, cells were washed in serum-free DMEM, and Xa generation over time was measured after addition of 10 nmol/L VIIa and 100 nmol/L X.
Quantitative real-time polymerase chain reaction and gene induction
Cells were stimulated with 10 nmol/L VIIa, with 0.5 nmol/L VIIa and 100 nmol/L X in the presence of 200 nmol/L NAPc2, or with10 nmol/L thrombin for 90 minutes. The mRNA was extracted with Trizol reagent from directly lysed cultured cells. After cDNA synthesis, mRNA levels were determined by quantitative polymerase chain reaction (PCR) on an ABI Prism Sequence Detection System (Molecular Devices, Sunnyvale, CA) using sequence-specific TaqMan primers designed using Primer Express version 2 software.
Tumor growth assay
All animal experiments were performed under approved protocols of the institutional animal use and care committee. MDA-MB-231mfp cells (2 × 106)32 or M24met cells (0.5 × 106)5 were mixed with 1 mg Mab-10H10, Mab-5G9, isotype matched mouse IgG1 (TIB115), mouse monoclonal anti–PAR1 ATAP2,34 rabbit anti–PAR2 IgG, normal rabbit IgG, or Fab′2 of rabbit anti-PAR2 in 100 μL PBS and injected subcutaneously into the flank of 6 week-old, female C.B-17 SCID mice (Taconic Farms, Germantown, NY). To characterize growth in the orthotopic tumor environment, breast cancer cells were injected into the murine mammary fat pad. Tumor volumes were measured with calipers. Tumor weights were determined at the time of sacrifice. Analysis of variance (ANOVA) was used to establish differences between groups, and significance levels were determined by nonparametric Kruskal-Wallis test. Frozen sections (5 μm) of tumors were fixed with PFA for 10 minutes, blocked with 10% goat serum, and stained with rat anti–mouse CD 31 (Clone 13.3; BD Pharmingen, San Diego, CA) for 1 hour, followed by detection with goat anti-rat Alexa Fluor 488 (Invitrogen). Nuclei were stained with 4,6-diamidino-2-phenylindole in the mounting medium (Vector Laboratories, Burlingame, CA). Images were taken with a Q imaging Retiga 2000 R camera (QImaging, Surrey, BC) attached to a Nikon-Eclipse TE 2000S microscope (Nikon, Melville, NY) using a 40×/0.56 or 16×/0.40 objective (Zeiss). Photographs were analyzed with QCapture Pro version 5.1.1.14 software (QImaging) and images were assembled using Adobe Photoshop CS2 version 9.0.2 software (Adobe Systems, San Jose, CA). Microvessels were counted under high magnification in at least 3 tumors for each experimental group.
Experimental metastasis assay
Luciferase-tagged MDA-MB-231 cells (4.5 × 105 in 200 μL) were mixed with 1 mg Mab-10H10, Mab-5G9, or buffer control and injected into the lateral tail vein of severe combined immunodeficient (SCID) mice. For intravital bioluminescence imaging at 4 hours and 1, 2, and 7 days after tumor-cell injection, luciferin (1.5 mg in 100 μL PBS) was injected intraperitoneally, and light emission was measured 5 minutes later with an IVIS 200 imaging system (Xenogen Biosciences, Cranbury, NJ). Quantification of the light signal was performed with the Living Image Software (Xenogen) to compare lung tumor burden at different time points and between experimental groups based on photon flux (photons per second per square centimeter).
Results
Anti-TF Mab-10H10 and Mab-5G9 had differential effects on hematogenous metastasis of human breast cancer cells
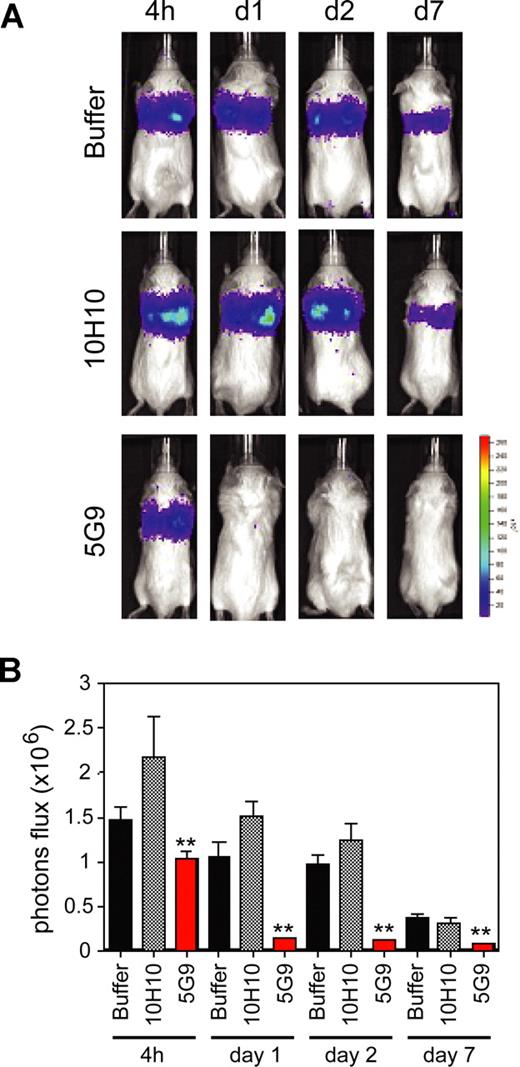
The role of TF-mediated coagulation activation in experimental hematogenous metastasis has been extensively studied, and blocking the macromolecular substrate exosite of the TF-VIIa complex with specific antibodies inhibits metastasis in several tumor models.5,35 We first evaluated the effect of signaling blockade by Mab-10H10 on hematogenous metastasis of the tumor model used in this study, MDA-MB-231 human breast cancer cells. Previous studies5,9,36,37 have shown that coagulation activation supports early events in metastasis after intravenous injection. Blockade of TF-dependent coagulation by Mab-5G9 consistently and dramatically diminished the load of surviving tumor cells in the lungs after 24 hours (Figure 1), but signaling blocking antibody Mab-10H10 produced no significant effect relative to buffer control for up to 7 days. Thus, TF-VIIa signaling does not contribute to the initial survival of arrested tumor cells in the lung. In addition, these data indicate that Mab-10H10 has no major activity to elicit antibody-directed killing—as expected for an antibody of the murine IgG1 subclass—even though TF antigen levels are high on MDA-MB-231 cells (Figure 2). Although these data did not indicate a necessary role for direct TF signaling in the early stages of metastatic arrest, more extensive treatment experiments would be required to rule out that subsequent growth of established metastases is not affected by blocking direct TF signaling.
Inhibition of breast cancer metastasis by coagulation blocking anti-TF Mab-5G9. Luciferase-tagged MDA-MB-231 cells were mixed with 1 mg of control IgG1 (TIB115), Mab-5G9, or Mab-10H10 and injected into the tail vein of SCID mice. Bioluminescence was measured (A) and quantified based on photon flux (photons per second per square centimeter). (B) 4 hours and 1, 2, and 7 days after tumor cell injection; n = 8, t test; **P < .05.
Inhibition of breast cancer metastasis by coagulation blocking anti-TF Mab-5G9. Luciferase-tagged MDA-MB-231 cells were mixed with 1 mg of control IgG1 (TIB115), Mab-5G9, or Mab-10H10 and injected into the tail vein of SCID mice. Bioluminescence was measured (A) and quantified based on photon flux (photons per second per square centimeter). (B) 4 hours and 1, 2, and 7 days after tumor cell injection; n = 8, t test; **P < .05.
Inhibition of breast cancer PAR2 signaling by anti-TF antibodies
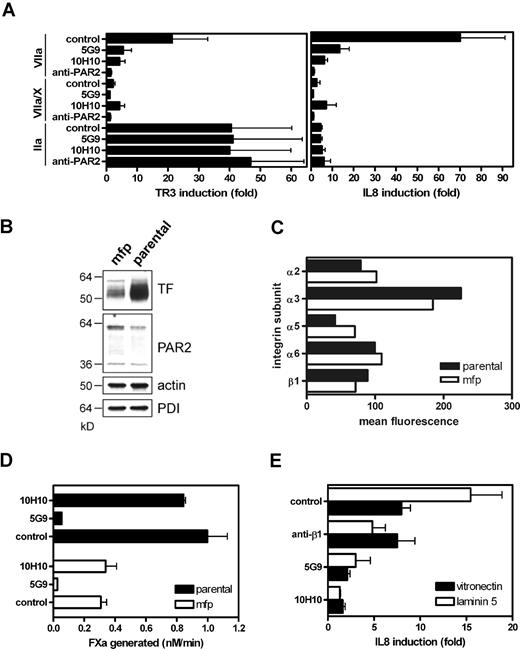
In noncancerous human keratinocytes, Mab-10H10 selectively blocked TF-VIIa mediated ERK1/2 phosphorylation, whereas Mab-5G9 had an effect only on signaling of the ternary TF-VIIa-Xa coagulation initiation complex.31 Inhibition of cancer cell TF signaling by these antibodies was studied by measuring induction of IL-8, a well established downstream effect of direct TF signaling in breast cancer cells,23,29 and of the nuclear orphan receptor TR3, a sensitive readout for PAR signaling in various cells.38 MDA-MB-231 cells were pretreated with antibody for 15 minutes, followed by stimulation with 10 nmol/L VIIa and 0.5 nmol/L VIIa plus factor X in the presence of NAPc2 to trap the signaling active ternary initiation complex,39 or thrombin. TF-VIIa signaling was the most potent inducer of IL-8 and TF-VIIa was efficiently inhibited by Mab-10H10 (Figure 2A). Unlike the earlier findings in the HaCaT keratinocyte model, Mab-5G9 inhibited TF-VIIa signaling, but less efficiently than Mab-10H10. In contrast, Mab-5G9, but not Mab-10H10, inhibited ternary complex signaling, in line with our previous conclusion that Mab-10H10 minimally perturbs the procoagulant cell surface pool of TF.31 TF-dependent signaling was blocked by anti-PAR2 antibody. In contrast, thrombin signaling was not inhibited by anti-PAR2, Mab-10H10 or Mab-5G9, demonstrating specificity. Although thrombin was less potent than TF-VIIa in inducing IL-8, thrombin up-regulated TR3 efficiently, excluding that lack of PAR1 expression or low signaling strength are responsible for inefficient IL-8 induction in breast cancer cells.
Inhibition of proteolytic PAR2 signaling by anti-TF antibodies on MDA-MB-231 human breast cancer cells. (A) Mab-10H10 and Mab-5G9 inhibit TF-VIIa signaling in MDA-MB-231 cells. Cells were preincubated for 15 minutes with 50 μg/mL Mab-10H10 or Mab-5G9 or 100 μg/mL polyclonal PAR2-cleavage-blocking antibody, followed by addition of 10 nmol/L VIIa, ternary complex (0.5 nmol/L VIIa, 100 nmol/L X, and 200 nmol/L NAPc2) or 10 nmol/L thrombin (IIa). TR3 and IL-8 mRNA induction over control after 90 minutes was determined by quantitative PCR. (B) TF and PAR2 expression in parental MDA-MB-231 and MDA-MD-231mfp cells by Western blotting using polyclonal anti-TF and anti-PAR2 with loading controls of abundant cellular proteins, PDI and actin. (C) Comparative FACS analysis for integrin expression by parental and mfp MDA-MB-231 cells. Averages for 2 experiments are shown. (D) Comparison of Xa generation by parental and mfp cells on monolayers seeded at equal cell density. (E) Matrix-dependent TF-VIIa signaling. MDA-MB-231mfp cells were grown on vitronectin or laminin 5-containing matrix. Cells were pretreated with 50 μg/mL anti-β1 AIIB2, Mab-5G9, or Mab-10H10 for 15 minutes before stimulation with VIIa (10 nmol/L) for 90 minutes.
Inhibition of proteolytic PAR2 signaling by anti-TF antibodies on MDA-MB-231 human breast cancer cells. (A) Mab-10H10 and Mab-5G9 inhibit TF-VIIa signaling in MDA-MB-231 cells. Cells were preincubated for 15 minutes with 50 μg/mL Mab-10H10 or Mab-5G9 or 100 μg/mL polyclonal PAR2-cleavage-blocking antibody, followed by addition of 10 nmol/L VIIa, ternary complex (0.5 nmol/L VIIa, 100 nmol/L X, and 200 nmol/L NAPc2) or 10 nmol/L thrombin (IIa). TR3 and IL-8 mRNA induction over control after 90 minutes was determined by quantitative PCR. (B) TF and PAR2 expression in parental MDA-MB-231 and MDA-MD-231mfp cells by Western blotting using polyclonal anti-TF and anti-PAR2 with loading controls of abundant cellular proteins, PDI and actin. (C) Comparative FACS analysis for integrin expression by parental and mfp MDA-MB-231 cells. Averages for 2 experiments are shown. (D) Comparison of Xa generation by parental and mfp cells on monolayers seeded at equal cell density. (E) Matrix-dependent TF-VIIa signaling. MDA-MB-231mfp cells were grown on vitronectin or laminin 5-containing matrix. Cells were pretreated with 50 μg/mL anti-β1 AIIB2, Mab-5G9, or Mab-10H10 for 15 minutes before stimulation with VIIa (10 nmol/L) for 90 minutes.
MDA-MB-231 cells show a considerable lag phase in tumor development when transplanted subcutaneously or orthotopically into the mammary fat pad (mfp) of immune-deficient mice. Poor in vivo growth is presumably due to tissue culture adaptation, because in vivo selection in the orthotopic mfp yielded a more aggressive cell line, MDA-MB-231mfp.32 These more aggressive breast cancer cells express lower levels of TF protein (Figure 2B) than the parental cells, but they express similar levels of PAR2 and several integrin subunits (Figure 2C). Reduced cell surface expression of TF also resulted in reduced rates of Xa generation on MDA-MB-231mfp cells (Figure 2D). Compared with parental cells, MDA-MB-231mfp cells showed somewhat reduced TF-VIIa-PAR2-mediated IL-8 induction, and no thrombin responses were detectable in these cells (data not shown). Thus, TF-VIIa signaling is the predominant protease pathway for IL-8 up-regulation in highly aggressive breast cancer cells.
Because TF is associated with integrins17 and cell adhesion has an effect on how cells sense extracellular signals,40 we addressed whether β1 integrin ligation influences proteolytic signaling of TF-VIIa. MDA-MB-231mfp cells were plated either on vitronectin, which is predominantly recognized by β3 and β5 integrins, or on a laminin 5-containing matrix that primarily engages β1 integrins. TF-VIIa-mediated induction of IL-8 was enhanced ∼2-fold on laminin 5 matrix and anti-β1 antibody specifically blocked enhanced IL-8 induction (Figure 2E). These data indicate that the extracellular matrix environment can enhance proteolytic cell signaling through PAR2. Mab-10H10 very effectively blocked TF-VIIa signaling under these conditions. Taken together, these data show that Mab-5G9 and Mab-10H10 had the expected specificity to block coagulation, but Mab-5G9 showed more pronounced inhibition of direct TF-VIIa signaling in cancer cells compared with previously characterized noncancerous epithelial cells.
TF-β1 integrin association was regulated by exogenous VIIa on keratinocytes
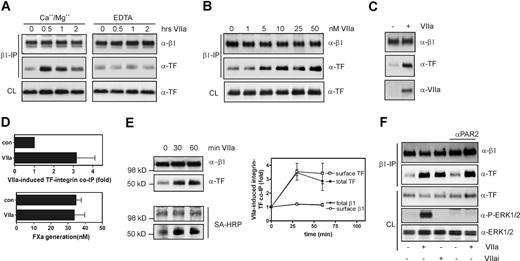
We further analyzed the inhibitory roles of anti-TF antibodies with respect to integrin pathways and first used the noncancerous HaCaT keratinocyte model to address how the interaction of TF with integrins is regulated and influenced by Mab-5G9 and Mab-10H10. HaCaT cells were stimulated with VIIa for defined times. Subsequently, cells were lysed in a Brij35-containing buffer, β1 integrins were immunoprecipitated, and immunoprecipitates were analyzed by Western blotting for TF and the β1 integrin subunit. Addition of 10 nmol/L VIIa led to a time-dependent, 3-fold increase of TF in β1 immunoprecipitates (Figure 3A) and coimmunoprecipitation of TF with integrin was dependent on the presence of Ca2+/Mg2+. Thus, the interaction required the cation-bound conformation of integrin and/or VIIa. The association of TF with β1 integrin required VIIa in the nanomoles per liter range, indicating that pools of TF with low affinity for VIIa are recruited (Figure 3B). Recovery of VIIa in β1 immunoprecipitates confirmed the association of the TF-VIIa complex with β1 integrin (Figure 3C).
VIIa-induces association of TF with β1 integrin in HaCaT nonmalignant human keratinocytes. (A) Time course of coimmunoprecipitation of TF with β1 integrin after addition of 10 nmol/L VIIa to HaCaT cells. Immunoprecipitations were from cells lysed with Brij35 buffers containing either 1 mmol/L CaCl2 and MgCl2, or 1 mmol/L EDTA. Western blots for β1 integrin and TF in the immunoprecipitates and in the cleared cell lysate (CL) before immunoprecipitation are shown. (B) Dose-dependence of VIIa-induced association of TF with β1 integrin after 1 hour of incubation. (C) Coprecipitation of VIIa in the β1 integrin-TF complex detected after 10 nmol/L VIIa addition for 1 hour. (D) VIIa-induced β1-associated TF is coagulant inactive. Cells were treated with VIIa for 60 minutes, and β1 was precipitated from the lysate. β1 and TF precipitation were assessed on Western blots, and coagulant activity of the immunoprecipitate was assessed by adding immunoprecipitates to an Xa generation assay; mean and standard deviations, n = 3. (E) TF associates with integrin on the cell surface. After addition of 10 nmol/L VIIa for the times indicated, cells were surface biotin-labeled. Biotinylated proteins are detected by horseradish peroxidase (HRP)-streptavidin (SA-HRP) relative to total immunoprecipitated TF and β1. Densitometry from 3 separate experiments showed similar ∼3-fold up-regulation in total and cell surface TF upon VIIa addition as depicted in the graph. (F) VIIa-induced TF-integrin interaction is independent of PAR2 activation and activity of VIIa. PAR2 activation was blocked with 100 μg/mL polyclonal anti-PAR2 in cells stimulated with 10 nmol/L VIIa or active site-inhibited VIIa (VIIai) was added for 1 hour.
VIIa-induces association of TF with β1 integrin in HaCaT nonmalignant human keratinocytes. (A) Time course of coimmunoprecipitation of TF with β1 integrin after addition of 10 nmol/L VIIa to HaCaT cells. Immunoprecipitations were from cells lysed with Brij35 buffers containing either 1 mmol/L CaCl2 and MgCl2, or 1 mmol/L EDTA. Western blots for β1 integrin and TF in the immunoprecipitates and in the cleared cell lysate (CL) before immunoprecipitation are shown. (B) Dose-dependence of VIIa-induced association of TF with β1 integrin after 1 hour of incubation. (C) Coprecipitation of VIIa in the β1 integrin-TF complex detected after 10 nmol/L VIIa addition for 1 hour. (D) VIIa-induced β1-associated TF is coagulant inactive. Cells were treated with VIIa for 60 minutes, and β1 was precipitated from the lysate. β1 and TF precipitation were assessed on Western blots, and coagulant activity of the immunoprecipitate was assessed by adding immunoprecipitates to an Xa generation assay; mean and standard deviations, n = 3. (E) TF associates with integrin on the cell surface. After addition of 10 nmol/L VIIa for the times indicated, cells were surface biotin-labeled. Biotinylated proteins are detected by horseradish peroxidase (HRP)-streptavidin (SA-HRP) relative to total immunoprecipitated TF and β1. Densitometry from 3 separate experiments showed similar ∼3-fold up-regulation in total and cell surface TF upon VIIa addition as depicted in the graph. (F) VIIa-induced TF-integrin interaction is independent of PAR2 activation and activity of VIIa. PAR2 activation was blocked with 100 μg/mL polyclonal anti-PAR2 in cells stimulated with 10 nmol/L VIIa or active site-inhibited VIIa (VIIai) was added for 1 hour.
To further address the functional state of TF in integrin complexes, we compared TF coagulant activity of immunoprecipitates. TF coimmunoprecipitation with β1 integrin increased 3-fold in the presence of VIIa, but TF-VIIa–dependent Xa generation was unchanged (Figure 3D). Although we cannot exclude the possibility that the detergent lysis induced the activation of baseline TF that is associated with integrin, TF that was recruited in response to VIIa did not increase X activation. Indirect evidence for recruitment of nonprocoagulant TF pools further comes from the fairly high concentrations of VIIa required to induce integrin association (Figure 3B), because cryptic and signaling pools are known to have low affinity for VIIa. To address whether TF and integrin associate at the cell surface or within intracellular compartments, cells were surface-biotinylated after stimulation and before cell lysis for immunoprecipitation. VIIa stimulation did not change surface biotinylation of β1 integrin, and biotinylated TF protein increased concordantly with total TF in the β1 integrin immunoprecipitate (Figure 3E), providing evidence that TF associated with integrin on the cell surface.
To address whether TF-VIIa-PAR2 signaling promotes TF-integrin association, HaCaT cells were exposed to either active site-inhibited VIIa or to active VIIa in the presence of a cleavage blocking anti-PAR2 antibody. As expected, ERK1/2 phosphorylation was not induced in either instance, but TF-integrin association was up-regulated. These data indicate that PAR2 signaling is not required for integrin-TF complex formation and that VIIa binding to TF is sufficient to promote integrin-TF interaction (Figure 3F).
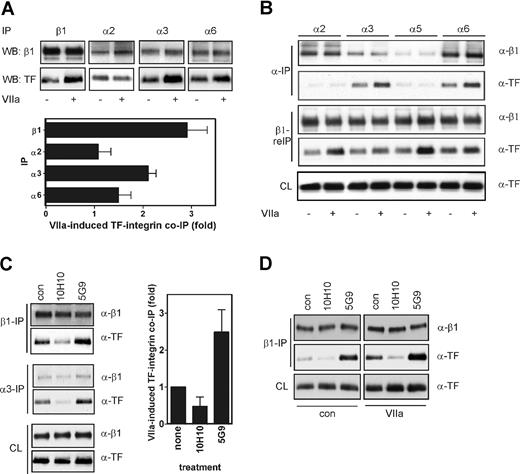
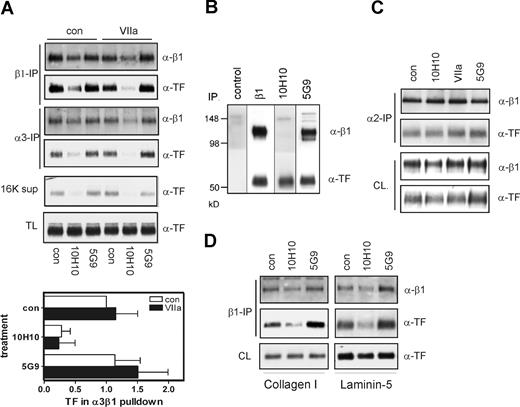
To identify β1 integrin heterodimers that associate with TF, we immunoprecipitated predominant integrin α subunits of HaCaT cells.17 VIIa enhanced coprecipitation with α3 and somewhat less pronounced with α6, but not α2, integrin (Figure 4A). Similarly, in endothelial cells that were transduced to express TF and PAR2, VIIa enhanced association between TF and integrin subunits α3 and α6 but not α2 and α5 (Figure 4B). Subsequent immunoprecipitation of β1 integrin from the α subunit-depleted lysates confirmed that TF-integrin complexes were unperturbed after depletion of α2β1 and α5β1, but depleted after precipitation of α3β1 and α6β1, confirming that TF is associated with the latter integrins.
Mab-10H10, but not Mab-5G9, dissociates the TF-α3β1 integrin complex in HaCaT cells. (A) TF associates with α3 and α6 in HaCaT cells. VIIa was added for 60 minutes, and lysates were immunoprecipitated with anti-β1, α2, α3, and α6. β1 was used as a control for the efficiency of immunoprecipitation of integrin heterodimers, and coprecipitation of TF was assessed by Western blotting. Immunoprecipitation of α5 yielded no signal, consistent with the low expression levels of this integrin subunit in HaCaT cells.17 Densitometric quantitation is given as mean and standard deviation, n = 3. (B) TF associates with α3 and α6 in endothelial cells. HUVECs were transduced to express TF and PAR2. VIIa was added for 60 minutes, and lysates were immunoprecipitated with anti-α2, α3, α5, and α6. The predepleted lysates were then reimmunoprecipitated with anti-β1. Coprecipitation of TF was assessed by Western blotting. β1 was assessed on blot as well as a control for the efficiency of immunoprecipitation of the β1 subunit as well as integrin heterodimers. (C) Mab-5G9 stimulates and Mab-10H10 inhibits TF association with α3 and β1 integrin in HaCaT cells. Cells were incubated for 60 minutes with 50 μg/mL Mab-10H10 or Mab-5G9 and integrin β1 and α3 were immunoprecipitated from lysates. β1 and TF in immunoprecipitates and supernatants were determined by Western blot. The graph shows mean and standard deviation for 3 β1 pulldown assays. (D) Mab-10H10 inhibits basal and VIIa-induced TF association with β1 integrin. HaCaT cells were incubated with 50 μg/mL Mab-10H10 or Mab-5G9 for 15 minutes and treated with VIIa for 60 minutes.
Mab-10H10, but not Mab-5G9, dissociates the TF-α3β1 integrin complex in HaCaT cells. (A) TF associates with α3 and α6 in HaCaT cells. VIIa was added for 60 minutes, and lysates were immunoprecipitated with anti-β1, α2, α3, and α6. β1 was used as a control for the efficiency of immunoprecipitation of integrin heterodimers, and coprecipitation of TF was assessed by Western blotting. Immunoprecipitation of α5 yielded no signal, consistent with the low expression levels of this integrin subunit in HaCaT cells.17 Densitometric quantitation is given as mean and standard deviation, n = 3. (B) TF associates with α3 and α6 in endothelial cells. HUVECs were transduced to express TF and PAR2. VIIa was added for 60 minutes, and lysates were immunoprecipitated with anti-α2, α3, α5, and α6. The predepleted lysates were then reimmunoprecipitated with anti-β1. Coprecipitation of TF was assessed by Western blotting. β1 was assessed on blot as well as a control for the efficiency of immunoprecipitation of the β1 subunit as well as integrin heterodimers. (C) Mab-5G9 stimulates and Mab-10H10 inhibits TF association with α3 and β1 integrin in HaCaT cells. Cells were incubated for 60 minutes with 50 μg/mL Mab-10H10 or Mab-5G9 and integrin β1 and α3 were immunoprecipitated from lysates. β1 and TF in immunoprecipitates and supernatants were determined by Western blot. The graph shows mean and standard deviation for 3 β1 pulldown assays. (D) Mab-10H10 inhibits basal and VIIa-induced TF association with β1 integrin. HaCaT cells were incubated with 50 μg/mL Mab-10H10 or Mab-5G9 for 15 minutes and treated with VIIa for 60 minutes.
Mab-5G9, but not Mab-10H10, was previously shown to promote α3β1-dependent cell migration of HaCaT cells.17 Mab-5G9 induced association of TF with integrins similar to VIIa. This enhanced association of TF with integrins may explain the migration promoting activities of this antibody. In contrast, Mab-10H10 blocked the basal interaction of TF with α3 and α6 (Figure 4C). In addition, Mab-10H10, but not Mab-5G9, prevented VIIa-induced association of TF with β1 integrin (Figure 4D). Thus, TF associates predominantly with α3 and α6, which form integrin heterodimers that bind laminin 5. These data are consistent with the finding that TF has a selective effect on HaCaT cell migration on laminin 5, but not on fibronectin.
TF-β1 integrin association was constitutive on cancer cells
We next determined the effect of TF antibodies on the TF-integrin interaction in highly aggressive MDA-MB-231mfp cells. In contrast to the noncancerous HaCaT keratinocyte cell line, TF-β1 integrin association was found to be constitutive and not regulated by VIIa in cancer cells (Figure 5A). Mab-10H10 essentially abolished coimmunoprecipitation of TF with α3 and β1 integrin from MDA-MB-231mfp cells, but Mab-5G9 had no effect. Mab-5G9, but not Mab-10H10, coimmunoprecipitated β1 integrin with TF, further demonstrating different effects of the antibodies on TF-integrin association (Figure 5B). Treatment with Mab-10H10 reduced the detergent soluble pool of TF and reduced recovery of β1 in α3, but not α2 immunoprecipitates (Figure 5C). The loss of TF from Brij35 soluble fractions is possibly caused by interruption of normal trafficking of TF, because addition of fluorescently tagged Mab-10H10 to cells in tissue culture resulted in perinuclear accumulation (data not shown). TF is known to rapidly recycle and in part to localize to the perinuclear Golgi.41,42 Consistent with the results in HaCaT and endothelial cells, the small amount of TF detectable in α2 integrin immunoprecipitates was also not influenced by the antibody or VIIa treatment of cancer cells. Mab-10H10 blocked TF-β1 integrin coimmunoprecipitation from cells grown on laminin 5- or collagen-containing matrices (Figure 5D) indicating that constitutive association of TF with integrin is not regulated by β1 integrin ligation on specific extracellular matrix. Thus, Mab-10H10 has broad inhibitory activity on all direct TF signaling pathways and is unique in its ability to prevent constitutive formation of a TF-integrin complex in Brij35-soluble fractions of breast cancer cells.
Constitutive association of TF with integrins in breast cancer cells. (A) TF-integrin interaction in breast cancer cells is VIIa independent. MDA-MB-231mfp cells were treated with 50 μg/mL antibody for 10 minutes before addition of 10 nmol/L VIIa. Coimmunoprecipitation with α3β1 was quantified in 4 separate experiments, mean and standard deviations are shown. (B) Mab-10H10 does not precipitate the TF-β1 integrin complex. Cells were lysed, and lysate was subjected to immunoprecipitation with control antibody, β1 antibody, Mab-10H10, or Mab-5G9. Immunoprecipitates were analyzed for the presence of β1 and TF by Western blotting. (C) VIIa, Mab-10H10, and Mab-5G9 do not influence TF coimmunoprecipitation with α2 integrin. MDA-MB-231mfp cells were treated with 50 μg/mL antibody for 10 minutes before addition of 10 nmol/L VIIa. Coimmunoprecipitation of TF with α2 was analyzed by Western blot. (D) Constitutive association of TF with β1 integrins is independent of the extracellular matrix. Cells were grown on collagen 1 or a laminin 5-rich matrix and immunoprecipitated after 1 hour Mab-10H10 or Mab-5G9 treatment.
Constitutive association of TF with integrins in breast cancer cells. (A) TF-integrin interaction in breast cancer cells is VIIa independent. MDA-MB-231mfp cells were treated with 50 μg/mL antibody for 10 minutes before addition of 10 nmol/L VIIa. Coimmunoprecipitation with α3β1 was quantified in 4 separate experiments, mean and standard deviations are shown. (B) Mab-10H10 does not precipitate the TF-β1 integrin complex. Cells were lysed, and lysate was subjected to immunoprecipitation with control antibody, β1 antibody, Mab-10H10, or Mab-5G9. Immunoprecipitates were analyzed for the presence of β1 and TF by Western blotting. (C) VIIa, Mab-10H10, and Mab-5G9 do not influence TF coimmunoprecipitation with α2 integrin. MDA-MB-231mfp cells were treated with 50 μg/mL antibody for 10 minutes before addition of 10 nmol/L VIIa. Coimmunoprecipitation of TF with α2 was analyzed by Western blot. (D) Constitutive association of TF with β1 integrins is independent of the extracellular matrix. Cells were grown on collagen 1 or a laminin 5-rich matrix and immunoprecipitated after 1 hour Mab-10H10 or Mab-5G9 treatment.
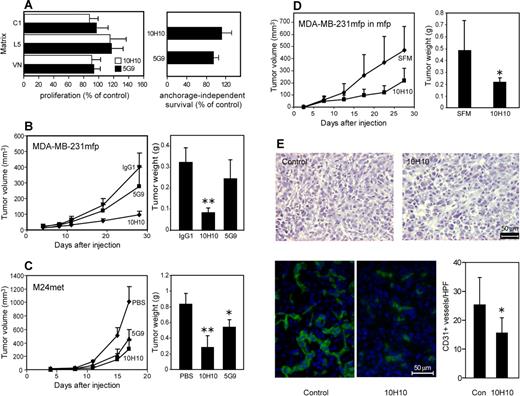
Inhibition of TF-VIIa signaling by Mab-10H10 was sufficient to inhibit tumor growth
Although Mab-10H10 had no apparent effect on arrested lung metastases in vivo, it was relevant to address whether Mab-10H10 inhibited proliferation of MDA-MD-231mfp cells. No effect of antibody treatment was observed when cells were plated on defined extracellular matrix proteins, and proliferation in serum was quantified after 48 to 72 hours (Figure 6A). We also measured survival of MDA-MD-231mfp cells in suspension culture in methylcellulose under serum-free conditions and found no evi-dence for enhanced anoikis in the presence of Mab-10H10 or Mab-5G9. These data are consistent with previous conclusions that TF expression on tumor cells is not required for in vitro proliferation, but specifically influences the biology of tumor growth in vivo.10,13
TF-VIIa signaling promotes tumor growth. (A) Mab-10H10 does not influence proliferation on different matrices. MDA-MB-231mfp cells were grown on collagen I, laminin 5, or vitronectin and treated with Mab-10H10 or Mab-5G9 (left graph). Cells were also plated in 0.5% methylcellulose for anchorage-independent cell growth (right graph). Proliferation was determined using an MTT assay, and anchorage-independent survival was quantified by cell counts. Values are expressed as percentage of control cells. (B) MDA-MB-231mfp subcutaneous tumor growth in the presence of 1 mg of control IgG1 (TIB115), Mab-5G9, or Mab-10H10 coinjected to achieve high local antibody concentrations in the flanks of SCID mice. Tumor weights were determined at sacrifice (n = 6, 2-sided ANOVA, Kruskal-Wallis; **P < .001). (C) Tumor growth of M24met melanoma cells injected subcutaneously without or with 1 mg of Mab-5G9 or Mab-10H10 (n = 8, 2-sided ANOVA, Kruskal-Wallis; **P < .001; *P < .01). (D) MDA-MB-231mfp tumor growth is inhibited in the mammary fat pad by treatment with 1 mg of coinjected Mab-10H10; SFM denotes serum-free medium control (n = 6, *P < .01). (E) Results of histologic examination of orthotopic MDA-MB-231mfp tumors grown in the presence or absence of Mab-10H10. Hematoxylin and eosin and CD31 staining of 5-μm frozen sections are shown. CD31 positive vessels were quantified, *, difference in vessel density between groups, t test, P < .005.
TF-VIIa signaling promotes tumor growth. (A) Mab-10H10 does not influence proliferation on different matrices. MDA-MB-231mfp cells were grown on collagen I, laminin 5, or vitronectin and treated with Mab-10H10 or Mab-5G9 (left graph). Cells were also plated in 0.5% methylcellulose for anchorage-independent cell growth (right graph). Proliferation was determined using an MTT assay, and anchorage-independent survival was quantified by cell counts. Values are expressed as percentage of control cells. (B) MDA-MB-231mfp subcutaneous tumor growth in the presence of 1 mg of control IgG1 (TIB115), Mab-5G9, or Mab-10H10 coinjected to achieve high local antibody concentrations in the flanks of SCID mice. Tumor weights were determined at sacrifice (n = 6, 2-sided ANOVA, Kruskal-Wallis; **P < .001). (C) Tumor growth of M24met melanoma cells injected subcutaneously without or with 1 mg of Mab-5G9 or Mab-10H10 (n = 8, 2-sided ANOVA, Kruskal-Wallis; **P < .001; *P < .01). (D) MDA-MB-231mfp tumor growth is inhibited in the mammary fat pad by treatment with 1 mg of coinjected Mab-10H10; SFM denotes serum-free medium control (n = 6, *P < .01). (E) Results of histologic examination of orthotopic MDA-MB-231mfp tumors grown in the presence or absence of Mab-10H10. Hematoxylin and eosin and CD31 staining of 5-μm frozen sections are shown. CD31 positive vessels were quantified, *, difference in vessel density between groups, t test, P < .005.
Mab-10H10 and Mab-5G9 are of the same IgG1 isotype and specifically react with human TF but not mouse TF. Therefore, these antibodies are excellent tools to specifically address the role of human tumor cell TF signaling in xenograft models. MDA-MB-231mfp cells implanted with Mab-10H10, but not Mab-5G9, showed significantly reduced final tumor sizes and tumor weights relative to tumors treated with isotype-matched control IgG1 (Figure 6B). Mab-5G9 slightly reduced tumor volumes which may be explained by Mab-5G9's partial inhibition of TF-VIIa mediated PAR2 signaling. However, to achieve substantial inhibition of this aggressive breast cancer model, inhibition of the TF-integrin interaction by Mab-10H10 appeared to be required.
We confirmed inhibition of tumor growth by Mab-10H10 in the M24met melanoma cell model. Mab-5G9 also retarded melanoma primary tumor growth, but Mab-10H10 was more potent and sufficient to reduce both final tumor volumes and weights (Figure 6C). We had previously shown in the melanoma model that Mab-5G9, but not Mab-10H10, inhibited lung metastases,5 similar to the data presented in Figure 1 for breast cancer cells. Thus, 2 different tumor models show that TF mainly serves as a procoagulant molecule to enhance early tumor cell arrest in the vasculature of the target organ, whereas primary tumor growth is sensitive to inhibition of direct TF signaling. Taken together, these data suggest that TF has distinct functional roles in tumor progression depending on the tumor microenvironment.
Breast cancer develops and spreads in the unique environment of the mammary gland. We therefore confirmed the inhibitory activity of Mab-10H10 on MDA-MB-231mfp cells placed in the mammary fat pad (Figure 6D). Breast cancer growth was attenuated similar to subcutaneous locations, confirming that TF-VIIa signaling also plays a crucial role in orthotopic sites of breast cancer development. In addition, Mab-10H10 treated tumors showed reduced microvessel density based on CD31 staining. Thus, inhibition of TF signaling reduced angiogenesis of tumors in orthotopic sites.
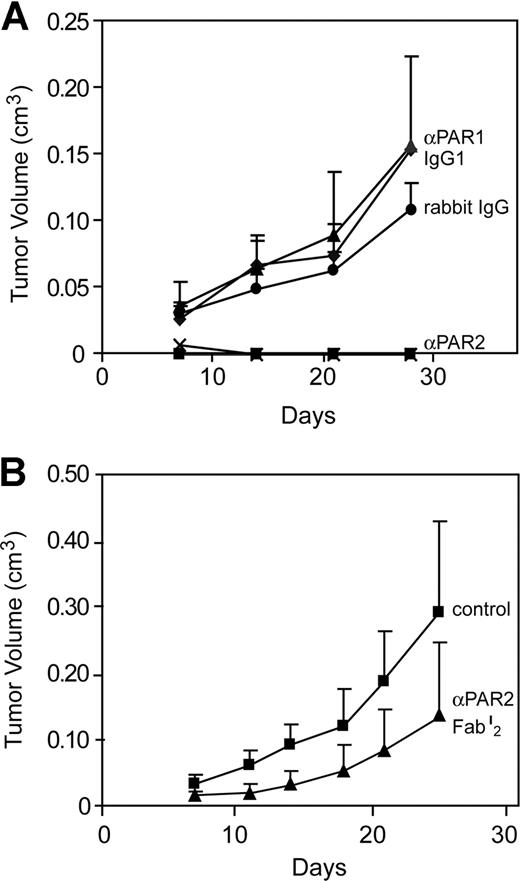
To further implicate TF-dependent PAR2 signaling, we implanted MDA-MB-231mfp cells with cleavage blocking antibodies to PAR1 or PAR2 (Figure 7A). Coinjection of anti-PAR2 com-pletely blocked tumor growth, whereas anti-PAR1, isotype-matched IgG1 control or preimmune IgG were without effect. The complete tumor eradication in anti-PAR2–treated animals indicated an additional effect of antibody-directed killing of PAR2-expressing tumor cells. To specifically define the effects of PAR2 signaling blockade, we treated another cohort of mice with purified Fab′2 fragments of the same polyclonal anti-PAR2 antibody, which also resulted in significant suppression of tumor growth (Figure 7B). These experiments confirmed that blockade of tumor cell PAR2 signaling suppressed breast cancer growth. Thus, 2 independent approaches implicate TF-VIIa-PAR2 signaling as a crucial mechanism that supports tumor growth in vivo.
Antibody inhibition of PAR2 but not PAR1 cleavage attenuated human breast cancer growth. (A) MDA-MD 231mfp cells were injected subcutaneously with 1 mg of rabbit anti-PAR2 polyclonal antibody, preimmune rabbit IgG, anti-PAR1 monoclonal antibody ATAP2 or murine IgG1 control (TIB115). A composite of 2 independent experiments is shown. Tumor volumes of anti-PAR2-treated groups were significantly different from all other groups by ANOVA, followed by Kruskal- Wallis, P < .001. (B) Inhibition of tumor growth in the absence of Fc-mediated killing is demonstrated by injecting tumor cells in the presence of 1 mg of anti-PAR2 Fab′2 fragment. The preparation of these experiments inhibited TF-VIIa-mediated activation of human PAR2 by more than 95%, but mouse PAR2 only marginally (<50%). Final tumor volumes were different at P < .05, t test.
Antibody inhibition of PAR2 but not PAR1 cleavage attenuated human breast cancer growth. (A) MDA-MD 231mfp cells were injected subcutaneously with 1 mg of rabbit anti-PAR2 polyclonal antibody, preimmune rabbit IgG, anti-PAR1 monoclonal antibody ATAP2 or murine IgG1 control (TIB115). A composite of 2 independent experiments is shown. Tumor volumes of anti-PAR2-treated groups were significantly different from all other groups by ANOVA, followed by Kruskal- Wallis, P < .001. (B) Inhibition of tumor growth in the absence of Fc-mediated killing is demonstrated by injecting tumor cells in the presence of 1 mg of anti-PAR2 Fab′2 fragment. The preparation of these experiments inhibited TF-VIIa-mediated activation of human PAR2 by more than 95%, but mouse PAR2 only marginally (<50%). Final tumor volumes were different at P < .05, t test.
Discussion
We here use TF antibodies with unique functional specificities to separate the contributions of TF-VIIa–dependent coagulation activation and direct cell signaling to tumor growth pathology in vivo. Mab-5G9 is a potent inhibitor of coagulation and ternary complex signaling, whereas Mab-10H10 selectively blocks TF-VIIa binary complex signaling without attenuating coagulation.31 We identify a new function that distinguishes the inhibitory profile of Mab-5G9 and Mab-10H10, which is perturbation of the TF-integrin interaction. These antibodies allowed us to clarify how the association between TF and integrins is regulated in noncancerous versus cancer cells. In keratinocytes and endothelial cells, VIIa promoted the interaction of TF with β1, α3, and α6, but not α2, integrin subunits. VIIa-induced association with integrins was independent of VIIa proteolytic activity or PAR2 signaling, consistent with a conformational effect of ligand VIIa binding to facilitate TF-integrin interaction. Likewise, the urokinase receptor associates with integrin α3β1 upon protease ligand binding.43 Active site-blocked VIIa has previously been shown to stimulate migration on certain tumor cells, but the underlying mechanism remained unclear.18 Our data suggest that VIIa-induced association with integrins may have triggered these protease-independent signaling effects by positioning the TF cytoplasmic domain near intracellular signaling adaptors of integrins. However, we consider it unlikely that VIIa binding alone is sufficient to induce the multiple steps required for cell motility in physiologic contexts.
Rather, we suggest that the association of the TF-VIIa with integrin serves to properly position the complex for activation of PAR2. Our data show that TF-VIIa recruited to integrins did not trigger coagulation. In addition, TF-VIIa-PAR2–mediated cytokine induction was enhanced by ligation of β1 integrins, suggesting that TF-VIIa and integrin signaling were linked. PAR2 activation stimulates cell motility through phosphatidylinositol 3-kinase and β-arrestin-dependent pathways.24,44,45 In the context of cell migration, recruitment of TF-VIIa to integrins may position PAR2 signaling optimally to relevant contacts with the extracellular matrix. Several studies have focused on the role of direct TF signaling in cell migration and invasion and variably implicated either binary TF-VIIa or ternary TF-VIIa-Xa complex signaling.22,23,46 The unique inhibitory profile of Mab-10H10 and the absence of detectable TF-VIIa-Xa signaling in highly aggressive breast cancer cells indicate that TF-VIIa signaling is sufficient for tumor promoting effects of TF in vivo. Tumor take and growth are probably dependent on a complex interplay of tumor cells with the extracellular matrix, as well as cross-talk of tumor and host cells via angiogenic regulators. Reduced vessel density in Mab-10H10–treated tumors indicated that the integrin-associated TF-VIIa-PAR2 signaling complex indeed regulated these tumor cell functions in the orthotopic tumor microenvironment.
In contrast to the regulated TF-integrin association on keratinocytes, TF was constitutively associated with integrins on highly aggressive breast cancer cells. This implies that cancer cells may have lost the ability to sense extracellular gradients of coagulation proteases and constitutively couple TF and integrin signaling. Although the precise mechanism for this coupling remains to be elucidated, ectopic synthesis of VIIa by tumor cells47 may contribute to the constitutive activation of PAR2 and integrin signaling in cancer cells. In addition to supporting cell migration, PAR2 signaling induces a diverse set of angiogenic regulators, chemokines, and antiapoptotic genes that are consistent with a wound-healing program.23,29 Constitutive synthesis of VIIa may sustain this program and contribute to the characteristic of the tumor microenvironment as a “wound that does not heal.”48
Several studies have shown that TF expression levels are correlated with clinical tumor progression.49,,–52 The reduced expression of TF in highly aggressive breast cancer cell line obtained by in vivo selection seems to contradict this notion. Although TF expression was reduced in these cells, PAR2 levels did not change and in vitro TF-VIIa signaling was only modestly attenuated in this aggressive line. This may indicate that cell culture adapted tumor cells up-regulate TF protein that is mainly nonfunctional or even inhibitory in vivo. Selection for aggressive tumor cells in vivo appears to maintain signaling active TF. This is in striking contrast to the finding in nontumorigenic cells, where differentiation induces down-regulation of TF and a concordant loss of TF-VIIa signaling.31 These data suggest that highly aggressive tumor cells have lost the ability to shut off the signaling pool of TF. Inhibition of tumor growth by Mab-10H10 provides evidence that interruption of the coupling of TF to integrins is sufficient to significantly attenuate the aggressive phenotype of tumor cells.
The presented data showed that direct TF-VIIa signaling was a major contributor to tumor growth in breast cancer. Inhibition of TF-VIIa may thus represent an effective approach to targeted cancer therapy. Although active site-directed inhibitors of VIIa are effective to inhibit both coagulation and direct signaling of the TF-VIIa complex, clinical application of potent anticoagulants remains associated with significant risks. For example, therapy with anticoagulants has previously been limited by the increased of risk of bleeding.53 Our data show that it is possible to selectively block direct TF-VIIa signaling without interfering with the coagulant activity of TF-VIIa. The possibility to safely inhibit TF signaling with minor effects on TF coagulant function provides an opportunity for aggressive intervention with direct TF signaling in cancer patients with impaired hemostasis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Pablito Tejada, Jennifer Royce, and Cindi Biazak for assistance.
This work was supported by National Institute of Health grant HL-60 742 (to W.R.) and Netherlands Scientific Organization grant S92-251 (to H.H.V.).
National Institutes of Health
Authorship
Contribution: H.H.V. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. F.S., M.K., H.H.P., and J.A. performed research and analyzed data. B.F.-H., and Y.T. provided vital reagents and resources. B.M.M. designed experiments and analyzed data. W.R. supervised the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfram Ruf, Department of Immunology, SP258, Scripps Research Institute, La Jolla, CA 92037; e-mail: ruf@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal