Abstract
The heparan sulfate proteoglycan syndecan-1 is expressed by myeloma cells and shed into the myeloma microenvironment. High levels of shed syndecan-1 in myeloma patient sera correlate with poor prognosis and studies in animal models indicate that shed syndecan-1 is a potent stimulator of myeloma tumor growth and metastasis. Overexpression of extracellular endosulfatases, enzymes which remove 6-O sulfate groups from heparan sulfate chains, diminishes myeloma tumor growth in vivo. Together, these findings identify syndecan-1 as a potential target for myeloma therapy. Here, 3 different strategies were tested in animal models of myeloma with the following results: (1) treatment with bacterial heparinase III, an enzyme that degrades heparan sulfate chains, dramatically inhibited the growth of primary tumors in the human severe combined immunodeficient (SCID-hu) model of myeloma; (2) treatment with an inhibitor of human heparanase, an enzyme that synergizes with syndecan-1 in promoting myeloma progression, blocked the growth of myeloma in vivo; and (3) knockdown of syndecan-1 expression by RNAi diminished and delayed myeloma tumor development in vivo. These results confirm the importance of syndecan-1 in myeloma pathobiology and provide strong evidence that disruption of the normal function or amount of syndecan-1 or its heparan sulfate chains is a valid therapeutic approach for this cancer.
Introduction
Syndecan-1 (CD138) is a cell-surface heparan sulfate–bearing proteoglycan that was first detected by polymerase chain reaction (PCR) in mRNA from human patients with myeloma and later confirmed by monoclonal antibody staining to be present on all myeloma tumors.1,2 Syndecan-1 is shed from the myeloma tumor cell surface and accumulates in the bone marrow and serum of patients. When present at high levels in the serum, syndecan-1 is an independent indicator of poor prognosis.3–5 However, high-serum syndecan-1 is more than simply an indicator of poor prognosis. Studies in animal models have shown that high levels of soluble syndecan-1 enhance both the growth and metastasis of tumors.6 Syndecan-1 exerts its growth-promoting effects by regulating the activity of many effector molecules important for myeloma growth and survival, including hepatocyte growth factor (HGF) and heparin-binding epidermal growth factor (HB-EGF) family members, among others.7,8 The high levels of heparan sulfate in the tumor microenvironment resulting from syndecan-1 shedding also act as positive regulators that condition the microenvironment for robust tumor growth. For example, heparan sulfate binds to and promotes the activity of important angiogenic growth factors such as fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF). This activity can occur in trans, indicating that shed syndecan-1 can contribute to the high level of angiogenesis seen in many patients with myeloma.9,10 In addition, the syndecan-1 that becomes embedded within the myeloma tumor stroma can serve as reservoir for storage and concentration of heparan sulfate–binding growth factors that can later be mobilized by cleavage of the heparan sulfate by heparanase.3,11 This mobilization of heparan sulfate–retained growth factors in the bone marrow likely contributes to the high rate of relapse among patients with myeloma
Because of its high level of expression on myeloma tumors, syndecan-1 has been explored as a candidate antigen for antibody targeting of toxins to the tumor cell surface.12–14 In addition, antibodies to syndecan-1 show promise as facilitators of myeloma immunotherapeutic approaches.15 However, specific strategies for disrupting the function of syndecan-1 or its heparan sulfate chains as a potential therapy for myeloma have not been reported. In general, targeting heparan sulfate proteoglycans as an approach to cancer therapy has been a topic of interest among cancer researchers, but testing of this approach in vivo has been limited.16 In a recent study, we demonstrated that high levels of expression of the extracellular endosulfatases HSulf-1 or HSulf-2 by a myeloma cell line attenuated tumor growth in vivo.17 These enzymes specifically remove 6-O sulfate groups from heparan sulfate chains, thereby altering signaling pathways of growth factors such as bone morphogenic protein (BMP), Wnt, and FGF-2.18–20 Our finding that enhanced HSulf expression alters heparan sulfate structure in vivo with a resulting impact on tumor growth provided the first evidence that heparan sulfate might be a target for myeloma therapy.
To test this idea further, we used 3 distinct approaches to target heparan sulfate or syndecan-1 in myeloma. These include directly targeting heparan sulfate for degradation with the enzyme bacterial heparinase III (HepIII); blocking the activity of human heparanase, an enzyme that activates heparan sulfate and stimulates myeloma growth and metastasis; and finally, by using shRNA to knockdown syndecan-1 expression by myeloma cells. All of these approaches, although distinct in their mechanism of action against heparan sulfate or syndecan-1, resulted in diminished myeloma growth in vivo. These studies confirm the importance of syndecan-1 in the pathobiology of myeloma and provide strong evidence that targeting syndecan-1 is a viable strategy for myeloma therapy.
Patients, materials, and methods
Myeloma cells
Myeloma cells were obtained from bone marrow aspirates of 8 patients of the Myeloma Institute for Research and Therapy (Little Rock, AR). This work was approved by the institutional review board of the University of Arkansas for Medical Sciences (Little Rock, AR). Signed institutional review board–approved consent forms are kept on record. Informed consent was obtained in accordance with the Declaration of Helsinki. The bone marrow samples were separated by Ficoll-Paque density centrifugation, and the proportion of myeloma cells present was determined by CD38/CD45 flow cytometry. Only samples having a minimum of 25% myeloma tumor cells present were used for the in vivo studies in human severe combined immunodeficient (SCID-hu) mice. The CAG myeloma cell line was established from a bone marrow aspirate taken from a patient with myeloma as previously described.21 CAG cells were transfected with empty vector only or vector containing the cDNA for human heparanase to generate the CAG heparanase–low and CAG heparanase–high cells, respectively. The methods for transfection and characterization of these cells have been previously published.22
In vivo models of myeloma
The SCID-hu model was constructed as previously described.6,23,24 Briefly, 5- to 6-week-old male CB.17 scid/scid mice were obtained from Harlan Sprague Dawley (Indianapolis, IN) and were housed and monitored in the animal facility at the University of Arkansas for Medical Sciences or the University of Alabama at Birmingham. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committees of the respective institutions. Human femora were cut into halves (approximately 5 × 5 × 10 mm) and implanted subcutaneously into each SCID mouse. At 6 to 8 weeks after implantation of bone, 1 × 107 cells of a bone marrow aspirate from a patient with myeloma were injected directly into the marrow cavity of the bone implanted in the SCID-hu host. Murine sera were collected weekly, and the levels of human immunoglobulin light chain (either kappa or lambda depending on the patient) were assessed as previously described as an indicator of tumor burden.6 Treatment was begun when human light chain present in the mouse serum reached a level, on average, of 36 μg/mL. Treatment with HepIII (15 mg/kg/day) or HepIII-generated fragments of heparan sulfate (0.8 mg/kg/day) was accomplished by twice-daily injections, one intraperitoneal and one subcutaneous, in the proximity of the human bone implant. For the subcutaneous model, 1 × 106 CAG cells were injected subcutaneously into the left flank of each mouse. For animals being treated with the modified heparin 100NA,RO-H (100% N-acetylated and 25% glycol-split heparin),25 10 days after injection of tumor cells, Alzet osmotic pumps (Durect Corporation, Cupertino, CA) were implanted on the right flank of each mouse. Pumps contained either the 100NA,RO-H at the indicated concentration or PBS as a control, and the solution was delivered continuously for 28 days. At the completion of the treatment period, animals were killed, and the wet weight of the subcutaneous tumors and the mean sera kappa level among the experimental groups were compared by the log-rank test (P ≤ .05 was considered statistically significant). For experiments with the syndecan-1 knockdown cells, 1 × 106 CAG control shRNA or syndecan-1 shRNA infected cells were injected subcutaneously and monitored for levels of kappa light chain present in the mouse serum. The experiment was terminated 8 weeks after injection of the tumor cells.
Preparation of recombinant HepIII, heparan sulfate fragments, and 100NA,RO-H
Recombinant HepIII was expressed, purified to homogeneity and cleared of endotoxins as described.26,27 Fragments of heparan sulfate generated by HepIII were collected by treating partially purified syndecan-1 that was isolated from the culture media of CAG cells overexpressing a form of syndecan-1 lacking its cytoplasmic and transmembrane domain, thus mimicking shed syndecan-1.28 Syndecan-1 (200 μg) in PBS was treated with HepIII (20 μg) at 37°C for 1 hour, followed by boiling for 15 minutes. Following sterile filtration, the concentration of heparan sulfate was determined by disaccharide analysis. Preparation and characterization of the heparanase inhibitor 100NA,RO-H was previously described in detail.25
Knockdown of syndecan-1 expression with shRNA
We used a sequence previously shown effective in siRNA knockout of syndecan-1 (1162AGGAGGAATTCTATGCCTGA1181)29 to design a synthetic double-stranded oligonucleotide sequence for shRNA knockdown studies (5′-CGCGTCCCCGGAGGAATTCTATGCCTGATTCAAGAGATCAGGCATAGAATTCCTCCTTTTTGGAAAT-3′). A control oligonucleotide sequence not matching any sequence in the human genome (5′-AATTCTCCGAACGTGTCACGT-3′; Qiagen, Valencia, CA)30 was used to design a control shRNA sequence (5′-CGCGTCCCCGTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACG TTCGGAGACTTTTTGGAAAT-3′). Both double-stranded shRNA sequences were obtained from Integrated DNA Technologies (Coralville, IA). The double-stranded oligonucleotides were cloned into pLVTHM, and virus was generated by cotransfection of 293FT cells with the pLVTHM vector and helper plasmids pMD2G and pCMV-dR8.91 (all kindly provided by Dr Didier Trono, University of Geneva, Switzerland). The crude lentivirus was filtered (0.45 μm), and viral titers were determined by measuring the percent of green fluorescent protein (GFP)–positive cells present 48 hours after infection of CAG cells. Stable cell lines expressing shRNA were achieved by infection at 50 multiplicity of infection (MOI). GFP+ CAG cells were sorted 2 times, after which cells were more than 99% positive. The reduction of syndecan-1 expression was confirmed by reverse transcription (RT)–PCR, flow cytometry, and Western blotting. For PCR, the forward and reverse primers were 5′-CTTCACACTCCCCACACAGA-3′ (forward) and 5′-TCCTGTTTGGTGGGCTTCTG-3′ (reverse) for syndecan-1 and 5′-ACCACAGTCCATGCCCATCAC-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). After initial denaturation at 95°C for 2 minutes, PCR was carried out for 32 cycles (95°C for 1 minute; 58°C for 1 minute; and 72°C for 1 minute). PCR products were separated by 1.2% agarose gel electrophoresis and visualized by ethidium bromide staining. The size of PCR product was 396 bp for syndecan-1 and 452 bp for GAPDH, respectively. For flow cytometry, cells were stained with antibody B-A38 (Serotec, Raleigh, NC), followed by anti-mouse conjugated to Alexa 647 (Invitrogen, Carlsbad, CA). For Western blotting, cell extracts were separated on 4% to 15% gradient SDS-PAGE, transferred to Nytran+ filters (Whatman/Schleicher & Schuell, Florham Park, NJ), probed with antibody B-A38 followed by a horseradish peroxidase–conjugated secondary anti-mouse antibody, and visualized by chemiluminescence (GE Healthcare, Pittsburgh, PA).
MicroCT analysis of bones
Upon termination of the experiment, the implanted human bones were removed from SCID-hu mice and fixed in phosphate-buffered 10% formalin (pH 7.4) for a minimum of 24 hours. Bones were then dehydrated successively in 70%, 95%, and 100% ethanol and analyzed using a microcomputed tomography (microCT) 40 (Scanco Medical, Bassersdorf, Switzerland) using a voxel size of 12 μm in all dimensions, as described previously31,32 Briefly, the region of interest selected for analysis was the entire excised specimen and comprised approximately 1000 transverse CT slices. Three-dimensional reconstructions were created by stacking the regions of interest from each two-dimensional slice, then applying a grayscale threshold and Gaussian noise filter specifically optimized for human bone.
Results
Treatment with HepIII inhibits growth of primary myeloma tumors in vivo
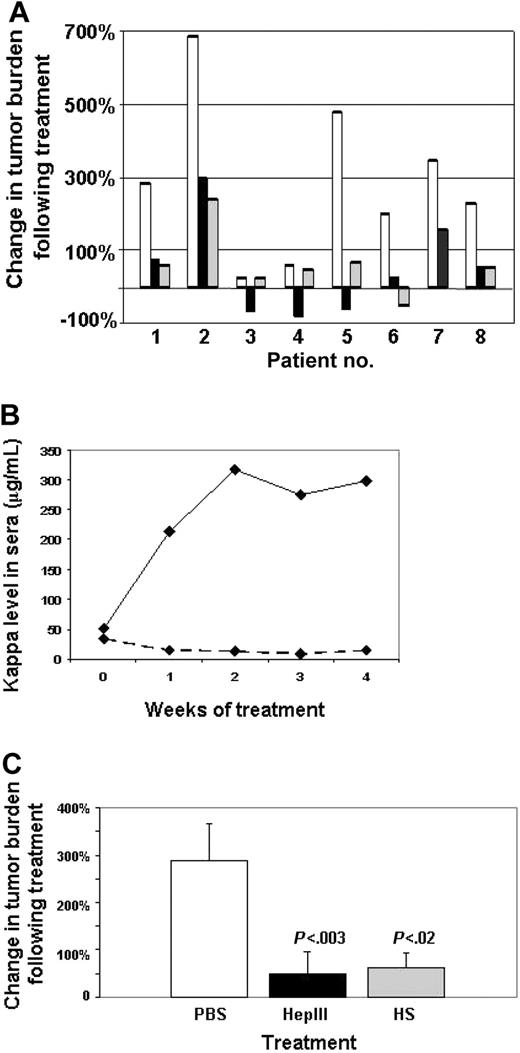
When administered in vivo, the heparan sulfate–degrading enzyme bacterial HepIII has been shown to inhibit the growth and metastasis of both melanoma and lung carcinoma tumors in animal models.26 This effect is thought to be due to HepIII-mediated release of heparan sulfate fragments that possess antitumor activity. Because of the abundant heparan sulfate in the myeloma microenvironment due to the presence of both cell-surface and shed syndecan-1, we tested the effects of this enzyme on established myeloma tumors. Daily injections of HepIII into the animals bearing subcutaneous myeloma tumors substantially inhibited tumor growth (data not shown). To extend these studies, we established tumors by injecting cells from the bone marrow of patients with myeloma into human bones implanted in SCID mice (SCID-hu model).23 Once engraftment of the human tumor was confirmed by detection of human light chain in the serum of the mice, animals were treated by injection of HepIII (15 mg/kg/day). At the end of the treatment period, light chain levels, as a measure of tumor burden, were assessed. As expected, among the 8 patient tumors tested, there is considerable variability in rates of tumor growth (Figure 1A). However, in all cases, tumor growth was attenuated in animals treated with HepIII. In the case of 3 tumors, levels of light chain were lower than at the start of treatment, suggesting that tumor growth was arrested by the HepIII treatment (patients 3, 4, and 5). Even the most aggressive tumor, which exhibited a 700% increase in light chain level, was substantially retarded in its growth by treatment with HepIII (patient 2). The most dramatic response was seen in the tumor established from patient 5, where light chain levels increased almost 500% in the control animal but were decreased by 58% in the HepIII-treated animal (Figure 1B).
HepIII inhibits growth of primary myeloma tumors in vivo. (A) Tumors formed by cells from patients with myeloma were established in the SCID-hu host and then treated for 28 days (patients 1, 2, 4, and 5), 21 days (patients 6-8), or 14 days (patient 3) by daily injection of either PBS (□), active recombinant HepIII enzyme (■), or HepIII-generated heparan sulfate fragments (▩). At the end of the treatment period, human light chain levels in the serum of the mice were analyzed and plotted as the percentage increase or decrease over the light chain level present at the time treatment was initiated. (B) Levels of kappa light chain measured at weekly intervals in animals bearing tumor from patient 5 during treatment with PBS (–) or HepIII (-------). (C) Percentage change in tumor burden following treatment as measured by levels of human light chain in the serum of mice. Bars show the combined mean percentage change of all 8 patients plus or minus SEM following treatment with PBS, HepIII, or heparan sulfate (HS) fragments generated ex vivo by HepIII.
HepIII inhibits growth of primary myeloma tumors in vivo. (A) Tumors formed by cells from patients with myeloma were established in the SCID-hu host and then treated for 28 days (patients 1, 2, 4, and 5), 21 days (patients 6-8), or 14 days (patient 3) by daily injection of either PBS (□), active recombinant HepIII enzyme (■), or HepIII-generated heparan sulfate fragments (▩). At the end of the treatment period, human light chain levels in the serum of the mice were analyzed and plotted as the percentage increase or decrease over the light chain level present at the time treatment was initiated. (B) Levels of kappa light chain measured at weekly intervals in animals bearing tumor from patient 5 during treatment with PBS (–) or HepIII (-------). (C) Percentage change in tumor burden following treatment as measured by levels of human light chain in the serum of mice. Bars show the combined mean percentage change of all 8 patients plus or minus SEM following treatment with PBS, HepIII, or heparan sulfate (HS) fragments generated ex vivo by HepIII.
As an alternative to treating tumors with the HepIII enzyme, we prepared fragments of heparan sulfate ex vivo by treating syndecan-1 isolated from CAG myeloma cells with HepIII.26 The fragments released by the enzyme were harvested and then injected into SCID-hu animals bearing myeloma patient tumor. Although not as potent as the enzyme itself, these heparan sulfate fragments exhibited significant antitumor activity (Figure 1A). When the increases in tumor burden from all 8 patients were combined, treatment with either HepIII or the HepIII-generated heparan sulfate fragments significantly inhibited the growth of the primary myeloma tumors as compared with controls (Figure 1C).
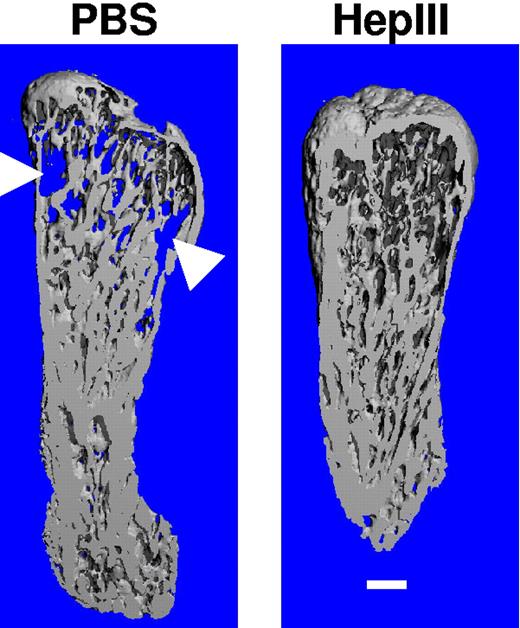
At termination of the experiment, microCT scanning of the human bones harvested from animals reveals protection by HepIII from the bone destruction associated with myeloma tumor growth (Figure 2). Not all patient tumors induced the same extent of osteolysis as the one shown in Figure 2, but in several cases when there was significant osteolysis in contols, in comparison, degradation was diminished in bones harvested from the HepIII-treated animals.
Treatment with HepIII protects implanted bones from osteolysis. At the termination of the experiment, implanted human bones were excised and imaged by microcomputed tomography (microCT). Shown are the three-dimensional reconstructions of the bones (sliced longitudinally through the midpoint of each specimen) injected with cells from patient 4 followed by treatment of the animal with either PBS (excessive bone resorption is seen) or HepIII (no bone resorption is observed, trabecular bone is intact). The areas of increased osteoclastic bone resorption in the PBS-treated sample are indicated by arrowheads. Note the appearance of the blue background behind the bone from the PBS-treated sample, indicative of resorption through the entire specimen. Scale bar equals 1 mm.
Treatment with HepIII protects implanted bones from osteolysis. At the termination of the experiment, implanted human bones were excised and imaged by microcomputed tomography (microCT). Shown are the three-dimensional reconstructions of the bones (sliced longitudinally through the midpoint of each specimen) injected with cells from patient 4 followed by treatment of the animal with either PBS (excessive bone resorption is seen) or HepIII (no bone resorption is observed, trabecular bone is intact). The areas of increased osteoclastic bone resorption in the PBS-treated sample are indicated by arrowheads. Note the appearance of the blue background behind the bone from the PBS-treated sample, indicative of resorption through the entire specimen. Scale bar equals 1 mm.
Inhibition of myeloma growth in vivo by an inhibitor of heparanase
As a second approach to test targeting of heparan sulfate in myeloma tumors, we used an inhibitor of human heparanase. Heparanase is distinct in its action from that of bacterial HepIII, and in contrast to the antitumor activity of HepIII, human heparanase has been shown to promote the growth and metastasis of many types of human tumors.11 In patients with myeloma, enzymatically active heparanase is present in the bone marrow and is associated with a high microvessel density and poor prognosis.33,34 Overexpression of heparanase in CAG myeloma cells substantially enhances their growth and spontaneous metastasis to bone.22 In addition, heparanase increases the synthesis and shedding of syndecan-1 by myeloma cells, thereby contributing to myeloma progression by elevating levels of syndecan-1 in the tumor microenvironment.34,35 Thus, we reasoned that inhibitors of heparanase activity may have a dramatic impact on the growth of myeloma tumors in vivo. Recently, a series of chemically modified heparins were shown to be potent inhibitors of heparanase in vitro.25 An important property of these modified heparins is that they lack anticoagulant activity and thus can be administered in vivo at relatively high doses. We chose to use a modified heparin called 100NA,RO-H that is 100% N-acetylated and 25% glycol-split. At a concentration of 0.2 μg/mL, this compound inhibits 93.8% of heparanase activity but does not displace extracellular matrix-bound FGF-2 or potentiate its growth promoting activity.25
A total of two experiments testing the effectiveness of this compound were performed, one using CAG myeloma cells expressing high levels of heparanase, and one using CAG myeloma cells expressing low levels of heparanase. We have previously demonstrated that the CAG cells expressing high levels of heparanase exhibit very aggressive growth and metastatic behavior in vivo as compared with control cells that express low levels of the enzyme.22 For the present studies, we used a subcutaneous tumor model similar to those that have been used extensively for testing new therapeutics for their efficacy against myeloma.36–38 Tumor cells were injected subcutaneously at a single site in each mouse.
After allowing 10 days for tumors to establish, Alzet osmotic pumps were inserted into animals to deliver either PBS or the 100NA,RO-H for 28 days. Results demonstrate a concentration-dependent antitumor effect of the modified heparin on both the heparanase-high and heparanase-low cells (Figure 3; Table 1,2). At the 36 mg/kg per day concentration tested in the very aggressive heparanase-high cells, tumor was found in only 1 of the 6 animals. The levels of serum kappa light chain demonstrated that in addition to the absence of tumor at the subcutaneous site, there was not widespread dissemination of tumor to other sites.
An inhibitor of heparanase activity inhibits myeloma tumor growth in vivo. Established subcutaneous tumors formed by CAG myeloma cells expressing heparanase at either high (HPSEhigh) or low (HPSElow) levels were treated for 28 days with PBS or 100NA,RO-H at doses of 18 mg/kg per day (both HPSEhigh and HPSElow cells) or 36 mg/kg per day (HPSEhigh cells only). At termination of the treatment period, tumors were harvested and photographed. X indicates no gross tumor detectable in the animal. Tumor wet weights and P values are shown in Tables 1 and 2.
An inhibitor of heparanase activity inhibits myeloma tumor growth in vivo. Established subcutaneous tumors formed by CAG myeloma cells expressing heparanase at either high (HPSEhigh) or low (HPSElow) levels were treated for 28 days with PBS or 100NA,RO-H at doses of 18 mg/kg per day (both HPSEhigh and HPSElow cells) or 36 mg/kg per day (HPSEhigh cells only). At termination of the treatment period, tumors were harvested and photographed. X indicates no gross tumor detectable in the animal. Tumor wet weights and P values are shown in Tables 1 and 2.
Effects of 100NA,RO-H on in vivo growth of CAG heparanase-high myeloma cells
| Mouse tag no. . | 100NA,RO-H, mg/kg per d . | Tumor wet weight,* mg . | Serum kappa light chain,† ng/mL . |
|---|---|---|---|
| OBOB | 0 | 1390 | 23 271 |
| OA14 | 0 | 350 | 4 796 |
| 1310 | 0 | 240 | 2 419 |
| 176D | 0 | 170 | 2 265 |
| 2A0C | 0 | 100 | 3 956 |
| 532B | 18 | 80 | 582 |
| 3D4A | 18 | 50 | 482 |
| 7003 | 18 | 30 | 34 |
| 956 | 18 | 20 | 101 |
| 283C | 18 | 10 | 26 |
| 6464 | 18 | 1 | 5 |
| 4972 | 18 | 0 | 0 |
| 6D6B | 36 | 5 | 197 |
| 5A67 | 36 | 0 | 0 |
| 737 | 36 | 0 | 2 |
| 7253 | 36 | 0 | 0 |
| 7670 | 36 | 0 | 5 |
| 7732 | 36 | 0 | 0 |
| Mouse tag no. . | 100NA,RO-H, mg/kg per d . | Tumor wet weight,* mg . | Serum kappa light chain,† ng/mL . |
|---|---|---|---|
| OBOB | 0 | 1390 | 23 271 |
| OA14 | 0 | 350 | 4 796 |
| 1310 | 0 | 240 | 2 419 |
| 176D | 0 | 170 | 2 265 |
| 2A0C | 0 | 100 | 3 956 |
| 532B | 18 | 80 | 582 |
| 3D4A | 18 | 50 | 482 |
| 7003 | 18 | 30 | 34 |
| 956 | 18 | 20 | 101 |
| 283C | 18 | 10 | 26 |
| 6464 | 18 | 1 | 5 |
| 4972 | 18 | 0 | 0 |
| 6D6B | 36 | 5 | 197 |
| 5A67 | 36 | 0 | 0 |
| 737 | 36 | 0 | 2 |
| 7253 | 36 | 0 | 0 |
| 7670 | 36 | 0 | 5 |
| 7732 | 36 | 0 | 0 |
Mean tumor weights: No 100NA,RO-H, 450 mg; 18 mg/kg per day group, 30 mg; 36 mg/kg per day group (1 tumor only), 5 mg. No 100NA,RO-H group versus 18 mg/kg per day group, P = .029. No 100NA,RO-H group versus 36 mg/kg per day group, P = .033.
Mean kappa levels at termination of treatment: No 100NA,RO-H = 7341 ng; 18 mg/kg per day group, 176 ng; 36 mg/kg per day group (1 tumor only), 34 ng. No 100NA,RO-H group versus 18 mg/kg per day group, P = .028. No 100NA,RO-H group versus 36 mg/kg per day group, P = .037.
Effects of 100NA,RO-H on in vivo growth of CAG heparanase-low myeloma cells
| Mouse tag no. . | 100NA,RO-H, mg/kg per d . | Tumor wet weight,* mg . | Serum kappa light chain,† ng/mL . |
|---|---|---|---|
| 502B | 0 | 400 | 7424 |
| 3F44 | 0 | 140 | 2676 |
| 133F | 0 | 200 | 2325 |
| 3648 | 0 | 110 | 1407 |
| 4B1F | 0 | 130 | 1432 |
| 611A | 0 | 100 | 3461 |
| 1949 | 18 | 50 | 523 |
| 793A | 18 | 30 | 6 |
| C573 | 18 | 0 | 2 |
| 353A | 18 | 0 | 0 |
| 7C23 | 18 | 0 | 4 |
| 5D4B | 18 | 0 | 116 |
| Mouse tag no. . | 100NA,RO-H, mg/kg per d . | Tumor wet weight,* mg . | Serum kappa light chain,† ng/mL . |
|---|---|---|---|
| 502B | 0 | 400 | 7424 |
| 3F44 | 0 | 140 | 2676 |
| 133F | 0 | 200 | 2325 |
| 3648 | 0 | 110 | 1407 |
| 4B1F | 0 | 130 | 1432 |
| 611A | 0 | 100 | 3461 |
| 1949 | 18 | 50 | 523 |
| 793A | 18 | 30 | 6 |
| C573 | 18 | 0 | 2 |
| 353A | 18 | 0 | 0 |
| 7C23 | 18 | 0 | 4 |
| 5D4B | 18 | 0 | 116 |
Mean tumor weights: No 100NA,RO-H, 180 mg; 18 mg/kg per day group, 40 mg (two tumors only). No 100NA,RO-H group versus 18 mg/kg per day group, P = .003.
Mean kappa levels at termination of treatment: No 100NA,RO-H, 3121 ng; 18 mg/kg per day group, 109 ng; No 100NA,RO-H S group versus 18 mg/kg per day group, P = .004.
Myeloma cells with reduced expression of syndecan-1 grow poorly in vivo
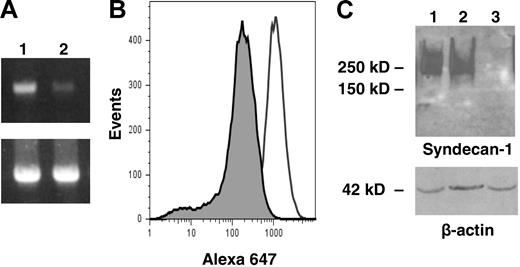
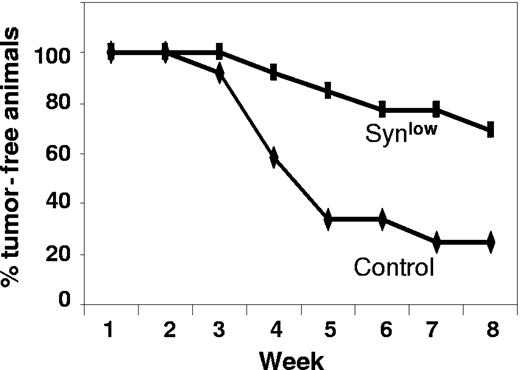
As final confirmation that syndecan-1 is a viable target for myeloma therapy, we used an shRNA to knock down expression of syndecan-1 in the CAG myeloma cell line. We hypothesized that a reduction in syndecan-1 expression would diminish the level of heparan sulfate at the cell surface and in the tumor microenvironment. A 10-fold reduction in the level of cell-surface syndecan-1 was demonstrated by flow cytometric analysis in the knockdown cells as compared with controls, and on Western blots, syndecan-1 from the knockdown cells was below the level of detection (Figure 4). When injected subcutaneously into SCID mice, cells with diminished levels of syndecan grew poorly. In the first experiment, 8 weeks after injection of cells with low syndecan-1 expression, none of the animals formed tumors at the site of inoculation (0 of 6 animals developed tumor; mean kappa light chain level, 0). In contrast, controls expressing normal levels of syndecan-1 formed tumors (4 of 6 animals developed tumor; mean kappa light chain level, 2533 ng/mL). In a second experiment, 4 of the 7 animals bearing cells with syndecan-1 knockdown did develop tumors, but the onset of tumor development was delayed compared with that of controls (data not shown). Figure 5 includes data from both experiments and reflects the overall decrease and delay in tumor development in the cells expressing low levels of syndecan-1 compared with that of controls. Interestingly, at termination of the second experiment, when tumors were removed from animals and immunostained for syndecan-1 expression, those formed from cells in which syndecan-1 expression had been knocked down exhibited strong expression of syndecan-1 (data not shown). Because the antibody used for staining syndecan-1 is human specific, this result indicates that the level of syndecan-1 expression eventually increased in the knockdown cells to relatively high levels.
shRNA reduces syndecan-1 expression on myeloma cells. (A) Expression of syndecan-1 mRNA by lentiviral-infected CAG cells was quantified by PCR (lane 1, control shRNA cells; lane 2, syndecan-1 shRNA cells). Bottom panel shows GAPDH control. Bands migrated to their predicted location relative to standards with sizes of 396 bp for syndecan-1 and 452 bp for GAPDH, respectively. (B) Flow cytometry showing cell-surface expression of syndecan-1 on shRNA control (open peak) or shRNA syndecan-1 (shaded peak) cells. (C) Western blot for syndecan-1 from CAG wild-type (lane 1), control shRNA (lane 2), and syndecan-1 shRNA cells (lane 3).
shRNA reduces syndecan-1 expression on myeloma cells. (A) Expression of syndecan-1 mRNA by lentiviral-infected CAG cells was quantified by PCR (lane 1, control shRNA cells; lane 2, syndecan-1 shRNA cells). Bottom panel shows GAPDH control. Bands migrated to their predicted location relative to standards with sizes of 396 bp for syndecan-1 and 452 bp for GAPDH, respectively. (B) Flow cytometry showing cell-surface expression of syndecan-1 on shRNA control (open peak) or shRNA syndecan-1 (shaded peak) cells. (C) Western blot for syndecan-1 from CAG wild-type (lane 1), control shRNA (lane 2), and syndecan-1 shRNA cells (lane 3).
shRNA inhibits and delays development of myeloma tumors. CAG cells infected with control shRNA (control) or syndecan-1 shRNA (Synlow) were injected subcutaneously into mice. Animals were considered positive for tumor formation when serum kappa light chain levels reached 50 ng/mL. The plot shows the percentage of animals that were free of tumor at weekly intervals for 8 weeks after injection of tumor cells.
shRNA inhibits and delays development of myeloma tumors. CAG cells infected with control shRNA (control) or syndecan-1 shRNA (Synlow) were injected subcutaneously into mice. Animals were considered positive for tumor formation when serum kappa light chain levels reached 50 ng/mL. The plot shows the percentage of animals that were free of tumor at weekly intervals for 8 weeks after injection of tumor cells.
Discussion
The data presented here demonstrate that myeloma growth is substantially inhibited in vivo by approaches that target either heparan sulfate or syndecan-1. In addition, our previously published work shows that modulation of the sulfation pattern within the heparan sulfate chains expressed by myeloma cells can also inhibit tumor growth.17 Collectively, these results provide strong proof of principle supporting the idea that targeting of heparan sulfate or syndecan-1 within the tumor microenvironment is a viable therapeutic approach for myeloma.
HepIII is an enzyme whose activity generates heparan sulfate fragments with antitumor activity.26 Myeloma may be a particularly good target for this enzyme because of the high levels of syndecan-1 heparan sulfate at the cell surface and shed into the tumor microenvironment. The activity of the enzyme on tumor-associated heparan sulfate would likely impact the tumor in 2 ways. First, as mentioned, antitumor heparan sulfate fragments would be generated. Second, because syndecan-1 with its heparan sulfate chains is a promoter of tumor growth and metastasis in vivo,6 its degradation by HepIII would diminish the positive growth effects of the proteoglycan. Importantly, our studies here used primary myeloma tumor cells growing in human bone, suggesting that HepIII can exert its antimyeloma effect even on an established tumor growing in a microenvironment where the tumor normally thrives. Growth inhibition was also accomplished by treating animals with heparan sulfate fragments generated by HepIII, indicating that in this myeloma model, the fragments have direct antitumor activity or are competing with the endogenous tumor heparan sulfate, thereby blocking their ability to promote tumor growth. Whatever the mode of action, these findings indicate that the ex vivo identification and characterization of the specific fragments of heparan sulfate with antitumor activity could provide a powerful new therapeutic tool and circumvent the problems associated with using the HepIII enzyme itself as a therapy.
The finding that 100NA,RO-H, an engineered heparin-based compound with no anticoagulant activity, can dramatically inhibit heparanase activity and myeloma growth in vivo provides yet another proteoglycan-based approach to treat this cancer. It is noteworthy that in these studies, the highly aggressive CAG cell line was used. Like many other myeloma cell lines, CAG cells cluster with the most aggressive of myeloma tumors as assessed by gene profiling.39 In addition, these cells are capable of growing outside the bone marrow microenvironment, a characteristic similar to many highly aggressive late-stage clinical myeloma tumors. In addition, to challenge the effectiveness of the antiheparanase compound, we used CAG cells overexpressing heparanase. These cells exhibit an enhanced ability to grow and spontaneously metastasize to bone.22 In spite of this overexpression of heparanase, 100NA,RO-H was a potent inhibitor of CAG tumor growth and metastasis and significantly inhibited tumor growth even at the lower concentration tested (18 mg/kg per day). At the higher concentration tested (36 mg/kg per day), tumor was found in only 1 animal of the 6 that were treated. Interestingly, in animals bearing tumors formed by the heparanase-low CAG cells, the 18 mg/kg per day concentration of 100NA,RO-H had a greater antitumor effect than it did on the heparanase-high cells (Figure 3; Tables 1,2). This suggests that the effect of 100NA,RO-H is related to its antiheparanase activity. Moreover, it indicates that this compound can be effective against tumors that express relatively low levels of the enzyme. Importantly, the treated animals showed no sign of adverse side effects, even though the modified heparin was delivered constantly and at high concentrations for 28 days.
Due to the correlation of heparanase expression with aggressive behavior in many tumor types, it has been widely speculated that heparanase represents an important therapeutic target.11,40–42 Numerous compounds with antiheparanase activity have been identified, and one, PI-88, is currently in clinical trials.43–45 The inhibitory action of 100NA, RO-H is thought to occur due to its tight binding to heparanase facilitated by the enhanced flexibility of the heparin afforded by the glycol splitting, which opens the sugar ring. In addition, once bound, the heparanase cannot cleave these heparins. The modified heparins such as the one used here provide several advantages as potential therapeutics. Heparin is readily available, and there is a considerable amount of information regarding its safety in humans. Chemical modifications can be controlled and analytical techniques to verify its composition are well established, thereby making scale-up production for human therapeutics feasible.25
Knockdown of syndecan-1 expression using shRNA dramatically diminished the ability of CAG cells to form subcutaneous tumors in vivo. This is particularly important because the ability of cell lines to grow outside of the bone marrow microenvironment reflects a robust propensity for growth and survival. Thus, the poor growth of these cells in vivo following a 10-fold reduction of syndecan-1 expression suggests that even very aggressive myeloma tumors need syndecan-1 for their growth in vivo. Interestingly, and in contrast to our findings in vivo, the in vitro growth rate of the CAG cells was not affected by diminished syndecan-1 expression (data not shown). These data suggest that the poor growth of low syndecan-1 expressers in vivo is related to the inability of the cells to condition the tumor microenvironment in a manner that supports tumor survival and subsequent growth. Due to the role of heparan sulfate as a coreceptor for proangiogenic growth factors such as VEGF and FGF-2, the poor growth of tumors with reduced syndecan-1 expression could reflect a poor angiogenic response of the host to the tumor.
The observation that syndecan-1 levels are relatively high in tumors that eventually do grow out from the syndecan-1 knockdown cells is also important. This suggests that after injection of the tumor cells, they either lose expression of the shRNA or that a subpopulation of the knockdown cells that retained relatively high levels of syndecan-1 expression are able to grow preferentially and eventually form tumors. It has been demonstrated that a small population of syndecan-1− cells is present in myeloma cell lines and clinical material, and that these negative cells have greater clonogenic potential than syndecan-1+ cells.46 In light of that finding, it is interesting that in our model, the syndecan-1− population within the CAG knockdown population does not emerge as the predominant cell type in the tumors that do form. One possibility is that the syndecan-1− cells do seed the tumor and eventually shift to a syndecan-1+ phenotype. However, the fact that many of the animals injected with syndecan-1 knockdown cells do not form tumors at all suggests that in the CAG cell line, the syndecan-1− cell population does not have the potential to form tumors in vivo.
Another possibility that would explain the delayed growth of the tumors forming from syndecan-1 knockdown cells is that over time, enough shed syndecan-1 accumulates in the tumor microenvironment to promote tumor growth. Although a reduction in heparan sulfate levels within the tumor due to diminished syndecan-1 expression likely has important effects, we cannot rule out the possibility that the syndecan-1 core protein also plays a role in promoting tumor growth. Interestingly, the core protein of syndecan-1 was recently shown to regulate activation of αvβ3 and αvβ5 integrins, which act as regulators of myeloma cell invasion and angiogenesis.29,47–49
Why does targeting syndecan-1 or enzymes that modify syndecan-1 heparan sulfate have such a dramatic impact on myeloma tumor growth and progression? Our working hypothesis is that syndecan-1 acts as a master regulator that promotes the activity of many signaling molecules within the tumor microenvironment. Via these multiple regulatory activities, syndecan-1 drives robust tumor growth and progression. For example, hepatocyte growth factor (HGF), a growth factor known to be up-regulated in many myeloma tumors,39 binds to the heparan sulfate of syndecan-1 and helps potentiate signaling via the cMET receptor with resulting cell proliferation.7 In addition to its activity on the myeloma cell surface, syndecan-1 is shed from myeloma tumor cells and can accumulate in the tumor microenvironment.3 High levels of shed syndecan-1 correlate with poor patient prognosis, and animal studies have demonstrated that myeloma tumor growth and dissemination are enhanced when levels of shed syndecan-1 are increased.4–6 The growth stimulatory effects of shed syndecan-1 are likely due to numerous interactions of the proteoglycan within the microenvironment. Heparan sulfate is known to regulate the activity of angiogenic growth factors such as FGF-2 and VEGF, and we have found that myeloma cells engineered to express high levels of soluble syndecan-1 form tumors with an elevated microvessel density compared with controls (unpublished data). This is consistent with published work showing a positive correlation between high levels of syndecan-1 in the serum of patients with myeloma and elevated tumor microvessel density.50 The heparan sulfate chains of syndecan-1 may also regulate bone turnover in myeloma due to their interaction with OPG and DKK, 2 heparin-binding molecules that are important modulators of myeloma bone disease.51–53 Thus, the heparan sulfate proteoglycans may aid in molecular events that trigger increased bone turnover, which further stimulates tumor growth. Collectively, these and other pathways regulated by syndecan-1 and/or heparan sulfate point to a major role for syndecan-1 in regulating myeloma tumor growth and progression.
Although syndecan-1 is the most abundant source of heparan sulfate in the myeloma tumor microenvironment, there are other heparan sulfate proteoglycans present. For example, endothelial cells have been shown to express multiple heparan sulfate proteoglycans that participate in regulating the angiogenic response.54,55 Using an antisense RNA approach, specific targeting of perlecan, the basement membrane heparan sulfate proteoglycan, was effective in blocking growth and angiogenesis of both colon carcinoma and melanoma.56 An advantage of the approach we use here with both HepIII and antiheparanase strategies is that they would target not only syndecan-1, but other heparan sulfates or heparan sulfate proteoglycans present in the myeloma microenvironment.
The finding that multiple strategies targeting the syndecan-1/heparan sulfate/heparanase axis are effective against myeloma provides strong proof of principle for the approach. Because there is a high degree of genetic chaos within most myeloma tumors, agents that target a single signaling pathway may have limited therapeutic value. The concept of targeting a master regulator of multiple signaling pathways such as syndecan-1 and its heparan sulfate chains may be especially effective against myeloma and thus provides a new therapeutic avenue for attacking this cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Didier Trono, University of Geneva, Switzerland, for plasmids used to construct lentiviral vectors.
This work was supported in part by grant P01 CA55819 (B.B., J.E., J.S., R.D.S.) and CA 103054 (R.D.S.) from the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: Y.Y. designed and performed the research and analyzed and interpreted the data; V.M. prepared constructs, transfected cell lines, and assisted with animal studies; Y.D. prepared shRNA constructs, infected cells, and performed shRNA animal studies; Y.S. performed shRNA animal studies; Z.S., G.V., and R.S. provided recombinant HepIII and assisted in design of HepIII-related experiments; A.N., G.T., and B.C. provided 100NA,RO-H and assisted in design of related experiments; I.V. characterized the antiheparanase activity of 100NA,RO-H; L.J.S. performed microCT analysis of bones; J.E. provided clinical material; S.Y. provided assistance and advice related to the SCID-hu model; J.D.S. provided clinical material; B.B. provided clinical material and clinical data; and R.D.S. conceptualized and designed the research, analyzed and interpreted the data, and wrote the paper.
Conflict of interest disclosure: Two of the authors (Z.S., G.V.) are employed by Momenta Pharmaceuticals, whose potential product was studied in the present work. One of the authors (R.S.) has declared a financial interest in Momenta Pharmaceuticals, whose potential product was studied in the present work. All other authors declare no competing financial interests.
Correspondence: Ralph D. Sanderson, Department of Pathology, University of Alabama at Birmingham, 814 SHEL, 1530 3rd Ave S, Birmingham, AL 35294; e-mail: sanderson@uab.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal