Abstract
Here we investigated the cytotoxicity of JS-K, a prodrug designed to release nitric oxide (NO•) following reaction with glutathione S-transferases, in multiple myeloma (MM). JS-K showed significant cytotoxicity in both conventional therapy-sensitive and -resistant MM cell lines, as well as patient-derived MM cells. JS-K induced apoptosis in MM cells, which was associated with PARP, caspase-8, and caspase-9 cleavage; increased Fas/CD95 expression; Mcl-1 cleavage; and Bcl-2 phosphorylation, as well as cytochrome c, apoptosis-inducing factor (AIF), and endonuclease G (EndoG) release. Moreover, JS-K overcame the survival advantages conferred by interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF-1), or by adherence of MM cells to bone marrow stromal cells. Mechanistic studies revealed that JS-K–induced cytotoxicity was mediated via NO• in MM cells. Furthermore, JS-K induced DNA double-strand breaks (DSBs) and activated DNA damage responses, as evidenced by neutral comet assay, as well as H2AX, Chk2 and p53 phosphorylation. JS-K also activated c-Jun NH2-terminal kinase (JNK) in MM cells; conversely, inhibition of JNK markedly decreased JS-K–induced cytotoxicity. Importantly, bortezomib significantly enhanced JS-K–induced cytotoxicity. Finally, JS-K is well tolerated, inhibits tumor growth, and prolongs survival in a human MM xenograft mouse model. Taken together, these data provide the preclinical rationale for the clinical evaluation of JS-K to improve patient outcome in MM.
Introduction
Multiple myeloma (MM) is a B-cell malignancy characterized by proliferation of monoclonal plasma cells in the bone marrow (BM). Despite the recent emergence of novel therapies including bortezomib,1,2 thalidomide,3,4 and lenalidomide,5 it remains incurable due to the development of drug resistance.5-7 Among the factors that lead to this resistance are defects in apoptotic signaling pathways and overexpression of the multidrug resistance protein (MRP) pumps that enhance drug efflux.8 In addition, the BM microenvironment confers drug resistance in MM via (1) secretion of cytokines such as interleukin 6 (IL-6) and insulin-like growth factor 1 (IGF-1), which mediate survival signals in MM cells,9-11 as well as (2) direct interaction with MM cells, which results in cell adhesion–mediated drug resistance.12,13 Despite recent progress, MM remains incurable, and new therapeutic agents with novel mechanism of actions are urgently needed.
JS-K (O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate) belongs to a diazeniumdiolate class of prodrug designed to release nitric oxide (NO•) when metabolized by glutathione S-transferases (GSTs; Figure 1A).14 GSTs are enzymes that catalyze the conjugation of xenobiotics with glutathione (GSH), thereby facilitating their subsequent efflux through MRP pumps.15 GSTs are frequently overexpressed in a broad spectrum of tumors.16,17 In the context of conventional chemotherapy, this provides tumor cells with a selective survival advantage over normal cells by enhancing drug efflux and thus decreasing therapeutic efficacy. In contrast, since JS-K uniquely requires GST for its optimal activity, it can potentially turn GST overexpression to the tumor's disadvantage by generating relatively high intracellular concentrations of cytotoxic NO•, specifically within tumor cells. Importantly, JS-K has recently been shown to inhibit tumor growth in both in vitro and in vivo models of human prostate cancer and human leukemia.14 Importantly, GSTs are overexpressed in 10% to 70% of patients with MM at diagnosis, and in 30% of patients at relapse.8 In addition, in our recent study comparing gene expression profiles of patient MM cells with normal plasma cells from a genetically identical twin, we observed that GST was overexpressed by 7-fold in MM cells.18 Furthermore, in our high-resolution genomic and expression profiling of primary tumor cells from 67 patients with MM and plasma cells from 12 healthy donors,19 33% and 39% of the MM cells overexpressed GSTP1 and GSTM1, respectively, when compared with plasma cell controls. To date, however, the biological effects of JS-K on MM cells have not been characterized.
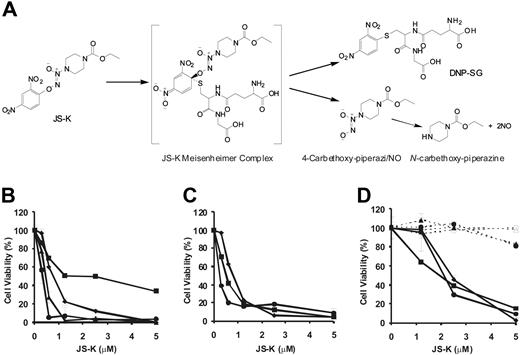
JS-K induces cytotoxicity in MM cells, but not in PBMCs and BMSCs. (A) JS-K reacts with GSH under the catalysis of GST enzymes. This reaction yields the intermediate JS-K Meisenheimer complex, which disintegrates into 4-carbethoxy-piperazi/NO and DNP-SG. Under physiologic conditions, 4-carbethoxy- piperazi/NO spontaneously generates NO•. (B) Conventional therapy–sensitive MM.1S (♦), OPM1 (●), OPM2 (▴), and RPMI-8226 (■) MM cell lines were cultured in the presence of JS-K for 48 hours. (C) Conventional therapy–resistant cell lines MM.1R (♦; Dex-resistant), RPMI-Dox40 (●; Dox-resistant), and RPMI-LR5 (■; Mel-resistant) were cultured in the presence or absence of JS-K for 48 hours. (D) MM cells from 3 patients (solid lines; ■, ●, and ♦), PBMCs derived from 2 healthy subjects (dashed lines; ● and ▴), and BMSCs isolated from 2 patients (dashed lines; ○ and ▵) were cultured with JS-K for 72 hours. In all cases, cell viability was assessed by MTT assay, and data represent means (± SD) of triplicate cultures. The final DMSO concentration in the cultures was 0.1% or less, which was found to be nontoxic (results not shown).
JS-K induces cytotoxicity in MM cells, but not in PBMCs and BMSCs. (A) JS-K reacts with GSH under the catalysis of GST enzymes. This reaction yields the intermediate JS-K Meisenheimer complex, which disintegrates into 4-carbethoxy-piperazi/NO and DNP-SG. Under physiologic conditions, 4-carbethoxy- piperazi/NO spontaneously generates NO•. (B) Conventional therapy–sensitive MM.1S (♦), OPM1 (●), OPM2 (▴), and RPMI-8226 (■) MM cell lines were cultured in the presence of JS-K for 48 hours. (C) Conventional therapy–resistant cell lines MM.1R (♦; Dex-resistant), RPMI-Dox40 (●; Dox-resistant), and RPMI-LR5 (■; Mel-resistant) were cultured in the presence or absence of JS-K for 48 hours. (D) MM cells from 3 patients (solid lines; ■, ●, and ♦), PBMCs derived from 2 healthy subjects (dashed lines; ● and ▴), and BMSCs isolated from 2 patients (dashed lines; ○ and ▵) were cultured with JS-K for 72 hours. In all cases, cell viability was assessed by MTT assay, and data represent means (± SD) of triplicate cultures. The final DMSO concentration in the cultures was 0.1% or less, which was found to be nontoxic (results not shown).
The purpose of the present study is to investigate the cytotoxicity of JS-K in human MM cells and to characterize the biochemical and cellular mechanisms by which JS-K induces tumor cell death. These studies provide the preclinical rationale for the clinical evaluation of JS-K to improve patient outcome in MM.
Patients, materials, and methods
JS-K and inhibitors
JS-K was synthesized as described previously.20 Stock solutions of JS-K (5 mM) were prepared in DMSO and stored at −20°C. The stock solutions were further diluted in RPMI for cell culture experiments. c-Jun NH2-terminal kinase (JNK) inhibitor II, z-VAD-fmk, N-acetyl-L-cysteine, and sulfasalazine were purchased from Calbiochem (San Diego, CA). Cobalamin and Cibacron Blue were purchased from Sigma-Aldrich (St Louis, MO). NO• indicator DAF-FM diacetate was purchased from Molecular Probes (Eugene, OR).
Cell culture and reagents
Dex-sensitive (MM.1S) and -resistant (MM.1R) human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). RPMI-8226 was obtained from American Type Culture Collection (Rockville, MD). Doxorubicin-resistant (RPMI-Dox40) and melphalan-resistant (RPMI-LR5) cells were kindly provided by Dr William Dalton (H. Lee Moffitt Cancer Center, Tampa, FL). The OPM1 and OPM2 cell lines were obtained from Dr Lief Bergsagel (Mayo Clinic, Scottsdale, AZ). All MM cell lines were cultured in RPMI 1640 media (Sigma-Aldrich) containing 10% fetal bovine serum (FBS), 2 mM l-glutamine (Gibco, Carlsbad, CA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). Blood samples collected from healthy volunteers were processed by Ficoll Hpaque gradient to obtain peripheral blood mononuclear cells (PBMCs), which were cultured in RPMI-1640 media containing 20% FBS. Patient MM and BM cells were obtained from BM samples after informed consent was obtained in accordance with the Declaration of Helsinki and after approval by the Institutional Review Board of the Dana-Farber Cancer Institute (Boston, MA). BM mononuclear cells were separated using Ficoll Hpaque density sedimentation, and plasma cells were purified (> 95% CD138+) by positive selection with anti-CD138 magnetic activated cell separation microbeads (Miltenyi, Auburn, CA).
Growth inhibition and proliferation assays
To evaluate the growth inhibitory effect of JS-K on MM cells, PBMCs, and bone marrow stromal cells (BMSCs), a colorimetric MTT assay (Chemicon, Temecula, CA) was performed, as described previously.21 Briefly, cells were incubated in 96-well plates in the presence of increasing concentrations of JS-K (or vehicle control) for 48 hours. MTT was added to the cultures during the last 4 hours of incubation. This was followed by the addition of isopropanol containing 0.04 N HCl to the wells and measurement of absorbance at a wavelength of 570 nm, with a reference wavelength of 630 nm.
To measure proliferation of MM cells and BMSCs, the rate of DNA synthesis was measured as described previously.21 Briefly, cells were incubated in 96-well plates with increasing concentrations of JS-K for 48 hours. During the last 8 hours of incubation, cells were pulsed with [3H]thymidine (0.5 μCi/well [0.0185 MBq/well]) and then harvested onto glass filters with an automatic cell harvester. Radioactivity was counted using the LKB Betaplate scintillation counter (Wallac, Waltham, MA).
To evaluate the effects of growth factors, 10 ng/mL recombinant IL-6 (R&D Systems, Minneapolis, MN) or 50 ng/mL IGF-1 (R&D Systems) was added to the wells with increasing concentrations of JS-K. To evaluate the effects of BMSCs on MM cell proliferation, BMSCs were incubated in 96-well culture plates (approximately 5000-10 000 BMSCs/well) for 24 hours. The medium was washed off, and MM cells were added to the wells (2.5 × 104 cells/well) with increasing concentrations of JS-K. Proliferation of MM cells was measured after 48 hours as described.
Western blotting
MM cells were cultured with the indicated concentrations of JS-K for the specified times, harvested, washed, and lysed using lysis buffer (radioimmunoprecipitation assay buffer, 2 mM Na3VO4, 5 mM NaF, 1 mM phenylmethyl sulfonyl fluoride, 5 mg/mL leupeptin, and 5 mg/mL aprotinin). Cell lysates were subjected to SDS-PAGE; transferred to polyvinylidene difluoride membrane; and immunoblotted with antibodies for poly(ADP-ribose) polymerase, caspase-8, caspase-9, caspase-3, caspase-7, Bcl-2, phospho(ser70)–Bcl-2, Bcl-xl, Bax, Bak, Mcl-1, apoptosis-inducing factor (AIF), endonuclease G (EndoG) (Axxora, San Diego, CA), phospho (ser20)-p53, p53, phospho(Ser317)-Chk1, Chk1, phospho(Thr68)-Chk2, Chk2, phospho(Thr183/Tyr185)-JNK, JNK (Santa Cruz Biotechnology, Santa Cruz, CA), phospho(Ser139)-H2AX, H2AX (Upstate Biotechnologies, Lake Placid, NY), and actin (Santa Cruz Biotechnology). All the antibodies were purchased from Cell Signaling (Beverly, MA) unless otherwise indicated.
Flow cytometry
For detection of apoptotic cells, cell-surface staining was performed with FITC-labeled anti–Annexin V antibody and PI (BD Pharmingen, San Diego, CA). For detection of surface CD95 levels, cells were stained with anti-CD95 antibody (Becton Dickinson, San Jose, CA). Isotype-matched antibodies were used as negative controls. For detection of intracellular NO• generation, cells were first treated with JS-K and then incubated with 5 μM DAF-FM diacetate for 30 minutes. A total of 40 000 stained cells were analyzed by a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Neutral single-cell gel electrophoresis (comet assay)
Neutral comet assays were performed using the Trevigen Comet Assay kit (Trevigen, Gaithersburg, MD) to asses JS-K–induced DNA double-strand breaks (DSBs). Cells at 1 × 105/mL were combined with molten LMAgarose (at 37°C) at a ratio of 1:10, and 50 μL were immediately pipetted onto a CometSlide (Trevigen). The slides were kept at 4°C for 10 minutes, lysed for 1 hour in prechilled lysis buffer (2.5 M sodium chloride/100 mM EDTA [pH 10]/10 mM Tris base/1% sodium lauryl sarcosinate/0.01% Triton X-100), and then electrophoresed at 30 V for 20 minutes. After staining with SYBY Green, cells were photographed using a Nikon E800 fluorescence microscope at 60×/1.0% (Nikon, Melville, NY) with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed with the Komet 4.2 Single Cell Gel Electrophoresis Analysis (Kinetic Imaging Limited, Liverpool, United Kingdom). Olive tail moment, defined as the product of percentage DNA in the tail and displacement between the position of the mean centers of mass in the heads and tails, was determined for at least 40 cells per sample.
Immunocytochemistry
Cytospin of JS-K–treated cells were prepared on glass slides and fixed with 50% methanol/50% acetone at −20°C. The slides were blocked with 5% FBS at 37°C, and then incubated with primary antibody for 1 hour and FITC-labeled secondary antibody for 30 minutes. Coverslips were then mounted on the glass slides with VectaShield antifade/DAPI (Vector Labs, Burlingame, CA) and analyzed by Nikon E800 fluorescence microscope at 60×/1.0% (Nikon), with a SPOT RT camera (Diagnostic Instruments).
Isobologram analysis
For combination studies of JS-K with bortezomib, MTT assay data were converted into values representing the fraction of growth affected (FA) and analyzed using CalcuSyn software (Biosoft, Ferguson, MO) to yield combination index (CI) values based on the Chou-Talalay method.
Xenograft murine model
NIH III mice (5 to 6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). All animal studies were conducted according to protocols approved by the Animal Ethics Committee of the Dana-Farber Cancer Institute. The mice were inoculated subcutaneously in the right flank with 3 × 107 OPM1 MM cells in 100 μL RPMI-1640 and 100 μL Matrigel basement membrane matrix (Becton Dickinson). When tumors were palpable, 9 mice were assigned into the treatment group receiving 4 μmol/kg JS-K intravenously (3 times per week), and 8 mice were assigned into the control group receiving vehicle alone. Caliper measurements of the longest perpendicular tumor diameters were performed every alternate day to estimate the tumor volume using the following formula representing the 3D volume of an ellipse: 4/3 × (width/2)2 × (length/2). Animals were killed when tumors reached 2 cm or if the mice appeared moribund. Survival was evaluated from the first day of treatment until death. Tumor growth was evaluated using caliper measurements from the first day of treatment until day of first killing, which was day 21 for control, and day 43 for the treatment groups.
For the ex vivo analysis of tumors, tumors were excised at the time of killing, 4 hours after the last drug injection, processed, and analyzed for propidium iodide (PI) staining, TUNEL assay, and immunohistochemistry for activated caspase-3 as described previously.22 Images were captured with a LEICA DM IL microscope connected to the LEICA DFC300 FX camera at 40×/0.60 (Leica, Heidelberg, Germany).
Results
JS-K is cytotoxic to MM cell lines and patient MM cells
The structure and reaction scheme of JS-K is shown in Figure 1A. The cytotoxic effects of JS-K on growth of conventional therapy-sensitive MM cell lines (MM.1S, RPMI8226, OPM1, and OPM2) were determined using MTT assay. JS-K was significantly cytotoxic in all 3 cell lines tested, with an IC50 of 0.3 to 1.2 μM at 48 hours (Figure 1B). In addition, JS-K was effective even in cell lines resistant to conventional chemotherapy, including dexamethasone-resistant MM.1R, doxorubicin-resistant RPMI-Dox40, and the melphalan-resistant RPMI-LR5, with an IC50 of 0.3 to 0.9 μM at 48 hours (Figure 1C). JS-K was also cytotoxic in multidrug-resistant patient MM cells, with an IC50 of 2 to 2.5 μM at 72 hours (Figure 1D). Most important, JS-K (up to 2.5 μM) was not cytotoxic to healthy donor PBMCs, with more than 20% cytotoxicity at 5 μM, a dose which was toxic to most patient MM cells (Figure 1D). In addition, no significant cytotoxicity of JS-K, at these doses, was observed in BMSCs isolated from patients (Figure 1D). These data demonstrate that JS-K has selective cytotoxicity in MM cells.
JS-K induces apoptosis associated with PARP, caspase-8, and caspase-9 cleavage
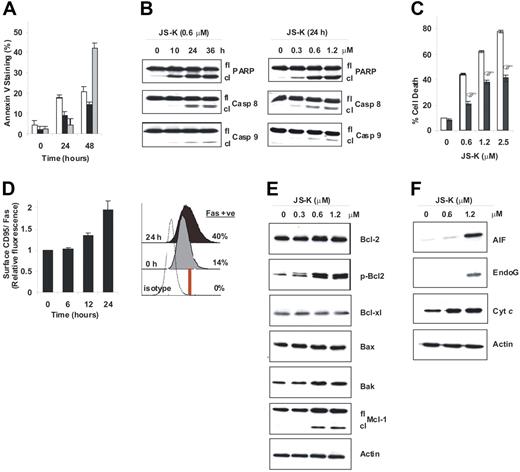
To determine whether JS-K induces apoptosis, MM.1S, OPM1, and RPMI-8226 cells were treated with JS-K at their IC50 values (0.6 μM, 0.3 μM, and 1.2 μM, respectively), and then analyzed by flow cytometry for the early apoptotic marker Annexin V. JS-K treatment (24 and 48 hours) significantly increased the proportion of cells that were positive for Annexin V in 3 different MM cell lines (Figure 2A). We further characterized apoptosis triggered by JS-K by examining cleavage of PARP as well as caspase-8 and caspase-9 in MM.1S cells. MM.1S cells were exposed to JS-K at the indicated doses and periods of time, and cell lysates were then examined by Western blotting. Both the time course (Figure 2B; left panel), and dose-response experiments (Figure 2B; right panel) showed that JS-K induced significant PARP, caspase-8, and caspase-9 cleavage. Finally, to confirm the role played by caspases in JS-K–induced apoptosis, MM.1S cells were pretreated with the pancaspase inhibitor z-VAD-fmk and then treated with JS-K (0-2.5 μM) for 48 hours. Z-VAD-fmk significantly, but only partially, inhibited JS-K–induced cell death (Figure 2C). Based on these results, we conclude that JS-K–induced apoptosis is, at least in part, mediated by caspases.
JS-K induces apoptosis in MM cells associated with the extrinsic and intrinsic apoptotic pathways. (A) MM.1S (□), RPMI-8226 (■), and OPM1 (□) cells were treated with JS-K at their IC50 values (0.6 μM, 1.2 μM, and 0.3 μM, respectively) for 0 to 48 hours, and apoptosis was then assessed by flow cytometry following Annexin V staining. Data represent means (± SD) of triplicate experiments. (B) Cleavage of PARP and the initiator caspase-8 and caspase-9 were determined by Western blotting of MM.1S cells treated with 0.6 μM JS-K for 0 to 36 hours (left panel), or with 0 to 1.2 μM JS-K for 24 hours (right panel). (C) MM.1S cells were treated with JS-K (0–2.5 μM) for 48 hours, with (■) or without (□) pretreatment by z-VAD-fmk (100 μM). Cell death was assessed by flow cytometry after PI staining. Data represent means (± SD) of triplicate experiments. *P < .01, as compared with no z-VAD-fmk pretreatment. (D) MM.1S cells were treated with 0.6 μM JS-K for 0 to 24 hours, and cell-surface CD95/Fas expression was then assessed by flow cytometry. Fold increase of mean surface CD95/Fas expression induced by JS-K is plotted relative to untreated control (left panel). Data represent means ± SD of triplicate experiments. *P < .01. The histogram plot shows the percentage of cells positive for cell-surface CD95/Fas at 0 hours and 24 hours after JS-K exposure (right panel). (E-F) MM.1S cells were treated with JS-K (0-1.2 μM) for 24 hours, followed by immunoblotting for Bcl-2 family proteins (E) or release of mitochondrial proteins into the cytosol (F).
JS-K induces apoptosis in MM cells associated with the extrinsic and intrinsic apoptotic pathways. (A) MM.1S (□), RPMI-8226 (■), and OPM1 (□) cells were treated with JS-K at their IC50 values (0.6 μM, 1.2 μM, and 0.3 μM, respectively) for 0 to 48 hours, and apoptosis was then assessed by flow cytometry following Annexin V staining. Data represent means (± SD) of triplicate experiments. (B) Cleavage of PARP and the initiator caspase-8 and caspase-9 were determined by Western blotting of MM.1S cells treated with 0.6 μM JS-K for 0 to 36 hours (left panel), or with 0 to 1.2 μM JS-K for 24 hours (right panel). (C) MM.1S cells were treated with JS-K (0–2.5 μM) for 48 hours, with (■) or without (□) pretreatment by z-VAD-fmk (100 μM). Cell death was assessed by flow cytometry after PI staining. Data represent means (± SD) of triplicate experiments. *P < .01, as compared with no z-VAD-fmk pretreatment. (D) MM.1S cells were treated with 0.6 μM JS-K for 0 to 24 hours, and cell-surface CD95/Fas expression was then assessed by flow cytometry. Fold increase of mean surface CD95/Fas expression induced by JS-K is plotted relative to untreated control (left panel). Data represent means ± SD of triplicate experiments. *P < .01. The histogram plot shows the percentage of cells positive for cell-surface CD95/Fas at 0 hours and 24 hours after JS-K exposure (right panel). (E-F) MM.1S cells were treated with JS-K (0-1.2 μM) for 24 hours, followed by immunoblotting for Bcl-2 family proteins (E) or release of mitochondrial proteins into the cytosol (F).
Both the extrinsic and intrinsic apoptotic pathway proteins are modulated during JS-K–induced apoptosis
To further characterize apoptosis induced by JS-K, we examined how the extrinsic and intrinsic apoptotic pathway proteins are modulated by JS-K. Caspase-8 activation (Figure 2B) suggests involvement of the death receptor pathways in apoptosis. Consistent with caspase-8 activation, flow cytometry experiments revealed that surface expression of Fas was also up-regulated in MM.1S cells during JS-K–induced apoptosis. Specifically, exposure to JS-K (0.6 μM) triggered an increase in the surface density of Fas at 12 hours, which peaked (approximately 2-fold) at 24 hours (Figure 2D; left panel). In addition, the ratio of cells expressing surface Fas relative to isotype control antibody increased by approximately 3-fold (from 14% to 40%) after 24 hours of treatment with JS-K (0.6 μM; Figure 2D; right panel). These results confirm involvement of the death receptor pathway in JS-K–induced apoptosis.
Induction of caspase-9 cleavage by JS-K (Figure 2B) suggested involvement of the mitochondrial apoptotic pathway. Therefore, we next explored the effects of JS-K on Bcl-2 family members. Although no significant changes were detected in expression of the proapoptotic family member Bax, nor of the antiapoptotic members Bcl-2 and Bcl-xl, a moderate increase in proapoptotic Bak levels and significant cleavage of the antiapoptotic Mcl-1 were detected after JS-K treatment (0.6 and 1.2 μM; Figure 2E). In addition, JS-K caused significant phosphorylation of Bcl-2 at serine 70 (Figure 2E), which has previously been shown to inhibit the antiapoptotic effects of Bcl-2.23 Given that apoptotic control depends on the fine balance between the proapoptotic and antiapoptotic Bcl-2 family members, these changes triggered by JS-K may be sufficient to cause a shift toward apoptosis in MM cells. Moreover, mitochondrial cytochrome c (cyt c), apoptosis-inducing factor AIF, and EndoG were released into the cytosol during JS-K (1.2 μM)–induced apoptosis (Figure 2F), confirming involvement of the mitochondrial pathway. Importantly, AIF and EndoG are mediators of the caspase-independent apoptotic pathway. These results, coupled with the fact that the pancaspase inhibitor z-VAD-fmk (100 μM) only partially inhibited JS-K–induced cell death (Figure 2C), suggest that JS-K–induced apoptosis involves both caspase-dependent and -independent pathways in MM cells.
JS-K overcomes the growth and survival advantages conferred by IL-6, IGF-1, and patient-derived BMSCs
MM cells are predominantly localized in the BM microenvironment, where interactions between tumor cells and BMSCs trigger the production of cytokines such as IL-6 and IGF-1. These cytokines, through autocrine and paracrine mechanisms, provide growth and survival signals to MM cells and confer protection against drug-induced apoptosis.11,24-29 Therefore, we next examined whether JS-K could overcome the growth and survival advantages conferred by IL-6 or IGF-1 using DNA thymidine incorporation assay. As can be seen in Figure 3A and 3B, exogenous IL-6 (10 ng/mL) and IGF-1 (50 ng/mL) triggered an approximately 2-fold and approximately 1.3-fold increase in MM.1S cell growth, respectively. Importantly, even in the presence of IL-6 and IGF-1, JS-K inhibited growth of MM cells in a dose-dependent fashion. To examine the effects of JS-K on MM cells in the BM microenvironment, MM.1S cells were next cocultured with patient-derived BMSCs, and then were treated with increasing doses of JS-K. Although coculture of MM.1S cells with BMSCs increased (approximately 2.5-fold) MM.1S cell growth, as detected by DNA thymidine incorporation, JS-K inhibited this response in a dose-dependent manner (Figure 3C). In contrast, JS-K treatment was not toxic to BMSCs as detected by MTT assay (Figure 1D), indicating that JS-K is selective against MM cells. Taken together, these results indicate that JS-K overcomes the growth and survival advantages conferred by IL-6, IGF-1, and BMSCs in MM cells.
JS-K overcomes the protective effects of IL-6 and IGF-1, and adherence to patient BMSCs. MM.1S cells were treated for 48 hours with JS-K (0–2.4 μM) in the absence (□) or presence (■) of IL-6 (10 ng/mL) (A) or IGF-1 (50 ng/mL) (B), and without (□) or with BMSCs derived from MM patients 1(■) and 2 (□) (C). DNA synthesis was determined by measuring [3H]-thymidine incorporation during the last 8 hours of 48-hour cultures. Data represent means (± SD) of triplicate experiments.
JS-K overcomes the protective effects of IL-6 and IGF-1, and adherence to patient BMSCs. MM.1S cells were treated for 48 hours with JS-K (0–2.4 μM) in the absence (□) or presence (■) of IL-6 (10 ng/mL) (A) or IGF-1 (50 ng/mL) (B), and without (□) or with BMSCs derived from MM patients 1(■) and 2 (□) (C). DNA synthesis was determined by measuring [3H]-thymidine incorporation during the last 8 hours of 48-hour cultures. Data represent means (± SD) of triplicate experiments.
JS-K–induced cytotoxicity is mediated via NO• in MM cells
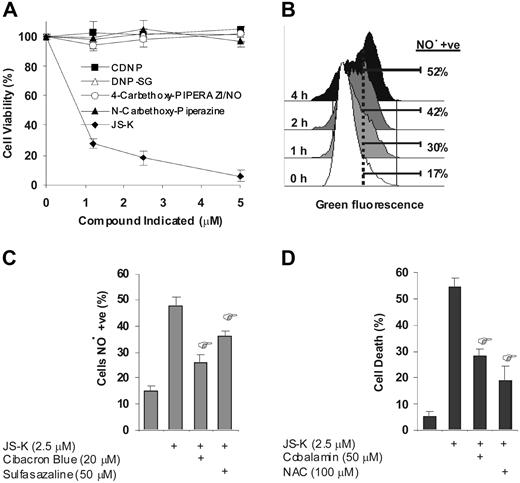
As shown in Figure 1A, JS-K is a prodrug that releases NO• intracellularly when it reacts with glutathione under catalysis of GSTs. As a result of this reaction, several other compounds (besides NO•) are also formed. These include 4-carbethoxy-piperazi/NO, N-carbethoxy-piperazine, and dinitrophenyl-glutathione (DNP-SG) (Figure 1A).14 To delineate the basis of JS-K–induced cytotoxicity in MM cells, we first determined whether these additional compounds formed besides NO• had any cytotoxic effects in MM cells. For this, MM cells were cultured with each of these compounds under identical conditions with JS-K. As seen in Figure 4A, 4-carbethoxy-piperazi/NO (which acts as a GST-independent, extracellular NO• generator), N-carbethoxy-piperazine, and DNP-SG were not cytotoxic in MM cells at concentrations (up to 5 μM) at which JS-K was cytotoxic. In addition, the control compound chloro-dinitrobenzene (CDNB), which reacts with GSTs and GSH in the same manner as JS-K but does not release NO•,14 was not cytotoxic to MM cells at doses up to 5 μM (Figure 4A). Altogether, these results suggest that the cytotoxicity of JS-K was mediated via NO•, but not via the additional reaction products formed. Next, we confirmed the intracellular release of NO• from JS-K in MM cells by using flow cytometry and the NO• indicator DAF-FM diacetate, a nonfluorescent reagent which fluoresces upon reaction with NO•. Significant NO• release was detectable in MM.1S cells within only a few hours of JS-K exposure. As can be seen in the histogram plot shown in Figure 4B, cells expressing detectable levels of NO• increased by approximately 3-fold (17%-52%) after 4 hours of JS-K treatment (2.5 μM). Importantly, NO• release by JS-K was significantly inhibited by GST inhibitors Cibacron Blue (by approximately 66%) and sulfasalazine (by approximately 36%), suggesting that the NO• release by JS-K, is at least in part, mediated by GSTs (Figure 4C). It is noteworthy that intracellular NO• release was not detected with the exogenous addition of the control compounds 4-carbethoxy-piperazi/NO, N-carbethoxy-piperazine, DNP-SG, and CDNB (data not shown). Most importantly, the cytotoxic effect after 24 hours of JS-K exposure (2.5 μM) was significantly inhibited by NO• scavengers cobalamin (by approximately 56%) and N-acetyl-L-cysteine (by approximately 75%) (Figure 4D). Together, these data suggest that the cytotoxicity of JS-K in MM cells is mediated by NO•.
JS-K induced cytotoxicity is mediated via NO•. (A) MM.1S cells were cultured with 0 to 5 μM of JS-K (♦), 4-carbethoxy-piperazi/NO (○), N-carbethoxy-piperazine (▴), DNP-SG (▵), or CDNB (■) for 48 hours. Cell viability was assessed by MTT assay, and data represent means (± SD) of triplicate cultures. The final DMSO concentration in the cultures was 0.1% or less, which was found to be nontoxic (results not shown). (B) MM.1S cells were treated with JS-K (2.5 μM), and then intracellular NO• was detected by flow cytometry using the NO• indicator DAF-FM diacetate. The histogram plot shows the percentage of cells that have detectable levels of NO• at 1, 2, and 4 hours. (C) MM.1S cells were cultured with JS-K (2.5 μM) for 4 hours after 1 hour of preincubation with GST inhibitors Cibacron Blue (20 μM), or sulfasalazine (50 μM). Percentage of NO•-positive cells were then assayed by flow cytometry. Data represents mean (± SD) of triplicate experiments. *P < .01 compared with JS-K (2.5 μM)–only control. (D) MM.1S cells were cultured with JS-K (2.5 μM) for 24 hours after 2 hours of preincubation with NO• scavengers cobalamin (50 μM) or NAC (100 μM). Cell death was assayed by flow cytometry after PI staining. Data represents mean (± SD) of triplicate cultures. *P < .01 compared with JS-K–only control.
JS-K induced cytotoxicity is mediated via NO•. (A) MM.1S cells were cultured with 0 to 5 μM of JS-K (♦), 4-carbethoxy-piperazi/NO (○), N-carbethoxy-piperazine (▴), DNP-SG (▵), or CDNB (■) for 48 hours. Cell viability was assessed by MTT assay, and data represent means (± SD) of triplicate cultures. The final DMSO concentration in the cultures was 0.1% or less, which was found to be nontoxic (results not shown). (B) MM.1S cells were treated with JS-K (2.5 μM), and then intracellular NO• was detected by flow cytometry using the NO• indicator DAF-FM diacetate. The histogram plot shows the percentage of cells that have detectable levels of NO• at 1, 2, and 4 hours. (C) MM.1S cells were cultured with JS-K (2.5 μM) for 4 hours after 1 hour of preincubation with GST inhibitors Cibacron Blue (20 μM), or sulfasalazine (50 μM). Percentage of NO•-positive cells were then assayed by flow cytometry. Data represents mean (± SD) of triplicate experiments. *P < .01 compared with JS-K (2.5 μM)–only control. (D) MM.1S cells were cultured with JS-K (2.5 μM) for 24 hours after 2 hours of preincubation with NO• scavengers cobalamin (50 μM) or NAC (100 μM). Cell death was assayed by flow cytometry after PI staining. Data represents mean (± SD) of triplicate cultures. *P < .01 compared with JS-K–only control.
JS-K induces DNA DSBs and activates DNA damage response pathways
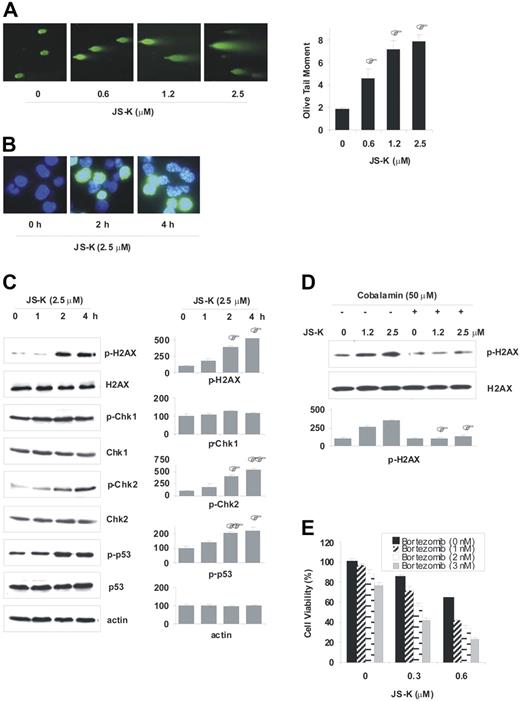
DSBs are the most cytotoxic DNA lesions that lead to apoptosis of mammalian cells.30-32 NO• has previously been shown to cause DNA DSBs in mammalian cells.33-36 Given that JS-K is a NO•-generating prodrug, we hypothesized that JS-K induces DNA DSBs in MM cells. We therefore next assayed for direct evidence of DSB formation using the neutral comet assay. MM.1S cells were treated with increasing concentrations of JS-K, and comet tails were analyzed for DSB formation and quantification. JS-K induced significant DSB formation even at low doses (0.6 μM) and short time intervals (2.5 hours) (Figure 5A). Since apoptosis was not observed during the first 8 hours of JS-K exposure at these doses, the formation of DSBs was not due to internucleosomal DNA cleavage in the apoptotic process.
JS-K induces DNA DSBs, and activates DNA damage response pathways. (A) MM.1S cells were treated with JS-K (0-2.5 μM) for 2.5 hours, and then assayed for formation of DSBs by the neutral comet assay. Representative images of the comet tails are shown (left panel), and the olive tail moments are plotted (right panel). To calculate the olive tail moments, at least 40 cells per sample were analyzed. Data represents the mean (± SD) of 3 independent experiments. *P < .05 compared with nontreated control. (B-C) MM.1S cells were dosed with 2.5 μM JS-K for 0 to 4 hours. Representative images for the immunocytochemistry assay performed with antiphospho(Ser139)-H2AX antibody is shown (B). Phosphorylation of the DNA damage response proteins H2AX (Ser139), Chk1 (Ser317), Chk2 (Thr68), and p53 (Ser20) was assessed by Western blotting (C). Representative western blots (left panel), and percentage of mean quantitative densitometric values (± SD) from 2 or 3 independent experiments (right panel) are shown. *P < .05; **P < .01 compared with 0-hour control. (D) MM.1S cells were cultured with JS-K (0 to 2.5 μM) for 2 hours, after 1 hour of preincubation with cobalamin (50 μM). Phosphorylation of H2AX was assayed by Western blotting. Representative western blots and percentage of mean quantitative densitometric values ± SD from 2 independent experiments are shown. *P < .01 compared with no-cobalamin control. (E) Low doses of bortezomib sensitize MM cells to JS-K. MM.1S cells were treated with bortezomib (0, 1, 2, or 3 nM) for 8 hours, which was followed by JS-K treatment (0, 0.3, or 0.6 μM). Cell viability was detected by MTT assay (48 hours). Data represents the mean (± SD) of 3 independent experiments.
JS-K induces DNA DSBs, and activates DNA damage response pathways. (A) MM.1S cells were treated with JS-K (0-2.5 μM) for 2.5 hours, and then assayed for formation of DSBs by the neutral comet assay. Representative images of the comet tails are shown (left panel), and the olive tail moments are plotted (right panel). To calculate the olive tail moments, at least 40 cells per sample were analyzed. Data represents the mean (± SD) of 3 independent experiments. *P < .05 compared with nontreated control. (B-C) MM.1S cells were dosed with 2.5 μM JS-K for 0 to 4 hours. Representative images for the immunocytochemistry assay performed with antiphospho(Ser139)-H2AX antibody is shown (B). Phosphorylation of the DNA damage response proteins H2AX (Ser139), Chk1 (Ser317), Chk2 (Thr68), and p53 (Ser20) was assessed by Western blotting (C). Representative western blots (left panel), and percentage of mean quantitative densitometric values (± SD) from 2 or 3 independent experiments (right panel) are shown. *P < .05; **P < .01 compared with 0-hour control. (D) MM.1S cells were cultured with JS-K (0 to 2.5 μM) for 2 hours, after 1 hour of preincubation with cobalamin (50 μM). Phosphorylation of H2AX was assayed by Western blotting. Representative western blots and percentage of mean quantitative densitometric values ± SD from 2 independent experiments are shown. *P < .01 compared with no-cobalamin control. (E) Low doses of bortezomib sensitize MM cells to JS-K. MM.1S cells were treated with bortezomib (0, 1, 2, or 3 nM) for 8 hours, which was followed by JS-K treatment (0, 0.3, or 0.6 μM). Cell viability was detected by MTT assay (48 hours). Data represents the mean (± SD) of 3 independent experiments.
It has recently been established that an early specific cellular response to DSBs in mammalian cells includes phosphorylation of the histone protein H2AX at Ser139 (γ-H2AX).37,38 Moreover, the respective foci formation by γ-H2AX is one of the essential steps for signaling DNA damage responses after DSB. We next examined whether JS-K induced H2AX phosphorylation and respective foci formation in MM cells. As shown in Figure 5B, immunocytochemical analysis in MM.1S cells showed that JS-K induces significant H2AX phosphorylation at Ser139 and respective foci formation as early as 2 hours after JS-K exposure. Phosphorylation of H2AX and organization of γ-H2AX into discrete foci not only indicates that JS-K causes DSB formation, but also shows that DNA damage response pathways are activated in MM cells. A multitude of other proteins have been identified as mediators of DNA damage responses, including the checkpoint kinases (ie, Chk1, Chk2) and p53.39-41 To further explore the activation of DNA damage responses induced by JS-K, we next assayed for activation of Chk1, Chk2, and p53 proteins by Western blotting. As seen in Figure 5C, Western blot analysis confirmed that JS-K induced significant H2AX phosphorylation at early time points, without significant changes in total H2AX protein levels. Western blot analysis further revealed that JS-K, at early time points (0-4 hours), induced significant phosphorylation of Chk2 (Thr68) without significant change in Chk1 phosphorylation or in the total protein levels of Chk1, Chk2, or p53. Ser20 of p53 has previously been shown to be a substrate of Chk2,42-44 and we detected significant phosphorylation of p53 at Ser20 as early as 2 hours after JS-K exposure. Taken together, these results indicate that JS-K induces DNA DSBs and activates DNA damage response pathways early, prior to the initiation of apoptotic events. Importantly, phosphorylation of H2AX was significantly inhibited by the NO• scavenger cobalamin, indicating that JS-K–induced DNA damage responses are also mediated via NO• (Figure 5D).
Low-dose bortezomib sensitizes MM cells to JS-K
It has previously been shown that bortezomib induces cleavage of DNA-PK, an important DNA DSB repair enzyme.45 Having shown that JS-K induces DSBs, we hypothesized that treatment of MM cells with bortezomib would enhance JS-K–induced cytotoxicity by inhibiting the repair of cytotoxic DSBs induced by JS-K. To examine this, MM cells were treated with low doses of bortezomib for 8 hours, which was followed by JS-K treatment. As seen in Figure 5E, low doses of bortezomib significantly enhanced JS-K–induced cytotoxicity. Based on the isobologram analysis, the CI values were 0.907 or greater for all combinations shown (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article), suggesting that low doses of bortezomib are synergistic with JS-K. These results provide the framework for combination trials of JS-K with bortezomib to increase therapeutic efficacy.
JS-K–induced apoptosis is mediated via JNK pathway
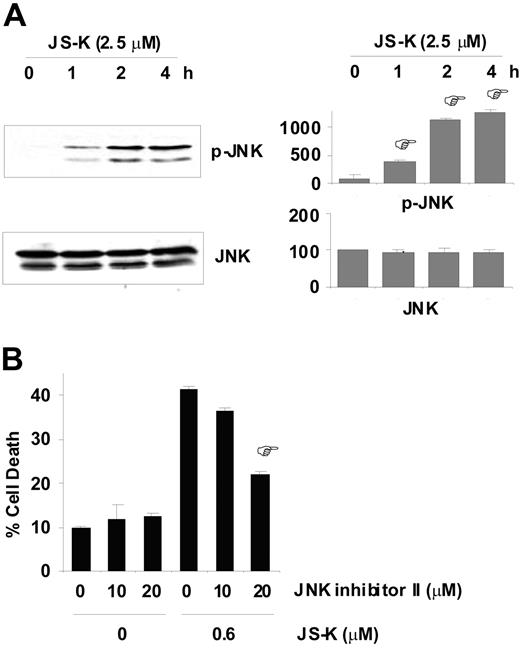
The JNK pathway has been shown to be activated by various genotoxic stresses.46,47 Most importantly, apoptosis induced by various DSB-generating agents, including topoisomerase inhibitors and γ-radiation, is mediated via the JNK pathway in mammalian cells.48,49 Therefore, we next determined whether JNK was activated by JS-K in MM cells, and conversely, whether MM cells were protected from apoptosis by JNK inhibition. Our results show that JS-K induced significant JNK activation in MM.1S cells early after exposure to JS-K (0.6 μM; Figure 6A). Notably, significant rescue from cell death was observed when MM.1S cells were treated with JNK inhibitor II prior to JS-K treatment. As shown in Figure 6B, 20 μM JNK inhibitor II reduced MM cell death by approximately 50% (from 41% to 22%). No cytotoxic effects of JNK inhibitor II on MM.1S cells were detected at the doses used. These results suggest that JNK functions as a proapoptotic effector kinase in JS-K–induced apoptosis in MM cells, and that JS-K–mediated apoptosis is, at least in part, mediated by the JNK pathway.
JS-K–induced apoptosis is mediated via the JNK pathway. (A) JS-K induces phosphorylation of JNK in MM.1S cells. MM.1S cells were exposed to 0.6 μM JS-K for 0 to 4 hours, and then whole-cell lysates were subjected to Western blotting with antiphospho(Thr183/Tyr185)-JNK antibody. Reblotting with anti-JNK antibody confirmed equal loading. Representative Western blots (left panel), and percentage of mean quantitative densitometric values (± SD) from 3 independent experiments (right panel) are shown. *P < .01 compared with 0-hour control. (B) MM.1S cells were cultured with (or without) JS-K (0.6 μM) for 48 hours after 2 hours of preincubation with JNK inhibitor II (0, 10, or 20 μM). Cells were then assayed for apoptosis by Annexin V/PI staining. Data represent mean (± SD) of triplicate experiments. *P < .01 compared with no JNK inhibitor II, JS-K (0.6 μM) control
JS-K–induced apoptosis is mediated via the JNK pathway. (A) JS-K induces phosphorylation of JNK in MM.1S cells. MM.1S cells were exposed to 0.6 μM JS-K for 0 to 4 hours, and then whole-cell lysates were subjected to Western blotting with antiphospho(Thr183/Tyr185)-JNK antibody. Reblotting with anti-JNK antibody confirmed equal loading. Representative Western blots (left panel), and percentage of mean quantitative densitometric values (± SD) from 3 independent experiments (right panel) are shown. *P < .01 compared with 0-hour control. (B) MM.1S cells were cultured with (or without) JS-K (0.6 μM) for 48 hours after 2 hours of preincubation with JNK inhibitor II (0, 10, or 20 μM). Cells were then assayed for apoptosis by Annexin V/PI staining. Data represent mean (± SD) of triplicate experiments. *P < .01 compared with no JNK inhibitor II, JS-K (0.6 μM) control
JS-K inhibits tumor growth in a mouse xenograft model of MM
Having shown the signaling mechanisms mediating the anti-MM effects of JS-K in vitro, we next determined whether JS-K mediates in vivo anti–human MM cell activity using a human xenograft mouse model. Previous studies have shown that JS-K can be administered intravenously in mouse models, 3 times per week, without any significant toxicity, including systemic hypotesion, up to 4 μmol/kg.14 This concentration corresponds to expected peak blood levels of 17 μM,14 which is well above the IC50 values we observed in MM cells in vitro. Therefore, this dose, frequency, and route of administration were used for a 9-week period in our studies to assess anti-MM activity.
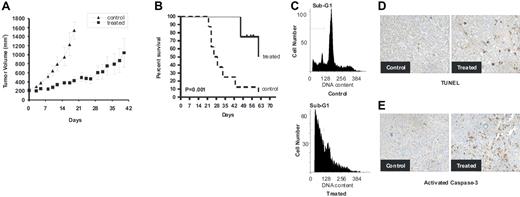
JS-K significantly reduced human MM cell growth in the treatment group (9 mice) when compared with control animals (8 mice) treated with vehicle only (Figure 7A). Using Kaplan-Meier curves and log-rank analysis, the mean overall survival (OS) was 28 days (95% confidence interval) in the control cohort versus 62 days (95% CI) in the treatment group. Statistically significant prolongation in mean OS compared with control mice was observed in the treated animals (P = .001; Figure 7B). Furthermore, these results underestimate the survival advantage of JS-K, since 4 treated mice (50%) were killed on days 52 to 63, before the tumors reached 2 cm, in order to further examine molecular events ex vivo. These mice had small tumors well controlled by JS-K. Finally, ex vivo analysis of tumors excised from mice showed significantly increased apoptosis in the JS-K–treated versus control cohorts, evidenced by propidium iodide staining (Figure 7C), TUNEL assay (Figure 7D), and immunohistochemistry for activated caspase-3 (Figure 7E). It is noteworthy that treatment with either vehicle or JS-K did not affect body weight. Taken together, these results demonstrate that JS-K inhibits tumor growth by inducing MM cell apoptosis in vivo, and significantly prolongs host survival.
JS-K inhibits human MM cell growth in vivo. NIH III mice were inoculated subcutaneously in the flank with 3 × 107 OPM1 cells. When tumors became palpable, JS-K (4 μmol/kg; n = 9) or vehicle (n = 8) was administered intravenously 3 times per week. (A) JS-K significantly inhibits MM tumor growth compared with the controls. Tumor volumes are represented as means (± SE). (B) JS-K markedly increases survival of the host. Survival was evaluated using Kaplan-Meier curves and log-rank analysis. JS-K significantly increased survival (P = .001) compared with the control group. (C-E). JS-K induces apoptosis in vivo. Mice were killed 4 hours after the last treatment, and tumors were excised for PI (C), TUNEL (D), or activated caspase-3 (E) analysis. Representative images captured at 40 ×/0.60 are shown.
JS-K inhibits human MM cell growth in vivo. NIH III mice were inoculated subcutaneously in the flank with 3 × 107 OPM1 cells. When tumors became palpable, JS-K (4 μmol/kg; n = 9) or vehicle (n = 8) was administered intravenously 3 times per week. (A) JS-K significantly inhibits MM tumor growth compared with the controls. Tumor volumes are represented as means (± SE). (B) JS-K markedly increases survival of the host. Survival was evaluated using Kaplan-Meier curves and log-rank analysis. JS-K significantly increased survival (P = .001) compared with the control group. (C-E). JS-K induces apoptosis in vivo. Mice were killed 4 hours after the last treatment, and tumors were excised for PI (C), TUNEL (D), or activated caspase-3 (E) analysis. Representative images captured at 40 ×/0.60 are shown.
Discussion
MM is currently an incurable hematologic malignancy, and novel biologically based treatment strategies that can overcome conventional drug resistance are urgently needed. In this report, we demonstrate that JS-K, a GST-activated generator of NO•, induces significant cytotoxicity in both conventional therapy sensitive and resistant MM cell lines, as well as multidrug-resistant patient MM cells, with an IC50 of 0.3 to 2.5 μM. Importantly, we observed no significant cytotoxicity of JS-K on PBMCs or BMSCs at these concentrations, which suggests selective cytotoxicity of JS-K on MM cells. In support of this, we showed that JS-K has significant efficacy in a mouse xenograft of MM without significant toxicity.
JS-K is a potent generator of NO•. NO•-induced apoptosis is complex, involving both the extrinsic and intrinsic apoptotic pathways, as well as the caspase-dependent and -independent pathways.50,51 Consistent with this paradigm, we show that JS-K modulates both the intrinsic and extrinsic apoptotic pathway proteins in MM, as evidenced by changes in the expression of Bcl-2 family members, as well as the death receptor Fas/CD95. In addition, although JS-K triggers caspase activation and z-VAD-fmk significantly inhibits JS-K–induced cell death, it also induces release of AIF and EndoG, mediators of caspase-independent apoptotic pathway, into the cytosol. These data therefore suggest involvement of both extrinsic and intrinsic apoptotic cascades, as well as both caspase-dependent and -independent pathways. Given the genetic and molecular heterogeneity of MM, this ability of JS-K to induce apoptosis via several different pathways may enhance its ability to overcome drug resistance resulting from defects in certain apoptotic pathways.
It has previously been shown that the BM microenvironment confers drug resistance in MM cells.52,53 Although the BM microenvironment consists of many components that may contribute to drug resistance, 2 mechanisms have been well established. First, cytokines such as IL-6 and IGF-1 are present in the BM milieu and induce Janus kinase 2/STAT3 and/or PI3-K/Akt signaling, which in turn mediates antiapoptosis and resistance to conventional and novel therapies.10,11,25,53 Secondly, adhesion of MM cells to BMSCs confers cell adhesion–mediated drug resistance.13 This is mediated by induction of p27Kip1 and activation of NF-κB family transcription factors.12 Accordingly, biologically based treatments targeting not only MM cells, but also MM cell–BM interactions may be required to overcome drug resistance. Importantly, in this study neither IL-6 or IGF-1, nor adherence of MM cells to BMSCs, abrogated JS-K–induced cytotoxicity, suggesting that JS-K can overcome BM microenvironment–induced drug resistance.
JS-K is a novel, targeted agent designed to release cytotoxic levels of intracellular NO• when metabolized by GSTs. As shown here, it is cytotoxic to MM cells through NO• generation, followed by DNA DSB formation. As such, its mechanisms of action are novel and unique compared with currently available classes of anti-MM agents. Several other NO• generators were shown to induce DNA DSBs in mammalian cells.33-36 However, in most cases, these compounds generated NO• extracellularly, and significant NO• is scavenged by the extracellular milieu, thereby decreasing the effective NO• concentration reaching DNA. Given that JS-K releases NO• intracellularly, we anticipated JS-K to be a very potent generator of DNA DSBs. Our results supported this hypothesis, since JS-K induced significant DSB formation in MM cells as early as 2.5 hours after drug exposure. Furthermore, in our studies, we show that NO• generation by JS-K is, at least in part, dependent on GSTs. Although a variety of other DNA damaging agents, including melphalan, cyclophosphamide, carmustine, and doxorubicin, are currently used to treat MM, these agents were not designed to specifically target tumor cells. Therefore JS-K, with its unique GST-based targeted design, may induce selective tumor cell cytotoxicity. Factors that may impinge on the sensitivity of the myeloma cells to JS-K include GST and GSH levels in the cells, as well as the levels of the antioxidant enzymes such as superoxide dismutases, glutathione peroxidases, and catalases. For example, the RPMI-8266 cell line has relatively lower levels of GSTs than the other MM cell lines (data not shown), which may account, at least in part, for the plateau observed in the viability of RPMI-8266 cells at 40% despite increasing doses of JS-K (Figure 1B). Ongoing studies are delineating the mechanisms of sensitivity versus resistance of MM cells to JS-K.
The DNA damage responses to DSBs involve the PIKK family member sensor proteins ATM and ATR.39 A multitude of DNA repair and checkpoint proteins have been identified as downstream substrates of ATM/ATR, including H2AX and the effector kinases Chk2 and Chk1.39-41 In this study, we showed that JS-K not only induces DSB in MM cells, but also up-regulates DNA damage responses. Specifically, we show that JS-K induces significant H2AX phosphorylation and foci formation, which is one of the most specific responses to DSB formation in cells.37,38 In addition, we show that JS-K induces Chk2 phosphorylation and downstream Ser20 phosphorylation of p53, which is a specific substrate of Chk2.42-44 These results suggest that the Chk2 pathway is involved in JS-K–induced DNA damage responses. Chk2, in addition to its cell-cycle checkpoint effects, can also induce apoptosis via p53, E2F1, and PML.49,54 Although the exact role played by Chk2 during JS-K–induced apoptosis remains under investigation, our results suggest that the Chk2 pathway is an important mediator of DNA damage responses induced by JS-K in MM cells.
Besides Chk1 and Chk2, JNK also functions as an effector kinase that transduces DNA damage signals for various anticancer drugs targeting genomic DNA.48,49 Our results show significant protection against JS-K–induced tumor cell death conferred by pretreatment with JNK inhibitor II. Importantly, although IL-6 is known to inhibit MM cell apoptosis by inhibiting the JNK/SAPK pathway,9 we showed that neither exogenous addition of IL-6 nor tumor cell adherence to BMSCs were able to overcome JS-K–induced apoptosis in MM cells. These data suggest that JNK is a proapoptotic effector kinase for JS-K, and further confirm that JS-K can overcome the protective effects of cytokines and the BM milieu.
Our results therefore demonstrate for the first time that JS-K, a GST-based generator of intracellular NO•, induces cytotoxicity in MM cells via formation of DNA DSBs. Importantly, JS-K is synergistic with bortezomib, and overcomes the protective effects of IL-6, IGF-1, and BMSCs. In addition, its complex and multiple apoptotic mechanisms further enhance its ability to overcome conventional drug resistance. Finally, JS-K is well tolerated and inhibits tumor cell growth in a MM xenograft mouse model via induction of apoptosis. Together, these results provide the preclinical rationale for the clinical evaluation of JS-K, alone and in combination with other agents, to improve patient outcome in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nikhil Munshi, Paul Richardson, Klaus Podar, Yu-Tzu Tai, Rory Coffey, Janice Jin, and Shweta Chhetri for their contributions. We thank Bevin Engelward for the critical reading of the manuscript and support.
This work was supported by the Yearley Family Research Fellowship (T.K.); National Institutes of Health (NIH) grants RO-1 A50947, PO-1 CA78373, and SPORE P50 CA100707; a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); Multiple Myeloma Research Foundation (MMRF) Senior Research Award (D.C.); National Cancer Institute contract N01-CO12400 with SAIC-Frederick (J.E.S.); the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (L.K.K.); The Myeloma Research Fund; and The Lobow Myeloma Fund.
National Institutes of Health
Authorship
Contribution: TK designed, performed, and analyzed research and wrote the manuscript. TH, KI, and NR participated in the design and interpretation of data. EMO and LC helped design and perform in vivo experiments. CQL, LT, and GNW contributed to the comet assay. HY and SV contributed to data generation. JLK performed and helped analyze immunohistology experiments. DC and CM participated in design of the study. JES, LKK, and PS performed the synthesis and purification of JS-K, and contributed to the design of the study. KCA participated in design, coordination, and performance of the study; assisted in writing the manuscript; and funded the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu; Tanyel Kiziltepe, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: tanyel_kiziltepe@dfci.harvard.edu.

![Figure 3. JS-K overcomes the protective effects of IL-6 and IGF-1, and adherence to patient BMSCs. MM.1S cells were treated for 48 hours with JS-K (0–2.4 μM) in the absence (□) or presence (■) of IL-6 (10 ng/mL) (A) or IGF-1 (50 ng/mL) (B), and without (□) or with BMSCs derived from MM patients 1(■) and 2 (□) (C). DNA synthesis was determined by measuring [3H]-thymidine incorporation during the last 8 hours of 48-hour cultures. Data represent means (± SD) of triplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-10-052845/4/m_zh80130703370003.jpeg?Expires=1765884143&Signature=Q2l8Cgub96fchbipa9HRvB0lUfDIYG9j0M1HD5ByGH96lzjBcJdOo35loNApp3WHsDkmWJ-wuPahQd~73~fsfShyrFydpXHSIgFG-9X~rtzC2txVt~8SNgI-skOaxxmBnToHbE2j~A5Y4cKVm8gFxZjTazH6G4i8~XbIjdw3kTp6NdaphYJUofhz3hi1zfmLmdkUqPeMOoppsh9EPimYNkVR8TQx3iTd5mDeeVPKpVaWrbPYCdZhLKYQfRAYR~Y8B7MDuARkc6qmc9jDSKzSOI32pdg1f9M8WuU5myzxtJY-QwwMkLXwiL6XQYzqOfAZhLskfshmTixP9cIglVkdtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal