Mutations of the TP53 tumor suppressor gene have been associated with poor survival in some series of diffuse large B-cell lymphoma (DLBCL) but not in other studies. The purpose of this study was to identify the frequency of TP53 alterations (mutations or deletions), characterize the gene expression of mutant/deleted cases, and determine the effects of mutations on survival. In a series of DLBCL that had previous gene expression profiling, we identified 24 mutations in 113 cases (21%). There was no difference in the frequency of mutations in the molecular subgroups of DLBCL. Twelve (50%) of the 24 cases had mutations localized to the DNA-binding codons in the core domain of TP53. The presence of any TP53 mutation correlated with poor overall survival (OS; P = .044), but DNA-binding mutations were the most significant predictor of poor OS (P < .001). Multivariate analysis confirmed that the International Prognostic Index, tumor size, and TP53 DNA-binding mutations were independent predictors of OS. Gene expression analysis showed that TRAILreceptor-2 (DR5) was the most differentially underexpressed gene in the TP53 mutated cases. Investigation is warranted into targeted therapy toward TRAIL receptor-2, to potentially bypass the adverse effect of mutated TP53 in DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive non-Hodgkin lymphoma and comprises 35% to 40% of adult lymphomas.1,2 This disease represents a clinically and genetically heterogeneous entity, and it develops either de novo or as a high-grade transformation from low-grade B-cell lymphoma. In the era before rituximab, DLBCL treated with conventional chemotherapy, with or without radiotherapy, showed progression-free survival of approximately 70% in early stage and 50% in advanced stage disease. However, because of disease progression, approximately half of all patients exhibit poor overall survival. Overall survival has been significantly improved in DLBCL by the addition of rituximab.3,,–6
The International Prognostic Index (IPI) has been widely used to predict the prognosis in DLBCL.7,8 However, the molecular events that are important in explaining the IPI values are only partially known.9 In our recent studies that used a complimentary DNA (cDNA) Lymphochip microarray, 3 distinct molecular subgroups of DLBCL were identified, including germinal center B-cell–like (GCB), activated B-cell–like (ABC), and primary mediastinal B-cell (PMBL), leaving a heterogeneous group of cases that are not classifiable.10,,–13 These results have shown that patients with DLBCL with GCB and PMBL subtypes have a significantly better overall survival (OS) than those with the ABC or unclassifiable subtypes.10,11,13,14 Alternatively, DLBCL analyzed with Affymetrix oligonucleotide microarrays has been subclassified into oxidative phosphorylation, B-cell receptor/proliferation, and host response molecular subgroups.15,16
To better understand these differences in survival of various types of DLBCL, the TP53 tumor suppressor gene is of particular interest because it plays a critical role in the regulation of cell proliferation and survival.17,18 Although the TP53 gene is inactivated in approximately 50% of human tumors because of structural alterations (mutation or deletion or both) or functional inhibition by other oncogenic factors,19,20 TP53 mutations are less frequent in hematologic malignancies, with a mean frequency of 14%.21 Mutations of the TP53 gene have been found to be associated with an unfavorable prognosis in transformed follicular lymphoma,22 mantle cell lymphoma,23 Burkitt lymphoma,24,25 and chronic lymphocytic leukemia.26,–28
In DLBCL, however, the role of an altered TP53 gene in predicting survival remains controversial. Early studies found that TP53 mutations were associated with a low rate of complete remission and were predicted for poor OS in the low or intermediate IPI risk groups of patients.29,–31 More recent studies have shown that TP53 mutation or deletion predicts treatment resistance and short survival in the plasmablastic/plasmacytoid variant of DL-BCL.32,33 However, other studies failed to show that TP53 mutations correlated with prognosis.34,35 In addition, no studies have reported the frequency of TP53 mutations in the molecular subgroups of DLBCL or described the gene expression pattern of DLBCL with TP53 mutations.
We hypothesized that the frequency of TP53 alterations would be higher in the ABC subtype of DLBCL and would provide a partial basis for the poor OS observed in that subtype. We also hypothesized that DNA-binding TP53 mutations may have the greatest effect on survival, as previously observed in solid tumors.36,–38 Therefore, to test these hypotheses and to clarify the previous conflicting observations, mutational analysis and fluorescence in situ hybridization (FISH) for TP53 were performed in a large series of DLBCL. Our goals were to determine (1) the differences in clinical characteristics and survival between DLBCL with or without TP53 alterations, (2) the frequency of TP53 mutations or deletions or both among the molecular subgroups of DLBCL, (3) the characteristic gene expression pattern of TP53 mutant cases, and (4) the predictive value of subsets of TP53 gene mutations on OS in patients with DLBCL.
Patients, materials, and methods
Patient information
These cases were derived from a series of 240 patients with DLBCL that were previously profiled with the Lymphochip cDNA microarray by the Lymphoma/Leukemia Molecular Profiling Project.11 The de novo cases in the series represented patients with newly diagnosed and untreated disease, including both nodal and extranodal cases, who received anthracycline-containing regimens without rituximab. After power calculations, 113 cases were chosen for mutation analysis from DNA available on the 240 cases. Of the 240 cases, 132 cases had paraffin material available for deletion studies. The recently published Bayesian classification system was used to define the molecular profiles of the DLBCL cases.12 Thirteen patients who did not receive a curative regimen and 4 patients with incomplete survival information were excluded from the analysis of clinical characteristics and survival. The Institutional Review Board of the University of Nebraska Medical Center approved this study.
Mutational analysis of TP53 by DHPLC
Genomic DNA was extracted simultaneously with RNA from the same frozen tissue samples. Exons 5 to 8 of the TP53 gene were amplified using previously published polymerase chain reaction (PCR) primer sequences,23 500 ng of genomic DNA, 1.5 U Amplitaq polymerase, 100mM dNTPs, 1.5mM MgCl2, 50μM primers in a reaction volume of 100 μL. The PCR reactions were performed in an auto thermocycler (Perkin Elmer Cetus, Norwalk, CT) with the following conditions: denatured at 94°C for 9 minutes, followed by 45 cycles, 94°C for 75 seconds, annealing at 60°C for exons 6 and 7 (58°C for exons 5 and 8) for 75 seconds, extension at 72°C for 30 seconds, followed by final extension at 72°C for 7 minutes. Mutations were then identified using denaturing high-performance liquid chromatography (DHPLC) in a WAVE DNA Fragment Analysis System (Transgenomic, Omaha, NE), which has a sensitivity of 0.1%.39,40 Briefly, the PCR products were analyzed for exon 5 at 66.7°C with 54% buffer B, exon 6 at 62.2°C with 52% buffer B, exon 7 at 64.9°C with 49% buffer B, and exon 8 at 64°C with 53% buffer B. The DNA samples that exhibited shifted peaks, as compared with wild-type DNA, were captured with a fraction collector, reamplified, and directly sequenced bidirectionally with the same unclamped TP53 primers, using the Big Dye Terminator Kit on the ABI 377 Sequencer (Applied Biosystems, Foster City, CA).39,40
Detection of chromosome 17p13.1 deletions by FISH
Among the 240 cases studied by gene expression profiling, 132 available cases were evaluated by an interphase FISH assay for chromosome 17p13.1 deletions on formalin-fixed, paraffin-embedded tissue sections according to a previously described procedure with minor modification.41 A LSI TP53 Spectrum Orange Probe (Vysis, Downers Grove, IL) was used to detect 17p13.1 deletions, and the CEP 18 Spectrum Aqua Probe (Vysis) was used simultaneously to evaluate the copy number of chromosome 17. A positive result was defined as more than 20% nuclei with deletions in this 17p13.1 FISH assay in our laboratory.
Immunohistochemistry of p53 and p21 proteins
For the tissue microarray (TMA), hematoxylin and eosin–stained sections from each paraffin-embedded, formalin-fixed block were used to define diagnostic areas, and 4 representative 0.6-mm cores were obtained from each case and inserted in a grid pattern into a recipient paraffin block using a tissue arrayer (Beecher Instruments, Silver Spring, MD). Immunohistochemical analysis of p53 protein was performed in 72 of 132 cases that had paraffin blocks available, and p21 protein expression was successfully evaluated in 63 of 72 patients with p53 immunohistochemical results. Sections (5 μm) were cut from each TMA and stained with the monoclonal antibody DO-7 for p53 protein (DAKO, Carpinteria, CA) or clone 6B6 for p21 protein (Pharmingen, San Diego, CA) by using the streptoavidin-biotin-peroxidase technique.23 After deparaffinization, heat-induced antigen retrieval was performed in 10mM citrate buffer at pH 6.0 for 30 minutes. The monoclonal antibody DO-7, which is recognized as an amino terminal epitope (residue 35–45) of the p53 protein, and the 6B6 antibody for p21, were each applied at a 1:50 concentration and incubated at room temperature for 30 minutes. Detection was completed in a Ventana ES instrument using a diaminobenzidine immunoperoxidase detection kit (Ventana Medical Systems, Tucson, AZ). Each case was evaluated independently by 2 pathologists (K.H.Y. and T.C.G.) for the percentage of tumor cells staining, and disagreements were resolved by joint review on a multihead microscope. Approximately 750 cells/slide were evaluated, and a positive result was recorded when the percentage of positively staining nuclei exceeded 5% of the tumor cells, a threshold previously described in normal paraffin-embedded tissues.22,23
Statistical analysis of mRNA expression in mutant TP53 cases
The global mRNA expression patterns were previously reported on this series of DLBCL.11 Supervised analysis was performed on the gene expression patterns of the TP53 mutated cases versus wild-type (WT) cases. Differentially expressed genes were identified using BRB ArrayTools (National Cancer Institute, Bethesda, MD). Random-variance t tests were used to find genes whose expression differed significantly between the 2 groups. Differential gene expression was considered statistically significant if the P value was less than .001. To confirm these results, P values for significant genes were computed based on 1000 random permutations. Only the genes that passed this permutation analysis are listed (P < .004). The chromosomal location of the differentially expressed genes was noted, and a chi-square goodness-of-fit test was used to determine whether the differentially expressed genes were randomly distributed across the chromosomes. The Lymphochip had 3 probes for each of the TP53 and CDKN1A (p21) genes, and the average value of the mRNA expression for the 3 gene probes was calculated for each gene. For each subgroup of cases (WT or mutant), the median expression was then compared for each gene by using the Wilcoxon rank sum test.
RT-PCR analysis of TRAIL receptor-2
Total RNA was re-extracted from frozen tissue of 3 cases with TP53 DNA-binding mutations and 3 wild-type cases with Lymphochip data. After homogenization of the tissue in Triazol (Invitrogen, Carlsbad, CA) and mixing with chloroform, the aqueous phase was used to extract RNA with an Rneasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's directions. Reverse transcriptase (RT) of 400 ng of total RNA was performed using the Superscript III first-strand synthesis system for RT-PCR (Invitrogen). Quantitative PCR was performed in triplicate for GAPDH (GAPDH forward: CCTGACCTGCCGTCTAGAAAA; GAPDH reverse: CCCTGTTGCTGTAGCCAAATT) with Finnzymes DyNAmo HS SYBR green qPCR kit (New England Biolabs, Ipswich, MA), and with 2 Assays on Demand systems (Hs00366278_ml and Hs01043171_ml) for TRAIL receptor-2 (Applied Biosystems). An initial denaturation at 95°C for 15 minutes was followed by GAPDH at 40 cycles of 95°C for 30 seconds and TRAIL receptor-2 at 45 cycles of 95°C for 15 seconds, with annealing at 58°C for GAPDH and 63°C for TRAIL receptor-2 for 1 minute in a DNA Engine Opticon 2 instrument (Bio-Rad Laboratories, Hercules, CA). Relative copy number of gene expression was determined by using the formula: Copy number = 2−ΔΔCT with GAPDH as a calibrator.42,43
Survival analysis
The Kaplan-Meier method was used to estimate overall survival (OS) and event-free survival (EFS) of the patients. The log-rank test was used to compare the differences in clinical markers and the survival differences between subgroups with and without TP53 mutation or deletion. OS was defined as the time from diagnosis to death resulting from any cause or, for patients remaining alive, the time from diagnosis to last contact. EFS was defined as the time from diagnosis to the occurrence of the first relapse or death from any cause or, for patients remaining alive and relapse free, the time from diagnosis to last contact. Fisher exact test for 2 × 2 tables and the Student t test were used for analysis of the distributions of categorical data between groups. Multivariate analysis using the Cox regression model was used to evaluate which variables were potential independent prognostic factors for OS. SAS software was used for the data analysis (SAS Institute, Cary, NC).
Results
TP53 gene mutations
Of the 113 analyzed cases, 24 cases (21%) contained TP53 mutations, including 22 cases with single-base missense mutations, one single-base deletion (codon 152), and one 10-base insertion (codon 282) (Table 1). The mutations included 6 in exon 5, 5 in exon 6, 7 in exon 7, and 6 in exon 8. A known polymorphism at codon 213 was identified in one case. For the ABC subtype, mutations were found in 11 (24%) of 45 cases, whereas 11 (30%) of 37 cases were mutated in the GCB subtype (P = .43). No mutations were seen in the 7 PMBL cases, but mutations were identified in 2 (8%) of 24 unclassifiable cases.
Mutations of TP53 in DLBCL
| Case . | Group . | Exon . | Codon . | TP53 domain . | Nucleotide change . | Amino acid change . | Follow-up, status (mo) . |
|---|---|---|---|---|---|---|---|
| 1 | GCB | 5 | 132 | S2 | AAG>AAT | Lys>Asn | Dead (132) |
| 2 | ABC | 5 | 152 | S3 | CCG>C-G | Pro>Arg* | Alive (101) |
| 3 | GCB | 5 | 158 | S4 | CGC>CAC | Arg>His | Dead (18) |
| 4 | ABC | 5 | 173 | L2 | GTG>GCG | Val>Ala | Alive (92) |
| 5 | ABC | 5 | 175 | L2 | CGC>CAC | Arg>His | Dead (5) |
| 6 | ABC | 5 | 176 | L2 | TGC>CGC | Cys>Arg | Dead (4) |
| 7 | GCB | 6 | 211 | S6 | ACT>ATT | Thr>Ile | Alive (97) |
| 8 | ABC | 6 | 213 | S6 | CGA>TGA | Arg>Stop | Dead (7) |
| 9 | ABC | 6 | 213 | S6 | CGA>TGA | Arg>Stop | Alive (137) |
| 10 | GCB | 6 | 215 | S7 | AGT>AGG | Ser>Arg | Dead (5) |
| 11 | GCB | 6 | 216 | S7 | GTG>ATG | Val>Met | Dead (8) |
| 12 | ABC | 7 | 229 | S8 | TGT>TGA | Cys>Stop | Dead (20) |
| 12Δ | — | 7 | 254 | S9 | ATC>AGC | Ile>Ser | — |
| 13 | ABC | 7 | 234 | S8 | TAC>AAC | Tyr>Asn | Dead (30) |
| 14 | GCB | 7 | 248 | L3 | CGG>CAG | Arg>Gln | Dead (5) |
| 15 | ABC | 7 | 248 | L3 | CGG>CAG | Arg>Gln | Dead (6) |
| 16 | Unclass | 7 | 248 | L3 | CGG>CAG | Arg>Gln | Alive (62) |
| 17 | Unclass | 7 | 248 | L3 | CGG>TGG | Arg>Trp | Dead (11) |
| 18 | GCB | 7 | 251 | L3 | ATC>TTC | Ile>Phe | Dead (10) |
| 19 | GCB | 8 | 272 | S10 | GTG>GAG | Val>Glu | N/A |
| 20 | GCB | 8 | 273 | S10 | CGT>CCT | Arg>Pro | Dead (8) |
| 21 | GCB | 8 | 273 | S10 | CGT>TGT | Arg>Cys | Dead (36) |
| 22 | GCB | 8 | 280 | H2 | AGA>AGT | Arg>Ser | Dead (8) |
| 23 | ABC | 8 | 280 | H2 | AGA>ATA | Arg>Ile | Dead (12) |
| 24 | ABC | 8 | 283 | H2 | CGC>(I)CGC | Arg>Leu† | Alive (49) |
| Case . | Group . | Exon . | Codon . | TP53 domain . | Nucleotide change . | Amino acid change . | Follow-up, status (mo) . |
|---|---|---|---|---|---|---|---|
| 1 | GCB | 5 | 132 | S2 | AAG>AAT | Lys>Asn | Dead (132) |
| 2 | ABC | 5 | 152 | S3 | CCG>C-G | Pro>Arg* | Alive (101) |
| 3 | GCB | 5 | 158 | S4 | CGC>CAC | Arg>His | Dead (18) |
| 4 | ABC | 5 | 173 | L2 | GTG>GCG | Val>Ala | Alive (92) |
| 5 | ABC | 5 | 175 | L2 | CGC>CAC | Arg>His | Dead (5) |
| 6 | ABC | 5 | 176 | L2 | TGC>CGC | Cys>Arg | Dead (4) |
| 7 | GCB | 6 | 211 | S6 | ACT>ATT | Thr>Ile | Alive (97) |
| 8 | ABC | 6 | 213 | S6 | CGA>TGA | Arg>Stop | Dead (7) |
| 9 | ABC | 6 | 213 | S6 | CGA>TGA | Arg>Stop | Alive (137) |
| 10 | GCB | 6 | 215 | S7 | AGT>AGG | Ser>Arg | Dead (5) |
| 11 | GCB | 6 | 216 | S7 | GTG>ATG | Val>Met | Dead (8) |
| 12 | ABC | 7 | 229 | S8 | TGT>TGA | Cys>Stop | Dead (20) |
| 12Δ | — | 7 | 254 | S9 | ATC>AGC | Ile>Ser | — |
| 13 | ABC | 7 | 234 | S8 | TAC>AAC | Tyr>Asn | Dead (30) |
| 14 | GCB | 7 | 248 | L3 | CGG>CAG | Arg>Gln | Dead (5) |
| 15 | ABC | 7 | 248 | L3 | CGG>CAG | Arg>Gln | Dead (6) |
| 16 | Unclass | 7 | 248 | L3 | CGG>CAG | Arg>Gln | Alive (62) |
| 17 | Unclass | 7 | 248 | L3 | CGG>TGG | Arg>Trp | Dead (11) |
| 18 | GCB | 7 | 251 | L3 | ATC>TTC | Ile>Phe | Dead (10) |
| 19 | GCB | 8 | 272 | S10 | GTG>GAG | Val>Glu | N/A |
| 20 | GCB | 8 | 273 | S10 | CGT>CCT | Arg>Pro | Dead (8) |
| 21 | GCB | 8 | 273 | S10 | CGT>TGT | Arg>Cys | Dead (36) |
| 22 | GCB | 8 | 280 | H2 | AGA>AGT | Arg>Ser | Dead (8) |
| 23 | ABC | 8 | 280 | H2 | AGA>ATA | Arg>Ile | Dead (12) |
| 24 | ABC | 8 | 283 | H2 | CGC>(I)CGC | Arg>Leu† | Alive (49) |
Δ case 12 has two TP53 mutations.
Unclass indicates unclassified; N/A, not applicable; I, CTTAACCCGG; and —, same as line above.
Stop at 169.
Stop at 308.
In 6 cases, mutations were localized in codons previously described as TP53 hot spots in non-Hodgkin lymphoma.30,33,44,45 When the mutation distribution pattern was analyzed on the TP53 molecule, 12 mutations (50%) were found in codons involved in DNA binding in the core domain. These included 4 cases with codons encoding Arg248 residues, which are believed to interact with the DNA minor groove, and 5 cases with residues (Asn273, Arg280, and Arg283) that interact with the DNA major groove. The remaining 3 mutations occurred in amino acid residues (Arg175, Cys176, and Ile251) that are believed to enhance the binding affinity of TP53 with the DNA helix at physiologic conditions.44,46,47
TP53 gene deletions
Similar frequencies and distribution patterns were also noted for TP53 gene deletion (24% in ABC and 26% in GCB subtypes, respectively; P = .52) (Table 2). The frequency of deletions was 14% and 9% in the PMBL and unclassifiable subtypes, respectively. However, because there are few cases in the PMBL subtype and the unclassifiable cases are heterogeneous, these subtypes will not be discussed in survival analysis of molecular subtypes. Alterations in TP53, which are defined as either mutation or chromosomal deletion occurred in 43 (47%) of 92 of cases.
Frequency of TP53 alterations in the molecular subtypes of DLBCL
| . | ABC, n/total (%) . | GCB, n/total (%) . | PMBL, n/total (%) . | Unclass, n/total (%) . | Total, n/total (%) . | P . |
|---|---|---|---|---|---|---|
| TP53 mutations | 11/45 (24) | 11/37 (30) | 0/7 (0) | 2/24 (8) | 24/113 (21) | .11 |
| 17p13.1 deletions | 11/46 (24) | 12/53 (23) | 1/10 (10) | 2/23 (9) | 26/132 (20) | .44 |
| . | ABC, n/total (%) . | GCB, n/total (%) . | PMBL, n/total (%) . | Unclass, n/total (%) . | Total, n/total (%) . | P . |
|---|---|---|---|---|---|---|
| TP53 mutations | 11/45 (24) | 11/37 (30) | 0/7 (0) | 2/24 (8) | 24/113 (21) | .11 |
| 17p13.1 deletions | 11/46 (24) | 12/53 (23) | 1/10 (10) | 2/23 (9) | 26/132 (20) | .44 |
Unclass indicates unclassified
mRNA expression pattern of TP53 mutant cases
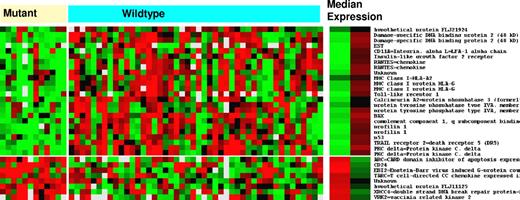
Supervised analysis of Lymphochip expression data showed that 135 genes were differentially expressed in the TP53 mutant cases (P < .001). Decreased expression with the greatest statistical difference was seen in TRAIL receptor-2 (DR5), followed by other TP53 target genes such as DDB2, XPC, CDKN1A (p21), and BAX (Table 3). TRAIL receptor-2 is also identified as the most differentially expressed gene, followed by TP53, BAX, and DDB2 in the DNA-binding subset of TP53 mutations (Figure 1). The decreased expression of TRAIL receptor-2 was confirmed by RT-PCR analysis in a representative sample of 3 cases with DNA-binding TP53 mutations compared with 3 wild-type cases (Table 4). We found that it is possible to construct a 22-gene predictor that has an accuracy of 70% (range, 60%-80%) in identifying cases with TP53 mutations, depending on the analytical tool used. T-cell receptor and other T-cell surface antigens tend to be underexpressed in TP53 mutant cases. In TP53 deleted cases, there was differential expression of 11 genes, with the greatest decrease in expression for TP53 followed by IFIT3, TRAIL receptor-2, and the DVL2 gene, which is colocalized with TP53 on chromosome 17p13 (Table 5).
Differential mRNA expression in DLBCL with TP53 mutations
| Gene symbol . | Location . | Gene description . | Fold difference . |
|---|---|---|---|
| TNFRSF10B (DR5) | 8p22-p21 | Tumor necrosis factor receptor superfamily, member 10b (TRAIL receptor 2) | −1.45* |
| LCP2 (SLP-76) | 5q33.1-qter | Tyrosine phosphoprotein SLP-76 | −1.91* |
| ARL7 | 2q37.1 | ADP-ribosylation factor-like 7 | −1.83* |
| AIF-1 | 6p21.3 | Allograft-inflammatory factor-1 | −1.74 |
| XPC | 3p25 | Xeroderma pigmentosum, complementation group C | −1.46* |
| CD11A | 16p11.2 | CD11A, Integrin, alpha L; LFA-1 alpha chain | −1.46 |
| DDB2 | 11p12-p11 | Damage-specific DNA binding protein 2 | −1.56* |
| TP53 | 17p13.1 | p53 Tumor suppressor | −1.58 |
| CABIN1 | 22q11.23 | Negative regulator for calcineurin signaling in T lymphocytes | −1.31 |
| TXNIP | 1q21.1 | Thioredoxin-interacting protein | −1.88 |
| LILRB1 | 19q13.4 | Leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 3 | −1.73 |
| ARHGEF1 | 19q13.13 | Rho guanine nucleotide exchange factor (GEF) 1 | −1.37 |
| ARRB2 | 17p13 | Arrestin beta 2 | −1.42* |
| FYN | 6q21 | Tyrosine protein kinase | −1.92* |
| FYB | 5p13.1 | SLAP-130, SLP-76 associated protein; FYN, binding protein | −1.85 |
| DPH2L | 17p13.3 | Similar to diphthamide biosynthesis gene DPH2 of Saccharomyces cerevisiae | −1.33 |
| CDKN1A (p21) | 6p21.2 | Cyclin kinase inhibitor, WAF1, CIP1 | −1.56 |
| FCGRT | 19q13.3 | IgG Fc receptor hFcRn | −1.73 |
| NSF | 17q21 | Ribosomal protein S7 | −1.69 |
| TCRA | 14q11.2 | T-cell receptor alpha chain | −1.99 |
| CD64 | 1q21.1 | High-affinity immunoglobulin gamma FC receptor I A | −3.36 |
| PSRY5 | 13q14 | Purinergic receptor P2Y5 | −2.30 |
| LSP1 | 11p15.5 | Lymphocyte-specific protein | −1.75* |
| BTG1 | 12q22 | B-cell translocation gene 1 | −1.61 |
| TOP3A | 17p12-p11.2 | Topoisomerase DNA III alpha | −1.36 |
| BAX | 19q13.3-4 | BCL2-associated X protein | −1.52 |
| GBP2 | 1p22.2 | Guanylate-binding protein 2, interferon-inducible | −1.93 |
| NK4 | 16p13.3 | Interleukin 32 | −2.08 |
| SPAG7 | 17p13.2 | Sperm-associated antigen | −1.35 |
| Gene symbol . | Location . | Gene description . | Fold difference . |
|---|---|---|---|
| TNFRSF10B (DR5) | 8p22-p21 | Tumor necrosis factor receptor superfamily, member 10b (TRAIL receptor 2) | −1.45* |
| LCP2 (SLP-76) | 5q33.1-qter | Tyrosine phosphoprotein SLP-76 | −1.91* |
| ARL7 | 2q37.1 | ADP-ribosylation factor-like 7 | −1.83* |
| AIF-1 | 6p21.3 | Allograft-inflammatory factor-1 | −1.74 |
| XPC | 3p25 | Xeroderma pigmentosum, complementation group C | −1.46* |
| CD11A | 16p11.2 | CD11A, Integrin, alpha L; LFA-1 alpha chain | −1.46 |
| DDB2 | 11p12-p11 | Damage-specific DNA binding protein 2 | −1.56* |
| TP53 | 17p13.1 | p53 Tumor suppressor | −1.58 |
| CABIN1 | 22q11.23 | Negative regulator for calcineurin signaling in T lymphocytes | −1.31 |
| TXNIP | 1q21.1 | Thioredoxin-interacting protein | −1.88 |
| LILRB1 | 19q13.4 | Leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 3 | −1.73 |
| ARHGEF1 | 19q13.13 | Rho guanine nucleotide exchange factor (GEF) 1 | −1.37 |
| ARRB2 | 17p13 | Arrestin beta 2 | −1.42* |
| FYN | 6q21 | Tyrosine protein kinase | −1.92* |
| FYB | 5p13.1 | SLAP-130, SLP-76 associated protein; FYN, binding protein | −1.85 |
| DPH2L | 17p13.3 | Similar to diphthamide biosynthesis gene DPH2 of Saccharomyces cerevisiae | −1.33 |
| CDKN1A (p21) | 6p21.2 | Cyclin kinase inhibitor, WAF1, CIP1 | −1.56 |
| FCGRT | 19q13.3 | IgG Fc receptor hFcRn | −1.73 |
| NSF | 17q21 | Ribosomal protein S7 | −1.69 |
| TCRA | 14q11.2 | T-cell receptor alpha chain | −1.99 |
| CD64 | 1q21.1 | High-affinity immunoglobulin gamma FC receptor I A | −3.36 |
| PSRY5 | 13q14 | Purinergic receptor P2Y5 | −2.30 |
| LSP1 | 11p15.5 | Lymphocyte-specific protein | −1.75* |
| BTG1 | 12q22 | B-cell translocation gene 1 | −1.61 |
| TOP3A | 17p12-p11.2 | Topoisomerase DNA III alpha | −1.36 |
| BAX | 19q13.3-4 | BCL2-associated X protein | −1.52 |
| GBP2 | 1p22.2 | Guanylate-binding protein 2, interferon-inducible | −1.93 |
| NK4 | 16p13.3 | Interleukin 32 | −2.08 |
| SPAG7 | 17p13.2 | Sperm-associated antigen | −1.35 |
Only known genes with a P value of < .0001 are listed in ranked order (P values not shown) from the total of 135 differentially expressed genes at P < .001.
Average of multiple values.
Differential mRNA gene expression between tumors with DNA-binding codon TP53 mutations and tumors with wild-type TP53. Median expression of each gene listed in the right column is provided for each of the 2 subgroups of tumors (mutant and wild-type). Red is increased and green is decreased.
Differential mRNA gene expression between tumors with DNA-binding codon TP53 mutations and tumors with wild-type TP53. Median expression of each gene listed in the right column is provided for each of the 2 subgroups of tumors (mutant and wild-type). Red is increased and green is decreased.
TRAIL receptor-2 mRNA expression
| Case . | Lymphochip 1 . | Lymphochip 2 . | RT-PCR 1 . | RT-PCR 2 . |
|---|---|---|---|---|
| Mutated 1 | 0.39 | 0.43 | 0.59 | 0.61 |
| Mutated 2 | 0.46 | 0.5 | 0.28 | 0.56 |
| Mutated 3 | 0.54 | 0.48 | 0.12 | 0.16 |
| Mean (± SD)* | 0.46 (± 0.08) | 0.47 (± 0.04) | 0.33 (± 0.24) | 0.44 (± 0.25) |
| Wild-type 1 | 1.49 | 1.53 | 2.72 | 3.44 |
| Wild-type 2 | 1.53 | 1.55 | 0.84 | 1.02 |
| Wild-type 3 | 1.63 | 2 | 1.54 | 2.07 |
| Mean (± SD)* | 1.55 (± 0.07) | 1.69 (± 0.27) | 1.70 (± 0.95) | 2.18 (± 1.21) |
| Case . | Lymphochip 1 . | Lymphochip 2 . | RT-PCR 1 . | RT-PCR 2 . |
|---|---|---|---|---|
| Mutated 1 | 0.39 | 0.43 | 0.59 | 0.61 |
| Mutated 2 | 0.46 | 0.5 | 0.28 | 0.56 |
| Mutated 3 | 0.54 | 0.48 | 0.12 | 0.16 |
| Mean (± SD)* | 0.46 (± 0.08) | 0.47 (± 0.04) | 0.33 (± 0.24) | 0.44 (± 0.25) |
| Wild-type 1 | 1.49 | 1.53 | 2.72 | 3.44 |
| Wild-type 2 | 1.53 | 1.55 | 0.84 | 1.02 |
| Wild-type 3 | 1.63 | 2 | 1.54 | 2.07 |
| Mean (± SD)* | 1.55 (± 0.07) | 1.69 (± 0.27) | 1.70 (± 0.95) | 2.18 (± 1.21) |
Lymphochip 1 and 2 represent relative mRNA expression data compared to control mRNA of pooled cell lines from 2 probes in Rosenwald et al.11 RT-PCR 1 and RT-PCR 2 represent relative copy number compared with GAPDH using 2 different probes.
Mean (± SD) of 3 cases.
Differential mRNA expression in DLBCL with TP53 deletions
| Gene symbol . | Location . | Gene description . | Fold difference . |
|---|---|---|---|
| TP53 | 17p13.1 | P53 tumor suppressor | −1.56* |
| IFIT3 | 10q24 | Interferon-induced protein with tetratricopeptide repeats 3 | −1.27 |
| TNFRSF10B | 8p22-p21 | TRAIL receptor 2 (death receptor 5) | −1.32 |
| CASP-7 | 10q25 | CASPASE-7, apoptosis-related cysteine protease | −1.48 |
| C3AR1 | 12p13.31 | Complement component 3a receptor 1 | −2.53 |
| DVL2 | 17p13.2 | Disheveled 2 | −1.39 |
| Gene symbol . | Location . | Gene description . | Fold difference . |
|---|---|---|---|
| TP53 | 17p13.1 | P53 tumor suppressor | −1.56* |
| IFIT3 | 10q24 | Interferon-induced protein with tetratricopeptide repeats 3 | −1.27 |
| TNFRSF10B | 8p22-p21 | TRAIL receptor 2 (death receptor 5) | −1.32 |
| CASP-7 | 10q25 | CASPASE-7, apoptosis-related cysteine protease | −1.48 |
| C3AR1 | 12p13.31 | Complement component 3a receptor 1 | −2.53 |
| DVL2 | 17p13.2 | Disheveled 2 | −1.39 |
Only known genes with a P value of < .0001 are listed from the total of 11 differentially expressed genes at P < .001.
Average of multiple values.
TP53 and CDKN1A (p21) mRNA expression
Significantly decreased TP53 mRNA was found in DLBCL with TP53 mutations (median = 0.62) or deletions (median = 0.58) compared with the corresponding wild-type cases for each assay (no mutation, median = 0.81; no deletion, median = 0.88; P < .001) (Table 6). The results of CDKN1A mRNA expression were similar to the TP53 results. The microarray data showed that DLBCL with TP53 mutations exhibited decreased CDKN1A mRNA expression (median = 0.53; P = .001), as did DLBCL marginally with TP53 deletions (median = 0.55; P = .058), compared with DLBCL with wild-type TP53 (medians = 0.70 and 0.68, respectively; Table 6).
Correlation of TP53 mutation and 17p13.1 deletion with TP53 and CDKN1A mRNA expression
| . | TP53 mRNA expression . | CDKN1A mRNA expression . |
|---|---|---|
| TP53 mutation (n = 24) | 0.62 | 0.53 |
| Wild-type TP53 (n = 89)* | 0.81 | 0.70 |
| P | <.001 | .001 |
| 17p13.1 deletion (n = 26) | 0.58 | 0.55 |
| 17p13.1 wild-type (n = 106)† | 0.88 | 0.68 |
| P | <.001 | .058 |
| . | TP53 mRNA expression . | CDKN1A mRNA expression . |
|---|---|---|
| TP53 mutation (n = 24) | 0.62 | 0.53 |
| Wild-type TP53 (n = 89)* | 0.81 | 0.70 |
| P | <.001 | .001 |
| 17p13.1 deletion (n = 26) | 0.58 | 0.55 |
| 17p13.1 wild-type (n = 106)† | 0.88 | 0.68 |
| P | <.001 | .058 |
Expression is stated as median values for all table entries.
Wild-type is defined as normal results in the dHPLC screening assay.
Wild-type is defined as normal results in the FISH assay.
p53 and p21 protein expression
Expression of the p53 protein was found in 40 (56%) of 72 cases. However, only 13 (32%) of the 40 protein-positive cases had an altered TP53 gene. Expression of p53 was seen in 13 (81%) of 16 mutant cases, but expression was also paradoxically seen in 27 (48%) of 56 cases with wild-type TP53 (Table 7). Expression of p21 protein was found in 28 (44%) of 63 cases, and most of these cases, 26 (93%) of the 28 had the WT TP53 gene (Table 8). Four different p53/p21 phenotypes (p53+/p21+, p53+/p21−, p53−/p21+, p53−/p21−) were analyzed for correlation with TP53 alterations. Simultaneous expression of p53 and p21 proteins (p53+/p21+) was observed in 20 cases. All of these dual-expressing cases, except one (mutation at codon 280), exhibited the WT TP53 gene. We found p53 protein expression to be dissociated from p21 protein expression (p53+/p21−) in 14 patients. The p53+/p21− phenotype, which may serve as an indicator for TP53 alterations, was found in a high proportion of patients, 10 (91%) of 11, with TP53 gene alterations. However, isolated p21 expression (p53−/p21+) was seen in 8 cases, of which only one case had a TP53 alteration. The p53−/p21− phenotype was identified in 21 cases, but only 2 (9.5%) of these 21 cases had TP53 gene alterations.
Correlation of TP53 alterations with p53 protein expression
| . | p53 Protein positive, n . | p53 Protein negative, n . | Concordance rate, % (n/total) . |
|---|---|---|---|
| TP53 alterations (n = 16) | 13 | 3 | 81 (13/16) |
| Wild-type TP53 (n = 56) | 27 | 29 | 52 (29/56) |
| Total, n/total (%) | 40/72 (55.6) | 32/72 (44.4) | — |
| . | p53 Protein positive, n . | p53 Protein negative, n . | Concordance rate, % (n/total) . |
|---|---|---|---|
| TP53 alterations (n = 16) | 13 | 3 | 81 (13/16) |
| Wild-type TP53 (n = 56) | 27 | 29 | 52 (29/56) |
| Total, n/total (%) | 40/72 (55.6) | 32/72 (44.4) | — |
TP53 alterations include either mutation or deletion. Protein expression was identified in paraffin-embedded tissue.
— indicates not applicable.
Correlation of p53/p21 protein expression with TP53 alteration status
| . | Protein expression . | |||
|---|---|---|---|---|
| p53 positive (34 cases) . | p53 negative (29 cases) . | |||
| p53+/p21+ . | p53+/p21− . | p53−/p21+ . | p53−/p21− . | |
| TP53 alterations (n = 14) | 1/11 | 10/11 | 1/3 | 2/3 |
| Wild-type (n = 49) | 19/23 | 4/23 | 7/26 | 19/26 |
| Total (n = 63) | 20/34 | 14/34 | 8/29 | 21/29 |
| . | Protein expression . | |||
|---|---|---|---|---|
| p53 positive (34 cases) . | p53 negative (29 cases) . | |||
| p53+/p21+ . | p53+/p21− . | p53−/p21+ . | p53−/p21− . | |
| TP53 alterations (n = 14) | 1/11 | 10/11 | 1/3 | 2/3 |
| Wild-type (n = 49) | 19/23 | 4/23 | 7/26 | 19/26 |
| Total (n = 63) | 20/34 | 14/34 | 8/29 | 21/29 |
TP53 alterations include either mutation or deletion. Protein expression was identified by immunostaining of paraffin-embedded tissue.
Clinical characteristics
The major clinical characteristics of 92 patients with DLBCL with both TP53 mutation and deletion data compared with cases with wild-type TP53 are summarized in Table S1 (available on the Blood website; see the Supplemental Table link at the top of the online article). The 2 groups did not differ significantly in clinical characteristics (Table S1). The median age at diagnosis overall was 62.6 years (range, 14.4-83 years). Sixty-two (73%) of 84 patients with data achieved a complete remission (CR), 11 (13%) had a partial response, and 13 (14%) had no response after initial treatment. There was no significant difference in the proportion of patients who achieved a CR based on TP53 status (WT [81%] versus mutation [62%]; P = .33). However, in contrast, the patients with TP53 mutations in the DNA-binding codons exhibited a lower complete remission rate (45%) than did those with WT TP53 (81%) (P = .049).
The clinical characteristics that were predictors of OS in the 90 patients with DLBCL with clinical data are summarized in Table S2. Univariate analysis showed that the number of extranodal sites, serum LDH levels, performance status, clinical stage, B symptoms, tumor size, and IPI risk groups were significant predictors of OS in DLBCL. A significant difference in OS and event-free survival (EFS) was noted in high-risk (3-5) IPI group compared with low-risk (0-2) IPI groups (P < .001).
Survival analysis of DLBCL cases with TP53 alterations
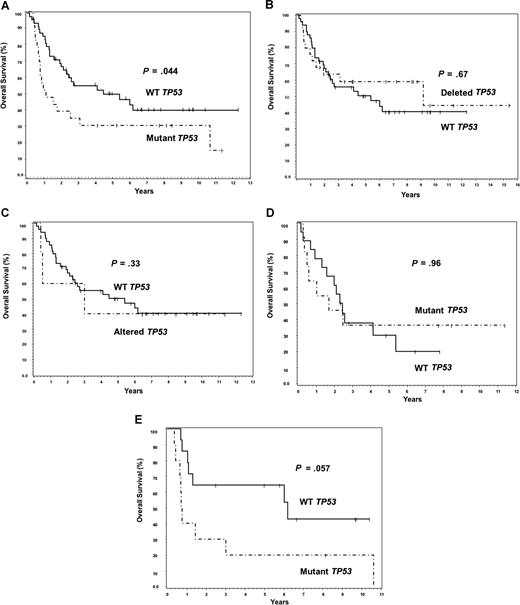
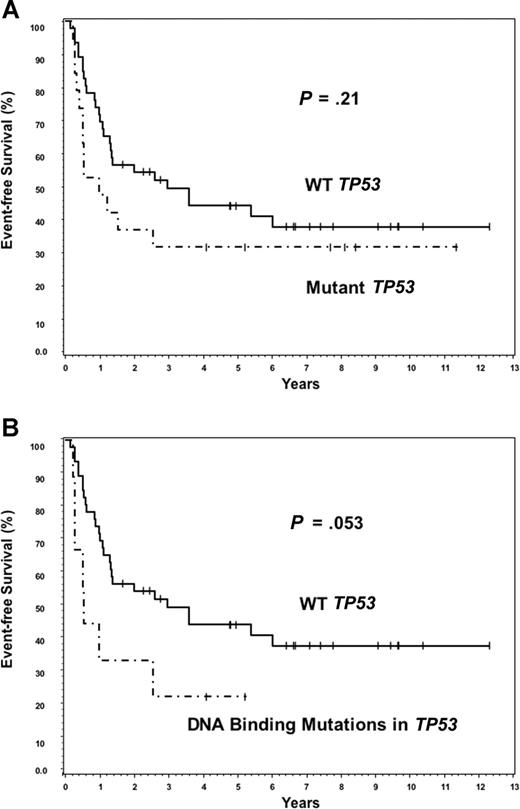
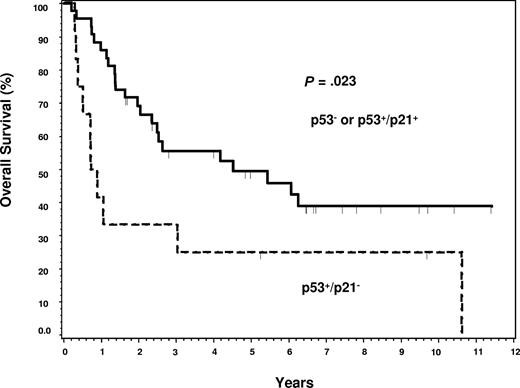
Twenty-three of 24 patients with TP53 mutations had survival data available for analysis. Although TP53 mutations predicted for poor OS (P = .044), there was no significant difference in survival between the deletion or combined alteration subgroups compared with the WT TP53 group (Figure 2A-C). TP53 mutations did not predict for poor survival in the ABC subtype (Figure 2D; P = .96). Although there was a suggestion of poor OS within the GCB subtype (Figure 2E; P = .057), it was not statistically significant. Because the presence of any TP53 mutation was mildly predictive for poor OS, we hypothesized that the distribution pattern of TP53 mutations in the core domain may be even more predictive. The 12 patients with TP53 mutations in DNA-binding codons had a significantly worse median OS (0.74 years) compared with the 11 patients with mutations in the non–DNA-binding codons (2.59 years), or the patients with WT TP53 (5.4 years) (Figure 3A,B). No significant difference on EFS was found between WT and mutant TP53 groups (Figure 4A). However, patients with DNA-binding domain mutations similarly had poor median EFS (0.54 years) (Figure 4B) compared with patients with wild-type TP53 (2.97 years), but it was not statistically significant (P = .053). This is most probably because EFS data were available on 6 fewer patients (55) than was available for OS (61 patients).
TP53mutation predicts for poor overall survival in DLBCL. (A) Overall survival of patients with TP53 mutations versus those with WT TP53. (B) Overall survival of patients with TP53 deletions versus those with WT TP53. (C) Overall survival of patients with TP53 alterations (combined mutations, deletions, or both) versus those with WT TP53. (D) Overall survival of mutant and WT TP53 patients within the ABC subtype of DLBCL. (E) Overall survival of patients with mutant and WT TP53 within the GCB subtype.
TP53mutation predicts for poor overall survival in DLBCL. (A) Overall survival of patients with TP53 mutations versus those with WT TP53. (B) Overall survival of patients with TP53 deletions versus those with WT TP53. (C) Overall survival of patients with TP53 alterations (combined mutations, deletions, or both) versus those with WT TP53. (D) Overall survival of mutant and WT TP53 patients within the ABC subtype of DLBCL. (E) Overall survival of patients with mutant and WT TP53 within the GCB subtype.
The subset of DNA-binding codon mutations accounts for poor overall survival in DLBCL. (A) Overall survival of patients with DNA-binding codon mutations is significantly worse than patients with WT TP53. (B) Overall survival of patients with non–DNA-binding mutations is no different from patients with WT TP53.
The subset of DNA-binding codon mutations accounts for poor overall survival in DLBCL. (A) Overall survival of patients with DNA-binding codon mutations is significantly worse than patients with WT TP53. (B) Overall survival of patients with non–DNA-binding mutations is no different from patients with WT TP53.
Event-free survival in DLBCL. (A) Event-free survival of patients with TP53 mutations versus those with WT TP53. (B) Event-free survival of patients with DNA-binding codon mutations versus patients with WT TP53.
Event-free survival in DLBCL. (A) Event-free survival of patients with TP53 mutations versus those with WT TP53. (B) Event-free survival of patients with DNA-binding codon mutations versus patients with WT TP53.
A stepwise Cox model incorporating clinical prognostic factors (ie, age, PS, stage, LDH level, number of extranodal sites, IPI, and the TP53 gene aberration status) identified high IPI (3-5) scores (P = .001), tumor size (P < .001), and the presence of TP53 mutations in the DNA-binding codons (P = .002) as independent predictors of decreased survival. To explore the effect of DNA-binding site mutations on survival in the 2 risk groups defined by the IPI scores (0-2 and 3-5), we performed an additional Cox model analysis and showed that the prognostic significance of TP53 mutations on survival was marginally independent of the IPI risk group (P = .048; data not shown).
Specific p53+/p21− immunophenotype predicts for survival
Patients with the p53+/p21− phenotype had a significantly worse OS compared with p53− or p53+/p21+ immunophenotypes (P = .023; Figure 5). However, multivariate analysis failed to confirm the p53+/p21− phenotype as an independent prognostic factor (P > .05; data not shown).
Predictive value of p53+/p21− phenotype for overall survival in DLBCL. Patients with the p53+/p21− phenotype have a significantly worse survival compared with those with other phenotypes (P = .023).
Predictive value of p53+/p21− phenotype for overall survival in DLBCL. Patients with the p53+/p21− phenotype have a significantly worse survival compared with those with other phenotypes (P = .023).
Discussion
In our study, the frequency of TP53 mutations (21%) in DLBCL is similar to that of the average frequency of 23% in previous reports in DLBCL.21,29,31,48,–50 TP53 mutations occurred with similar frequencies in the ABC and GCB subtypes (24% versus 30%), indicating that TP53 mutagenesis most probably constitutes a common secondary defect in DLBCL, as often seen in other human cancers.17,21,51
Our studies identified 7 new TP53 mutations, which have not been previously reported in DLBCL. Three mutations were found in exons 6 and 7, including missense mutations of codon 215 (serine to arginine), and 216 (valine to methionine), and one missense mutation of codon 229 (cystine to stop codon). All of these residues are located in the central hydrophobic core of the protein. The substitution of apolar valine (or neutrally polar serine) by methionine (or arginine) results in a p53 protein with a structural defect. Two of these 3 patients exhibited decreased TP53 mRNA expression and no p53 protein, and their survival was short (5 and 8 months, respectively). The other new mutation identified in codon 229 (cystine to stop codon) results in a truncated p53 protein. The lymphoma was unresponsive to chemotherapy, and the survival time of this patient was only 20 months.
No previous study has examined the gene expression profile of DLBCL with a mutant TP53. Expected downstream p53 gene targets were found to be decreased in TP53 mutant cases in this study, with TRAIL receptor-2 (DR5/KILLER/TNFRSF10B) showing the greatest differential expression. TRAIL receptor-2 initiates the extrinsic apoptotic or death receptor pathway after activation by TRAIL,52 an important molecule in inhibiting lymphogenesis as shown in a knockout mouse model.53 In human tumors, Cillessen et al54 observed that TRAIL receptor-2 levels are lower in TRAIL-resistant DLBCL, but no data on TP53 were provided in their study. Wagner et al55 have previously shown, in a microarray analysis of chemoresistant follicular lymphoma cell lines with TP53 mutations, that the abnormal p53 protein cannot up-regulate TRAIL receptor-2 after cells encountered stress from either etoposide or doxorubicin treatment in vitro, which does occur in cell lines with WT TP53. Similarly, Rosenwald et al28 showed that the level of TRAIL receptor-2 is not up-regulated in a lymphoblastoid cell line with a TP53 mutation treated with fludarabine or irradiation, or in a fludarabine-resistant chronic lymphocytic leukemia with p53 overexpression. Our study also shows that p53 mRNA is decreased in TP53 mutated cases. Therefore, TRAIL receptor-2 may be decreased by 2 mechanisms, a lack of up-regulation by mutant TP53 and a dosage effect by deletion of one allele in other cases.
In vitro studies have shown that the use of exogenous TRAIL or antibodies to TRAIL receptor-2 will stimulate the receptor with the regain of normal apoptosis in lymphoma cell lines with TP53 mutations,54,–56 including cell lines derived from DLBCL.54 This is in contrast to chronic lymphocyte leukemia or mantle cell lymphoma in which TRAIL receptor 1 (DR4) is activated, but TRAIL receptor-2 is not up-regulated.57 CP-31398 treatment of cell lines has been shown to up-regulate TRAIL receptor-2.58 Other investigators have shown that small molecules can increase TRAIL receptor-2 and CDKN1A (p21) in TP53-null colon cancer cell lines.59 Although Georgakis et al56 attempted to determine which patients with lymphoma might benefit from human agonistic antibodies to TRAIL death receptors, they did not examine the TP53 mutation status. In summary, our findings suggest that up-regulation of TRAIL receptor-2 may potentially be useful to investigate for therapy to bypass the mutated TP53 pathway in DLBCL.
Similar to our previous observation of decreased expression of genes colocalized on 17p13 in mantle cell lymphoma with deletion of 17p13,39 there was decreased expression of TP53 and the DVL2 gene in DLBCL cases with deletion of 17p13. Therefore, it is important to realize that the gene expression pattern may also reflect structural chromosome changes and not just downstream targets of TP53. The decreased TP53 mRNA expression in the group with TP53 mutations reflects the effect of nonsense mutations or coexistent 17p deletion of the second allele.
Increased p53 protein detected by immunohistochemistry is the result of either a TP53 gene mutation with prolonged p53 protein half-life or accumulation of WT p53 that is induced by stress or other mechanisms.18 There was a high concordance rate (81%) of p53 protein expression in DLBCL with TP53 gene alterations. The p21 protein is a direct target of the p53 protein, and the p21 protein is responsible for p53-mediated G1 cell-cycle arrest.60 Several studies in other tumors have suggested that the p53+/p21− immunophenotype is frequently associated with TP53 gene alterations, which we confirmed in 10 (71%) of 14 cases with TP53 mutations. However, the coexpression of the p21 protein with p53, (p53+/p21+), which has been reported to be a marker of WT TP53 status,31,48,61 performed better in predicting wild-type TP53 (95%) than the p53+/p21− phenotype did in predicting TP53 alterations (71%). Our findings are consistent with previous reports in DLBCL31,61,–63 and suggest that p53 protein expression often may not reflect a genetic abnormality of TP53. However, the p53+/p21− phenotype should be used cautiously to predict mutant status because some cases had WT TP53, which we observed in 4 (29%) of 14 cases. Alternatively, the level of positive expression of TP53 was set too low. p53 Protein expression was often found in wild-type cases, which characteristically had high mRNA levels. This indicates that up-regulation of p53 expression may occur by other mechanisms. This reinforces the concept that p53 protein expression must be studied in association with p21 protein expression on paraffin-embedded tissue for better, but still incomplete, prediction of the TP53 mutation status.
Previous studies suggest that the biologic effects of various TP53 mutations are heterogeneous, leading to different OS in DLBCL. Certain mutations may render tumors resistant to chemotherapy, whereas, in some cases, the product of the mutations behaves like the wild-type protein and does not affect OS.64 Structural analysis has shown that more than 50% of TP53 mutations affect the central core domain of the protein encoded by exons 5 to 8, particularly the DNA-binding codons in exons 5, 7, and 8.17,46,47 Previous studies have shown that mutations in the zinc-binding domains (L2 and L3 loops) are significantly associated with a poor prognosis and chemoresistance in patients with breast cancer36,37 and in colorectal cancer.38 Correlation of the location of TP53 mutations with clinical outcome has been suggested in DLBCL but has not been established because of an insufficient number of cases.31 The current study shows a strong relation between the location of the mutations and clinical behavior. Interestingly, it appeared that TP53 mutations in the DNA-binding domains (loops L2, L3, and helix H2) are associated with a poor survival. Five (42%) of 12 patients carrying such mutations died within 1 year, whereas only 2 (18%) of 11 patients with a TP53 mutation located outside these regions died (P < .01). Five of 12 patients carrying mutations in the DNA-binding domain exhibited disease progression with partial or no response to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)–based chemotherapy, whereas only 1 of 11 patients with a TP53 mutation located in other codons showed disease progression. Mutations that cause structural defects in TP53 seem to contribute to a more aggressive biologic behavior. Structural studies have revealed that Arg175 and Arg248, comprising the major part of L2 and L3 loops, are responsible for the binding with the DNA minor groove, whereas a loop-sheet-helix motif composed of residues Arg273, Cys277, Arg280, Asp281, Arg282, and Arg283 interacts to bind with the DNA major groove.46 Most of the mutations in these regions will result in loss of DNA-binding capacity. The results of this study support the conclusion that the location of TP53 mutations in exons 5 to 8 plays a pivotal role in determining the biologic behavior of DLBCL. Recent studies have also indicated that TP53 mutations continue to be predictive of poor OS with rituxan-CHOP therapy.65
In conclusion, this report shows 2 new findings. First, the DNA-binding mutations in the core domain of TP53 are significantly associated with poor overall survival in DLBCL. Second, TRAIL receptor-2 may be a potential target to investigate for therapeutic intervention in patients with DLBCL with p53 mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Deb Lytle for performance of the mutation analysis, Diane Pickering for FISH analysis, Xiao Li for statistical consultation, Leo Kinarsky and Sherman Simon for consultation support on p53 structural analysis, and Katrina Matthews for manuscript preparation. Analyses were performed with BRB ArrayTools developed by Dr Richard Simon and Amy Peng Lam.
This work was supported by grants from the US Public Health Service (CA36727 and CA84967) awarded by the National Cancer Institute, Department of Health and Human Services, and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. T.C.G. is a Lymphoma Research Foundation Mantle Cell Lymphoma Program Research Grantee.
National Institutes of Health
Authorship
Contribution: K.H.Y. performed research, collected data, analyzed data, and wrote the paper; B.D. performed research and collected data; D.D.W. analyzed data and wrote the paper; L.S. and J.I. analyzed data; W.S., E.C., J.D. R.D.G., G.O., L.R., H.K.M.-H., and E.S.J. collected data; A.R., L.M.S., and W.C.C. analyzed data; T.C.G. designed research, contributed vital new reagents or analytical tools, collected data, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy C. Greiner, Department of Pathology and Microbiology, University of Nebraska Medical Center, 983135 Nebraska Medical Center, Omaha, NE 68198-3135; e-mail:tgreiner@unmc.edu.