The TCL1/MTCP1 oncogenes were identified on the basis of their involvement in T-cell prolymphocytic leukemia (T-PLL). TCL1 and MTCP1 proteins directly interact with AKT and modulate the AKT signal-transduction pathway, but the relevance of this mechanism in leukemogenesis remains unclear. We investigate the biologic functions of TCL1 in the T-cell lineage using various cell lines, and primary malignant and normal lymphocytes. In the Jurkat cell line, expression of TCL1 had no effect in unstimulated cells, whereas it abrogated activation-induced cell death (AICD). These cellular effects were concomitant with a major inhibition by TCL1 of PKCθ and ERK pathways. Secondly, the TCL1-driven T-cell leukemia cell line SUP-T11 was shown to have impaired PKCθ and ERK phosphorylation upon stimulation, which were restored by TCL1 inhibition using RNA interference. Finally, defects in these pathways were also observed in primary malignant (T-PLL) and transduced normal T lymphocytes expressing TCL1. Altogether, our data demonstrated that TCL1 inhibits AICD in T cells by blocking PKCθ and ERK activation, upon cellular activation.
Introduction
The MTCP1 (mature T-cell proliferation-1) and TCL1 (T-cell leukemia-1) genes were initially cloned as the genes deregulated by t(X;14) and t(14;14)/inv(14) chromosomal rearrangements, respectively.1,2 These translocations, and hence expression of either of these oncogenes, are associated with T-cell prolymphocytic leukemia (T-PLL), an adult mature T-cell leukemia.3,–5 TCL1 is also overexpressed in other human malignancies, including a variety of germinal center and post–germinal center B-cell–derived leukemias and lymphomas (for review, see Teitell6 ). The oncogenic properties of MTCP1 and TCL1 were demonstrated in transgenic animal models by their ability to reconstitute T-cell and B-cell leukemia/lymphoma that resemble corresponding human diseases.7,–9 MTCP1, TCL1, and the subsequently identified TCL1B/TML1 genes encode small intracellular, nonenzymatic protein products with significant sequence homologies.1,2,10,11 In addition, MTCP1 and TCL1 demonstrate similar 8-stranded antiparallel β barrel structures.12,13
The prevailing view of the role of TCL1/MTCP1 proteins in tumorigenesis is related to their involvement in the AKT signal-transduction pathway. Biochemical studies using cell lines overexpressing TCL1 showed that TCL1 binds the pleckstrin homology (PH) domain of AKT and causes its hetero-oligomerization.14,–16 Functional and structural studies support a model in which TCL1 binds and stabilizes AKT at the cell membrane, and in which TCL1 and phosphatidyl inositide triphosphate interact simultaneously on opposite faces of the PHAKT domain, concomitant to TCL1 homodimerization.17,18 The mechanism by which TCL1 augments AKT activity is not completely resolved and remains controversial.6,19 In this regard, the absence of phospho-AKT in TCL1-expressing CD4+/CD56+ hematodermic neoplasms and T-PLLs does not argue in favor of a major role for the AKT pathway in TCL1/MTCP1-driven leukemogenesis, without excluding possible roles at earlier steps of the malignant process or independent of AKT phosphorylation status.20
Cellular functions of TCL1 were also investigated through the generation of deficient animal models. Analyses of Tcl1 transgenic and knock-out animals, as well as in vitro analyses, supported the idea of a role for TCL1 in T- and B-cell growth and survival, including resistance to receptor-induced apoptosis.14,21,22 To maintain central and peripheral tolerance, and T-cell homeostasis, activated lymphocytes are physiologically eliminated by apoptosis. This process is known as activation-induced cell death (AICD) and occurs concomitantly with G1 phase cell-cycle arrest.23,24 Overexpression experiments in Jurkat cell line and studies of Pkcθ−/− mice have supported an important role of PKCθ in promoting AICD in T lymphocytes.25,,–28
PKCθ, a member of the Ca2+-independent novel-PKC subfamily, is more specifically expressed in T cells and has emerged in recent years as a key enzyme involved in T-cell activation.29,30 This central role of PKCθ in T-cell physiology is supported by T-cell impairment in PKCθ-deficient mice associated with deficient activation of its downstream pathways, such as NF-κB, ERK, AP-1, and NF-AT.31,–33 Engagement of the T-cell receptor (TCR) upon its recognition of the presented antigen triggers an intracellular cascade of biochemical events. One of the earliest events is the activation of protein tyrosine kinases of the Src family, and subsequently of the Syk family, including ZAP70. A large multicomponent complex is then formed, which will recruit or activate numerous substrates, including enzymes such as phospholipase Cγ-1 (PLCγ-1). PLCγ-1 catalyzes inositol phospholipids into diacylglycerol (DAG) and inositol triphosphate. DAG is then responsible for the translocation of PKC to the plasma membrane and its activation. One of the hallmarks of PKCθ activation is its phosphorylation, presumably by PDK1, on threonine 538 (Thr538) located in its activation loop.34,35
In the present study, we show that TCL1 expression conferred resistance to activation-induced cell death (AICD) and growth arrest, by inhibiting the ERK pathway concomitant, and probably due to, an impairment of PKCθ activation.
Materials and methods
Reagents
The antibodies used in this study include the following: for Western blotting, anti-p44/42 MAP kinase (ERK1/2), anti–phospho-ERK1/2 (Thr202/Tyr204), anti-PKCθ, anti–phospho-PKCθ (Thr538), anti–phospho-AKT (Ser473), anti–phospho-IκBα (Ser32), anti–phospho-ZAP-70 (Thr319) (all from Cell Signaling Technology, Denver, MA), and anti–β-actin (Sigma-Aldrich, Saint Quentin Fallavier, France); for immunofluorescence, anti-ERK1/2 (Upstate, Lake Placid, NY) and anti-PKCθ (Becton Dickinson, San Jose, CA). Rabbit antisera were generated against purified glutathione S-transferase-TCL1 and -MTCP1 fusion proteins.5,36 The fluorescein isothiocyanate (FITC)–conjugated anti-CD69 was from Becton Dickinson. The human CD3-ϵ–specific UCHT1 monoclonal antibody was kindly given by C. Hivroz (Institut Curie, France). Phorbol myristate acetate (PMA) was from Sigma-Aldrich. The kinase inhibitors used in this study include rottlerin, a novel-PKC inhibitor; Gö6850, a pan-PKC inhibitor; Bay 11–7085, a NF-κB inhibitor (all from Calbiochem, San Diego, CA); U0126, a MEK1/2 inhibitor (Promega, Madison, WI); and LY294002, a PI3K inhibitor (Sigma-Aldrich).
Generation of TCL1-overexpressing Jurkat cell lines
Human TCL1 and MTCP1 open reading frames were amplified by polymerase chain reaction (PCR) and cloned into a pBICEP vector (Sigma-Aldrich). All constructs were generated using standard cloning procedures and verified by restriction enzyme analyses and DNA sequencing. Jurkat cells (107) were electroporated with 10 μg TCL1 or control vectors at 960 μF/250 V using an electroporator (Bio-Rad, Marnes-La-Coquette, France). Polyclonal populations of Jurkat-TCL1 and Jurkat-C were selected in RPMI 1640 complete medium containing 1 mg/mL G418 sulfate (Invitrogen, Cergy Pontoise, France) for 2 weeks. In transient transfection experiments, Jurkat cells (107) were electroporated with 10 μg TCL1, MTCP1, or control vectors and 2 μg of a GFP-expression vector under the same conditions and incubated for 18 hours at 37°C. Subsequently, GFP-positive Jurkat cells (Jurkat-TCL1*, Jurkat-MTCP1* or Jurkat-C*) were sorted using a FACSVantage cell sorter (Becton Dickinson).
Cell culture
Cell lines.
The Jurkat cell line was maintained in RPMI 1640 complete medium containing 2.5% fetal calf serum (FCS), 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2. Derivative Jurkat cell lines were maintained in the same conditions in presence of 1 mg/mL G418 sulfate. SUPT-11, a leukemic T-cell line that carries a t(14;14)(q11;q32) chromosomal translocation leading to an overexpression of TCL1, was grown in a complete medium containing 5% FCS.2,37
Primary samples.
Primary T-PLL samples were thawed from our cryopreserved T-PLL cell collection. Tumor cells were isolated on Histopaque gradient (Sigma-Aldrich), assessed for viability by trypan blue staining, and maintained for short-term cultures in RPMI 1640 complete medium containing 2.5% FCS. Normal peripheral blood mononuclear cells (PBMCs) were obtained from adult blood buffy coats after Histopaque gradient and maintained in complete medium as described.
Cell stimulation
Fifteen hours before treatment, cell lines and primary samples were diluted at appropriate concentration in fresh complete medium. PMA or UCHT1 was used for the durations and concentrations indicated in the figures. Where indicated, the cells were incubated for 1 hour at 37°C prior to the stimulation, with rottlerin (10 μM), Gö6850 (1 μM), U0126 (10 μM), Bay 11-7085 (0.5-1 μM), or LY294002 (10 μM) inhibitors.
Flow cytometry
Cells (106) were diluted in 50 μL phosphate-buffered saline (PBS)–bovine serum albumin (BSA) 0.5% and incubated with FITC-conjugated anti-CD69 for 20 minutes in the dark at room temperature. Cells were washed and resuspended in PBS-BSA 0.5% and analyzed with FACSCalibur and with Cell Quest Pro software (Becton Dickinson).
For analysis of cell-cycle distribution, PMA-stimulated cells were collected and washed with ice-cold PBS, after which they were fixed for 1 hour in 70% ethanol at 4°C. Cells were then pelleted by centrifugation and washed once with ice-cold PBS. Cells were stained with propidium iodide (PI; Sigma-Aldrich) by incubation for 15 minutes in PBS containing 50 μg/mL PI and 25 μg/mL RNAse A (Roche Diagnostics, Meylan, France). Cell-cycle profiles were determined using a FACSCalibur and analyzed using ModFit software (Becton Dickinson).
To evaluate apoptosis, cells were labeled using annexin V–FITC apoptosis detection kit (Becton Dickinson) following the manufacturer's recommendations.
Whole-cell extract preparation and immunoblotting analysis
Cells were collected by centrifugation at 600g for 5 minutes at 4°C and washed in cold PBS. Cell pellets were lysed by adding radioimmunoprecipitation assay (RIPA) buffer (100 mM Tris-HCl, 0.1 M NaCl, 1 mM EDTA, 1% Triton, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) supplemented with a cocktail of protease and phosphatase inhibitors, incubated on ice for 10 minutes, and centrifuged at 13 000 rpm for 15 minutes at 4°C. Protein supernatants were collected, and equal amounts of protein (60 μg) were separated by SDS–polyacrylamide gel electrophoresis (PAGE, 10% gel) and then electrotransferred to nitrocellulose membrane. Nonspecific antibody-binding sites were blocked by incubation in blocking buffer (PBS, 0.1% Tween 20 [PBS-T] with 5% wt/vol nonfat dry milk) for 1 hour at room temperature. After PBS-T washes, membranes were incubated overnight at 4°C with primary antibodies diluted according to the manufacturer's instructions in PBS-T with 5% BSA. After washes with PBS-T, membranes were incubated with horseradish peroxidase–conjugated secondary antibodies diluted in blocking buffer for 1 hour at room temperature. Proteins were detected by incubating the membrane with Super Signal West Pico (Pierce, Rockford, IL) detection reagent. Where indicated, the protein expression level was analyzed and quantified using a luminescent image analyzer (LAS-1000plus; Fujifilm, Saint Quentin en Yvelines, France).
Electrophoretic mobility shift assay
Nuclear extracts were prepared and analyzed as previously described.38 Jurkat cells were solubilized for 5 minutes at 4°C in electrophoretic mobility shift assay (EMSA) I buffer (50 mM Tris-HCl [pH 7.9], 10 mM KCl, 1 mM EDTA, 0.2% NP-40, 10% glycerol) supplemented with protease and phosphatase inhibitors. After centrifugation and extensive washes in EMSA I buffer, the pelleted nuclei were incubated for 20 minutes at 4°C with EMSA II buffer (400 mM NaCl, 20% glycerol, 20 mM HEPES [pH 7.9], 10 mM KCl, 1 mM EDTA) and protease and phosphatase inhibitors. The extracts were centrifuged at 14 000g for 10 minutes, and supernatants were used for the mobility shift assay. The following partially double-stranded KBF1 oligonucleotide probe was used for the mobility shift assays: GATCTGGGGATTCCCCAT; ACCCCTAAGGGGTACTAG.
Mobility shift assays were performed in a total volume of 20 μL in the following buffer: 4% Ficoll, 20 mM HEPES [pH 7.5], 70 mM NaCl, 2 mM dithiothreitol, 0.01% BSA, and 0.01% NP-40. Each reaction (mixtures also contained 1 μL 32P end-labeled probe and 1 μL poly(dI-dC) [Pharmacia]) was initiated by the addition of 10 μg nuclear extract, and mixtures were incubated at room temperature for 20 minutes prior to electrophoretic analysis on a 5% native polyacrylamide gel in 0.5× Tris-borate-EDTA buffer. Specificity controls included competition with 100-fold excess of cold KBF1 probe, and use of Bay 11–7085, a NF-κB inhibitor. EMSAs were quantified using a Storm PhosphorImager and the ImageQuant software (GE Healthcare, Munich, Germany).
Immunofluorescence
Jurkat cells immobilized on polylysine-coated slides were stimulated with UCHT1 at concentrations and durations indicated in the figures. Jurkat cells were then incubated for 10 minutes in ice-cold PBS containing 10 mM glycine, washed in PBS, fixed 20 minutes with 3.7% paraformaldehyde, and permeabilized and blocked for 45 minutes with 0.1% Tween–2.5% horse serum in PBS. The cells were stained with anti–phospho-AKT (1/50), anti-ERK1/2 (1/50), or anti-PKCθ (1/50), followed by cyanin 3– or FITC-conjugated antimouse or antirabbit antibody (Dako, Trappes, France). The cells were mounted with Vectashield (Vector Laboratories, Burlingame, CA) with DAPI (Boehringer Mannheim, Germany), and imaged with an Axioplan 2 microscope (Zeiss, LePecq, France) using 40×/1.3 and 100×/1.3 NA oil objectives equipped with appropriate filters and a Quantix camera (Photometrics, Tucson, AZ) and analyzed with Smart Capture version 2.1.1 software (Digital Scientific, Cambridge, United Kingdom). Intensity of immunofluorescence was quantified as pixel density using IPlab Spectrum software (Sigma-Aldrich).
Construction of expression and shRNA lentiviral vectors
Sense (63 nt, GATCCCAGGATAGGTTACAGTTACGTTCAAGA-GACGTAACTGTAACCTATCCTTTTTTGGAAA) and antisense (63 nt, AGCTTTTCCAAAAAAGGATAGGTTACAGTTACGTCTCTTGAACG-TAACTGTAACCTATCCTGG) oligonucleotides were designed in the coding region of the human TCL1 gene (shown in bold). They were annealed and cloned into the pTER plasmid, in a position 3′ of the polIII H1 promoter.39 The H1-shRNA-TCL1 DNA fragment was then subcloned into the pTRIP/ΔU3-EF1αGFP lentiviral vector40 (shRNA(GFP)-TCL1). A shRNA(GFP)-control (shRNA(GFP)-C) was designed using a sequence with no significant homology in the human genome, as determined by BLAST search41 (shown in bold) (sense: CGATCCCCTTCTCCGAA-CGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATT-TTTGGAAG and antisense: CTAGCTTCCAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGGGAT).
Human TCL1 open reading frame was also amplified by PCR and cloned into pCDH1-MCS1-EF1-Puro vector (System Biosciences, Mountain View, CA). The construct (pCDH1-TCL1) was generated using standard cloning procedures and verified by restriction enzyme analyses and DNA sequencing.
Transduction
Production of both shRNA(GFP)-TCL1 and shRNA(GFP)-C lentiviral vectors and cell line transduction were performed as described.42,43 Briefly, cells were transduced with shRNA vectors for 48 hours in complete medium containing 2.5% FCS. Cells were then washed and assayed for TCL1 expression. Transduction efficiency was more than 90% of the cells, as assessed for GFP-positive cells by flow cytometry.
In another series of experiments with the SUP-T11 cell line, shRNA vectors from the Mission human shRNA TCL1A clone sets were used to allow puromycin selection (shRNA-TCL1(1): 107141; shRNA-TCL1(3): 107143; shRNA-TCL1(4): 107144; and appropriate control vector: shRNA-C: SHC002; Sigma-Aldrich).
For lentiviral transduction of PBMCs, cells were stimulated by 1× phytohemagglutinin M (Invitrogen) and IL-2 (100 U/mL) for 48 hours in RPMI 1640 complete medium containing 10% FCS, transduced for 48 hours, and maintained at least 48 hours before experiments in complete medium containing 10% FCS and puromycin (1 μg/mL) to allow selection of transduced lymphocytes.
Results
TCL1 inhibits PMA-induced cell death and growth arrest
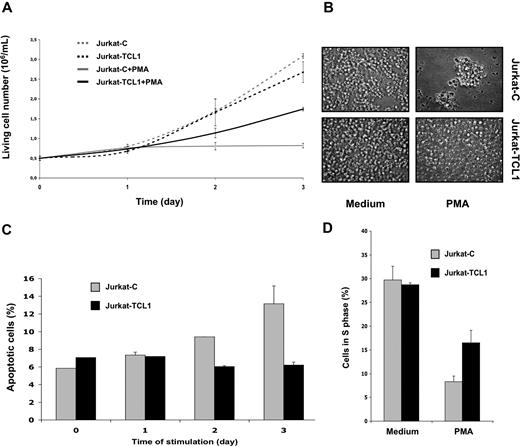
To investigate the biologic function of TCL1 in the T-cell lineage, we established stably transfected polyclonal Jurkat cells expressing TCL1 (Jurkat-TCL1) at a level similar to that of TCL1-associated T-PLLs. We then measured live cell numbers of Jurkat control (Jurkat-C) and Jurkat-TCL1 lines grown under standard culture conditions. Cells (0.5 × 106) were initially seeded. After 3 days of culture, Jurkat-TCL1 had not proliferated significantly more than the control line; the live cell numbers were 2.68 (± 0.26 means and SD) × 106 (5.4-fold increase) and 3.10 (± 0.06 means and SD) × 106 (6-fold increase) for Jurkat-TCL1 and Jurkat-C, respectively (Figure 1A). Microscopic examination of cell culture of Jurkat-TCL1 compared with Jurkat-C showed no detectable difference (Figure 1B).
TCL1 inhibits PMA-induced cell death and growth arrest. (A) Viable cell number of Jurkat-C (gray line)– and Jurkat-TCL1 (black line)–treated (solid line) or untreated (dashed line) with PMA was determined at different times, as indicated in the figure, using a cell viability analyzer (Vi-cell XR; Beckman, Hialeah, FL). Data are expressed as mean (± SE) of duplicate samples. Three additional experiments gave similar results. (B) Photomicroscopy of Jurkat-C or Jurkat-TCL1, treated or untreated with PMA for 3 days. Two additional experiments gave similar results. (C) Apoptotic cell fraction of Jurkat-C (gray box) or Jurkat-TCL1 (black box) induced with PMA was determined by flow cytometry as the annexin V–FITC–positive/PI-negative percentage at different times, as indicated in the figure. Data are expressed as mean (± SE) of duplicates samples. Two additional experiments gave similar results. (D) Cell-cycle distribution (S phase) using flow cytometry after PI staining was determined at 24 hours for Jurkat-C (gray box) or Jurkat-TCL1 (black box) induced or not induced with PMA. Three additional experiments gave similar results.
TCL1 inhibits PMA-induced cell death and growth arrest. (A) Viable cell number of Jurkat-C (gray line)– and Jurkat-TCL1 (black line)–treated (solid line) or untreated (dashed line) with PMA was determined at different times, as indicated in the figure, using a cell viability analyzer (Vi-cell XR; Beckman, Hialeah, FL). Data are expressed as mean (± SE) of duplicate samples. Three additional experiments gave similar results. (B) Photomicroscopy of Jurkat-C or Jurkat-TCL1, treated or untreated with PMA for 3 days. Two additional experiments gave similar results. (C) Apoptotic cell fraction of Jurkat-C (gray box) or Jurkat-TCL1 (black box) induced with PMA was determined by flow cytometry as the annexin V–FITC–positive/PI-negative percentage at different times, as indicated in the figure. Data are expressed as mean (± SE) of duplicates samples. Two additional experiments gave similar results. (D) Cell-cycle distribution (S phase) using flow cytometry after PI staining was determined at 24 hours for Jurkat-C (gray box) or Jurkat-TCL1 (black box) induced or not induced with PMA. Three additional experiments gave similar results.
The Jurkat line is a proliferating T-cell line, in which TCR-mediated or phorbol myristate acetate (PMA) activation lead to apoptosis and growth arrest.44,45 This property has made Jurkat a widely used model of T-cell activation-induced cell death (AICD). AICD was thus investigated in our cellular model. When treated by PMA (20 ng/mL) during 3 days of culture as described above, live cell numbers were 1.74 (± 0.04) means and SD × 106 (3.5-fold increase) and 0.82 (± 0.06) means and SD × 106 (1.6-fold increase) for Jurkat-TCL1 and Jurkat-C, respectively (Figure 1A). Gross morphologic differences were also readily observed under microscopic examination after 3 days of PMA stimulation, including reduction of the cell number and presence of clumps in the control culture, which were virtually absent in the Jurkat-TCL1 cell line (Figure 1B).
The effects of the TCL1 on apoptosis and G1 cell-cycle phase arrest were further investigated. Apoptotic cell fraction was determined as the percentage of annexin V–FITC–positive/propidium iodide (PI)–negative cells determined by flow cytometry (Figure 1C). As expected following PMA stimulation, the percentage of apoptotic cells in Jurkat-C increased from 5.9% (± 0.8%) in the unstimulated Jurkat-C to 13.2% (± 2.0%) after 3 days of stimulation. Interestingly, upon the same stimulation, the percentage of apoptotic cells in Jurkat-TCL1 remained unchanged (7.1% ± 0.8% and 6.2% ± 0.3% in unstimulated and stimulated Jurkat-TCL1, respectively; Figure 1C).
Cell distribution was then determined using PI staining and flow cytometry. As shown in Figure 1C, 28.7% and 29.7% of the cells were in S phase in unstimulated Jurkat-TCL1 and Jurkat-C cells, respectively. After 24 hours of PMA stimulation, the percentage of cells in S phase decreased to 16.5% and 8.3% in stimulated Jurkat-TCL1 and Jurkat-C cells, respectively (3.6- and 1.7-fold decrease, respectively) (Figure 1D), with corresponding increases in G1 phase (data not shown).
Thus, TCL1 expression was not associated with any detectable effect in unstimulated Jurkat cells. However, TCL1 confers resistance to both PMA-induced cell death and PMA-induced growth arrest in this cell line.
TCL1 inhibits PKCθ activation
As PMA is a PKC activator, we then investigated PKCθ, the key PKC isoform in T-cell activation. T-cell receptor (TCR) stimulation of the Jurkat cell lines was mimicked using an anti-CD3 monoclo-nal antibody (UCHT1). In Jurkat-C, UCHT1 induced a phosphorylation of PKCθ on Thr538 at 60 minutes, as detected by immunoblotting. By contrast, no significant PKCθ phosphorylation was observed in Jurkat-TCL1 upon the same stimulation (Figure 2A). Likewise, PMA stimulation induced a weak and early PKCθ phosphorylation in Jurkat-C, but no significant phosphorylation in Jurkat-TCL1 (data not shown). To functionally assess PKCθ activation following PMA stimulation, we analyzed the expression of CD69, a known target of PKCθ.31 The induction of CD69 expression was analyzed by FACS after 7 hours of PMA activation. Fluorescence patterns indicated a lower induction of CD69 in Jurkat-TCL1 compared with Jurkat-C (Figure 2B).
TCL1 inhibits PKCθ activation. (A) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 for different times, as indicated. Thr538-PKCθ phosphorylation (p-PKCθ, top) and TCL1 expression (bottom) were determined by Western blotting. Total PKCθ expression was used as a loading control (middle). A representative experiment of the 3 performed is shown. (B) Jurkat-C (gray line) and Jurkat-TCL1 (black line) were stimulated with PMA for 7 hours and CD69 expression was assessed by flow cytometry. A representative experiment of the 3 performed is shown. (C) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 for 10 minutes at different concentrations. ZAP70 phosphorylation (p-ZAP70) and TCL1 expression were determined by Western blotting. β-Actin expression was used as loading control. A representative experiment of the 2 performed is shown.
TCL1 inhibits PKCθ activation. (A) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 for different times, as indicated. Thr538-PKCθ phosphorylation (p-PKCθ, top) and TCL1 expression (bottom) were determined by Western blotting. Total PKCθ expression was used as a loading control (middle). A representative experiment of the 3 performed is shown. (B) Jurkat-C (gray line) and Jurkat-TCL1 (black line) were stimulated with PMA for 7 hours and CD69 expression was assessed by flow cytometry. A representative experiment of the 3 performed is shown. (C) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 for 10 minutes at different concentrations. ZAP70 phosphorylation (p-ZAP70) and TCL1 expression were determined by Western blotting. β-Actin expression was used as loading control. A representative experiment of the 2 performed is shown.
Concordant results observed upon PMA and UCHT1 stimulations were indicative of an effect of TCL1 downstream of the TCR pathway. Furthermore, phosphorylation of ZAP70 induced by UCHT1, one early event after TCR activation, was not impaired in Jurkat-TCL1 compared with Jurkat-C (Figure 2C). Secondary to activation of key kinases including ZAP70, activation of phospholipase-Cγ1 induces PKCθ translocation from cytosol to the membrane, before its phosphorylation.34,35 After 10 minutes of UCHT1 stimulation, PKCθ was mostly localized at the cell membrane in both Jurkat-TCL1 and Jurkat-C (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) at similar levels verified by pixel density quantification of fluorescence intensity.
Therefore, TCL1 impaired PKCθ activation and function, without detectable modification of the signal transduction upstream of PKCθ phosphorylation.
TCL1 inhibits NF-κB activation
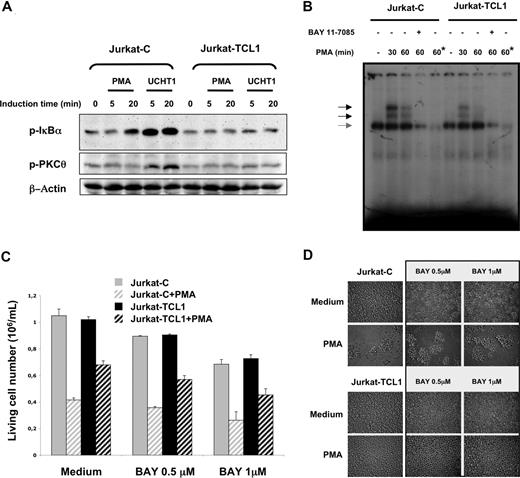
PKCθ activity has an important role in the phosphorylation cascade responsible for IκBα phosphorylation and subsequently for NF-κB activation.30,31,46 Indeed, Jurkat-C cells showed an induction of IκBα phosphorylation following PMA or UCHT1 stimulations. In accordance with the pattern of PKCθ phosphorylation described above, no induction of IκBα phosphorylation was detected in Jurkat-TCL1 cells upon both stimulations (Figure 3A). NF-κB pathway was further investigated by EMSA. The NF-κB DNA-binding activity strongly and transiently induced by PMA in Jurkat-C cells was dramatically reduced in Jurkat-TCL1 (2-fold and 5-fold reduction at 30 and 60 minutes, respectively; Figure 3B).
TCL1 inhibits NF-κB activation. (A) Jurkat-C and Jurkat-TCL1 were stimulated with PMA or UCHT1 at different times indicated in the figure. IκBα (p-IκBα, top) and PKCθ (p-PKCθ, middle) phosphorylation was determined by Western blotting. β-Actin expression was used as a loading control (bottom). A representative experiment of the 3 performed is shown. (B) Band shift assay of nuclear extracts from Jurkat-C and Jurkat-TCL1. Cells were either untreated or stimulated for 30 and 60 minutes with PMA in presence or absence of Bay 11-7085 as indicated in the figure. A constitutive DNA binding of NF-κB with the same intensity in Jurkat-C and Jurkat-TCL1 is indicated by a gray arrow. Asterisk (*) indicates lane containing a 100-fold excess of cold KBF1 oligonucleotide competitor. (C) Jurkat-C (▒) and Jurkat-TCL1 (■) were seeded at 0.5 × 106 cells/mL, and treated (□) or untreated (plain box) with PMA (20 ng/mL) in presence or absence of Bay 11-7085 added 1 hour before and during the stimulation period at indicated concentrations. The viable cell number was determined at 48 hours, using a cell viability analyzer (Vi-cell XR; Beckman). Data are expressed as mean (± SE) of duplicates samples. One additional experiment gave similar results. (D) Photomicroscopy of Jurkat-C and Jurkat-TCL1, treated or untreated with PMA for 3 days in presence or absence of Bay 11-7085 at indicated concentrations. Two additional experiments gave similar results.
TCL1 inhibits NF-κB activation. (A) Jurkat-C and Jurkat-TCL1 were stimulated with PMA or UCHT1 at different times indicated in the figure. IκBα (p-IκBα, top) and PKCθ (p-PKCθ, middle) phosphorylation was determined by Western blotting. β-Actin expression was used as a loading control (bottom). A representative experiment of the 3 performed is shown. (B) Band shift assay of nuclear extracts from Jurkat-C and Jurkat-TCL1. Cells were either untreated or stimulated for 30 and 60 minutes with PMA in presence or absence of Bay 11-7085 as indicated in the figure. A constitutive DNA binding of NF-κB with the same intensity in Jurkat-C and Jurkat-TCL1 is indicated by a gray arrow. Asterisk (*) indicates lane containing a 100-fold excess of cold KBF1 oligonucleotide competitor. (C) Jurkat-C (▒) and Jurkat-TCL1 (■) were seeded at 0.5 × 106 cells/mL, and treated (□) or untreated (plain box) with PMA (20 ng/mL) in presence or absence of Bay 11-7085 added 1 hour before and during the stimulation period at indicated concentrations. The viable cell number was determined at 48 hours, using a cell viability analyzer (Vi-cell XR; Beckman). Data are expressed as mean (± SE) of duplicates samples. One additional experiment gave similar results. (D) Photomicroscopy of Jurkat-C and Jurkat-TCL1, treated or untreated with PMA for 3 days in presence or absence of Bay 11-7085 at indicated concentrations. Two additional experiments gave similar results.
The contribution of the NF-κB pathway to the cellular phenotype observed in the Jurkat model was then investigated using Bay 11-7085, a NF-κB inhibitor. Bay 11-7085, at a concentration inhibiting NF-κB activity in EMSA (Figure 3B), had a moderate dose-dependent effect on cellular proliferation (Figure 3C). However, PMA-induced cell growth arrest and PMA-induced clumps in Jurkat cells were not modified by Bay 11-7085 (Figure 3C,D), suggesting that NF-κB is not a major actor of AICD in Jurkat cells. On the other hand, inhibition of the NF-κB pathway did not modify the impairment of AICD by TCL1 expression in Jurkat cells (Figure 3C,D).
TCL1 inhibits ERK activation
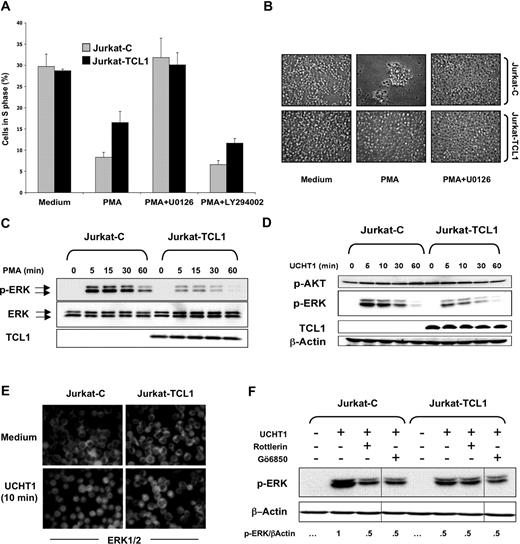
Previous studies have suggested that MEK/ERK pathway activation is an early event during AICD.47,48 The Jurkat cellular model was then investigated using U0126, an inhibitor of MEK/ERK pathway. Indeed, U0126 abolished PMA-induced cell growth arrest and the presence of clumps in Jurkat cells (Figure 4A,B). This suggested that inhibition of AICD by TCL1 expression could involve the MEK/ERK pathway. On the other hand, LY294002, an inhibitor of the PI3K/AKT pathway, did not significantly affect PMA-induced cell growth arrest (Figure 4A), although its efficiency was controlled by immunofluorescence using an anti–phospho-specific AKT antibody (data not shown).
TCL1 inhibits ERK activation. (A) Jurkat-C (gray box) and Jurkat-TCL1 (black box) were either treated or untreated with PMA in presence or absence of U0126, added 1 hour before and during the stimulation period. Cells were collected after 24 hours, stained with PI, and analyzed for cell cycle distribution (S phase) using flow cytometry. Three additional experiments gave similar results. Error bars represent SD. (B) Photomicroscopy of Jurkat-C and Jurkat-TCL1, treated or untreated with PMA for 3 days in presence or absence of U0126. Two additional experiments gave similar results. (C) Jurkat-C and Jurkat-TCL1 were stimulated with PMA at different times, as indicated in the figure. ERK phosphorylation (p-ERK) and TCL1 expression were determined by Western blotting. Total ERK expression was used as a loading control. A representative experiment of 5 performed is shown. Two polyclonal populations from independent transfections were tested with similar results (data not shown). (D) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 at different times, as indicated in the figure. ERK phosphorylation (p-ERK), AKT phosphorylation (p-AKT), and TCL1 expression were determined by Western blotting. β-Actin expression was used as a loading control. A representative experiment of 4 performed is shown. (E) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 for 10 minutes, and ERK was analyzed by immunofluorescence using an anti-ERK (FITC). Representative images of 3 independent experiments are shown. (F) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 in presence or absence of rottlerin (10 μM) or Gö6850 (1 μM), added 1 hour before and during the stimulation period. ERK phosphorylation was determined by Western blotting. After quantification, the p-ERK/β-actin ratio was determined as indicated at the bottom. Vertical lines have been inserted to indicate a repositioned gel lane. A representative experiment of 2 performed is shown.
TCL1 inhibits ERK activation. (A) Jurkat-C (gray box) and Jurkat-TCL1 (black box) were either treated or untreated with PMA in presence or absence of U0126, added 1 hour before and during the stimulation period. Cells were collected after 24 hours, stained with PI, and analyzed for cell cycle distribution (S phase) using flow cytometry. Three additional experiments gave similar results. Error bars represent SD. (B) Photomicroscopy of Jurkat-C and Jurkat-TCL1, treated or untreated with PMA for 3 days in presence or absence of U0126. Two additional experiments gave similar results. (C) Jurkat-C and Jurkat-TCL1 were stimulated with PMA at different times, as indicated in the figure. ERK phosphorylation (p-ERK) and TCL1 expression were determined by Western blotting. Total ERK expression was used as a loading control. A representative experiment of 5 performed is shown. Two polyclonal populations from independent transfections were tested with similar results (data not shown). (D) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 at different times, as indicated in the figure. ERK phosphorylation (p-ERK), AKT phosphorylation (p-AKT), and TCL1 expression were determined by Western blotting. β-Actin expression was used as a loading control. A representative experiment of 4 performed is shown. (E) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 for 10 minutes, and ERK was analyzed by immunofluorescence using an anti-ERK (FITC). Representative images of 3 independent experiments are shown. (F) Jurkat-C and Jurkat-TCL1 were stimulated with UCHT1 in presence or absence of rottlerin (10 μM) or Gö6850 (1 μM), added 1 hour before and during the stimulation period. ERK phosphorylation was determined by Western blotting. After quantification, the p-ERK/β-actin ratio was determined as indicated at the bottom. Vertical lines have been inserted to indicate a repositioned gel lane. A representative experiment of 2 performed is shown.
A kinetic analysis of ERK activation was determined by immunoblotting for the dually phosphorylated activated forms of ERK1/2. In the Jurkat-C cells, ERK phosphorylation upon PMA stimulation persisted at high levels from 5 to 30 minutes and decreased at 60 minutes (Figure 4C). However, a weaker ERK phosphorylation was observed in Jurkat-TCL1 at 5 and 30 minutes after the same stimulation, which approached the baseline at 60 minutes. In addition, we examined the dose response for ERK phosphorylation after 10 minutes of PMA stimulation. More than 10-fold higher concentration of PMA was required to obtain comparable ERK phosphorylation between Jurkat-TCL1 and Jurkat-C (data not shown). Activation of ERK following CD3 ligation using UCHT1 was similarly impaired in Jurkat-TCL1 compared with Jurkat-C (Figure 4D). TCR ligation causes phosphorylation and activation of ERK and its translocation from the cytosol to the nucleus.49 Immunofluorescence studies showed that after 10 minutes of UCHT1 stimulation, nuclear translocation of ERK was observed in nearly all Jurkat-C cells, and only a minority of Jurkat-TCL1 cells (Figure 4E). In contrast, upon stimulation, AKT phosphorylation on Ser473 was comparable in Jurkat-TCL1 and Jurkat-C (Figure 4D), and phospho-AKT was localized at the cell membrane at equal levels in both Jurkat-TCL1 and Jurkat-C (Figure S1B).
Both PKC-dependent and -independent ERK activation are mediated by TCR activation.50,51 Two widely used pharmacologic PKC inhibitors, Gö6850 (an inhibitor of most classes of PKCs) and rottlerin (a selective inhibitor of θ and δ novel-PKCs), were used to explore at which level TCL1 inhibited ERK phosphorylation. After pretreatment with both PKC inhibitors, levels of UCHT1-stimulated ERK phosphorylation in Jurkat-C were reduced to those observed in Jurkat-TCL1. Interestingly, PKC inhibitors did not further reduce the level of ERK phosphorylation in Jurkat-TCL1 (Figure 4F), suggesting that PKC-independent ERK activation was unmodified. TCL1 could thus specifically inhibit the PKC-dependent ERK activation.
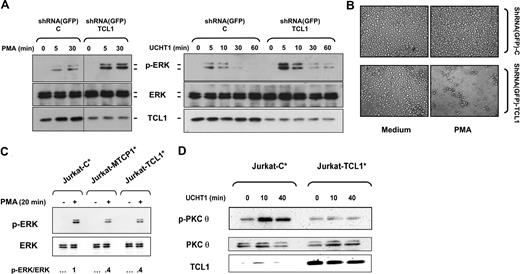
To validate that the cellular and biochemical effects observed were primarily due to the expression of TCL1, a series of experiments were performed. First, TCL1 expression was inhibited by RNA interference using shRNA lentiviral vector. shRNA(GFP)-TCL1 induced more than 70% decrease of the expression of TCL1 in Jurkat-TCL1 cells (Figure 5A). The decreased TCL1 expression in Jurkat-TCL1 dramatically enhanced the ERK phosphorylation in response to PMA and UCHT1 (Figure 5A) and restored cellular effects of PMA (Figure 5B), compared with shRNA(GFP)-C–transduced Jurkat-TCL1. Second, we analyzed the effect of transient TCL1 transfection. After PMA stimulation, the Jurkat cells overexpressing TCL1 induced less than half the amount of ERK phosphorylation compared with Jurkat cells transfected with a control plasmid (Figure 5C). Similarly, after UCHT1 stimulation, transient TCL1 expression dramatically reduced PKCθ phosphorylation (Figure 5D). Furthermore, transient expression of MTCP1, a second member of the TCL1 oncoprotein family, inhibited ERK phosphorylation induced by PMA in Jurkat cells in a similar fashion to TCL1 (Figure 5C).
TCL1 is the actor of cellular and biochemical phenotypes observed in Jurkat cells. (A) Jurkat-TCL1 cells were transduced with shRNA(GFP)-C and shRNA(GFP)-TCL1 vectors and stimulated with PMA (left) or UCHT1 (right) at the times indicated in the figure. ERK phosphorylation and TCL1 expression were determined by Western blotting. Total ERK expression was used as a loading control. A vertical line has been inserted to indicate a repositioned gel lane. A representative experiment of 2 performed is shown. (B) Photomicroscopy of Jurkat-TCL1 transduced with shRNA(GFP)-C and shRNA(GFP)-TCL1 vectors and treated or untreated with PMA for 3 days. (C) Jurkat cells were cotransfected with TCL1, MTCP1, or control vector and GFP-expression vector. Sorted GFP-positive transient transfected Jurkat cells (Jurkat-C*, Jurkat-TCL1*, and Jurkat-MTCP1*) were stimulated with PMA for 20 minutes (left). ERK phosphorylation and ERK expression were determined by Western blotting. After quantification, the p-ERK/total ERK ratio was determined as indicated at the bottom. (D) Transient transfected cells Jurkat-C* and Jurkat-TCL1* were stimulated with UCHT1. PKCθ phosphorylation and TCL1 expression were determined by Western blotting. Total PKCθ expression was used as a loading control.
TCL1 is the actor of cellular and biochemical phenotypes observed in Jurkat cells. (A) Jurkat-TCL1 cells were transduced with shRNA(GFP)-C and shRNA(GFP)-TCL1 vectors and stimulated with PMA (left) or UCHT1 (right) at the times indicated in the figure. ERK phosphorylation and TCL1 expression were determined by Western blotting. Total ERK expression was used as a loading control. A vertical line has been inserted to indicate a repositioned gel lane. A representative experiment of 2 performed is shown. (B) Photomicroscopy of Jurkat-TCL1 transduced with shRNA(GFP)-C and shRNA(GFP)-TCL1 vectors and treated or untreated with PMA for 3 days. (C) Jurkat cells were cotransfected with TCL1, MTCP1, or control vector and GFP-expression vector. Sorted GFP-positive transient transfected Jurkat cells (Jurkat-C*, Jurkat-TCL1*, and Jurkat-MTCP1*) were stimulated with PMA for 20 minutes (left). ERK phosphorylation and ERK expression were determined by Western blotting. After quantification, the p-ERK/total ERK ratio was determined as indicated at the bottom. (D) Transient transfected cells Jurkat-C* and Jurkat-TCL1* were stimulated with UCHT1. PKCθ phosphorylation and TCL1 expression were determined by Western blotting. Total PKCθ expression was used as a loading control.
By these experiments, we demonstrated that TCL1 is the actor of the cellular and biochemical phenotype observed in the Jurkat cell model.
Decreased TCL1 expression in SUP-T11 cell line restores PKCθ pathway activation
A series of RNA interference experiments were then performed in SUP-T11, a TCL1-driven T-cell leukemia cell line bearing genetic hallmarks of human T-cell prolymphocytic leukemia (T-PLL).2,37 In SUP-T11 cells transduced with a control shRNA vector (shRNA-C), phosphorylation of ERK and PKCθ was barely detected upon PMA and UCHT1 stimulation, respectively (Figure 6A,B). To test if TCL1 was responsible for this poor activation in SUPT-11, 3 shRNA-TCL1 vectors (shRNA-3, shRNA-1, and shRNA-4) were selected, which induced approximately 75%, 85%, and 95% decreases of the expression of TCL1, respectively. Phosphorylation of ERK and PKCθ was dramatically enhanced in shRNA-TCL1–transduced SUP-T11 cells upon PMA and UCHT1 stimulation, respectively (Figure 6A,B). Interestingly, enhancement of ERK and PKCθ phosphorylation upon induction was paralleled to TCL1 extinction. Therefore, impairment of activation-induced ERK and PKCθ phosphorylation in SUP-T11 cells is due to endogenous TCL1 expression.
Decreased TCL1 expression in SUP-T11 cell line restores PKCθ pathway activation. (A) SUPT-11 cells were transduced with shRNA-C, shRNA-TCL1(1), and shRNA-TCL1(4) vectors and stimulated with PMA (10 ng/mL) at the times indicated in the figure. ERK phosphorylation and TCL1 expression were determined by Western blotting. Total ERK expression was used as loading control. A representative experiment of 3 performed is shown. After quantification, the p-ERK/ERK ratio (top panel, white box: t = 0 minute; gray box: t = 5 minutes; black box, t = 30 minutes of PMA induction) and TCL1 expression inhibition (bottom panel, a white box represents the mean plus or minus SE of the 3 time points for each shRNA) were determined and related to shRNA control and represented as histograms. A representative experiment of 2 performed is shown. (B) SUPT-11 cells were transduced with shRNA-C, shRNA-TCL1(1), and shRNA-TCL1(3) vectors and stimulated with UCHT1 at the times indicated in the figure. PKCθ phosphorylation and TCL1 expression were determined by Western blotting. β-Actin expression was used as loading control. After quantification, the pPKCθ/β-actin ratio (top panel, white box: t = 0 minute; gray box: t = 5 minutes; black box, t = 30 minutes of PMA induction) and TCL1 expression inhibition (bottom panel, a white box represents the mean plus or minus SE of the 3 time points for each shRNA) were determined and related to shRNA control and represented as histograms. A representative experiment of 2 performed is shown.
Decreased TCL1 expression in SUP-T11 cell line restores PKCθ pathway activation. (A) SUPT-11 cells were transduced with shRNA-C, shRNA-TCL1(1), and shRNA-TCL1(4) vectors and stimulated with PMA (10 ng/mL) at the times indicated in the figure. ERK phosphorylation and TCL1 expression were determined by Western blotting. Total ERK expression was used as loading control. A representative experiment of 3 performed is shown. After quantification, the p-ERK/ERK ratio (top panel, white box: t = 0 minute; gray box: t = 5 minutes; black box, t = 30 minutes of PMA induction) and TCL1 expression inhibition (bottom panel, a white box represents the mean plus or minus SE of the 3 time points for each shRNA) were determined and related to shRNA control and represented as histograms. A representative experiment of 2 performed is shown. (B) SUPT-11 cells were transduced with shRNA-C, shRNA-TCL1(1), and shRNA-TCL1(3) vectors and stimulated with UCHT1 at the times indicated in the figure. PKCθ phosphorylation and TCL1 expression were determined by Western blotting. β-Actin expression was used as loading control. After quantification, the pPKCθ/β-actin ratio (top panel, white box: t = 0 minute; gray box: t = 5 minutes; black box, t = 30 minutes of PMA induction) and TCL1 expression inhibition (bottom panel, a white box represents the mean plus or minus SE of the 3 time points for each shRNA) were determined and related to shRNA control and represented as histograms. A representative experiment of 2 performed is shown.
Expression of TCL1 in primary transformed and untransformed T lymphocytes inhibits PKCθ and ERK activation
Activation of PKCθ and ERK was then analyzed in 2 primary T-PLL cases overexpressing TCL1, and compared with normal human peripheral blood mononuclear cells (PBMCs). In PBMCs, PMA induced a high level of phosphorylation of ERK, while this phosphorylation was much weaker in T-PLL cells (Figure 7A). Similarly, ERK phosphorylation induced by UCHT1 in PBMCs was absent in T-PLL cells (Figure 7A). Furthermore, PBMCs showed a marked induction of PKCθ phosphorylation upon UCHT1, while this phosphorylation was much weaker in T-PLL cells (Figure 7A). Thus, analysis of these 2 T-PLLs showed an inhibition of PKCθ and ERK phosphorylation compared with PBMCs. Similar results were observed with 4 additional T-PLL samples (data not shown). This phenomenon was not due to alterations of TCR transduction molecules upstream of PKCθ, as after UCHT1 stimulation, PKCθ translo-cated to the cell membrane at equal levels in both PBMCs and T-PLL cells (Figure S1C). Of note, in these conditions of cell stimulation, no detectable p-AKT was observed by immunofluorescence (Figure S1D) and Western blotting in both PBMCs and T-PLL cells (data not shown).
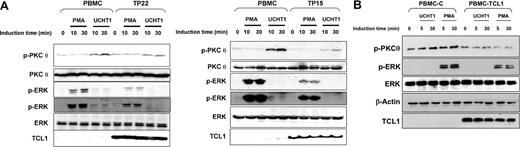
Expression of TCL1 in primary transformed and untransformed T lymphocytes inhibits PKCθ and ERK activation. (A) Human normal PBMCs and T-PLL cells overexpressing TCL1 (TP22 on left and TP15 on right) were stimulated with PMA or UCHT1 at different times as indicated in the figure. PKCθ phosphorylation (top), ERK phosphorylation (2 exposures are shown to visualize the weaker signal in UCHT1 stimulation) (middle), and TCL1 expression (bottom) were determined by Western blotting. Total ERK and PKCθ expression were used as loading controls. A representative experiment of the 2 performed is shown. (B) PBMCs were transduced with pCDH1 (PBMC-(C) and pCDH1-TCL1 (PBMC-TCL1) vectors and stimulated with UCHT1 or PMA at the times indicated in the figure. ERK and PKCθ phosphorylation, and TCL1 expression were determined by Western blotting. Total ERK and β-actin expression were used as loading control. A representative experiment of 2 performed is shown.
Expression of TCL1 in primary transformed and untransformed T lymphocytes inhibits PKCθ and ERK activation. (A) Human normal PBMCs and T-PLL cells overexpressing TCL1 (TP22 on left and TP15 on right) were stimulated with PMA or UCHT1 at different times as indicated in the figure. PKCθ phosphorylation (top), ERK phosphorylation (2 exposures are shown to visualize the weaker signal in UCHT1 stimulation) (middle), and TCL1 expression (bottom) were determined by Western blotting. Total ERK and PKCθ expression were used as loading controls. A representative experiment of the 2 performed is shown. (B) PBMCs were transduced with pCDH1 (PBMC-(C) and pCDH1-TCL1 (PBMC-TCL1) vectors and stimulated with UCHT1 or PMA at the times indicated in the figure. ERK and PKCθ phosphorylation, and TCL1 expression were determined by Western blotting. Total ERK and β-actin expression were used as loading control. A representative experiment of 2 performed is shown.
We then investigated if overexpression of TCL1 could confer similar phenotypes in primary lymphocytes. Normal human PBMCs were stimulated for a short period with phytohemagglutinin to allow lentivirus infection. After selection in puromycin antibiotic, transduced lymphocytes were stimulated with PMA or UCHT1. TCL1 expression in primary lymphocytes was thus obtained at levels similar to those observed in T-PLLs. As shown above in cellular models and primary T-PLLs, expression of TCL1 in human normal lymphocytes inhibited ERK and PKCθ phosphorylation after PMA and UCHT1 stimulation, respectively (Figure 7B).
Thus, inhibition of ERK and PKCθ pathways by TCL1 could be evidenced in established lymphoid lines as well as in primary cells, and in transformed as well as in normal lymphocytes.
Discussion
Despite recent advances, the role of TCL1 oncoprotein is still only partially defined.6,19 In a first approach, we investigated its biologic function in the widely used Jurkat cell line. In unstimulated conditions, proliferation and apoptosis were unmodified in Jurkat cells expressing TCL1 compared with the control cell line. However, major biologic changes were observed between these cell lines after PMA stimulation. TCL1 expression inhibited cell growth arrest and activation-induced cell death (AICD) normally induced by PMA in Jurkat cells. This effect was consistent with previous observations such as the prevention of apoptosis upon T-cell activation in transduced T-cell hybridoma,14 or the resistance to CD40-mediated FASL-induced apoptosis in TCL1-transgenic B lymphocytes.22 Furthermore, cellular expansion upon stimulation observed in TCL1-transgenic T cells could also result from inhibition of apoptosis, although this was not specifically investigated.52
The main effect of PMA is the activation of PKCs by mimicking their natural ligand, the diacylglycerol, leading us to investigate PKCθ, the PKC isoform required for T-cell activation and AICD.25,,–28 It should be noted that the consequences of Pkcθ inactivation on AICD were not univocal depending of the animal models and the precise experimental protocols used (for review, see Hayashi and Altman53 ). The catalytic activity of PKCθ is controlled in part by phosphorylation of the activation-loop residue Thr538 by PDK1.34,35 In our study, phospho-Thr538 PKCθ induced after T-cell stimulation in control Jurkat cells was dramatically inhibited in TCL1-expressing Jurkat cells. Because this effect was observed in an established cell line, we then demonstrated that this inhibition was indeed related to TCL1 expression by showing a reversion of this phenotype using shRNA lentiviral strategies. Furthermore, the phenotype was also observed in transient TCL1 expression in Jurkat.
However, TCL1 was ectopically expressed in Jurkat cells, which do not normally express any of the 3 TCL1/TCL1B/MTCP1 isoforms. We then analyzed SUP-T11, the only available T-cell line in which endogenous TCL1 overexpression is related to a t(14;14)(q11;q32) chromosomal translocation.2,37 Although initially derived from an acute T-cell lymphoid leukemia, its expression of TCL1 and the presence of other genetic abnormalities recurrent in T-PLL (E.L.T., unpublished data, June 2006) relate this atypical leukemia to T-PLL disease. As in TCL1-expressing Jurkat cells, TCR stimulation of SUP-T11 induced only a weak phosphorylation of PKCθ. Strikingly, lowering TCL1 expression using shRNA led to a major enhancement of PKCθ phosphorylation. We then examined if similar PKCθ inhibition was found in primary lymphocytes expressing TCL1. Indeed, primary T-PLL samples demonstrated a poor PKCθ phosphorylation upon stimulation compared with peripheral normal lymphocytes. Primary leukemic cells, as well as established cell lines, are genetically unstable and contain chromosome copy number changes, rearrangements, and mutations that may modify TCL1 effects onto signal transduction. We thus successfully expressed TCL1 in primary T lymphocytes using lentiviral infection. Level of expression was in the same order to that observed in primary T-PLL samples. After short-term culture allowing infection and selection of transduced cells, TCR stimulation and PMA again led to a weaker phosphorylation of PKCθ in TCL1-transduced lymphocytes compared with control lymphocytes. Thus, inhibition of PKCθ phosphorylation by TCL1 is dependent neither on cell line establishment nor on other genetic alterations.
Paradoxically, increased PKC-dependent T-cell signaling in T-cell leukemia line 2B4 overexpressing TCL1 was recently reported.52 However, different phosphorylated residues of PKCθ were investigated by Hoyer et al52 than the p-Thr538 characterized in this study. In addition, differences in experimental protocols and reagents, cell types and origins, and levels of oncogene expression could account for the different phenotypes induced by TCL1. However, the phenotype conferred by TCL1 described here was consistent in all our experimental models.
In parallel with PKCθ impairment, 3 PKCθ downstream targets, NF-κB, ERK, and CD69, were found poorly activated in TCL1-expressing Jurkat upon stimulation. Pharmacological inhibitors were then used to unravel which of the downstream pathways inhibited by TCL1 played a role in the activation-induced cellular phenotype observed in Jurkat cells. A NF-κB inhibitor had no effect on PMA-induced cell growth arrest and on PMA-induced clumps in Jurkat cells, suggesting that inhibition of AICD by TCL1 expression did not result from inhibition of the NF-κB pathway.
Another downstream pathway inhibited by TCL1 is the MEK/ERK pathway. ERK pathway has been previously shown to be a important activator of AICD,50,51 playing a role in the induction of NR4A1/TR3/NUR77, an orphan nuclear steroid receptor, and FASLG, the ligand of the death receptor FAS.47,48 In Jurkat cell line, a MEK/ERK inhibitor totally abrogates AICD observed upon activation. Furthermore, inhibition of the ERK phosphorylation/activation correlated with TCL1 expression in Jurkat and SUP-T11 cell lines, as well as in primary TCL1-expressing malignant and nonmalignant T lymphocytes. Both PKC-dependent and -independent ERK activation are mediated by TCR activation.50,51 Data obtained with PKC inhibitors supported a model in which TCL1 inhibited PKC-dependent ERK activation. Thus, TCL1 could inhibit AICD through its impairment of PKC-dependent ERK activation.
Based on our pharmacologic inhibition experiments, the proapoptotic ERK pathway appeared to be dominant upon the protective role of NF-κB,54 in susceptibility to AICD of Jurkat cells. TCL1-dependent PKCθ inhibition thus impaired AICD in these cells, in concordance with the resistance to AICD observed in Pkcθ−/− lymphocytes.28 However, PKCθ controls many important pathways in T cells, including NF-κB, NF-AT, MAPK/ERK, and AP-1 pathways, which are proapoptotic or antiapoptotic, and proproliferative or antiproliferative, depending on the type of stimulation and the cellular context. It is therefore likely that TCL1-dependent PKC inhibition could have different consequences upon other cellular stress.
The mechanism by which TCL1 inhibited PKCθ phosphorylation was then investigated. After TCR engagement, PKCθ is concomitantly autophosphorylated on several residues and translocated to the membrane through interaction of its C1 domain with DAG, and phosphorylated through protein-protein interactions with its activation kinase PDK1. However, the exact chronology of these events is incompletely elucidated.35 Importantly, the integrity of TCR-dependent transduction upstream of PKCθ was verified in the different cellular models and primary leukemic samples, as UCHT1 stimulation normally induced the phosphorylation of ZAP70 and the translocation of PKCθ to the cell membrane. This strongly argues for a role of TCL1 during the PKCθ phosphorylation process. Whether TCL1 exerts its function through direct interaction with a PKCθ complex is presently unknown. However, coimmunoprecipitation failed to detect such a TCL1-PKCθ complex in stimulated or unstimulated TCL1-expressing cells, whereas TCL1-AKT interaction was easily confirmed (G. D., unpublished data, January 2006). In contrast, cellular effects of TCL1 expression were independent of AKT phosphorylation, which was reported to be directly modulated by TCL1.14,–16 Indeed, AKT phosphorylation was similar in kinetics and intensity in TCL1-expressing and nonexpressing Jurkat cells, p-AKT was barely detectable in stimulated and unstimulated primary T-PLL samples and in SUP-T11 cell line, and efficient inhibition of this pathway by LY294002 had no significant effect on the cellular function of TCL1. However, our data do not exclude that the AKT-TCL1 interaction, independent of AKT phosphorylation status, plays a role in the TCL1-dependent PKCθ inhibition, potentially through AKT and PKCθ interactions with PDK1. Another hypothesis could be an action of TCL1 onto the subsequent down-regulation of PKC activation mediated by serine/threonine phosphatases such as PP2A.55,56 Treatment with okadaic acid or calyculin A, 2 PP2A inhibitors, did not restore the induction of PKCθ phosphorylation in TCL1-expressing cells (unpublished data). Thus, the biochemical mechanism by which TCL1 inhibits the PKCθ pathway remains to be elucidated.
An apparent paradox raised by our results is that a major effect of the TCL1 oncoprotein is down-regulation of several pathways, for which activation is critical to many oncogenic processes. However, T-cell expansion after cell stimulation is the result of a subtle balance between cell proliferation and apoptosis (for review, see Lenardo et al57 ). Our data support that in TCL1-driven T-cell leukemia, inhibition of PKCθ confers resistance to apoptosis and overall results in growth advantage, as it was previously proposed in TCL1-driven B-cell leukemogenesis.22 We here further linked this resistance to AICD to impaired activation of PKCθ and ERK pathways. Whether TCL1 protects leukemic cells from AICD in response to still-unknown external stimulatory signals or promotes leukemogenesis through modifying the proliferation/apoptosis balance remains to be investigated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by funds from Inserm and Institut Curie, Section de Recherche. G.D. is a recipient of grants from the Fondation de France, the Canceropole-Ile-de-France, and the Association Pour la Recherche sur l'Ataxie-Télangiectasie (APRAT). M.J. and E.L.T. are recipients of Fellowships from the Ministère de la Recherche et de la Technologie (MRT).
We thank C. Hivroz for helpful discussions, technical advice, and gift of reagents; C. Ghirelli for gift of reagents; D. Williamson for critical reading of the paper; K. Laud, F. Pflumio, and A. Dubard-Kupperschmitt for their expertise in lentivirus vector transduction; and O. Delattre for his support.
Authorship
Contribution: G.D. performed research, analyzed data, and wrote the paper; M.J. performed research and analyzed data; E.L.T. performed research; R.W. provided critical reagents and his expertise in NF-κB experiments; M.H.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc-Henri Stern, Inserm U830, Institut Curie, Centre de Recherche, 26 rue d'Ulm, 75248 Paris cedex 05, France; e-mail:marc-henri.stern@curie.fr.