Abstract
Life and death of peripheral lymphocytes is strictly controlled to maintain physiologic levels of T and B cells. Activation-induced cell death (AICD) is one mechanism to delete superfluous lymphocytes by restimulation of their immunoreceptors and it depends partially on the CD95/CD95L system. Recently, we have shown that hematopoietic progenitor kinase 1 (HPK1) determines T-cell fate. While full-length HPK1 is essential for NF-κB activation in T cells, the C-terminal fragment of HPK1, HPK1-C, suppresses NF-κB and sensitizes toward AICD by a yet undefined cell death pathway. Here we show that upon IL-2–driven expansion of primary T cells, HPK1 is converted to HPK1-C by a caspase-3 activity below the threshold of apoptosis induction. HPK1-C se-lectively blocks induction of NF-κB–dependent antiapoptotic Bcl-2 family members but not of the proapoptotic Bcl-2 family member Bim. Interestingly, T and B lymphocytes from HPK1-C transgenic mice undergo AICD independently of the CD95/CD95L system but involving caspase-9. Knock down of HPK1/HPK1-C or Bim by small interfering RNA shows that CD95L-dependent and HPK1/HPK1-C–dependent cell death pathways complement each other in AICD of primary T cells. Our results define HPK1-C as a suppressor of antiapoptotic Bcl-2 proteins and provide a molecular basis for our understanding of CD95L-independent AICD of lymphocytes.
Introduction
During an adaptive immune response, naive and memory T cells proliferate and fulfill their effector function. The expansion phase is followed by the contraction phase in which T-cell numbers decline and go back to normal levels. This process is highly regulated and requires a switch between life and death of activated T cells.1 T-cell receptor (TCR) restimulation of activated T lymphocytes induces activation-induced cell death (AICD), which contributes to the deletion of T cells during the contraction phase. AICD involves death receptors (eg, CD95)2,3 that execute cell death via the extrinsic cell death pathway. T cells can also undergo apoptosis by the intrinsic cell death pathway involving the mitochondria and BH3-only Bcl-2 family members.4 The contribution of the intrinsic cell death pathway in AICD of peripheral T cells and its underlying molecular mechanisms are not well characterized. Interestingly, in primary T cells, the blocking of only 1 of the 2 pathways either due to transgenic expression of Bcl-2 or due to mutation of the CD95L (ligand, gld mice) does not or not completely block TCR-induced AICD.4,5 It is tempting to speculate that both pathways play a redundant role in AICD. In contrast, several generally used T-cell models like Jurkat T cells are exclusively dependent on CD95L-mediated AICD.6
Similar to T cells, the deletion of autoreactive B cells is mediated by B-cell receptor (BCR)–induced apoptosis.7 But in contrast to T cells, BCR-mediated cell death is mainly controlled by the mitochondria and the BH3-containing Bcl-2 family member Bim.8,9
The NF-κB pathway is known to be a modulator of lymphocyte cell death.10-12 Recently, NF-κB was suggested to regulate AICD in T lymphocytes.13 A molecular link between NF-κB activation and AICD in T lymphocytes is the hematopoietic progenitor kinase 1 (HPK1). We have shown that HPK1 is an essential activator of the IKK complex.14 Upon activation and expansion of primary T cells, proteolytic cleavage of HPK1 generates the HPK1 C-terminus, HPK1-C. HPK1-C suppresses NF-κB by inhibiting the IKK complex and sensitizes primary T cells toward AICD.14,15
HPK1 is composed of an N-terminal catalytic domain with homology to the Sterile 20 kinase of Saccharomyces cerevisiae and a C-terminal regulatory domain called citron homology domain.16 In lymphocytes, HPK1 kinase activity is induced by antigen receptor cross-linking.17,18 HPK1 activation involves adaptor protein binding, recruitment to the plasma membrane, and phosphorylation by protein kinase D1.19-21 HPK1 activities are implicated in monocytic differentiation22 and in negative regulation of the immune response.23 Upon activation and expansion of primary T cells, proteolytic cleavage of HPK1 separates the N-terminal kinase domain from the regulatory C-terminus HPK1-C.
In this study, we show that a caspase-3 activity below the threshold of apoptosis induction in expanded primary T cells results in proteolytic processing of HPK1 and generation of HPK1-C. By the recently described suppression of the NF-κB pathway in activated T cells,14 HPK1-C blocks expression of antiapoptotic NF-κB target genes and promotes AICD. We show that HPK1-C is supporting AICD in vitro and increases AICD in vivo. Unexpectedly, HPK1-C–mediated AICD is independent of CD95L but involves caspase-9. Knock down of HPK1/HPK1-C or Bim by small interfering RNA (siRNA) in primary T cells shows that HPK1-C– and CD95L-mediated mechanisms cooperate in AICD. Our work suggests that the conversion of HPK1 to HPK1-C acts as a molecular switch to regulate AICD by the intrinsic pathway in primary T and B cells. Our results give substantial new insights into the cross talk of the extrinsic and the intrinsic cell death pathway in activated lymphocytes.
Materials and methods
Antibodies and Western blotting
The HRPO-conjugated antibodies (Abs) antimouse IgG1 or IgG2b or antirabbit IgG were purchased from Southern Biotechnology Associates (Birmingham, AL) or Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit sera anti-HPK1 number 7 have been described previously.15 The Abs used were anti-Erk1/2 (Cell Signaling Technology, Boston, MA), anti–α-tubulin (Sigma, St Louis, MO), anti–Bcl-2 (N-19) and anti–Bcl2-A1 (FL-175; both from Santa Cruz Biotechnology), anti-Bim/BOD (Stressgen, San Diego, CA), anti–mouse IgM (Southern Biotechnology Associates/Biozol), anti–human CD95L (NOK1; Pharmingen, Heidelberg, Germany), anti–mouse CD95L (MFL3; Pharmingen), anti–human CD3 (OKT3; BD Bioscience, Heidelberg, Germany), and anti–mouse CD3 (145-2C11; Pharmingen). Fluorochrome-coupled Abs anti–mouse TCRβ (H57-597), B220 (CD45R), CD4 (L3T4), and CD8 (Ly-2) were from BD Bioscience. The CD95-agonistic antibody anti–Apo-1 was published previously.24 All chemicals used were purchased from Merck (Darmstadt, Germany) or Sigma. Proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Hybond nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ), incubated with 5% nonfat dry milk in PBS/T (PBS with 0.05% Tween-20) for 1 hour, washed with PBS/T, and incubated with the primary antibodies diluted in PBS/T for 18 hours at 4°C. Blots were developed using chemiluminescence following the manufacturer's protocol (Perkin Elmer Life Sciences, Waltham, MA).
Primary lymphocyte preparation and cell culture
Human and murine T cells were prepared and cultivated as described previously.25,26 For activation and expansion, human T cells were stimulated with 1 μg/mL phytohemagglutinin (PHA) and murine T cells were stimulated with 5 μg/mL concanavalin A (Con A; Pharmacia, Uppsala, Sweden) for 18 hours. The culture medium was supplemented with 25 U/mL IL-2 and the cells were kept at 2 × 106 cells/mL. The purity of the T cells obtained by either method was greater than 90%, as determined by flow cytometry. Primary B cells were affinity purified from mouse splenocytes, preactivated by LPS (0.5 μg/mL; Sigma) for 18 hours, and cultivated for 2 days prior to restimulation with plate-bound anti-IgM. HPK1-C transgenic (tg) mice, human Jurkat T-cell clone J16-145, and HPK1-C stable Jurkat T cells have been described previously.14 WEHI-231 B cells (107) were transfected by electroporation with 25 μg of pEGFP:HPK1-C or pEGFP in 300 μL of RPMI medium (without fetal calf serum) using one 300-mV pulse of 10 ms. Forty-eight hours later, transfected cells were divided in 2 fractions and were either stimulated with 10 μg/mL of anti-IgM antibodies for 12 hours or left nonstimulated. To analyze specific apoptosis, the relative decrease of green fluorescent protein (GFP) positive cells in stimulated versus nonstimulated cells was measured by flow cytometry.
Coculture of activated primary T cells with target cells
Cells (4 × 105/mL) of the CD95L-sensitive and anti-CD3–resistant thymoma cell line 308 were stained with 0.1 μM carboxy-fluorescein diacetate succinimidyl ester (CFSE) for 10 minutes at 37°C. Primary mouse T cells (2 × 105) at day 5 after stimulation were activated by 0.1 μg/mL of plate-bound anti-CD3 antibodies and used in coculture with the CFSE-labeled thymoma cell line 308 as target cells. T cell–to–target cell ratios were 1:1 and 5:1. MFL3 (10 μg/mL) was used to block CD95L. Specific cell death of the target cells was measured by the decrease of CFSE intensity compared with the control by flow cytometry as reported previously.27
Apoptosis assays
For TCR-mediated apoptosis induction, Jurkat T cells, primary human T cells, or primary mouse T cells were seeded in triplicates on culture plates coated with 0.1 to 10 μg/mL antihuman CD3 Abs or with 0.1 to 10 μg/mL antimouse CD3 Abs, respectively. Cell death was quantified by the forward scatter/side scatter (FSC/SSC) ratio or by staining with 2.5 μg/mL propidium iodide and measuring by flow cytometry.28 Blocking experiments were performed with 10 μg/mL MFL3 or 10 μg/mL NOK1. For caspase inhibitors, the following concentrations were used: z-DEVD-fmk (Bachem, Weil am Rhein, Germany), 0.5 to 50 μM; z-LEHD-fmk (R&D Systems, Minneapolis, MN), 20 μM to 80 μM; qVD-oph (Imgenex, San Diego, CA), 10 μM. Analysis of mitochondrial membrane potential was performed as follows. Untreated cells or cells (2 × 105/mL to 5 × 105/mL) stimulated with plate-bound anti-CD3 for 8 hours were incubated with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; 0.5 μg/mL; Invitrogen/Molecular Probes, Karlsruhe, Germany) for 20 minutes at room temperature in the dark and analyzed by flow cytometry. CD95L was described previously.29 Specific apoptosis was calculated as follows: (percentage of induced apoptosis − percentage of spontaneous apoptosis)/(100 − percentage of spontaneous apoptosis) × 100. Values given are mean with the standard deviations depicted for triplicate measurements. Caspase activity measurements were performed by coincubation of cell lysates with AFC-conjugated substrates according to the manufacturer's instructions (Invitrogen/Molecular Probes).
Reverse transcriptase–polymerase chain reaction
Total RNA was extracted from purified primary human T cells at the indicated time points using Trizol (Invitrogen) and reverse transcribed using Superscript II (Invitrogen) and oligo (dT)15 primers (MWG Biotech, Ebersberg, Germany). A standard PCR reaction with Taq polymerase (Sigma) was performed using the following primers: actin, TGACGGGGTCACCCACAATGTGCCCATCTA and CTAGAAGCATTTGCGGTGGACGATGGAGGG; Bcl-2, CGACTTCGCCGAGATGTCCAGCCAG and ACTTGTGGCCCAGATAGGCACCCAG; Bcl-xL, GAGCTGGTGGTCGACTTTCTC and CCTGGATCCAAGGCTCTAGG; c-FLIP, CTGATGGAGATTGGTGAGAGC and GAGCGAAGCCTGGAGAGTATT; cIAP1, GCTGGGAATCTGGAGATGAC and CACTGAACCGTCTGTCTCAC; CD95L, AGCCCATGAATTACCCATGTC and CCATATGTGTCTTCCCATTCC.
siRNA-mediated knock down and in vivo AICD model
Primary human T cells were transfected by nucleofection (AMAXA, Koeln, Germany) or lipofection (HiPerfect; Qiagen, Hilden, Germany) with negative control or validated siRNA oligonucleotides specific for human HPK1 (Qiagen; MAP4K1_5_HP) or mouse Bim (Quiagen; Mm_Bcl2l11_1, Mm_Bcl2l11_2, and Mm_Bcl2l11_3 used as mix). siRNA (4 μg; 1500 nM) was used for nucleofection of 5 × 106 cells, and 0.4 μg (150 nM) was used for lipofection of 2 × 105 cells. Transfected cells were rested for 48 hours before subjecting to further analysis. The mouse model of in vivo AICD was performed as described previously25 and was approved by the institutional review board for animal studies. Briefly, anti-CD3 antibody (10 μg/mouse, 145-2C11; Pharmingen) was injected intravenously into the tail vein of 8- to 10-week-old HPK1-C tg or non-tg mice (C57BL/6). Splenocytes were harvested 20 hours later and the CD4+ T cell–to–B cell ratio and the CD8+ T cell–to–B cell ratio were determined by flow cytometry. The T cell–to–B cell ratio of CD3-treated mice was expressed relative to the T cell–to–B cell ratio of control mice set to 1.
Results
HPK1-C sensitizes toward activated T-cell death in vivo
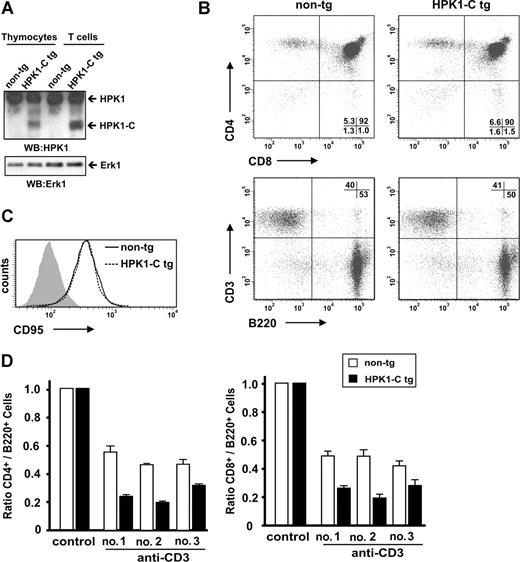
Recently, we have characterized HPK1-C to sensitize primary T cells ex vivo toward AICD.14 To further address the physiologic role of HPK1-C in activated T-cell death we used HPK1-C transgenic (tg) mice, which express HPK1-C constitutively in lymphocytes (Figure 1A; see also Figure 5D). Presence of HPK1-C does not affect development of CD4+ or CD8+ T cells in the thymus or the T cell–to–B cell ratio in the spleen (Figure 1B). Total numbers of T cells or B cells from the spleen and the expression of CD95 on T cells do not differ between HPK1-C tg and non-tg littermates (Figure 1C).
HPK1-C sensitizes toward activated T-cell death in vivo. (A) Thymocytes and naive T cells from non-tg and HPK1-C tg mice were analyzed for expression of HPK1 and HPK1-C by Western blotting (WB). (B) CD4 and CD8 (top panels) or CD3 and B220 (bottom panels) surface expression of thymocytes (top panels) or spleenocytes (bottom panels) from non-tg or HPK1-C tg mice was analyzed by flow cytometry. The percentage of cells in the respective quadrants is indicated. (C) Activated T cells of HPK1-C tg mice or non-tg littermates were analyzed by flow cytometry for CD95 surface expression. The filled histogram shows the isotype control. (D) HPK1-C tg mice or non-tg littermates were analyzed for AICD in vivo by intravenous injection of 10 μg of anti-CD3 antibodies or isotype control antibodies (control). After 24 hours, splenocytes were stained with anti-TCRβ, anti-B220, anti-CD4, or anti-CD8 antibodies and analyzed by flow cytometry. Depicted bars correspond to individual mice of 3 independent experiments (nos. 1, 2, and 3) with standard deviations given for triplicate measurements.
HPK1-C sensitizes toward activated T-cell death in vivo. (A) Thymocytes and naive T cells from non-tg and HPK1-C tg mice were analyzed for expression of HPK1 and HPK1-C by Western blotting (WB). (B) CD4 and CD8 (top panels) or CD3 and B220 (bottom panels) surface expression of thymocytes (top panels) or spleenocytes (bottom panels) from non-tg or HPK1-C tg mice was analyzed by flow cytometry. The percentage of cells in the respective quadrants is indicated. (C) Activated T cells of HPK1-C tg mice or non-tg littermates were analyzed by flow cytometry for CD95 surface expression. The filled histogram shows the isotype control. (D) HPK1-C tg mice or non-tg littermates were analyzed for AICD in vivo by intravenous injection of 10 μg of anti-CD3 antibodies or isotype control antibodies (control). After 24 hours, splenocytes were stained with anti-TCRβ, anti-B220, anti-CD4, or anti-CD8 antibodies and analyzed by flow cytometry. Depicted bars correspond to individual mice of 3 independent experiments (nos. 1, 2, and 3) with standard deviations given for triplicate measurements.
Initially, we compared activated T-cell death between HPK1-C tg and non-tg mice after intravenous injection of anti-CD3 antibodies, which serves as a model for AICD in vivo.25,30 As expected, T cells of HPK1-C tg mice showed increased sensitization toward activated T-cell death resulting in a stronger decrease of the CD4+ T cell–to–B cell ratio (Figure 1D left) and the CD8+ T cell–to–B cell ratio (Figure 1D right) compared with non-tg littermates. These data underline the in vivo relevance of HPK1-C as a regulator of AICD.
A low caspase-3 activity cleaves HPK1 in expanded primary T cells
Stimulation of primary human T cells with PHA and expansion in the presence of IL-2 leads to cleavage of full-length HPK1 and generation of HPK1-C in proliferating T cells.14 HPK1-C is best seen at day 6 in culture after stimulation, when the expanded T cells show the highest sensitivity toward AICD (Figure 2A). In the early phase after T-cell activation (eg, at day 1 in culture after stimulation), HPK1-C is not yet detectable and T cells are resistant toward AICD.
A low caspase-3 activity cleaves HPK1 in expanded primary T cells. (A) Expansion of T cells leads to increased HPK1 expression and conversion to HPK1-C. Presence of full-length HPK1 and its processed fragment HPK1-C was visualized by Western blotting (WB) in primary human T cells at day 1 or day 6 after stimulation. Erk1 is shown as a control. (B) Cell lysates of primary human T cells at day 1 or day 6 after stimulation with PHA and expansion with IL-2 were incubated with in vitro–translated (i.v.tr.) [35S]-labeled HPK1 and resolved by SDS-PAGE. Full-length HPK1 and the caspase cleavage fragments HPK1-N and HPK1-C were detected by autoradiography. As a control, apoptotic processing of [35S]-labeled HPK1 is shown by incubation with lysates from SKW6.4 cells stimulated for 3 hours with anti–Apo-1 (+) or left nonstimulated (−). (C) Primary human T cells isolated from peripheral blood were stimulated with PHA and expanded with IL-2. Samples were taken at the indicated culture day and lysates were resolved by SDS-PAGE. Full-length caspase-3 and its processed fragments p20, p19, and p17 were visualized by Western blotting. Presence of HPK1 and processing toward HPK1-C were analyzed by Western blotting. Expression of actin is shown as control. Viability of the expanded T cells at the time points when processing of caspase-3 and HPK1 was detected was higher compared with the time points when no processing of caspase-3 or HPK1 was detected. Lysates of Jurkat T cells stimulated for 3 hours with anti–Apo-124 (+) or left nonstimulated (−) are shown to control for apoptotic processing of caspase-3. (D) The experiment was performed as in panel B, with cell lysates of primary human T cells at day 6 of culture in the absence or presence of increasing concentrations of the caspase-3/-7–specific inhibitor z-DEVD-fmk. T-cell lysate was omitted in lane 1 (input). (E,F) To block the cleavage of HPK1 toward HPK1-C, primary mouse T cells were incubated at day 3 after stimulation with Con A and expanded in the presence of the 5 μM panspecific caspase inhibitor qVD-oph (E) or 50 μM caspase-3–specific inhibitor z-DEVD-fmk (F) for 72 hours. Cells were washed to remove residual inhibitors, divided into 2 fractions, and subjected either to Western blotting (inset) using antibodies against HPK1 (top) or Erk1 (bottom) or to analysis of AICD. Therefore, cells were further analyzed by incubation with 0.1 μg/mL of plate-bound anti-CD3 antibodies or 2.5 ng/mL of CD95L for 18 hours. Viability of the expanded T cells was not altered by addition of qVD-oph or z-DEVD-fmk. Standard deviation is given for triplicate measurements. The experiment was repeated 3 times with similar outcome.
A low caspase-3 activity cleaves HPK1 in expanded primary T cells. (A) Expansion of T cells leads to increased HPK1 expression and conversion to HPK1-C. Presence of full-length HPK1 and its processed fragment HPK1-C was visualized by Western blotting (WB) in primary human T cells at day 1 or day 6 after stimulation. Erk1 is shown as a control. (B) Cell lysates of primary human T cells at day 1 or day 6 after stimulation with PHA and expansion with IL-2 were incubated with in vitro–translated (i.v.tr.) [35S]-labeled HPK1 and resolved by SDS-PAGE. Full-length HPK1 and the caspase cleavage fragments HPK1-N and HPK1-C were detected by autoradiography. As a control, apoptotic processing of [35S]-labeled HPK1 is shown by incubation with lysates from SKW6.4 cells stimulated for 3 hours with anti–Apo-1 (+) or left nonstimulated (−). (C) Primary human T cells isolated from peripheral blood were stimulated with PHA and expanded with IL-2. Samples were taken at the indicated culture day and lysates were resolved by SDS-PAGE. Full-length caspase-3 and its processed fragments p20, p19, and p17 were visualized by Western blotting. Presence of HPK1 and processing toward HPK1-C were analyzed by Western blotting. Expression of actin is shown as control. Viability of the expanded T cells at the time points when processing of caspase-3 and HPK1 was detected was higher compared with the time points when no processing of caspase-3 or HPK1 was detected. Lysates of Jurkat T cells stimulated for 3 hours with anti–Apo-124 (+) or left nonstimulated (−) are shown to control for apoptotic processing of caspase-3. (D) The experiment was performed as in panel B, with cell lysates of primary human T cells at day 6 of culture in the absence or presence of increasing concentrations of the caspase-3/-7–specific inhibitor z-DEVD-fmk. T-cell lysate was omitted in lane 1 (input). (E,F) To block the cleavage of HPK1 toward HPK1-C, primary mouse T cells were incubated at day 3 after stimulation with Con A and expanded in the presence of the 5 μM panspecific caspase inhibitor qVD-oph (E) or 50 μM caspase-3–specific inhibitor z-DEVD-fmk (F) for 72 hours. Cells were washed to remove residual inhibitors, divided into 2 fractions, and subjected either to Western blotting (inset) using antibodies against HPK1 (top) or Erk1 (bottom) or to analysis of AICD. Therefore, cells were further analyzed by incubation with 0.1 μg/mL of plate-bound anti-CD3 antibodies or 2.5 ng/mL of CD95L for 18 hours. Viability of the expanded T cells was not altered by addition of qVD-oph or z-DEVD-fmk. Standard deviation is given for triplicate measurements. The experiment was repeated 3 times with similar outcome.
Up to now it is unclear how HPK1 is converted into its cleavage fragments in expanded activated T cells. To address this question, we incubated [35S]-labeled HPK1 together with T-cell lysates of day 1 or day 6 T cells (Figure 2B). As expected, processing of HPK1 was found in lysates of expanded day 6 T cells, indicating an HPK1-specific protease activity in expanded T cells. Interestingly, the detected fragments, HPK1-N and HPK1-C, correspond well to the cleavage pattern of HPK1 upon addition of CD95-stimulated, apoptotic SKW6.4 cell lysates containing activated caspases (Figure 2B). The generation of HPK1-C does not result from background apoptosis, as cell viability at day 6 is always higher compared with day 1 (data not shown). Previously it has been shown that HPK1 can be cleaved by caspase-3 in vitro.15,31 This raises the possibility that the identified caspase-3 cleavage site of HPK1 might be used in primary day 6 T cells.
In a first step, we investigated the processing of caspase-3 during activation and expansion of primary T cells (Figure 2C top panel). Interestingly, we only detected caspase-3 processing in expanded primary T cells starting from culture of day 4 on but not in short-time–activated T cells (up to day 3). Compared with the CD95-stimulated Jurkat T-cell control, we identified the p20 fragment as the prominent processed form of caspase-3 in expanded normal T cells. Alongside with p20, we could not detect the further-processed capase-3 forms p19/p17, probably due to the low amount of active caspase-3. Processing of caspase-3 in primary T cells is paralleled by cleavage of HPK1 toward HPK1-C, which also can be detected starting from culture of day 4 on (Figure 2C bottom panels).
To test, if a caspase-3 activity is needed to cleave [35S]-labeled HPK1, we applied the inhibitor z-DEVD-fmk to lysates of expanded primary human day 6 T cells (Figure 2D). Already, 0.5 μM of z-DEVD-fmk selectively blocked HPK1 cleavage. This implies that a low caspase-3–like activity is sufficient to cleave HPK1 in primary T cells. This result shows an intermediate caspase activity below the apoptotic threshold in expanded primary human T cells and suggests that HPK1 is hypersensitive for caspase-3–like cleavage under survival conditions.
To address the requirement of a caspase activity in primary T cells for the sensitization toward AICD, we expanded activated T cells in the presence of the pan-caspase inhibitor quinolyl-valyl-O-methylaspartyl-[2,6-difluorophenoxy]-methyl ketone (qVD-oph). After 3 days of treatment, the accumulation of the caspase cleavage product HPK1-C was largely prevented (Figure 2E inset), which confirms that blocking of an endogenous caspase activity in primary T cells was successful. After washing off remaining qVD-oph, treated T cells showed decreased AICD induction (Figure 2E). This was not caused by direct caspase inhibition by remaining qVD-oph, as CD95-induced cell death was not affected. To control this result, we repeated the experiment using the capase-3/-7–specific inhibitor z-DEVD-fmk, which also led to a specific decrease of AICD (Figure 2F). Under these conditions, z-DEVD-fmk was less potent to interfere with AICD, which might reflect the different affinities of qVD-oph and z-DEVD-fmk for the inhibition of caspases.
Therefore, caspase activity during expansion of T cells led to sensitization toward AICD by an indirect mechanism. Further, we confirmed that HPK1 is cleaved by a caspase activity in expanded T cells. It is possible that the absence of HPK1-C in treated T cells causes the decreased AICD sensitivity. This interpretation is in line with previous work, where siRNA-mediated knock down of HPK1/HPK1-C led to diminished AICD in primary human T cells.14 Taken together, our data demonstrate a caspase-3–like activity below the threshold of apoptosis induction in expanded primary T cells, which converts full-length HPK1 to HPK1-C and, thus, determines sensitization toward AICD.
HPK1-C initiates AICD independent of CD95L
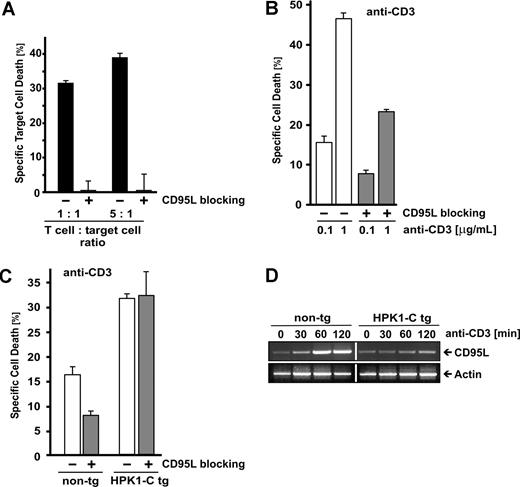
While HPK1-C increases the sensitivity of T cells toward AICD in vivo (Figure 1), the involved mechanisms are elusive. As AICD is partially dependent on the CD95/CD95L system,3,32 we addressed the question of whether HPK1-C–mediated sensitization would involve CD95L. To assay this, we established conditions under which application of CD95L-blocking antibody MFL3 efficiently inhibited CD95L-dependent cell death (Figure 3A). We used as a positive control a coculture system of activated primary T cells and CD95L-sensitive, anti-CD3–resistant, CFSE-labeled target cells and found that application of 10 μg/mL MFL3 completely abrogated CD95L-mediated cell death. Identical concentrations of MFL3 were used in subsequent experiments to completely suppress CD95L-dependent cell death of activated T cells. It is therefore possible to measure the impact of CD95L in HPK1-C–mediated AICD sensitization. Interestingly, blocking of CD95L during AICD of primary mouse T cells reduced cell death only by half, indicating that the remaining cell death might be CD95 independent (Figure 3B gray bars).
HPK1-C initiates AICD independent of CD95L. (A) To control for the capacity of the CD95L-blocking antibody MFL3 to efficiently inhibit CD95L-dependent cell death, primary mouse T cells at day 5 after stimulation were activated with 0.1 μg/mL of plate-bound anti-CD3 antibodies and cocultured with CD3-deficient, but CD95L-sensitive, CFSE-labeled target cells at the given ratios in the absence or presence of 10 μg/mL of the CD95L-blocking antibody MFL3. (B) Primary mouse T cells were analyzed at day 5 after stimulation for activation-induced cell death (AICD) after incubation with 0.1 or 1 μg/mL of plate-bound anti-CD3 antibodies for 18 hours in the absence (□) or the presence (▩) of 10 μg/mL of the CD95L-blocking antibody MFL3. Standard deviation is given for triplicate measurements. The experiment presented is representative of 5 repeats. (C) Primary mouse T cells of HPK1-C transgenic (tg) mice or non-transgenic (non-tg) littermates were analyzed at day 5 after stimulation with Con A and expansion with IL-2 for AICD by incubation with 0.1 μg/mL of plate-bound anti-CD3 antibodies for 18 hours in the presence (▩) or the absence (□) of 10 μg/mL of the CD95L-blocking antibody MFL3. Standard deviation is given for triplicate measurements. The experiment was repeated 5 times with similar outcome. (D) Primary mouse T cells of HPK1-C tg mice or non-tg littermates were stimulated by anti-CD3 antibodies for the indicated time points or left nonstimulated. RNA was extracted and subjected to reverse transcriptase–polymerase chain reaction (RT-PCR) analysis using primers specific for CD95L. Expression of actin is shown as a control.
HPK1-C initiates AICD independent of CD95L. (A) To control for the capacity of the CD95L-blocking antibody MFL3 to efficiently inhibit CD95L-dependent cell death, primary mouse T cells at day 5 after stimulation were activated with 0.1 μg/mL of plate-bound anti-CD3 antibodies and cocultured with CD3-deficient, but CD95L-sensitive, CFSE-labeled target cells at the given ratios in the absence or presence of 10 μg/mL of the CD95L-blocking antibody MFL3. (B) Primary mouse T cells were analyzed at day 5 after stimulation for activation-induced cell death (AICD) after incubation with 0.1 or 1 μg/mL of plate-bound anti-CD3 antibodies for 18 hours in the absence (□) or the presence (▩) of 10 μg/mL of the CD95L-blocking antibody MFL3. Standard deviation is given for triplicate measurements. The experiment presented is representative of 5 repeats. (C) Primary mouse T cells of HPK1-C transgenic (tg) mice or non-transgenic (non-tg) littermates were analyzed at day 5 after stimulation with Con A and expansion with IL-2 for AICD by incubation with 0.1 μg/mL of plate-bound anti-CD3 antibodies for 18 hours in the presence (▩) or the absence (□) of 10 μg/mL of the CD95L-blocking antibody MFL3. Standard deviation is given for triplicate measurements. The experiment was repeated 5 times with similar outcome. (D) Primary mouse T cells of HPK1-C tg mice or non-tg littermates were stimulated by anti-CD3 antibodies for the indicated time points or left nonstimulated. RNA was extracted and subjected to reverse transcriptase–polymerase chain reaction (RT-PCR) analysis using primers specific for CD95L. Expression of actin is shown as a control.
To test the role for HPK1-C in CD95L-dependent cell death in primary T cells, we took advantage of HPK1-C transgenic (tg) mice.14 T cells from HPK1-C tg mice show increased AICD compared with the nontransgenic (non-tg) littermates (Figure 3C blank bars). As already seen in Figure 3B, blocking of CD95L by anti-CD95L antibodies reduced TCR-induced cell death of T cells from non-tg littermates by half. Surprisingly, CD95L blockage did not show any reduction of cell death in T cells from HPK1-C tg mice (Figure 3C). To further confirm this result, we tested expression of CD95L by RT-PCR. Upon TCR stimulation, non-tg T cells up-regulated CD95L expression, but TCR stimulation did not significantly induce CD95L expression in T cells of HPK1-C tg mice (Figure 3D). Taken together, these data emphasize a HPK1-C–mediated mechanism that leads to increased AICD of primary T cells independent of CD95L. This indicates that HPK1-C does not sensitize toward cell death by modulating the CD95 pathway.
HPK1-C sensitizes for activated T-cell death via the mitochondrial pathway
To further confirm that AICD independent of CD95L can occur, we used T cells from gld mice, which express a mutated, nonfunctional CD95L.33 Interestingly, restimulation of primary T cells from gld mice also resulted in AICD nearly comparable to the level seen in wild-type T cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The cell death detected in gld T cells was accompanied by apoptotic DNA fragmentation. This indicates that AICD can occur independently of CD95L.
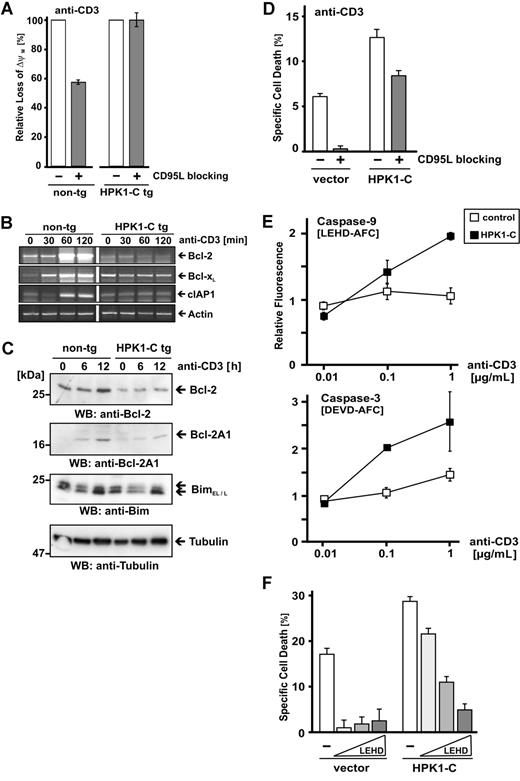
One of the main apoptosis pathways is mediated by the mitochondria. To investigate the involvement of the mitochondrial cell death pathway in HPK1-C–mediated AICD, we compared the loss of mitochondrial membrane potential (ΔψM) of primary T cells of HPK1-C tg mice to the one of their non-tg littermates. As expected, neutralization of CD95L by blocking antibodies did not significantly reduce the loss of ΔψM in HPK1-C tg T cells (Figure 4A). Loss of ΔψM in non-tg T cells was partially dependent on the CD95/CD95L system as reflected by partial blocking of TCR-induced cell death (Figure 3B). This result shows that HPK1-C leads to mitochondria depolarization, which is independent of CD95L. It is tempting to speculate that upon TCR stimulation HPK1-C activates the intrinsic cell death in primary T cells. Recently, it has been shown that HPK1-C suppresses TCR-induced NF-κB activity14 and that NF-κB activity controls AICD.13 It is well established that NF-κB positively influences expression of several antiapoptotic Bcl-2 family members.11 We therefore analyzed TCR-mediated induction of NF-κB–dependent survival genes on the transcriptional level (Figure 4B). In accordance with the function of HPK1-C as a suppressor of NF-κB, Bcl-2, Bcl-xL, and cIAP1 were not induced in primary T cells from HPK1-C tg mice. To confirm this finding on the protein level, we used Western blotting and detected significantly reduced induction of NF-κB–dependent Bcl-2 and Bcl-2A1 in primary T cells from HPK1-C tg mice, whereas expression of the proapoptotic NF-κB–independent Bcl-2 family member Bim was not altered (Figure 4C). Therefore, we propose that HPK1-C interferes with the expression of prosurvival genes and sensitizes toward the mitochondrial intrinsic cell death pathway by shifting the balance between proapoptotic and antiapoptotic Bcl-2 family members toward the proapoptotic proteins. Proapoptotic Bcl-2 family members, like Bim, are activated in response to stimulation, and as a consequence of the loss of antiapoptotic Bcl-2, family members lead to loss of mitochondrial membrane potential and subsequent execution of apoptosis.
HPK1-C sensitizes for activated T-cell death via the mitochondrial pathway. (A) Primary mouse T cells of HPK1-C transgenic (tg) mice or non-transgenic (non-tg) littermates were analyzed at day 5 after activation with Con A and expansion with IL-2 for loss of mitochondrial membrane potential (ΔψM) by JC-1 staining after incubation with 1 μg/mL plate-bound anti-CD3 antibodies for 18 hours in the presence of 10 μg/mL CD95L-blocking antibody MFL3. All values represent mean and standard deviation of triplicate measurements and are expressed relative to the loss of ΔψM without CD95L-blocking antibody set to 100%. (B) Primary mouse T cells of HPK1-C tg mice or non-tg littermates were stimulated by anti-CD3 antibodies for the indicated time or left nonstimulated. RNA was extracted and subjected to RT-PCR analysis using primers specific for the indicated NF-κB target genes Bcl-2, Bcl-xL, and cIAP1. Expression of actin is shown as control. (C) The experiment was carried out essentially as in panel B and cell lysates were resolved by SDS-PAGE. Expression of Bcl-2, Bcl2-A1, and BimEL/L was visualized by Western blotting (WB). Tubulin is shown as a control. (D) Jurkat T cells with stable integration of either empty vectors or HPK1-C expression vectors, respectively,14 were subjected to cell death induction by incubation with 1 μg/mL plate-bound anti-CD3 antibodies for 24 hours in the absence (□) or the presence (▩) of 10 μg/mL of the CD95L-blocking antibody NOK1. The experiment presented is representative of 4 repeats. Standard deviation is given for triplicate measurements. (E) T cells with stable integration of either empty vectors or HPK1-C expression vectors were stimulated with increasing amounts of plate-bound anti-CD3 antibodies for 8 hours. Subsequently, cells were lysed and caspase 9 (top panel) and caspase 3 (bottom panel) activities were analyzed by a fluorometric substrate cleavage assay. Standard deviation is given for triplicate measurements. (F) Jurkat T cells with stable integration of either empty vectors or HPK1-C expression vectors were incubated with increasing amounts of the caspase-9–specific inhibitor z-LEHD-fmk (20 μM, 40 μM, 80 μM) and subjected to TCR-induced cell death by incubation with 1 μg/mL plate-bound anti-CD3 antibodies for 24 hours. Cell death was quantified by flow cytometry using FSC/SSC.
HPK1-C sensitizes for activated T-cell death via the mitochondrial pathway. (A) Primary mouse T cells of HPK1-C transgenic (tg) mice or non-transgenic (non-tg) littermates were analyzed at day 5 after activation with Con A and expansion with IL-2 for loss of mitochondrial membrane potential (ΔψM) by JC-1 staining after incubation with 1 μg/mL plate-bound anti-CD3 antibodies for 18 hours in the presence of 10 μg/mL CD95L-blocking antibody MFL3. All values represent mean and standard deviation of triplicate measurements and are expressed relative to the loss of ΔψM without CD95L-blocking antibody set to 100%. (B) Primary mouse T cells of HPK1-C tg mice or non-tg littermates were stimulated by anti-CD3 antibodies for the indicated time or left nonstimulated. RNA was extracted and subjected to RT-PCR analysis using primers specific for the indicated NF-κB target genes Bcl-2, Bcl-xL, and cIAP1. Expression of actin is shown as control. (C) The experiment was carried out essentially as in panel B and cell lysates were resolved by SDS-PAGE. Expression of Bcl-2, Bcl2-A1, and BimEL/L was visualized by Western blotting (WB). Tubulin is shown as a control. (D) Jurkat T cells with stable integration of either empty vectors or HPK1-C expression vectors, respectively,14 were subjected to cell death induction by incubation with 1 μg/mL plate-bound anti-CD3 antibodies for 24 hours in the absence (□) or the presence (▩) of 10 μg/mL of the CD95L-blocking antibody NOK1. The experiment presented is representative of 4 repeats. Standard deviation is given for triplicate measurements. (E) T cells with stable integration of either empty vectors or HPK1-C expression vectors were stimulated with increasing amounts of plate-bound anti-CD3 antibodies for 8 hours. Subsequently, cells were lysed and caspase 9 (top panel) and caspase 3 (bottom panel) activities were analyzed by a fluorometric substrate cleavage assay. Standard deviation is given for triplicate measurements. (F) Jurkat T cells with stable integration of either empty vectors or HPK1-C expression vectors were incubated with increasing amounts of the caspase-9–specific inhibitor z-LEHD-fmk (20 μM, 40 μM, 80 μM) and subjected to TCR-induced cell death by incubation with 1 μg/mL plate-bound anti-CD3 antibodies for 24 hours. Cell death was quantified by flow cytometry using FSC/SSC.
To further confirm a regulatory role of HPK1-C in AICD, we used Jurkat T cells stably expressing HPK1-C or a control vector, respectively (Figure 4D). AICD of the Jurkat T cells used here is exclusively dependent on CD95L activity, and AICD can be completely blocked by CD95L-neutralizing antibodies.6 However, the CD95L-dependent cell death in Jurkat T cells involves parallel activation of intrinsic and extrinsic cell death pathways. As previously reported, stably expressing HPK1-C Jurkat T cells show a higher sensitivity toward TCR-mediated cell death compared with the control Jurkat T cells.14 Addition of CD95L-blocking antibodies rescued control Jurkat T cells from TCR-mediated cell death (Figure 4D vector bars). This is not the case in HPK1-C stably expressing Jurkat T cells, which show substantial cell death in the presence of CD95L-neutralizing antibodies (Figure 4D HPK1-C bars). This result indicates that HPK1-C increases the proportion of CD95L-independent cell death after TCR stimulation.
Accordingly, the elevated cell death of HPK1-C Jurkat T cells correlates with elevated caspase-9 and caspase-3 activities (Figure 4E), which indicates predominant involvement of the intrinsic cell death pathway. Consequently, the higher caspase-9 activity required application of higher amounts of the caspase-9 inhibitor z-LEHD-fmk for blocking of AICD in HPK1-C Jurkat T cells compared with control Jurkat T cells (Figure 4F). This result supports regulation of AICD by HPK1-C via the intrinsic cell death pathway.
HPK1-C increases AICD of primary B cells by the intrinsic cell death pathway
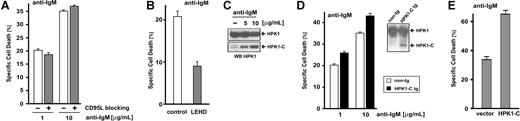
As we postulate a role for HPK1-C in regulating the intrinsic cell death pathway, we investigated how B-cell AICD is influenced by HPK1-C. Similar to AICD in T cells, deletion of activated B cells also involves AICD but is mainly controlled by the intrinsic cell death pathway.8 To test sensitization toward cell death by HPK1-C in splenic B cells, we first reconfirmed that CD95L is not involved in B-cell receptor (BCR)–mediated apoptosis (Figure 5A). As expected, cell death of primary B cells by BCR cross linking with anti-IgM antibodies was not blocked by CD95L-neutralizing antibodies. Furthermore, BCR-mediated AICD involves caspase-9 and can be blocked by the caspase-9 inhibitor z-LEHD-fmk (Figure 5B). As seen in primary T cells, activation of WEHI-231 B cells by stimulation of the BCR leads to accumulation of HPK1-C (Figure 5C). Therefore, BCR-mediated AICD also uses a CD95L-independent intrinsic cell death pathway involving caspase-9 and processing of HPK1 toward HPK1-C.
HPK1-C increases AICD of B cells by the intrinsic cell death pathway. (A) Mouse splenic B cells were analyzed for AICD by incubation with 1 or 10 μg/mL of plate-bound anti-IgM antibodies for 18 hours in the presence (▩) or the absence (□) of 10 μg/mL of the CD95L-blocking antibody MFL3. Standard deviation is given for triplicate measurements. (B) Splenic B cells were analyzed for AICD by incubation with 1 μg/mL of plate-bound anti-IgM antibodies in presence or absence of the caspase-9–specific inhibitor z-LEHD-fmk (40 μM) for 18 hours. Standard deviation is given for triplicate measurements. (C) WEHI-231 B cells were activated by BCR cross linking using 5 or 10 μg/mL of plate-bound anti-IgM antibodies. Cells were lysed and presence of HPK1 and processing toward HPK1-C were analyzed by Western blotting. (D) Splenic B cells of HPK1-C transgenic (tg) mice or non-transgenic (non-tg) littermates were analyzed for AICD by incubation with 1 or 10 μg/mL of plate-bound anti-IgM antibodies for 18 hours. Standard deviation is given for triplicate measurements. The inset shows expression levels of HPK1 and HPK1-C as detected by Western blotting. (E) A GFP:HPK1-C fusion protein19 (HPK1-C) or GFP alone (vector) was expressed in WEHI 231 B cells by transient transfection and GFP-positive cells were analyzed for cell death upon incubation with 10 μg/mL of anti-IgM antibodies for 12 hours. Standard deviation is given for triplicate measurements.
HPK1-C increases AICD of B cells by the intrinsic cell death pathway. (A) Mouse splenic B cells were analyzed for AICD by incubation with 1 or 10 μg/mL of plate-bound anti-IgM antibodies for 18 hours in the presence (▩) or the absence (□) of 10 μg/mL of the CD95L-blocking antibody MFL3. Standard deviation is given for triplicate measurements. (B) Splenic B cells were analyzed for AICD by incubation with 1 μg/mL of plate-bound anti-IgM antibodies in presence or absence of the caspase-9–specific inhibitor z-LEHD-fmk (40 μM) for 18 hours. Standard deviation is given for triplicate measurements. (C) WEHI-231 B cells were activated by BCR cross linking using 5 or 10 μg/mL of plate-bound anti-IgM antibodies. Cells were lysed and presence of HPK1 and processing toward HPK1-C were analyzed by Western blotting. (D) Splenic B cells of HPK1-C transgenic (tg) mice or non-transgenic (non-tg) littermates were analyzed for AICD by incubation with 1 or 10 μg/mL of plate-bound anti-IgM antibodies for 18 hours. Standard deviation is given for triplicate measurements. The inset shows expression levels of HPK1 and HPK1-C as detected by Western blotting. (E) A GFP:HPK1-C fusion protein19 (HPK1-C) or GFP alone (vector) was expressed in WEHI 231 B cells by transient transfection and GFP-positive cells were analyzed for cell death upon incubation with 10 μg/mL of anti-IgM antibodies for 12 hours. Standard deviation is given for triplicate measurements.
To analyze HPK1-C in this pathway, we used splenic B cells of HPK1-C tg mice and found increased sensitization toward activated B-cell death (Figure 5D). These results demonstrate that (1) HPK1-C also sensitizes primary B cells toward AICD; (2) sensitization by HPK1-C is dependent on the intrinsic, mitochondrial cell death pathway; and (3) sensitization toward cell death by HPK1-C is a common mechanism for activated T and B lymphocytes.
In order to reconfirm the primary B-cell data by an independent approach, we used WEHI-231 B cells to transiently express an EGFP:HPK1-C fusion protein, which was previously shown to be identical in function to the endogenous HPK1-C.19 BCR cross linking of WEHI-231 B cells leads to induction of AICD as in primary splenic B cells, and compared with EGFP alone, the EGFP:HPK1-C fusion protein led to increased sensitivity toward AICD in WEHI-231 B cells (Figure 5E). By using B cells that are dependent on the intrinsic cell death pathway, we confirmed that HPK1-C can sensitize T and B cells toward AICD by the CD95L-independent intrinsic cell death pathway.
CD95L-dependent and HPK1-C– mediated, CD95L-independent mechanisms synergize in AICD of primary human T cells
Loss of mitochondrial membrane potential is accompanied by caspase-9 activation.34 Consequently, under CD95L-independent conditions (treatment with CD95L-blocking antibodies), the caspase-9–specific inhibitor z-LEHD-fmk reduces TCR-induced AICD in primary human T cells (Figure 6A). This again shows the involvement of the CD95/CD95L-independent intrinsic cell death pathway in AICD. Furthermore, it implies that CD95-dependent and CD95-independent mechanisms synergize in TCR-induced AICD in primary human T cells.
CD95L-dependent and HPK1-C–mediated, CD95L-independent mechanisms synergize in AICD of primary human T cells. (A) Primary human T cells were analyzed for AICD at day 6 after stimulation with PHA and expansion with IL-2. Cells were incubated with 1 μg/mL of plate-bound anti-CD3 antibodies for 18 hours with or without caspase-9–specific inhibitor z-LEHD-fmk (20 μM) in the presence (▩) or the absence (□) of 10 μg/mL of the CD95L-blocking antibody NOK-1. (B) The experiment was carried out similar to panel A, but primary human T cells were transfected 48 hours before with siRNA oligonucleotides comprising a HPK1-specific (siHPK1) or nonspecific sequence (control). The experiments presented are representative of 3 repeats. The siRNA-mediated down regulation of HPK1 was compared with actin by Western blotting (inset). (C) T cells from non-tg or HPK1-C tg littermates were activated with Con A and expanded in the presence of IL-2. At day 3 after activation, cells were transfected with siRNA oligonucleotides comprising a Bim (siBim) or a nonspecific sequence (control). Forty-eight hours following transfection, T cells were subjected to TCR-induced AICD by incubation with 0.1μg/mL anti-CD3 antibodies. The experiments presented are representative of 3 repeats. The siRNA-mediated down-regulation of HPK1 was compared with actin by Western blotting (inset). Standard deviation is given for triplicate measurements. (D) Model for the role of HPK1-C in the regulation of activation-induced cell death (AICD). HPK1-C suppresses the induction of antiapoptotic NF-κB target genes Bcl-2, Bcl-xL, and cIAP1. Stimulation of the T-cell receptor (TCR) in preactivated T cells leads to activation of CD95 by newly synthesized CD95L involving caspase-8. In parallel, TCR stimulation leads to CD95-independent activation of the mitochondrial death pathway and caspase-9, which is augmented by HPK1-C. Stimulation of the B-cell receptor (BCR) leads to AICD primarily via the mitochondrial pathway, which is also augmented by the presence of HPK1-C.
CD95L-dependent and HPK1-C–mediated, CD95L-independent mechanisms synergize in AICD of primary human T cells. (A) Primary human T cells were analyzed for AICD at day 6 after stimulation with PHA and expansion with IL-2. Cells were incubated with 1 μg/mL of plate-bound anti-CD3 antibodies for 18 hours with or without caspase-9–specific inhibitor z-LEHD-fmk (20 μM) in the presence (▩) or the absence (□) of 10 μg/mL of the CD95L-blocking antibody NOK-1. (B) The experiment was carried out similar to panel A, but primary human T cells were transfected 48 hours before with siRNA oligonucleotides comprising a HPK1-specific (siHPK1) or nonspecific sequence (control). The experiments presented are representative of 3 repeats. The siRNA-mediated down regulation of HPK1 was compared with actin by Western blotting (inset). (C) T cells from non-tg or HPK1-C tg littermates were activated with Con A and expanded in the presence of IL-2. At day 3 after activation, cells were transfected with siRNA oligonucleotides comprising a Bim (siBim) or a nonspecific sequence (control). Forty-eight hours following transfection, T cells were subjected to TCR-induced AICD by incubation with 0.1μg/mL anti-CD3 antibodies. The experiments presented are representative of 3 repeats. The siRNA-mediated down-regulation of HPK1 was compared with actin by Western blotting (inset). Standard deviation is given for triplicate measurements. (D) Model for the role of HPK1-C in the regulation of activation-induced cell death (AICD). HPK1-C suppresses the induction of antiapoptotic NF-κB target genes Bcl-2, Bcl-xL, and cIAP1. Stimulation of the T-cell receptor (TCR) in preactivated T cells leads to activation of CD95 by newly synthesized CD95L involving caspase-8. In parallel, TCR stimulation leads to CD95-independent activation of the mitochondrial death pathway and caspase-9, which is augmented by HPK1-C. Stimulation of the B-cell receptor (BCR) leads to AICD primarily via the mitochondrial pathway, which is also augmented by the presence of HPK1-C.
To investigate the physiologic role of HPK1-C as regulator of the intrinsic cell death pathway, we used siRNA-mediated knockdown, which we had applied previously to prevent generation of HPK1-C in primary human T cells.14 In accordance with these data, knock down of HPK1 in expanded activated (day 6) T cells reduced TCR-induced cell death (Figure 6B). Interestingly, blocking of the extrinsic cell death pathway by anti-CD95L antibodies and simultaneous siRNA-mediated knock down of HPK1-C leads to an efficient additive inhibition of AICD (Figure 6B). This additive inhibition shows that the extrinsic and intrinsic cell death pathways cooperate in AICD and identify HPK1-C as a crucial regulator of CD95/CD95L-independent AICD in T cells.
To finally clarify the role of Bim during AICD involving HPK1-C, we used siRNA to knock down Bim expression in primary mouse T cells (Figure 6C inset). AICD was only partially inhibited in T cells from nontransgenic mice (Figure 6C). In contrast, AICD in T cells from HPK1-C tg mice was completely blocked in the absence of Bim. This result confirms our previous findings and identifies HPK1-C as an inducer of the intrinsic cell death pathway depending on the proapoptotic Bcl-2 family member Bim.
Discussion
Our data suggest that HPK1-C is an important physiologic regulator for AICD of primary T and B lymphocytes. HPK1-C suppresses the stimulation-dependent up-regulation of antiapoptotic Bcl-2 family members and thereby decreases the inhibition of the proapoptotic BH3-only Bcl-2 family members (eg, Bim). As a consequence, the loss of mitochondrial membrane potential and the subsequent caspase-9 activation is independent of the CD95/CD95L system (Figure 6D). This function of HPK1-C can explain some of the controversial findings as to its lack of relevance in the CD95/CD95L-dependent (extrinsic) cell death pathways and its definitive role in the CD95/CD95L-independent (intrinsic) cell death pathways. Depending on the presence of HPK1-C during lymphocyte activation, extrinsic or intrinsic AICD could be prevalent. Accumulation of HPK1-C shifts cell death toward intrinsic AICD involving Bim and suggests a regulation of the NF-κB pathway by HPK1/HPK1-C as the basis for cross-regulation between extrinsic and intrinsic cell death.
The role of CD95 in the regulation of lymphocyte homeostasis is shown by the finding that decreased expression of CD95 in lpr or a mutation of CD95L in gld mice leads to autoantibody production and lymphoproliferative disease.33,35 In contrast, T- and B-cell–specific ablation of CD95 leads to lymphocyte depletion and pulmonary fibrosis.36 Thus, CD95 inactivation in T and B cells alone is not sufficient for the pathogenesis of lymphoproliferation. Furthermore, T cell–specific loss of CD95 can result in chronically activated autoimmune T cells but does not impair T-cell deletion in response to strong antigenic stimulation.37 These data suggest the involvement of a CD95/CD95L-independent cell death pathway in AICD. Until now, the molecular basis for CD95/CD95L-independent AICD was not known. Here our data suggest that HPK1-C regulates CD95/CD95L-independent AICD via the intrinsic cell death pathway in primary T and B lymphocytes.
Bcl-2 family proteins were found to mediate activated T-cell death via the intrinsic cell death pathway.38 The fate of antigen-specific T cells is controlled by both activation-induced cell death (AICD) and activated T-cell autonomous death (ACAD).10 ACAD is likely to be mediated exclusively by the intrinsic cell death pathway and depends on the proapoptotic Bcl-2 family member Bim. Bcl-2 and Bcl-xL are constitutively associated with Bim at the mitochondria.39 While expression of Bcl-2 and Bcl-xL are largely controlled by TCR-mediated NF-κB activation, up-regulation of Bim by TCR triggering merely depends on p38 and JNK activities.40 Interestingly, p38 was also reported to mediate a CD95-dependent mitochondrial death in T cells.41 Our data show a physiologic role for HPK1-C as a potent mediator of the intrinsic cell death pathway involving Bim and suggest that HPK1-C determines the ratio between CD95/CD95L-dependent versus -independent AICD in T cells.
Recent studies have provided evidence for a role of HPK1 in the regulation of T-cell apoptosis42 and have shown that HPK1 can determine sensitivity toward AICD in primary T cells.14 The sensitization toward AICD was suggested to involve suppression of IKK activity by HPK1-C, which leads to NF-κB inhibition. This explanation is in line with several studies that show that suppression of NF-κB is essential for AICD.43-46 The potential of HPK1-C to suppress NF-κB activation is evident from studies using various cellular systems, including T and B cells.14,15,20,42 Therefore, HPK1-C interferes with known prosurvival mechanisms and results in blocked expression of NF-κB–dependent antiapoptotic Bcl-2 family proteins. The reduced protection by antiapoptotic Bcl-2 family members renders the cell sensitive toward AICD induction by proapoptotic BH3-only proteins (eg, Bim). To this end, HPK1-C links inhibition of the NF-κB survival pathway to induction of CD95/CD95L-independent AICD. This is even more evident, as HPK1-C blocks induction of CD95L expression after TCR stimulation (Figure 4D). This is in accordance with the requirement of NF-κB activation for expression of the CD95L gene.47
Apoptotic responses upon antigen receptor stimulation involve production of reactive oxygen species (ROS) and calcium flux. Accumulation of ROS is linked to suppression of Bcl-2 expression and induction of CD95L and, therefore, has a pivotal role in apoptosis.48-50 In addition, suppression of NF-κB was reported as a prerequisite for ROS accumulation.51 Therefore, it is likely that NF-κB inhibition by HPK1-C results in increased ROS accumulation and sensitization to AICD.
A strong stimulation-dependent intracellular calcium flux is linked to cell death induction, and high Bcl-2 levels can prevent TCR-mediated apoptosis by binding to the inositol 1,4,5-trisphosphate (IP3) receptors on the endoplasmic reticulum blocking calcium flux.52 Therefore, low Bcl-2 levels in the presence of HPK1-C allow for substantial calcium flux upon stimulation and might result in induction of Bim as reported for negative selection.53
Additionally, we have shown that HPK1 is cleaved by a capase-3 activity in AICD-sensitive primary human T cells and blocking of HPK1 cleavage decreased the sensitivity toward AICD. The HPK1-cleaving caspase activity is below the apoptotic threshold and was recently also found during monocytic differentiation to modulate HPK1 signaling.22 For T-cell function, caspase activities below the apoptotic threshold were reported earlier.54-56 Further investigation is needed to clarify which cellular pathways initiate this subapoptotic caspase activity.
Recently, hyperproliferation was described for T cells from HPK1−/− mice and suggested involvement of HPK1 in down-regulation of the immune response.23 This result could be partially explained by increased cell death resistance of activated T cells in the absence of HPK1-C in HPK1−/− mice. We also had observed that siRNA-mediated knock down of HPK1 led to AICD resistance of expanded primary T cells (Figure 6B), suggesting escape from cell death during the deletion phase of the immune response in the absence of HPK1-C.
Taken together, we have shown that (1) CD95/CD95L-dependent and -independent pathways work together in AICD of primary T cells; (2) HPK1-C is a novel common regulator of the intrinsic cell death pathway in primary T and B lymphocytes; and (3) the HPK1/HPK1-C system is responsible for sensitization of T cells toward AICD under physiologic conditions in vivo. Our findings defined the cross talk between the 2 major cell death pathways and suggest that a dysregulation of the HPK1/HPK1-C system might be a potential cause for disease progression in lymphocyte-based disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank S. Röhling and J. Jebsen for expert technical assistance. We are grateful to H. Sauter for expert secretary assistance and D. Klemke for taking blood samples.
This work was supported by grants from Deutsche Forschungsgemeinschaft, Wilhelm Sander-Stiftung, Deutsche Krebshilfe, European Community, and DKFZ-MOST (Ministry of Science, Culture and Sport) cooperation in cancer research.
Authorship
Contribution: D.B. and R.A. performed research, designed research, analyzed data, and wrote the manuscript; A.G. and D.M. designed research; M.B., W.M., C.R.F., and R.N. performed research; F.K. provided reagents; P.H.K. provided reagents and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rüdiger Arnold, German Cancer Research Center (DKFZ), INF 280, D-69120 Heidelberg, Germany; e-mail: r.arnold@dkfz.de.

![Figure 2. A low caspase-3 activity cleaves HPK1 in expanded primary T cells. (A) Expansion of T cells leads to increased HPK1 expression and conversion to HPK1-C. Presence of full-length HPK1 and its processed fragment HPK1-C was visualized by Western blotting (WB) in primary human T cells at day 1 or day 6 after stimulation. Erk1 is shown as a control. (B) Cell lysates of primary human T cells at day 1 or day 6 after stimulation with PHA and expansion with IL-2 were incubated with in vitro–translated (i.v.tr.) [35S]-labeled HPK1 and resolved by SDS-PAGE. Full-length HPK1 and the caspase cleavage fragments HPK1-N and HPK1-C were detected by autoradiography. As a control, apoptotic processing of [35S]-labeled HPK1 is shown by incubation with lysates from SKW6.4 cells stimulated for 3 hours with anti–Apo-1 (+) or left nonstimulated (−). (C) Primary human T cells isolated from peripheral blood were stimulated with PHA and expanded with IL-2. Samples were taken at the indicated culture day and lysates were resolved by SDS-PAGE. Full-length caspase-3 and its processed fragments p20, p19, and p17 were visualized by Western blotting. Presence of HPK1 and processing toward HPK1-C were analyzed by Western blotting. Expression of actin is shown as control. Viability of the expanded T cells at the time points when processing of caspase-3 and HPK1 was detected was higher compared with the time points when no processing of caspase-3 or HPK1 was detected. Lysates of Jurkat T cells stimulated for 3 hours with anti–Apo-124 (+) or left nonstimulated (−) are shown to control for apoptotic processing of caspase-3. (D) The experiment was performed as in panel B, with cell lysates of primary human T cells at day 6 of culture in the absence or presence of increasing concentrations of the caspase-3/-7–specific inhibitor z-DEVD-fmk. T-cell lysate was omitted in lane 1 (input). (E,F) To block the cleavage of HPK1 toward HPK1-C, primary mouse T cells were incubated at day 3 after stimulation with Con A and expanded in the presence of the 5 μM panspecific caspase inhibitor qVD-oph (E) or 50 μM caspase-3–specific inhibitor z-DEVD-fmk (F) for 72 hours. Cells were washed to remove residual inhibitors, divided into 2 fractions, and subjected either to Western blotting (inset) using antibodies against HPK1 (top) or Erk1 (bottom) or to analysis of AICD. Therefore, cells were further analyzed by incubation with 0.1 μg/mL of plate-bound anti-CD3 antibodies or 2.5 ng/mL of CD95L for 18 hours. Viability of the expanded T cells was not altered by addition of qVD-oph or z-DEVD-fmk. Standard deviation is given for triplicate measurements. The experiment was repeated 3 times with similar outcome.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/12/10.1182_blood-2007-01-071167/4/m_zh80240710030002.jpeg?Expires=1769139068&Signature=IiUn-QHMJOFz7URIoOh3zAh36LRLVtbvxTsFkGEUdzDgYlJ-Xkm98rDaJJ4njCU-2W1AdhGFu8N98XP8VXCEjNIt1S03ktAxdz60VGe0i2~oPbZFTkuoTTq4zeVDPxABoxpMwfF01CyO~D2H0y9BJd39a9wr7aogwlYVF6rYlMuFayGyV3QhSmoWdyW4y1gAIRa9IJUg6YlcePyzziPiG4y0p~ISbycOrN7gsYIttjQY-dHJX2pZluL2p1bTulAtyM8mygq3egsFLmPpeQT0GVB-xKtJJ2xJDuzKVvc-icvjCkm2ulP9xM7P0gIgr93VoavR05hRLrMZuFsLu3YNSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal