Abstract
Removal of pathogenic B lymphocytes by depletion of monoclonal antibodies (mAbs) or deprivation of B-cell survival factors has demonstrated clinical benefit in both oncologic and immunologic diseases. Partial clinical responses and emerging data demonstrating incomplete B-cell depletion after immunotherapy fuels the need for improved therapeutic modalities. Lessons from the first generation of therapeutics directed against B-cell-specific antigens (CD20, CD22) are being applied to develop novel antibodies with additional functional attributes. We describe the generation of a novel class of B-cell-directed therapy (anti-BR3 mAbs) that combines the depleting capacity of a therapeutic mAb and blockade of B-cell-activating factor (BAFF)–BR3 B-cell survival. In mice, treatment with antagonistic anti-BR3 antibodies results in quantitatively greater reduction in some B-cell subsets and qualitatively different effects on bone marrow plasma cells compared with BR3-Fc BAFF blockade or with anti-CD20 treatment. Comparative analysis of BR3-Fc and anti-BR3 mAb reveals a lower B-cell dependence for BAFF-mediated survival in nonhuman primates than in mice. This novel class of B-cell-targeted therapies shows species characteristics in mice and primates that will guide translation to treatment of human disease.
Introduction
B-cell immunotherapy has shown clinical benefit in the treatment of human B-cell malignancies and autoimmune diseases.1-4 Since approval of a depleting anti-CD20 monoclonal antibody (mAb) (rituximab [Genentech, South San Francisco, CA; Biogen-IDEC, Cambridge, MA; Roche, Basel, Switzerland)] in 1997 for the treatment of non-Hodgkin lymphoma (NHL), almost 1 million patients have been treated with rituximab as a first or second line therapy, either alone or in combination with chemotherapy. In addition, rituximab maintenance therapy significantly prolongs tumor remission and patient survival in patients with indolent B-cell NHL or chronic lymphocytic leukemia (CLL).5,6 More recently, rituximab has demonstrated clinical benefit in a variety of autoimmune diseases including rheumatoid arthritis, pemphigus vulgaris, immune thrombocytopenia and autoimmune hemolytic anemia.4,7,8 As a result, understanding the contribution of B lymphocytes to human autoimmune diseases received revived interest, and several mechanisms have been postulated to participate in disease pathogenesis, including autoantibody production, B-cell antigen presentation, cytokine generation, and lymphorganogenesis.2,3,9,10 Inhibition of different combinations of these mechanisms is probably responsible for clinical benefit
The success of anti-CD20 B-cell immunotherapy spearheaded a large number of preclinical and clinical efforts to understand in vivo mechanisms of drug activity. Direct elimination of malignant B cells through antigen-dependent cell-mediated cytotoxicity (ADCC), complement-mediated cytotoxicity (CDC), and apoptosis have been demonstrated as the main mechanisms of action in a variety of systems including mouse xenotumors and normal mouse and nonhuman primate (NHP) B-cell subsets.11-15 Two major determinants affecting normal mouse B-cell depletion have been identified.13,16 First, the kinetics of B-cell recirculation determines the speed and magnitude of anti-CD20 mAb-mediated B-cell depletion. Cells with higher recirculatory kinetics from blood, lymph nodes, and spleen follicular areas are depleted faster and more completely than cells with lower recirculatory kinetics (eg, peritoneal cavity [PEC], marginal zone [MZ], germinal centers [GC]). Second, the local microenvironment influences the extent of B-cell depletion. Marginal zone, Peyer patches (PP), germinal center, memory, and peritoneal cavity B cells exhibit greater resistance to depletion in mice and nonhuman primates. Reduced recruitment of effector mechanisms in the peritoneal cavity as well as intrinsic B1 B cells properties appear to cause the slower kinetics and B-cell reduction after anti-CD20 mAb treatment.16 Differences across mouse strains and epitopes recognized by anti-CD20 antibodies used for depletion might explain the differential effects seen on mouse splenic marginal zone B cells.13,16 Small but consistent numbers of residual B cells can be detected in most lymphoid organs in mice and primates treated with anti-CD20 mAbs. One possibility of achieving a more complete B-cell reduction would be to block B-cell survival signals in addition to administration of a B-cell-depleting reagent. B-cell-activating factor (BAFF) (also known as BLyS) and a proliferation-inducing ligand (APRIL), 2 related tumor necrosis factor (TNF) family members, and their receptors—BLyS receptor 3 (BR3; also known as BAFFR), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and B-cell maturation antigen (BCMA)—constitute a well characterized system involved in B-cell development and survival.17-21 Of these, BAFF/BR3 is the main axis transducing B-cell survival signals, and interference with this pathway using blocking reagents (anti-BAFF antibodies or BR3-, TACI-, or BCMA-Fc fusion proteins) results in B-cell reductions in mice, nonhuman primates, and humans.22,23 Recent clinical trials with an anti-BAFF antibody have shown clinical benefit in rheumatoid arthritis (RA) and positive trends in retrospective subset analysis in patients with systemic lupus erythematosus (SLE).24,25 In mice, BAFF blockade with BR3-Fc appears to act synergistically with anti-CD20, resulting in more complete B-cell depletion at doses suboptimal as monotherapy.13 It is noteworthy that serum BAFF levels increase when B cells are lacking (ie, rag-deficient mice) or after depletion with rituximab in patients with RA, SLE, or Sjögren syndrome, probably because of a decrease in receptor-mediated clearance and possibly other mechanisms.26-30 Thus, increased serum BAFF could provide additional survival signals for cells that were not removed during the depletion phase and for emerging new B cells during the recovery phases of B-cell immunotherapy.
Strategies targeted at enhancing intrinsic properties of anti-CD20 antibodies (eg, increased ADCC, CDC, and affinity to antigen) represent one modality by which to achieve greater clinical benefit compared with existing B-cell immunotherapy.31-35 Other efforts are driven toward testing antibodies directed against other B-cell surface molecules (CD19, CD22).36-39 Finally, conjugates of radioactive isotypes (ibritumomab tiuxetan [Zevalin], tositumomab [Bexxar]) and toxins (calicheamicin-antiCD22, anti-CD79 antibody-drug conjugates) represent a third strategy to enhance B-cell targeting.40-42 In addition to intrinsic molecule improvements, combinations of B-cell-depleting agents with molecules enhancing other arms of the immune system including effector mechanisms for B-cell depletion (eg, interferon-α, granulocyte–colony-stimulating factor, granulocyte macrophage–colony-stimulating factor, interleukin 2) are being attempted in B-cell malignancies with variable success.43-46
Based on the demonstrated synergy between B-cell depletion and blockade of B-cell survival through BR3, we developed anti-BR3 antibodies that combine, in one molecule, the abilities to deplete B cells and to block BAFF-mediated B-cell survival. These antibodies combine 2 mechanisms of action and show increased in vivo B-cell reduction in some subsets compared with anti-CD20 mAb-mediated B-cell depletion or BAFF-dependent survival blockade on their own. As a result, therapy with this novel type of antibody has the potential to translate into increased clinical benefit in diseases with B-cell-mediated pathogenic mechanisms.
Materials and methods
Anti-BR3 antibodies and in vivo dosing
mCB2 (mIgG2a) and mCB2 DANA, phage-displayed synthetic antibody, were generated as described previously.47 PIH11 (anti-mouse BR3, nonblocking antibody; Biogen-IDEC, Cambridge, MA), mBR3-Fc, and mouse anti-human CD20 (mouse IgG2a version of anti-human CD20 mAb 2H7) were described previously.13,48 Anti-ragweed mouse IgG2a was used as the isotype control in all mouse studies. Final endotoxin levels of antibodies for all in vivo studies were less than 0.2 EU/mg. To be able to compare among molecules, mice were dosed in all experiments to ensure maximal B-cell reduction based on in vivo titrations of anti-BR3, BR3-Fc and anti-CD20 reagents. Dose regimes were determined to achieve similar trough concentrations for anti-BR3 and BR3-Fc in all experiments. Anti-BR3 at a 500-μg single dose achieves blood drug levels at day 15 similar to 200-μg weekly multidose trough concentrations. BR3-Fc at 150 μg, 3 times per week, achieves a 72-hour trough in a similar range. Target trough concentrations were determined to achieve maximal B-cell reduction in splenic B-cell subsets based on a series of single-dose pharmacokinetic/pharmacodynamic experiments. For example, at day 4, anti-BR3 blood concentrations reached after a 20-μg dose did not achieve maximal B-cell depletion, whereas those reached after a 200-μg dose did (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The half-life is 5 days for anti-CD20 and anti-BR3 antibodies and 3 days for mouse BR3-Fc. Mouse anti-human BR3 (clone 9.1; Biogen-IDEC49 ) was humanized on a human IgG1 framework and used in NHP studies. All in vitro properties (eg, BR3 binding, BAFF antagonism, ADCC) were similar between the parent and humanized antibody.
Animals
Eight- to 12-week-old BALB/c mice (The Jackson Laboratory, Bar Harbor, Maine) and C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used for depletion studies. hCD20tgBAC FvB and C57BL/6 mice were described previously.13 NZB/W F1 female mice (The Jackson Laboratory), an SLE-prone model, were ∼7 to 8 months old and weighed 35 to 50 g when they were enrolled in therapeutic studies. Urine was tested for proteinuria by semiquantitatively dipsticks (Multistick; Thermo Fisher Scientific, Waltham, MA) every 4 weeks. The baseline protein levels at the start of the studies were between 30 and 300 mg/dL. All experimental animal studies were conducted according to protocols that were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) of Genentech Lab Animal Research (LAR).
NHP cynomolgus monkey studies were conducted at contract research organizations (CROs) according to their standard operating procedures and in compliance with applicable regulations concerning the use of laboratory animals. Naive monkeys, 2 to 5 years old, were acclimated to the study rooms for 28 days before the initiation of the study.
Tissue collection
Whole spleen, lymph nodes, Peyer patches, and kidneys were collected for analysis. Single-cell suspensions were prepared by grinding organs between frosted glass slides. The total cell counts were measured using fluorescence beads. Cells were filtered through a 70-μm nylon cell strainer and used for flow cytometry analysis and spot enzyme-linked immunosorbent assays. Blood samples were collected from the retro-orbital sinus (100 μL) at trough and by cardiac puncture (while the animals were anesthetized with 3% isoflurane anesthesia, 600∼800 μL) before the mice were euthanized.
Flow cytometry and antibodies
Spleen, lymph nodes, Peyer patches, peritoneal cavity lavage, and peripheral blood cells were analyzed for B and T cell subsets using conjugated antibodies as fluorescence-activated cell sorting (FACS) reagents: anti-mouse CD3, CD4, CD8, CD5, CD23, CD21, CD69, B220, CD38, CD138, CD44, CD62L, and anti-human CD19, CD5, and cynomolgus monkey cross-reactive CD20, CD40, CD21, and CD27, all from Pharmingen (San Diego, CA) and BD Biosciences (San Jose, CA). Polyclonal goat anti-mouse IgM, IgG1, IgG2a, and IgA were purchased from Southern Biotechnology Associates (Birmingham, AL). FACS analyses were conducted on a FACSCalibur (BD Biosciences). B-cell subsets were identified as described in each figure legend. Statistical analyses relative to the control group were performed using the Dunnett test.
BrdU labeling & staining
One week before take down, each mouse was received 2 mg of bromodeoxyuridine (BrdU; Sigma, St. Louis, MO) in phosphate-buffered saline (PBS) via intraperitoneal (i.p.) injection every other day for a week.
BrdU staining was performed using a BrdU flow kit (BD Biosciences). First, B lineage surface antigens B220 and CD138 were stained, followed by fixation and permeabilization of cells using BD Cytofix/Cytoperm buffer. The cells were then treated with DNase to expose BrdU epitopes. Cycling S phase cells were detected by staining cells with fluorochrome conjugated anti-BrdU and anti-Ig isotype antibodies and then analyzing by flow cytometry.
Immunofluorescence and immunohistochemistry staining
Five-micrometer cryosections of mouse spleen were stained with 7-amino-4-methylcoumarin-3-acetic acid–anti-mouse IgM (Vector Laboratories, Burlingame, CA), FITC-anti-mouse Thy1 (Pharmingen), and MOMA-1 mAb followed by horse anti–rat IgG/biotin and Cy3-Streptavidin (Jackson Immunoresearch, West Grove, PA). Fluorescently labeled sections were imaged on an Olympus BX-51 microscope (Olympus USA, Center Valley, PA) equipped with filter cubes for DAPI/AMCA, FITC/Cy2 and TRITC/Cy3. Twelve-bit monochrome images were acquired with a 10× objective using a Hamamatsu ORCA CCD camera (Olympus USA) driven by Meta-Morph version 6.1 imaging software (Universal Imaging, Downingtown, PA). Image files were transferred to Adobe Photoshop CS2 (Adobe, Mountain View, CA), adjusted for contrast, and merged to create 24-bit color images.
Formalin-fixed, paraffin-embedded cynomolgus monkey tissues were sectioned at 4 to 6 μm and processed by standard techniques for immunohistochemistry. Immunohistochemistry for B cells was accomplished using mouse IgG2a anti-human CD20 (clone L26; Dako, Carpinteria, CA) or an irrelevant IgG2a antibody (clone C1.18.4; BD Pharmingen) and detection system using ABC-Peroxidase Elite with Vector Blue (Vector Laboratories). Immunohistochemistry for T cells was accomplished using mouse IgG1 anti-CD3 (clone SP34–2; BD Pharmingen) or an irrelevant IgG1 antibody (clone MOPC-21; BD Pharmingen) and detection system using ABD-Peroxidase Elite with diaminobenzidine (Vector Laboratories).
Anti-DNA spot enzyme-linked immunosorbent assay
Sheared calf thymus DNA (Invitrogen, Carlsbad, CA) was coated overnight onto 96-well microtiter plates (Dynex Technologies, Chantilly, VA) at 10 μg/mL. After washing with PBS, plates were blocked for 1 hour with 0.5% bovine serum albumin in PBS at room temperature. Mouse spleen cells (3 × 106 cells/well) or kidney suspension cells (5 × 106 cells/well) in RPMI 1640 media with 10% fetal calf serum were added into the first row of the plates and serially diluted at 1:2. Plates were cultured overnight at 37°C, cells were aspirated, and plates were washed 6 times followed by incubation with goat anti-mouse IgG (Southern Biotechnology Associates) at 1 μg/mL for 2 hours at 37°C. The plates were then washed 6 times with 0.05% Tween 20 in PBS. Streptavidin-alkaline phosphatase was added to each well at 1:2000, and plates were incubated at room temperature for 1 hour. After wash, the plates were incubated with a mixture of 2-amino-2-methyl-1-propanol/5-bromo-4-chloro-3 indolyl phosphate) and 0.6% agarose gel overnight at 4°C. Anti-ds/ssDNA IgG antibody-forming cells were enumerated as blue spots using an inverted microscope and recorded as the number of antibody-forming cells per spleen or per kidney.
Results
Anti-BR3 mAb treatment resulted in greater mouse B-cell reduction than BR3-Fc
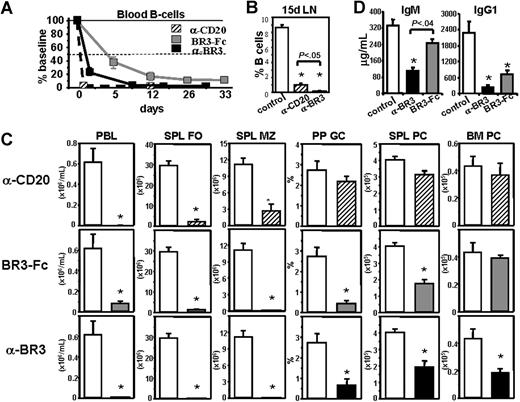
Antibodies against mouse and human BR3 were generated by both classic hybridoma and phage display technologies and selected based on their ability to bind BR3 with high affinity, block BAFF binding to BR3, and prevent BAFF-mediated B-cell survival and proliferation in B-cell cultures.47,49 A phage-derived antibody (CB2) was selected for further in vivo analysis.47 Fifteen days after in vivo administration of anti-BR3 (CB2) mAb in BALB/c mice, B cells were reduced in all lymphoid organs tested (Figure 1A-C). In blood, splenic follicles (FO; CD23+CD21int), splenic MZ (CD23loCD21hi), and lymph nodes, treatment with anti-BR3 mAb resulted in profound B-cell reduction (90%-99%) compared with isotype control-treated mice. In PP, FO cells (B220+CD38+) were reduced similarly to other recirculating blood, spleen, and lymph node compartments, whereas GC (B220+CD38lo) B cells were reduced to a lesser extent (Figure 1A,C). In the peritoneal cavity (PEC), B2 cells (B220+CD23+) and B1 cells (B220+CD23−) were reduced by 90% and 70%–80%, respectively. Compared with the in vivo effects of BR3-Fc fusion protein, anti-BR3 mAb treatment caused a significantly higher level of B-cell reduction in blood, spleen follicles, lymph nodes, and other compartments that contain recirculating cells (PEC, PP follicles). In contrast, spleen marginal zone B cells and plasma cells (PC) that do not recirculate and have shown survival dependence on BAFF are reduced to similar levels (Figure 1A,C). These findings were confirmed by immunofluorescence on spleen sections, which shows complete depletion of FO and MZ regions in the anti-BR3 treated group with some residual cells left in the follicles after BR3-Fc treatment (Figure 1B). Bright plasma cells (IgM+) in the red pulp are reduced in both BR3-Fc- and anti-BR3–treated groups.
Anti-BR3 mAb reduces mouse B-cell subsets more efficiently than BR3-Fc. BALB/c mice were treated with anti-BR3 (500 μg), BR3-Fc (150 μg × 3/week) or isotype control (500 μg); 15 days later, tissues were analyzed by flow cytometry (A,C) and immunofluorescence (B). Percentages of cells in B-cell subsets are displayed near each gate as follows: Spleen: follicular (FO) B cells (CD23+CD21+), marginal zone (MZ) B cells (CD23−CD21hi), and plasma cells (PC, CD138+B220lo); Lymph nodes: B cells (CD23+CD21+), blood (BL) B cells (CD23+CD21+), Peyer patches (PP), follicular B cells (B220+CD38+), and germinal center (GC) B cells (B220+CD38lo); peritoneal cavity (PEC): B2 cells (B220+CD23+) and B1 cells (B220+CD23−) (A,C). B cells (blue) and T cells (green) are stained in spleen sections after treatment with control, BR3-Fc or anti-BR3 mAb. Marginal zone and follicular B-cell areas are separated by the layer of metallophilic macrophages (MM [red]) (B). See “Immunofluorescence and immunohistochemistry staining” for image acquisition details. Absolute B-cell numbers or percentage of lymphocyte gate belonging to a defined subset are shown as mean plus or minus standard error. Statistical significance compared with the control treated group is indicated with asterisk (C). When 2 treatment groups are significantly different from each other, the P value is displayed. These data are representative of more than 10 independent experiments done with several maximally depleting doses of anti-BR3, using at least 5 mice/group.
Anti-BR3 mAb reduces mouse B-cell subsets more efficiently than BR3-Fc. BALB/c mice were treated with anti-BR3 (500 μg), BR3-Fc (150 μg × 3/week) or isotype control (500 μg); 15 days later, tissues were analyzed by flow cytometry (A,C) and immunofluorescence (B). Percentages of cells in B-cell subsets are displayed near each gate as follows: Spleen: follicular (FO) B cells (CD23+CD21+), marginal zone (MZ) B cells (CD23−CD21hi), and plasma cells (PC, CD138+B220lo); Lymph nodes: B cells (CD23+CD21+), blood (BL) B cells (CD23+CD21+), Peyer patches (PP), follicular B cells (B220+CD38+), and germinal center (GC) B cells (B220+CD38lo); peritoneal cavity (PEC): B2 cells (B220+CD23+) and B1 cells (B220+CD23−) (A,C). B cells (blue) and T cells (green) are stained in spleen sections after treatment with control, BR3-Fc or anti-BR3 mAb. Marginal zone and follicular B-cell areas are separated by the layer of metallophilic macrophages (MM [red]) (B). See “Immunofluorescence and immunohistochemistry staining” for image acquisition details. Absolute B-cell numbers or percentage of lymphocyte gate belonging to a defined subset are shown as mean plus or minus standard error. Statistical significance compared with the control treated group is indicated with asterisk (C). When 2 treatment groups are significantly different from each other, the P value is displayed. These data are representative of more than 10 independent experiments done with several maximally depleting doses of anti-BR3, using at least 5 mice/group.
Taken together, these data demonstrate that treatment with a depleting antagonistic anti-BR3 antibody results in superior B-cell reduction compared with BR3-Fc. These results were further confirmed by more frequent and longer-term treatments (data not shown).
Anti-BR3 treatment reduced mouse B cells more slowly but more profoundly in certain subsets than anti-CD20
To compare the effects of anti-BR3 and anti-CD20 mAbs on B-cell depletion, we used human CD20BACTg mice.13 Analysis of the kinetics of blood B-cell depletion with anti-BR3 revealed a 75% reduction within 1 day and 99% over 8 days (Figure 2A). In contrast, treatment with anti-CD20 mAb was faster at reducing blood B cells (75% reduction within 1 hour and 99% in 1 day), whereas the effect of BR3-Fc was slower than both anti-BR3 and anti-CD20 (Figures 2A, S2). In lymph nodes, B-cell reduction achieved by anti-BR3 mAb occurred at a rate slower than that induced by anti-CD20 but became superior in magnitude by day 15 (Figures 2B, S2).
Kinetics and comparative mouse B-cell reduction after treatment with anti-BR3 mAb, BR3-Fc, or anti-CD20 mAb. Mice (≥ 5/group) treated with anti-BR3 mAb (200 μg/week), BR3-Fc (150 μg × 3/week), or anti-CD20 mAb (200 μg/week) were analyzed at different times, with B-cell numbers and subsets evaluated by flow cytometry. Anti-BR3 treatment reduced blood B cells (expressed as percentage of baseline absolute cell counts) faster and more profoundly than BR3-Fc but slower than anti-CD20 treatment (A). By day 15 of treatment, the extent of B-cell reduction in the lymph node in response to anti-BR3 treatment appeared greater than that resulting from anti-CD20 mAb treatment (B). B-cell subsets were quantified using similar definitions as in Figure 1. Spleen and bone marrow plasma cells were defined as CD138+B220lo/−. Analysis of different B-cell subsets after 8 weeks of continuous treatment (200 μg/week anti-BR3 and anti-CD20; 150 μg × 3/week BR3-Fc) shows significantly higher MZ B-cell, spleen, and BM plasma cell reduction in anti-BR3 mAb compared with anti-CD20 mAb treatment (C). Serum IgM and IgG1 were measured by ELISA after 3 months of continuous treatment (D). These data are representative of 3 independent experiments. Data are shown as mean plus or minus standard error. Statistical significance compared with the control treated group is indicated with an asterisk. When 2 treatment groups are significantly different from each other, the P value is displayed.
Kinetics and comparative mouse B-cell reduction after treatment with anti-BR3 mAb, BR3-Fc, or anti-CD20 mAb. Mice (≥ 5/group) treated with anti-BR3 mAb (200 μg/week), BR3-Fc (150 μg × 3/week), or anti-CD20 mAb (200 μg/week) were analyzed at different times, with B-cell numbers and subsets evaluated by flow cytometry. Anti-BR3 treatment reduced blood B cells (expressed as percentage of baseline absolute cell counts) faster and more profoundly than BR3-Fc but slower than anti-CD20 treatment (A). By day 15 of treatment, the extent of B-cell reduction in the lymph node in response to anti-BR3 treatment appeared greater than that resulting from anti-CD20 mAb treatment (B). B-cell subsets were quantified using similar definitions as in Figure 1. Spleen and bone marrow plasma cells were defined as CD138+B220lo/−. Analysis of different B-cell subsets after 8 weeks of continuous treatment (200 μg/week anti-BR3 and anti-CD20; 150 μg × 3/week BR3-Fc) shows significantly higher MZ B-cell, spleen, and BM plasma cell reduction in anti-BR3 mAb compared with anti-CD20 mAb treatment (C). Serum IgM and IgG1 were measured by ELISA after 3 months of continuous treatment (D). These data are representative of 3 independent experiments. Data are shown as mean plus or minus standard error. Statistical significance compared with the control treated group is indicated with an asterisk. When 2 treatment groups are significantly different from each other, the P value is displayed.
To evaluate maximal B-cell reduction reached with these therapies in various B-cell subsets, we compared courses of 8 weeks of continuous treatment with each reagent. Blood B cells were reduced by 99% by both anti-CD20 and anti-BR3 mAbs and by 90%-95% in the BR3-Fc–treated mice (Figure 2C). In spleen and lymph nodes, FO cells were virtually eliminated by anti-BR3 mAb, whereas small residual subsets were seen after treatment with either anti-CD20 mAb (mostly MZ, few FO) or BR3-Fc (few FO). Reduction of PP GC cells and spleen plasma cells were comparable in mice treated with either anti-BR3 mAb or BR3-Fc (∼75% and 50%, respectively). In contrast, no significant reduction in PP GC or spleen plasma cells was observed after treatment with anti-CD20 mAb, suggesting that decrease in these 2 subsets was probably due to BAFF blockade.
The numbers of bone marrow (BM) PCs, which are enriched in long-lived PCs, were not altered by long-term treatment with either anti-CD20 mAb or BR3-Fc. However, BM PCs showed a significant decrease (∼50%) with anti-BR3 mAb treatment. These effects on BM plasma cells translated into significant decreases in serum IgM and IgG after anti-BR3 mAb treatment, whereas only IgG1 changed after 3 months of BR3-Fc treatment (Figure 2D and data not shown). Anti-CD20 mAb caused no reduction in serum immunoglobulin even after 1 year of continuous treatment.13 Because bone marrow plasma cells express low levels of BR3 but not CD2050 in both mice and humans (Figure S3), anti-BR3 mAbs may provide a therapeutic modality to target BM PCs.
The B-cell reduction profile of anti-BR3 was distinct from those of anti-CD20 and BR3-Fc in NHP
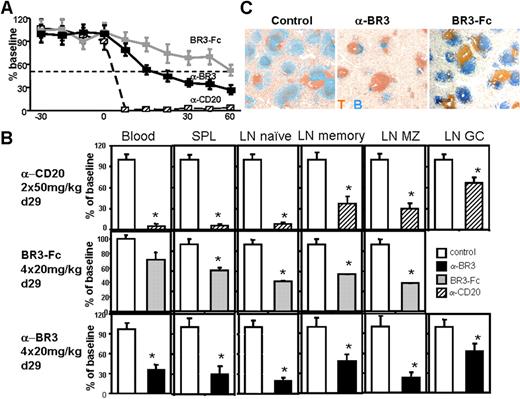
B-cell reduction profiles elicited by BAFF-targeting reagents from mice and primates are different.17,19,20 Although mouse B cells are exquisitely sensitive to BAFF targeting and more than 90% are eliminated within 2 weeks of treatment, NHP and human studies have shown a maximum of only 50% overall reduction after 1 to 6 months of treatment. This is due in part to different B-cell subset sensitivities for BAFF blockade (memory B cells, which comprise a larger part of the primate repertoire, appear less sensitive) but also to other unknown factors that might differ between rodents and primates. To evaluate and help translate the anti-BR3 findings from mouse to humans, we performed an NHP study in cynomolgus monkeys with a humanized antagonistic anti-BR3 antibody (h9.1). This clone is a surrogate of the mouse antibody (high affinity for human and cynomolgus monkey BR3, BAFF-blocking in vitro cellular assays, capable of ADCC, data not shown) while binding a different epitope on BR3.47 Monkeys were treated with anti-BR3, BR3-Fc, or anti-CD20 (in parallel experiments), and their B cells were compared with a control group. In contrast to mice, in which 99% of blood B cells were eliminated within 1 to 2 weeks after anti-BR3 mAb treatment, blood B cells in cynomolgus monkeys decreased by approximately 50% after 2 weeks (Figure 3A). Maximum blood B-cell reduction of approximately 75% was reached after 1 month and remained near this level for the rest of the treatment. Lymphoid organ analysis at a 4-week timepoint using flow cytometry and immunohistochemistry showed that in spleen and lymph nodes, B cells were decreased by approximately 80% (Figure 3B,C). Reduction of naive B cells in blood and lymph nodes (both FO and MZ) was faster and more profound compared with memory B cells (Figure 3B and data not shown), suggesting a differential sensitivity for BAFF blockade for these subsets in primates. Consistent with this lower primate sensitivity to BAFF blockade compared with mice, serum immunoglobulins did not significantly change after the first 3 months of anti-BR3 mAb treatment (data not shown).
NHP B-cell reduction effects of anti-BR3 mAb, BR3-Fc, and anti-CD20 mAb. Comparison of blood B cell numbers from cynomolgus monkeys treated with anti-BR3 mAb (20 mg/kg/week: days 0-29, n = 16; days 29-60, n = 10; terminal d29, n = 6), BR3-Fc (20 mg/kg/week: days 0-29, n = 12; days 29-60, n = 4; terminal d29, n = 8), anti-CD20 (2 × 50 mg/kg: days 0 and 14, n = 4), and vehicle control (same numbers as active agent groups) (A). Anti-BR3 appeared to reduce blood and spleen B cells faster and more profoundly than BR3-Fc but slower and less completely compared with anti-CD20 mAb. Naive lymph node B cells (CD20+CD21+CD27−) were reduced similarly by anti-BR3 and anti-CD20 treatments and less by BR3-Fc. Other lymph node B-cell subsets—memory (CD20+CD21+CD27+), marginal zone (CD20+CD21hiCD27−/+), germinal center (CD20+CD21−) cells—were reduced similarly by all treatments (germinal center cells were not assayed in the BR3-Fc-treated experiment) (B). Statistical significance compared with the control treated group is indicated with an asterisk. Because treatment groups were in separate experiments, statistical analysis was always performed against internal vehicle control group. Treatment with anti-BR3 or BR3-Fc result in spleen B-cell reduction measured by immunohistochemistry. B cells, blue (anti-CD20); T cells, orange (anti-CD3) (C). See “Immunofluorescence and immunohistochemistry staining” for image acquisition details.
NHP B-cell reduction effects of anti-BR3 mAb, BR3-Fc, and anti-CD20 mAb. Comparison of blood B cell numbers from cynomolgus monkeys treated with anti-BR3 mAb (20 mg/kg/week: days 0-29, n = 16; days 29-60, n = 10; terminal d29, n = 6), BR3-Fc (20 mg/kg/week: days 0-29, n = 12; days 29-60, n = 4; terminal d29, n = 8), anti-CD20 (2 × 50 mg/kg: days 0 and 14, n = 4), and vehicle control (same numbers as active agent groups) (A). Anti-BR3 appeared to reduce blood and spleen B cells faster and more profoundly than BR3-Fc but slower and less completely compared with anti-CD20 mAb. Naive lymph node B cells (CD20+CD21+CD27−) were reduced similarly by anti-BR3 and anti-CD20 treatments and less by BR3-Fc. Other lymph node B-cell subsets—memory (CD20+CD21+CD27+), marginal zone (CD20+CD21hiCD27−/+), germinal center (CD20+CD21−) cells—were reduced similarly by all treatments (germinal center cells were not assayed in the BR3-Fc-treated experiment) (B). Statistical significance compared with the control treated group is indicated with an asterisk. Because treatment groups were in separate experiments, statistical analysis was always performed against internal vehicle control group. Treatment with anti-BR3 or BR3-Fc result in spleen B-cell reduction measured by immunohistochemistry. B cells, blue (anti-CD20); T cells, orange (anti-CD3) (C). See “Immunofluorescence and immunohistochemistry staining” for image acquisition details.
Compared with the anti-CD20 mAb and BR3-Fc studies, anti-BR3 mAb was intermediate between the 2 in both kinetics and degree of B-cell reduction in blood and spleen (Figure 3A-C). BR3-Fc does not reduce blood B cells significantly in the first 4 weeks, whereas anti-BR3 and anti-CD20 mAbs do, suggesting that cellular depletion, not survival blockade, is the main mechanism responsible in this time frame for blood B-cell reduction. Reduction of lymph node B cells by anti-BR3 treatment seems to be similar to that induced by anti-CD20 mAb (Figure 3B). These findings in NHP show clear differences of the BAFF/BR3 system compared with rodents and have important consequences in guiding translatability of studies in rodents to treatment of human diseases.
Anti-BR3 reduced some B-cell subsets more profoundly than BR3-Fc in the NZB/W lupus-prone mouse strain
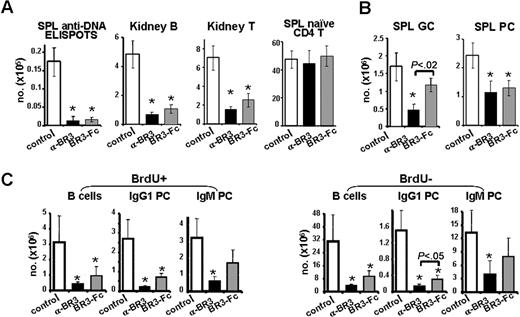
To examine the effects of anti-BR3 immunotherapy, we evaluated the potency on autoimmune B-cell reduction of this dual targeting agent after treatment in the lupus-prone NZB/W mouse strain. Seven- to 8-month-old NZB/W mice with established proteinuria (30-100 mg/dL) were treated with control, anti-BR3 mAb, or BR3-Fc continuously for up to 5.5 months. Treatment with anti-BR3 mAb decreased total B cells in blood and spleen to a greater extent compared with BR3-Fc (Figure S5). Although overall B-cell depletion in diseased NZB/W mice was efficient, the reduction was less than that observed in normal healthy mice used as control group in the same experiment (data not shown). As early as 2 weeks (data not shown), and maximal at 6 weeks, anti-BR3 mAb and BR3-Fc treatments decreased spleen anti-DNA-producing plasma cells, total plasma cells, and kidney-infiltrating B and T cells but left naive T cells unchanged (Figure 4A). Spleen germinal center B cells and noncycling IgG1 plasma cells were reduced more with anti-BR3 mAb than BR3-Fc treatment (Figure 4B,C; detailed below).
Anti-BR3 mAb decreases pathogenic B-cell subsets in the NZB/W F1 SLE mouse model. Therapeutic treatment of proteinuric NZB/W F1 mice (15/group) for 6 weeks with anti-BR3 mAb (600 μg/week) or BR3-Fc (300 μg × 3/week) reduced splenic anti-DNA-producing cells, kidney-infiltrating B cells (B220+), and T cells (CD3+) but not naive spleen T cells (A). Splenic germinal center B cells (B220+CD38lo) were decreased only by anti-BR3 mAb treatment, whereas plasma cells (CD138+B220lo) were decreased by both treatments (B). After 1 week, anti-BR3 mAb and BR3-Fc treatment showed significant reduction in both cycling and noncycling splenic B cells (B220+) and IgG1 plasma cells (cytoplasmic IgG1+CD138+), but only anti-BR3 mAb treatment resulted in significant reduction in IgM plasma cells (cytoplasmic IgM+CD138+) (C). Statistical significance compared with the control treated group is indicated with an asterisk. When 2 treatment groups are significantly different from each other, the P value is displayed.
Anti-BR3 mAb decreases pathogenic B-cell subsets in the NZB/W F1 SLE mouse model. Therapeutic treatment of proteinuric NZB/W F1 mice (15/group) for 6 weeks with anti-BR3 mAb (600 μg/week) or BR3-Fc (300 μg × 3/week) reduced splenic anti-DNA-producing cells, kidney-infiltrating B cells (B220+), and T cells (CD3+) but not naive spleen T cells (A). Splenic germinal center B cells (B220+CD38lo) were decreased only by anti-BR3 mAb treatment, whereas plasma cells (CD138+B220lo) were decreased by both treatments (B). After 1 week, anti-BR3 mAb and BR3-Fc treatment showed significant reduction in both cycling and noncycling splenic B cells (B220+) and IgG1 plasma cells (cytoplasmic IgG1+CD138+), but only anti-BR3 mAb treatment resulted in significant reduction in IgM plasma cells (cytoplasmic IgM+CD138+) (C). Statistical significance compared with the control treated group is indicated with an asterisk. When 2 treatment groups are significantly different from each other, the P value is displayed.
To evaluate the impact of anti-BR3 mAb or BR3-Fc treatment on cycling versus noncycling B-cell compartments, we performed BrdU pulse experiments. In contrast to cyclophosphamide (Cytoxan), which reduces mainly cycling cells, both therapies decreased cycling and noncycling B-cell compartments effectively after 11 days (Figures 4C, S5). Likewise, both cycling and noncycling plasma cells were reduced; the isotype-switched plasma cells were more profoundly decreased than IgM-positive plasma cells after therapy. Trends toward greater effects from anti-BR3 mAb compared with BR3-Fc treatment were observed in all compartments, but statistical significance between the 2 groups was reached only for IgG1 plasma cells. Effects of treatment on the plasma cell compartment were paralleled by decreases in total serum IgM and IgG isotypes, as well as of IgG anti-DNA antibodies (data not shown). In all these readouts, anti-BR3 mAb was superior to BR3-Fc, suggesting that, similar to normal mice, effects on the autoimmune plasma cell compartment are more pronounced with anti-BR3 mAb than BR3-Fc.
This series of experiments showed that anti-BR3 mAb reduced certain subsets (GC B cells, IgG1 plasma cells) more profoundly than BR3-Fc, although in all other B-cell subsets, the 2 therapies have similar effects.
Fc-mediated B-cell depletion and survival blockade contributed to the mechanism of anti-BR3 mAb action
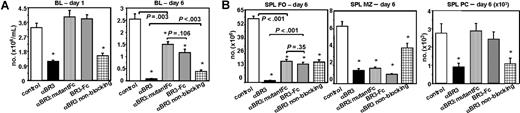
To further dissect in vivo the mechanisms by which anti-BR3 (CB2) mAb mediates B-cell reduction, we compared the wild-type mAb with a D265A/N297A anti-BR3 mutant mAb incapable of Fc receptor binding and ADCC but still capable of BAFF/BR3 blockade.51 This anti-BR3 Fc mutant molecule had the same affinity for BR3 and blocked BAFF-BR3 indistinguishably from the wild-type molecule (data not shown). We further compared the effects due to treatment with a nonblocking anti-BR3 mAb (PIH11) capable of inducing ADCC, but that does not prevent BAFF/BR3, whereas a final comparison group was treated with BR3-Fc, which blocks BAFF binding to all its receptors without any B-cell-directed ADCC. One day after therapeutic administration, both blocking and nonblocking anti-BR3 mAbs showed significant peripheral B-cell reduction, whereas the anti-BR3 mutant mAb and BR3-Fc did not (Figure 5A). These data indicate that early peripheral B-cell reduction appeared to result solely from Fc-mediated mechanisms. After 6 days of treatment, a greater degree of peripheral B-cell reduction was observed in mice treated with wild-type anti-BR3 (CB2) mAb than in all other groups. Hence, both Fc-mediated cell killing and BAFF/BR3 survival blockade function in vivo and contribute to B-cell reduction.
Anti-BR3 combines 2 mechanisms of action. BALB/c mice (5/group) were treated with the wild-type BAFF-blocking anti-BR3 mAb (CB2), anti-BR3 Fc mutant mAb (CB2-DANA), anti-BR3 nonblocking mAb (PIH11), or BR3-Fc and evaluated for B-cell reduction at days 1 and 6. In blood at day 1, only treatments that had the ability to engage Fcγ receptors and induce ADCC showed B-cell reduction, whereas at day 6, ADCC and survival blockade appear to combine for maximal effects (A). In spleen FO cells, both mechanisms appeared to contribute to B-cell reduction, whereas in MZ, the dominant mechanism appears to be BAFF/BR3-dependent survival blockade. At day 6, the spleen plasma cell compartment was reduced only by ADCC competent molecules (B). Statistical significance compared with the control-treated group is indicated with an asterisk; P values between groups are shown numerically.
Anti-BR3 combines 2 mechanisms of action. BALB/c mice (5/group) were treated with the wild-type BAFF-blocking anti-BR3 mAb (CB2), anti-BR3 Fc mutant mAb (CB2-DANA), anti-BR3 nonblocking mAb (PIH11), or BR3-Fc and evaluated for B-cell reduction at days 1 and 6. In blood at day 1, only treatments that had the ability to engage Fcγ receptors and induce ADCC showed B-cell reduction, whereas at day 6, ADCC and survival blockade appear to combine for maximal effects (A). In spleen FO cells, both mechanisms appeared to contribute to B-cell reduction, whereas in MZ, the dominant mechanism appears to be BAFF/BR3-dependent survival blockade. At day 6, the spleen plasma cell compartment was reduced only by ADCC competent molecules (B). Statistical significance compared with the control-treated group is indicated with an asterisk; P values between groups are shown numerically.
For spleen and lymph node FO B cells, the best reduction was again achieved with the blocking anti-BR3 (CB2) mAb, whereas all other groups showed lower but significant decreases in B-cell populations (Figure 5B). In contrast, the MZ compartment, which is extremely BAFF dependent for survival but does not recirculate, demonstrated comparable degrees of B-cell reduction with anti-BR3 (CB2) mAb, mutant anti-BR3 (mutant CB2), and BR3-Fc (Figure 5B). That BAFF blockade represents a major mechanism of B-cell reduction by anti-BR3 (CB2) mAb is further supported by the lesser reduction in MZ B cells by a nonblocking anti-BR3 (PIH11) mAb.
Finally, at day 6, spleen plasma cells were decreased by treatment with depleting blocking and nonblocking anti-BR3 (CB2 and PIH11) but not by mutant anti-BR3 or BR3-Fc (Figure 5B). This suggests that Fc-mediated depletion functions to decrease tissue-resident plasma cells. Because longer treatments with BR3-Fc reduce this compartment in the spleen (Figures 1C,2C) but not in bone marrow, Fc-mediated cell depletion confers additional qualitative properties distinct from BAFF survival blockade.
These data, taken together, demonstrate that a blocking anti-BR3 antibody reaches maximal efficiency to reduce B cells by combining Fc-mediated cell killing with survival blockade of the BAFF/BR3 pathway.
Discussion
In an attempt to create a therapeutic strategy combining B-cell depletion with survival factor blockade, we generated anti-BR3 mAbs with dual attributes—depletion and blockade of survival signals. In both mice and primates, these antibodies were shown to use a dual mechanism in reducing different B-cell subsets. In mice, the effect of anti-BR3 was slower than anti-CD20, faster than BR3-Fc, and ultimately resulted in a maximal B-cell reduction profile superior to anti-CD20 in particular B-cell subsets. This was evident in subsets that are relatively resistant to anti-CD20 depletion (eg, spleen marginal zone, Peyer patch germinal centers, spleen, and bone marrow plasma cells). It is important to mention that although these conclusions reflect comparisons between representative anti-BR3 and anti-CD20 mAbs, other specific antibodies against different CD20 epitopes and with additional or enhanced depleting properties could behave somewhat differently. In NHP, similar to mice, anti-BR3 mAb B-cell depletion was faster than BR3-Fc and slower than anti-CD20. However, the maximal B-cell reduction obtained after 1 month of treatment was approximately 80% in certain subsets (naive, follicular, and marginal zone) and less in others (memory, germinal center, and plasma cells). This reduction was superior to previously reported anti-BAFF reagents (anti-BAFF antibodies, BR3-Fc, TACI-Fc), showing that anti-BR3 mAb, in addition to blocking survival, is also depleting B cells in NHP. Compared with anti-CD20, which entirely depletes NHP blood and spleen B cells and incompletely depletes lymph node B cells, anti-BR3 B-cell reductions appear less in blood and spleen and comparable in lymph nodes.52 The faster kinetics of B-cell reduction of anti-CD20 is probably due to the higher density of CD20 surface expression compared with BR3 as well as capping differences between these molecules after mAb binding (data not shown). Although anti-CD20 depletion translates relatively well from rodents to primates, blockade of the BAFF/BR3 system does not. It is likely that this is due in part to a reduced dependence for BAFF survival signals of memory, germinal center B cells, and plasma cells compared with naive B cells. Because the primate immune system is populated at steady state with a much larger proportion of memory, germinal center, and plasma cells, compared with that in pathogen-free mice, primates could be expected to be less affected by BAFF blockade therapy than mice. Supporting this hypothesis, B-cell reduction after treatment with anti-BR3 mAb in lupus-prone mice, which have an activated immune system, is inferior to that seen in normal BALB/c, C57BL/6, or FvB mice. In addition, unknown primate B-cell survival characteristics could also contribute to this apparent discrepancy between species. Initial results from mice deficient in members of the BAFF/BR3-induced alternative nuclear factor κB (NF-κB) 2 pathway suggested that this pathway was indispensable for B-cell development and survival. However, more recent studies have demonstrated that the classic and alternative NF-κB pathways can replace each other in certain conditions during B-cell ontogeny.53,54 Considering the large number of cues feeding into these pathways during B-cell development (eg, BCR, BR3, CD40, TLR, interleukins), it is possible that during evolution, mice and primates developed different optimal combinations of survival and maturation signals. Further research in dissecting the developmental cues in various B-cell subsets in mice versus primates should shed light on this question.
Another aspect of therapeutical targeting relates to the B-cell subsets that are affected differentially with various strategies. Clinical and preclinical data shows that marginal zone B cells, as well as memory, germinal center, and plasma cells, are deregulated in human rheumatologic disorders. Marginal zone B cells associated with high levels of circulating BAFF have been described in both human Sjögren syndrome salivary gland infiltrates as well as in a Sjögren syndrome mouse model.28,55 Likewise, subsets of patients with memory B cells, germinal center, and plasma cells with different organization patterns in the joint have been described in patients with rheumatoid arthritis.56 Recent data from arthritis patients treated with anti-CD20 therapy show that B-cell depletion in the bone marrow and joints is incomplete, although the correlation of this finding with clinical response has not been studied yet.57,58 A more direct parallel between incomplete malignant B-cell depletion by immunotherapy and clinical relapse is better understood in NHL.5 In this light, for clinical benefit, different human diseases with B-cell deregulation could require distinct levels of B-cell reduction better achieved through an appropriate but distinct B-cell immunotherapy strategy.
Regardless of B-cell depletion level, because of the demonstrated dual mechanism of action of anti-BR3 mAbs, surviving residual B cells will be in an environment without a functional BAFF/BR3 pathway. This is important for potential therapy because both in oncology (CLL, NHL) and immunology (RA, SLE, Sjögren syndrome), increased BAFF levels signaling through its receptors contribute to pathogenic B-cell survival.59,60
BR3 was expressed at different levels on the surface of both the malignant and normal residual B cells in a series of 16 patients with CLL who varied widely with respect to CD20 expression (3/16 were negative as evaluated by flow cytometry; Figure S6). It was suggested that some of the in vitro BAFF survival effects on CLL B cells result through non-BR3 receptor (TACI) signaling and classic NF-κB activation.59 However, a functional BR3 survival pathway does exist in CLL B cells, and its in vivo inhibition could be beneficial in addition to the anti-BR3 mAb cellular depletion mechanism. Compared with normal B cells, CLL B cells have lower levels of CD20 expression, which probably contributes to the relative lower efficacy of anti-CD20 immunotherapy in CLL compared with NHL.61 Patients with negligible CD20 on CLL B cells that express significant BR3 constitute prime candidates for potential therapeutic benefit from anti-BR3 treatment. It is important to point out that our data show properties of anti-BR3 mAb largely in normal and mouse autoimmune B cells. Additional experiments will be required to evaluate this therapy in the context of malignant B cells.
Finally, reduction of BM plasma cells after anti-BR3 mAb treatment in mice parallels the synergistic effect seen with combining anti-CD20 mAb and BR3-Fc.13 If this effect translates even partially to humans, it should have therapeutic value in subsets of autoimmune patients with pathogenic long-lived autoantibody components. Future clinical trials in both oncology and immunology will test the translatability and therapeutic value of B-cell reduction through anti-BR3 mAbs in human disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the hard work and dedication of the in vivo immunology group in dosing, tracking down, and analyzing a very large number of studies, Wesley Cheng and Ronald Ferrando for immunofluorescence, and the development sciences FACS laboratory for the NHP FACS.
Authorship
Contribution: W.Y.L, Q.G., D.S., Z.L., W.P.L., K.L., S.Y., L.E.D., Q.O., S.Y., E.S., J.S., M.B., M.A.S., and F.M. performed research, collected, analyzed and interpreted data; K.H., M.L., S.I., J.B., D.D., and T.G. analyzed and interpreted data; CW.L., J.F., J.S.T., C.A., and A.E. contributed vital new reagents; T.G., M.B., M.A.S., and F.M. designed research; and FM drafted the manuscript.
Conflict-of-interest disclosure: The authors are employees of Genentech, with the exception of J.S.T. and C.A., who are employees of Biogen-IDEC.
Correspondence: Flavius Martin, Department of Immunology, Genentech, Inc, 1 DNA Way, MS34, 12-286, South San Francisco, CA 94080; e-mail: flavius@gene.com.

![Figure 1. Anti-BR3 mAb reduces mouse B-cell subsets more efficiently than BR3-Fc. BALB/c mice were treated with anti-BR3 (500 μg), BR3-Fc (150 μg × 3/week) or isotype control (500 μg); 15 days later, tissues were analyzed by flow cytometry (A,C) and immunofluorescence (B). Percentages of cells in B-cell subsets are displayed near each gate as follows: Spleen: follicular (FO) B cells (CD23+CD21+), marginal zone (MZ) B cells (CD23−CD21hi), and plasma cells (PC, CD138+B220lo); Lymph nodes: B cells (CD23+CD21+), blood (BL) B cells (CD23+CD21+), Peyer patches (PP), follicular B cells (B220+CD38+), and germinal center (GC) B cells (B220+CD38lo); peritoneal cavity (PEC): B2 cells (B220+CD23+) and B1 cells (B220+CD23−) (A,C). B cells (blue) and T cells (green) are stained in spleen sections after treatment with control, BR3-Fc or anti-BR3 mAb. Marginal zone and follicular B-cell areas are separated by the layer of metallophilic macrophages (MM [red]) (B). See “Immunofluorescence and immunohistochemistry staining” for image acquisition details. Absolute B-cell numbers or percentage of lymphocyte gate belonging to a defined subset are shown as mean plus or minus standard error. Statistical significance compared with the control treated group is indicated with asterisk (C). When 2 treatment groups are significantly different from each other, the P value is displayed. These data are representative of more than 10 independent experiments done with several maximally depleting doses of anti-BR3, using at least 5 mice/group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/12/10.1182_blood-2007-04-088088/4/m_zh80230709560001.jpeg?Expires=1767707955&Signature=xLPHG527dKeDtVyxuaQkv8EZvhv9-JYYKqyfLqf~wVugoq~bD9RDQeI8DYEVrfydMPW~nFUCsCiIdnuiAKG9qr8T6KdBj8G-OTU1Q3ftyMKocvSQfcvQvoH~z2HhdahhZXd-4m8dmwVjgWKZmHixSO2hRpuVoMlpmaNsSI8m~y1B3U1BH4XX0~hHDgXYWfOm~NT709iTUKX4YFYXB-WbzEKC3Q1cjaYHe7u79GQ06cpM86CLlBxdZlKfcHsskh9jN2ld8SRLthhRHwEdg6qJX75XsE8-aHT5f7Xs~-yvTzFMRBya422TRtGVRD5sU17XiqXxLmHBYkY9UHd2X-v7yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal