Abstract
Recent studies have indicated that activated protein C (APC) may exert its cytoprotective and anti-inflammatory activities through the endothelial protein C receptor (EPCR)-dependent cleavage of protease-activated receptor 1 (PAR-1) on vascular endothelial cells. Noting that (1) the activation of protein C on endothelial cells requires thrombin, (2) relative to APC, thrombin cleaves PAR-1 with approximately 3 to 4 orders of magnitude higher catalytic efficiency, and (3) PAR-1 is a target for the proinflammatory activity of thrombin, it is not understood how APC can elicit a protective signaling response through the cleavage of PAR-1 when thrombin is present. In this study, we demonstrate that EPCR is associated with caveolin-1 in lipid rafts of endothelial cells and that its occupancy by the γ-carboxyglutamic acid (Gla) domain of protein C/APC leads to its dissociation from caveolin-1 and recruitment of PAR-1 to a protective signaling pathway through coupling of PAR-1 to the pertussis toxin–sensitive Gi-protein. Thus, when EPCR is bound by protein C, the PAR-1 cleavage-dependent protective signaling responses in endothelial cells can be mediated by either thrombin or APC. These results provide a new paradigm for understanding how PAR-1 and EPCR participate in protective signaling events in endothelial cells.
Introduction
Activated protein C (APC) is a plasma serine protease that down-regulates thrombin generation by degrading the procoagulant cofactors Va and VIIIa by limited proteolysis.1-3 APC is generated when thrombin forms a complex with thrombomodulin on endothelial cell surface to activate the zymogen protein C.1 The anticoagulant function of APC in degradation of both cofactors is stimulated by protein S.3,4 The importance of APC in regulation of blood coagulation can be illustrated by the observation that a heterozygous protein C deficiency is associated with high risk of venous thrombosis, and its homozygous deficiency causes purpura fulminans, which is fatal unless treated by protein C replacement therapy.5 In addition to its anticoagulant role, APC also possesses anti-inflammatory properties,6-12 which have led to the FDA approval of recombinant APC as a therapeutic drug for treating severe sepsis.13 The mechanism of the anti-inflammatory function of APC is not well understood; however, it has been hypothesized that complex formation of APC with endothelial protein C receptor (EPCR) renders the protease capable of cleaving protease-activated receptor 1 (PAR-1), thereby eliciting protective signaling responses in endothelial cells.8,9,14 However, it is known that thrombin can cleave PAR-1 with at least 3 orders of magnitude higher catalytic efficiency than APC to initiate proinflammatory events in endothelial cells.14-16 Because thrombin is the only known physiologic activator of protein C, there is controversy as to whether APC can exert a protective activity through the cleavage of PAR-1 when thrombin is also present in the same environment.15
The EPCR- and PAR-1-dependent anti-inflammatory activities of APC have been extensively studied in lung endothelial cells,10 primary human umbilical vein endothelial cells (HUVECs), and transformed HUVECs (EA.hy926 cells).7,8,17 These and other previous studies have established that thrombin signaling through PAR-1 enhances permeability and initiates a proapoptotic cycle in cultured endothelial cells.10,17,18 On the other hand, the cleavage of PAR-1 by the APC-EPCR complex exerts an opposite effect, thus restoring the permeability to a baseline state and also inhibiting endothelial cell death.10,17 It is noteworthy that picomolar concentrations of thrombin exhibits, similarly to APC, a protective effect in endothelial cells, and high concentrations of APC enhances permeability, similarly to thrombin, leading to the hypothesis that the level of PAR-1 activation may dictate the type of the response, with a low-dose receptor activation by APC invoking protective and a high-dose receptor activation by thrombin invoking barrier-disruptive responses.17,19
To investigate whether the level of receptor activation by thrombin and APC determines the type of response in endothelial cells, we engineered a chimeric meizothrombin in which the γ-carboxyglutamic acid (Gla) domain of the thrombin intermediate was substituted with the corresponding domain of APC. This meizothrombin derivative retained its high specific activity toward PAR-1 and interacted with EPCR with normal affinity. We discovered that PAR-1 cleavage by this meizothrombin derivative elicits a protective response in endothelial cells, suggesting that the binding of Gla-domain of APC to EPCR determines the type of response. To investigate the mechanism of this effect, we studied the effect of PAR-1 cleavage by thrombin in endothelial cells that had been treated with the catalytically inactive Ser-195-to-Ala substitution mutant of protein C. The results revealed that when endothelial EPCR is occupied by its ligand protein C, the cleavage of PAR-1 by thrombin elicits only protective signaling responses in endothelial cells.
Materials and methods
Construction, expression, and purification of recombinant proteins
Construction and expression of protein C and its Ser-195-to-Ala (PC-S195A) (chymotrypsinogen numbering)20 variant in human embryonic kidney 293 cells has been described previously.21 The prothrombin mutant in which the protease cleavage sites Arg-155, Arg-271, and Arg-286 were eliminated by their substitutions with Ala has been described.22 Upon activation by Taipan snake venom, this mutant yields an active product named meizothrombin.23 A prothrombin chimera in the background of this mutant was constructed in which the Gla-domain was substituted with the corresponding domain of human protein C (PC-Gla/Proth/155,271,286A). The prothrombin mutant was activated by the Taipan snake venom, and the active product (PC-Gla/meizothrombin) was separated from the activator using a fast-performance liquid chromatography Mono Q (GE Health Care, Piscataway, NJ) ion exchange column as described previously.24 Protein C was activated by thrombin and APC was purified as described previously.21,24 The concentration of APC was determined by its absorbance at 280 nm and stoichiometric titration of the enzyme with known concentrations of recombinant protein C inhibitor as described previously.25 The concentration of PC-Gla/meizothrombin chimera was determined by an amidolytic activity assay and by stoichiometric titration with known concentrations of antithrombin as described previously.26
Antibodies blocking (H-111) and not blocking (S-19) the activation of PAR-1 on HUVECs,21 and the anti-PAR-2 (SAM-11) and anti-PAR-4 (H-120) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and function-blocking EPCR antibody (clone RCR-252) was purchased from Cell Sciences (Canton, MA). The antibody concentrations in the blocking assays were 25 μg/mL in all experiments. Anti-caveolin-1 antibody was purchased from BD Biosciences (San Jose, CA). Pertussis toxin (PTX), staurosporine, bacterial lipopolysaccharide (LPS), and hirudin were purchased from Sigma (St Louis, MO). Thrombin receptor agonist peptide (TRAP) for PAR-1 (H-Ser-Phe-Leu-Leu-Arg-Asn-NH2) was purchased from Bachem Bioscience (Torrance, CA).
PAR-1 cleavage assay
The cleavage rate of PAR-1 at the endothelial cell surface by different proteases was evaluated using an alkaline phosphatase-PAR-1 (ALP-PAR-1) reporter plasmid transiently transfected to EA.hy926 cells (kindly provided by Dr C. Edgell, University of North Carolina at Chapel Hill, NC) as described previously.21 Results are expressed as mean plus or minus SEM, and all experiments were repeated 3 times.
Apoptosis Assay
The protective effect of different proteases (APC, 0-100 nmol/L; thrombin and meizothrombin, 0-20 nmol/L) in EA.hy926 cells in the absence or presence of PC-S195A (0-80 nmol/L) was evaluated in a staurosporine-induced apoptosis assay as described previously.9,21 The number of apoptotic cells was expressed as the percentage of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive cells of the total number of nuclei determined by Hoechst staining as described previously.21 Results are expressed as mean plus or minus SEM, and all experiments were repeated 3 times. All APC assays were carried out in the presence of 4 units/mL hirudin.
Permeability assay
EA.hy926 cell permeability in response to thrombin (5 nmol/L for 10 minutes) after treatment with increasing concentrations of APC (0-100 nmol/L plus 4 units/mL hirudin for 3 h) or meizothrombin derivatives (0-10 nmol/L for 3 hours) was quantitated by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional EA.hy926 cell monolayers using a modified 2-compartment chamber model as described previously.17,21 For the function-blocking antibody treatments of the monolayers, medium was removed and antibodies were added for 30 minutes in serum-free medium followed by analysis of the permeability as described previously.21 Results are expressed as mean plus or minus SEM, and all experiments were repeated at least 3 times.
The same assay was used to evaluate the effect of thrombin (0-10 nmol/L for 3 hours) or TRAP (0-2 mmol/L for 3 hours) on permeability of EA.hy926 cells pretreated with PC-S195A (0-80 nmol/L) for 15 minutes. The cell permeability assays were also carried out in presence of PTX. In this case, endothelial cells were pretreated with the toxin (100 ng/mL) for 16 hours before their incubation with PAR-1 agonists. EA.hy926 cell permeability in response to LPS was carried out using the same methods except that instead of 5 nmol/L thrombin for 10 minutes, LPS (10 ng/mL) for 4 hours was used to induce permeability.
RNA interference
EA.hy926 cell permeability in response to thrombin (0-10 nmol/L) or APC (0-100 nmol/L) was evaluated after the knockdown of PAR-4 expression by a pool of 3 target-specific 20- to 25-nucleotide siRNAs (Santa Cruz Biotechnology) according to the manufacturer's instruction. A nontargeting 20- to 25-nucleotide siRNA obtained from the same company was used as a negative control.
Immunoprecipitation, SDS-PAGE, and Western blotting
Total cellular proteins were extracted by sonication with PBS containing 25 μmol/L proteosome inhibitor MG132 (Sigma-Aldrich) and complete protease inhibitor cocktail (Roche Molecular Systems, Summerville, NJ) as described.19 Lysates were combined with 3 μg of each specific antibody (H-111 for PAR-1, SAM-11 for PAR-2, and H-120 for PAR-4), and incubated for 2 hours at 4°C. Immunoprecipitates were collected with protein A/G Agarose (Santa Cruz, CA), fractionated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE), transferred to membranes, and subjected to Western blotting with appropriate primary and horseradish peroxidase-conjugated secondary antibodies as described previously.21 The immunoreactive protein bands were visualized by SuperSignal West Pico (Pierce, Rockford, IL).
Isolation of lipid rafts/caveolae
Lipid rafts/caveolae proteins were isolated by slight modification of procedures as described previously.27 In brief, confluent cells in 150-mm Petri dishes were washed with ice-cold phosphate-buffered saline and scraped into Triton X-100 containing Tris lysis buffer (25 mmol/L Tris-HCl, 250 mmol/L sucrose, 2 mmol/L EDTA (ethylenediaminetetraacetic acid), and 1% Triton X-100). Cell pellets were then homogenized with a tight-fitting Dounce homogenizer followed by 3 20-second bursts of an ultrasonic sonicator on ice. The lysate was adjusted to 45% sucrose by the addition of an equal volume of 90% sucrose in 25 mmol/L MES (2-[N-morpholino]ethanesulfonic acid)-buffered saline, pH 6.5, and placed at the bottom of an ultracentrifuge tube. Two solutions (1.7 mL each) of 35% and 5% sucrose were laid sequentially on the top of the 45% sucrose solution. After ultracentrifugation at 150 000g with a SW Ti55 rotor (Beckman Coulter, Fullerton, CA) for 16 to 20 hours, 10 0.5-mL fractions were collected from the top of tubes, and a portion of each fraction was analyzed by SDS-PAGE, transferred to nitrocellulose membranes, and subjected to Western blotting with appropriate primary and secondary antibodies.
Interaction with EPCR
The affinity of PC-Gla/meizothrombin for interaction with EPCR was evaluated by an enzyme-linked immunosorbent assay (ELISA)-based binding assay using HPC4-tagged recombinant soluble EPCR (kindly provided by Dr Charles Esmon, Oklahoma Medical Research Foundation, Oklahoma City, OK) as described previously.21
Results
PC-Gla/meizothrombin interaction with EPCR and cleavage of PAR-1
PC-Gla/meizothrombin interacted with soluble EPCR with a normal affinity (Figure 1A), exhibiting a Kd(app) of approximately 35 plus or minus 1 nmol/L that is similar to the value obtained for the interaction of APC with the receptor (Kd(app) = 34 ± 1 nmol/L). APC cleaved the Arg-41 scissile bond of PAR-1 with approximately 3 orders of magnitude lower catalytic efficiency than thrombin as determined by an alkaline phosphatase activity assay using a PAR-1 cleavage reporter plasmid transfected to EA.hy926 cells (Figure 1B). Both meizothrombin and PC-Gla/meizothrombin cleaved PAR-1 with an efficiency that was only about 20% lower than the cleavage rate by thrombin, suggesting that the exosite-1 of meizothrombin derivatives can interact with the hirudin-like sequence of PAR-1 to effectively catalyze the cleavage of the Arg-41 peptide bond on the PAR-1 exodomain (Figure 1B).
Binding of protein C and PC-Gla/meizothrombin to soluble EPCR and the cleavage of PAR-1 exodomain by the proteases. (A) An ELISA-based assay using the monoclonal antibody HPC4 (1 μg/mL) was used to measure the affinity of either protein C (○) or PC-Gla/meizothrombin (■) for interaction with soluble EPCR (0.5 μmol/L). (B) EA.hy926 cells were transiently transfected with ALP-PAR-1 reporter plasmid and incubated with increasing concentrations of thrombin (○), meizothrombin (□), PC-Gla/meizothrombin (■) and APC (▵) for 1 hour. The activity of soluble alkaline phosphatase (ALP) was measured as described under “PAR-1 cleavage assay.” The activity of 10 nmol/L thrombin is presented as 100%. OD, optical density. Error bars represent SD.
Binding of protein C and PC-Gla/meizothrombin to soluble EPCR and the cleavage of PAR-1 exodomain by the proteases. (A) An ELISA-based assay using the monoclonal antibody HPC4 (1 μg/mL) was used to measure the affinity of either protein C (○) or PC-Gla/meizothrombin (■) for interaction with soluble EPCR (0.5 μmol/L). (B) EA.hy926 cells were transiently transfected with ALP-PAR-1 reporter plasmid and incubated with increasing concentrations of thrombin (○), meizothrombin (□), PC-Gla/meizothrombin (■) and APC (▵) for 1 hour. The activity of soluble alkaline phosphatase (ALP) was measured as described under “PAR-1 cleavage assay.” The activity of 10 nmol/L thrombin is presented as 100%. OD, optical density. Error bars represent SD.
Barrier protective effect of PC-Gla/meizothrombin in endothelial cells
Previous studies have indicated that both thrombin17 and tumor necrosis factor-α19 disrupt the permeability barrier of EA.hy926 cells and that APC exerts a potent protective effect. Paradoxically, both the permeability-enhancing effect of thrombin and the barrier-protective effect of APC require the cleavage of PAR-1 by these proteases.17,19 However, the protective effect of APC requires its interaction with EPCR.9,17 This assay was used to evaluate the nature of the signaling effect of both meizothrombin, which cannot interact with EPCR, and PC-Gla/meizothrombin, which exhibits a normal affinity for the receptor. In agreement with previous results, treatment of EA.hy926 cells with thrombin resulted in a partial protective effect at approximately 50 pmol/L thrombin,17 but an enhanced permeability effect above 50 pmol/L that was effectively reversed by APC in a concentration-dependent manner, reaching saturation at 10 nmol/L APC (Figure 2A). Meizothrombin exhibited an effect that was essentially identical to that observed with thrombin (Figure 2A). On the other hand, PC-Gla/meizothrombin functioned like APC except that its barrier protective effect was observed at much lower concentrations of the protease (0.5-2 nmol/L). Like an enhanced permeability induced by the higher concentrations of APC,17,19 PC-Gla/meizothrombin also exhibited a similar effect when its concentrations exceeded 2 nmol/L (Figure 2A). Function-blocking antibodies to either EPCR or PAR-1 eliminated the barrier protective effects of both APC and PC-Gla/meizothrombin, confirming that the EPCR-dependent cleavage of PAR-1 mediates the cellular effects of both proteases (data not shown).
The permeability barrier enhancing and proapoptotic effects of thrombin are reversed by the ligand occupancy of EPCR. (A) EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin + anti-PAR-1 antibody (●), meizothrombin (□), PC-Gla/meizothrombin (■), thrombin + PC-S195A (50 nmol/L) (▴), and APC (▵) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. (B) The same as (A) except that after incubation of endothelial cells with increasing concentrations of thrombin (○), thrombin + PC-S195A (▴), and APC (▵), the permeability was induced with LPS (10 ng/mL) for 4 hour. (C) EA.hy926 cells were treated with increasing concentrations of thrombin (○), thrombin + PC-S195A (▴), and APC (▵) for 24 hours, followed by induction of apoptosis with staurosporine (5 μmol/L) for 4 hours. The number of apoptotic cells is expressed as the percentage of TUNEL-positive cells of the total number of nuclei. The number of TUNEL-positive cells in the absence of staurosporine was 10%-15%. (D) The same as (A) except that the concentration dependence of the protective effect of PC-S195A in the presence of 2 nmol/L thrombin (○) is measured in the permeability assay. The extent of the protective effect of 10 nmol/L APC (▵) is also presented. OD, optical density. Error bars represent SD.
The permeability barrier enhancing and proapoptotic effects of thrombin are reversed by the ligand occupancy of EPCR. (A) EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin + anti-PAR-1 antibody (●), meizothrombin (□), PC-Gla/meizothrombin (■), thrombin + PC-S195A (50 nmol/L) (▴), and APC (▵) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. (B) The same as (A) except that after incubation of endothelial cells with increasing concentrations of thrombin (○), thrombin + PC-S195A (▴), and APC (▵), the permeability was induced with LPS (10 ng/mL) for 4 hour. (C) EA.hy926 cells were treated with increasing concentrations of thrombin (○), thrombin + PC-S195A (▴), and APC (▵) for 24 hours, followed by induction of apoptosis with staurosporine (5 μmol/L) for 4 hours. The number of apoptotic cells is expressed as the percentage of TUNEL-positive cells of the total number of nuclei. The number of TUNEL-positive cells in the absence of staurosporine was 10%-15%. (D) The same as (A) except that the concentration dependence of the protective effect of PC-S195A in the presence of 2 nmol/L thrombin (○) is measured in the permeability assay. The extent of the protective effect of 10 nmol/L APC (▵) is also presented. OD, optical density. Error bars represent SD.
Based on these results, we hypothesized that the occupancy of EPCR by its ligand alters the specificity of the PAR-1 signaling pathway in endothelial cells. To test this hypothesis, we evaluated the effect of thrombin in the presence of PC-S195A, which can interact with normal affinity with EPCR, but cannot cleave PAR-1 to elicit a cellular response.9,21 It is noteworthy that thrombin in the presence of the mutant zymogen elicited a protective response identical to that observed with PC-Gla/meizothrombin (Figure 2A); a similar level of protective effect for thrombin in the presence of PC-S195A was observed if LPS was used to induce hyperpermeability, suggesting that when EPCR is ligated, thrombin plays a protective role in response to other proinflammatory stimuli (Figure 2B).
Activity of APC and thrombin in the apoptosis assay
Thrombin, proinflammatory cytokines, and the kinase inhibitor staurosporine are known to be able to induce apoptosis in endothelial cells that can be reversed by APC by an EPCR and PAR-1-dependent mechanism.7,9,19 As presented in Figure 2C, the treatment of EA.hy926 cells with staurosporine induced apoptosis, and APC inhibited cell death in a concentration-dependent manner. As with the permeability assay, thrombin exhibited a partial cytoprotective effect at low picomolar concentrations, but in the presence of PC-S195A, the signaling effect by thrombin was cytoprotective for up to 2 nmol/L protease (Figure 2C). These results support the hypothesis that the ligand occupancy of EPCR recruits PAR-1 to a cytoprotective pathway in endothelial cells.
The concentration dependence of the protective effect of PC-S195A was studied by a permeability assay. As presented in Figure 2D, the protective effect of thrombin reached saturation at 10 nmol/L mutant zymogen. These results clearly suggest that the occupancy of EPCR by its ligand determines the specificity of PAR-1 signaling and not the type of the protease that is cleaving PAR-1.
Protective effect of thrombin in the presence of PC-S195A can be mimicked by TRAP
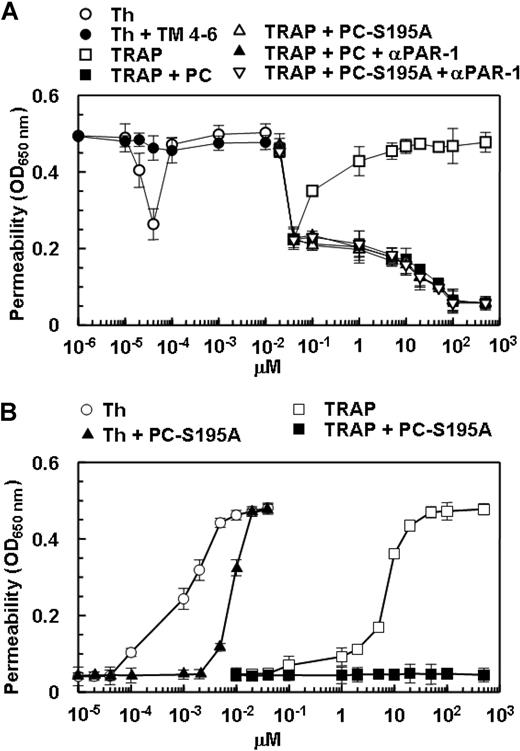
The activation of PAR-1 by the TRAP peptide is known to mimic the proinflammatory effect of thrombin in HUVECs.17,28 As presented in Figure 3A, TRAP enhanced the permeability barrier in endothelial cells. As with the protective effect of thrombin at picomolar concentrations, TRAP also elicited a similar response at relatively low concentrations (Figure 3A). As observed with thrombin, the treatment of endothelial cells with either protein C or PC-S195A switched the specificity of signaling by TRAP from a permeability-enhancing to a barrier-protective response, confirming the conclusion that when EPCR is occupied, PAR-1 activation elicits a protective response in endothelial cells (Figure 3A). To ensure that the protective effects of low picomolar concentrations of thrombin are mediated through activation of PAR-1, the permeability assay was also carried out after treatment of endothelial cells with thrombin in complex with a saturating concentration of TM456 (100 nmol/L), which blocks the exosite-1-dependent interaction of thrombin with PAR-1. The barrier-protective effect of low picomolar concentrations of thrombin was abolished in the presence of TM456 (Figure 3A). The same results were obtained if endothelial cells were incubated with the function-blocking anti-PAR-1 antibody, indicating that the protective effect of very low concentrations of thrombin is mediated through the activation of PAR-1 (Figure 3A).
The barrier-enhancing effect of TRAP and its reversal by the ligand occupancy of EPCR. (A) EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin + thrombomodulin fragments 456 (TM456) (●), TRAP (□), TRAP + wild-type protein C (■), TRAP + PC-S195A (▵), TRAP + wild-type protein C + blocking anti-PAR-1 (H-111) (▴), and TRAP + PC-S195A + anti-PAR-1 (H-111) (▿) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. (B) The same as (A) except that the permeability was directly monitored as a function increasing concentrations of thrombin (○), thrombin + PC-S195A (▴), TRAP (□), and TRAP + PC-S195A (■) by incubation of EA.hy926 cells with different agonists for 3 hours (the 5 nmol/L thrombin step for inducing permeability has been omitted in this assay). The first data point on the y-axis represents the baseline permeability level. OD, optical density. Error bars represent SD.
The barrier-enhancing effect of TRAP and its reversal by the ligand occupancy of EPCR. (A) EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin + thrombomodulin fragments 456 (TM456) (●), TRAP (□), TRAP + wild-type protein C (■), TRAP + PC-S195A (▵), TRAP + wild-type protein C + blocking anti-PAR-1 (H-111) (▴), and TRAP + PC-S195A + anti-PAR-1 (H-111) (▿) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. (B) The same as (A) except that the permeability was directly monitored as a function increasing concentrations of thrombin (○), thrombin + PC-S195A (▴), TRAP (□), and TRAP + PC-S195A (■) by incubation of EA.hy926 cells with different agonists for 3 hours (the 5 nmol/L thrombin step for inducing permeability has been omitted in this assay). The first data point on the y-axis represents the baseline permeability level. OD, optical density. Error bars represent SD.
In all permeability assays described above, endothelial cells were initially treated with different PAR-1 agonists for 3 hours before inducing permeability with 5 nmol/L thrombin. In a variation of this assay, we directly measured the effect of thrombin and TRAP on cell permeability in the absence and presence of PC-S195A after 3-hour incubation of cells with different concentrations of each agonist, omitting the 5 nmol/L thrombin treatment step. As presented in Figure 3B, both thrombin and TRAP dramatically enhanced the permeability barrier in a concentration-dependent manner with the maximal effects reaching saturation at 2 nmol/L thrombin and approximately 20 μmol/L TRAP. Similar to results presented above, PC-S195A switched the signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response for up to 2 nmol/L thrombin, whereas the zymogen mutant switched the effect of TRAP at both low and high concentrations so that it could no longer elicit a permeability-enhancing response (Figure 3B). These results strongly suggested that 2 nmol/L thrombin is sufficient to cleave all available cell surface PAR-1 in endothelial cells and that PAR-1 signaling is protective in HUVECs if EPCR is occupied by its ligand. This hypothesis is derived from the observation that, unlike the high concentration of thrombin, a high concentration of TRAP in the presence the zymogen mutant did not exhibit a hyperpermeability effect (Figure 3B). These results raised the possibility that a high concentration of thrombin in the presence of the mutant zymogen elicits a barrier disruptive response through the cleavage of other candidate PARs present at the surface of endothelial cells.
The permeability-enhancing effect of higher concentrations of both thrombin and APC are mediated via the cleavage of PAR-4
In addition to PAR-1, HUVECs are also known to express PAR-2 and PAR-4.29 To determine whether the permeability-enhancing effect of a higher concentration of thrombin is mediated through activation of PAR-2, we conducted the permeability assay for thrombin and TRAP (Figure 3B) in the presence of a function-blocking anti-PAR-2 (SAM-11) antibody. The ability of SAM-11 antibody to block PAR-2 signaling was first confirmed by its ability to block the PAR-2-dependent barrier protection in endothelial cells mediated by factor Xa, as reported previously (data not shown).30 The results (not shown) were essentially identical to those presented in Figure 3B, suggesting that the permeability-enhancing effect of higher than 2 nmol/L thrombin in the presence of PC-S195A is not mediated through the activation of PAR-2. Because we did not know whether the commercial anti-PAR-4 antibody is a function-blocking antibody, we decided to use the siRNA approach to investigate the contribution of the PAR-4 cleavage to the hyperpermeability effect of high concentrations of either thrombin in the presence of PC-S195A or APC in endothelial cells. The knockdown of PAR-4 in endothelial cells by siRNA was very efficient with no detectable expression of the receptor (Figure 4A). It is noteworthy that although the control siRNA had no effect on the cellular responses elicited by thrombin in the absence and presence of PC-S195A, the specific PAR-4 siRNA eliminated the hyperpermeability effect induced by a high concentration of thrombin in the presence of the mutant zymogen, suggesting that the permeability-enhancing effect of higher than 2 nmol/L thrombin is mediated through the cleavage of PAR-4 (Figure 4B). The observation that thrombin in the presence of PAR-4 siRNA (in the absence of PC-S195A) enhanced the permeability of endothelial cells implies that the thrombin cleavage of PAR-1 on these cells is proinflammatory unless EPCR has been occupied by its ligand. The hyperpermeability effect of a high and nonphysiologic concentration of APC was also found to be mediated through the cleavage of PAR-4, because siRNA also inhibited this response (Figure 4C). Similar studies indicated that the loss of antiapoptotic activity observed at thrombin concentrations of above 2 nmol/L (Figure 2B) was also due to the thrombin activation of PAR-4 (data not shown). The PAR-4-dependent thrombin enhancement of cell permeability does not require protein C because thrombin can induce similar hyperpermeability in the presence of function-blocking anti-PAR-1 antibody in the absence of protein C (Figure 2A, filled circles).
The effect of thrombin on the permeability of endothelial cells in the presence of protein C S195A and siRNA for PAR-4. (A) EA.hy926 cells were transiently transfected with siRNA for PAR-4 or control siRNA (1 μg each). After 3 days of incubation at 37°C, total cellular proteins were extracted, separated on 10% SDS-PAGE, and Western-blotted with antibodies directed to either PAR-4 or PAR-1. An antibody against actin was used as internal control. (B) EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin with prior treatment of cells for 3 days with control siRNA (●), thrombin with prior treatment of cells with siRNA for PAR-4 (□), thrombin + PC-S195A (■), thrombin + PC-S195A with prior treatment of cells with control siRNA (▵), and thrombin + PC-S195A with prior treatment of cells with siRNA for PAR-4 (▴) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. (C) The same as (B) except that the barrier-protective effect of APC was monitored in the absence (▵) and presence of control siRNA (●) or siRNA for PAR-4 (□). (D) The barrier-protective effect of thrombin + PC-S195A in endothelial cells is sensitive to PTX. EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin with prior treatment of cells with 100 ng/mL PTX for 16 hours (●), APC (□), APC with prior treatment of cells with 100 ng/mL PTX for 16 hours (■), thrombin + PC-S195A (▵), and thrombin + PC-S195A with prior treatment of cells with PTX (▴) for 3 hours before inducing permeability with 5 nmol/L thrombin. Error bars represent SD.
The effect of thrombin on the permeability of endothelial cells in the presence of protein C S195A and siRNA for PAR-4. (A) EA.hy926 cells were transiently transfected with siRNA for PAR-4 or control siRNA (1 μg each). After 3 days of incubation at 37°C, total cellular proteins were extracted, separated on 10% SDS-PAGE, and Western-blotted with antibodies directed to either PAR-4 or PAR-1. An antibody against actin was used as internal control. (B) EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin with prior treatment of cells for 3 days with control siRNA (●), thrombin with prior treatment of cells with siRNA for PAR-4 (□), thrombin + PC-S195A (■), thrombin + PC-S195A with prior treatment of cells with control siRNA (▵), and thrombin + PC-S195A with prior treatment of cells with siRNA for PAR-4 (▴) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. (C) The same as (B) except that the barrier-protective effect of APC was monitored in the absence (▵) and presence of control siRNA (●) or siRNA for PAR-4 (□). (D) The barrier-protective effect of thrombin + PC-S195A in endothelial cells is sensitive to PTX. EA.hy926 cells were incubated with increasing concentrations of thrombin (○), thrombin with prior treatment of cells with 100 ng/mL PTX for 16 hours (●), APC (□), APC with prior treatment of cells with 100 ng/mL PTX for 16 hours (■), thrombin + PC-S195A (▵), and thrombin + PC-S195A with prior treatment of cells with PTX (▴) for 3 hours before inducing permeability with 5 nmol/L thrombin. Error bars represent SD.
The ligand occupancy of EPCR couples PAR-1 to Gi protein
PAR-1 can signal via coupling to different G proteins.18,31,32 It is known that the signaling function of Gi is sensitive to PTX.31 To understand the mechanism by which EPCR switches the signaling specificity of thrombin, we investigated the cellular effect of thrombin in the absence and presence of PC-S195A in endothelial cells pretreated with PTX. We found that the permeability-enhancing effect of thrombin is mediated via coupling of PAR-1 to a non-Gi protein, because PTX did not influence the response of endothelial cells to thrombin (Figure 4D). On the other hand, the protective effect of APC was totally abolished by PTX, suggesting that the EPCR-bound APC signals via PAR-1 coupled to Gi protein (Figure 4D). It is noteworthy that, similar to APC, the protective signaling by thrombin was PTX-sensitive in the presence of PC-S195A (Figure 4D), clearly suggesting that the occupancy of EPCR by its ligand couples PAR-1 to Gi protein, thereby altering the signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response.
EPCR is associated with caveolin-1 in lipid rafts
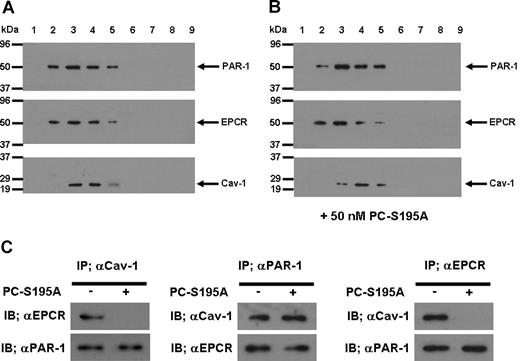
To understand the mechanism by which the occupancy of EPCR by its ligand recruits PAR-1 to a protective pathway, we isolated lipid rafts of non-treated and PC-S195A treated endothelial cells and analyzed the protein contents of different low and high buoyancy fractions by immunoblotting, using antibodies against EPCR, PAR-1, and caveolin-1. As presented in Figure 5, all 3 proteins were found to be colocalized within the cholesterol rich lipid rafts/caveolae membrane microdomains of endothelial cells in both the absence (Figure 5A) and presence of PC-S195A (Figure 5B). To explore a possible association between these molecules, we next immunoprecipitated the total protein extract of endothelial cells with anti-caveolin-1, anti-PAR-1, or anti-EPCR antibodies and subjected them to a 10% reducing SDS-PAGE. Western blot analyses of immunoprecipitates indicated that the antibody specific for either protein coimmunoprecipitates the other 2 proteins from the extract derived from nontreated endothelial cells (Figure 5C), suggesting that they are located in the same membrane microdomains. Immunoblotting of membrane lipid rafts derived from primary HUVECs using the same anti-PAR-1 and anti-EPCR antibodies yielded identical results (data not shown). It is noteworthy that when endothelial cells were treated with PC-S195A, whereas both EPCR and PAR-1 pairs and caveolin-1 and PAR-1 pairs remained localized to the same microdomains, EPCR and caveolin-1 were not coimmunoprecipitated by the antibody pairs (Figure 5C), suggesting that the occupancy of EPCR with its ligand leads to its dissociation and/or its migration out of the caveolar compartment, a process that appears to couple PAR-1 to Gi protein, thereby changing the specificity of the PAR-1 signaling from a permeability-enhancing to a barrier-protective response independent of the protease cleaving the receptor. Thus, thrombin cleavage of PAR-1 in endothelial cells would be protective as long as they expressed EPCR and there was sufficient ligand (protein C) to occupy the receptor.
SDS-PAGE, immunoblotting, and coimmunoprecipitation of lipid raft/caveolae preparations derived from EA.hy926 cells with and without treatment with PC-S195A. (A) After SDS-PAGE of membrane fractions prepared by discontinuous sucrose gradient ultracentrifugation, they were immunoblotted with antibodies directed to PAR-1, EPCR, and caveolin-1. (B) The same as (A) except that the fractions were derived from EA.hy926 cells treated with 50 nmol/L PC-S195A. (C) Total cellular proteins from nontreated and PC-S195A-treated EA.hy926 cells were immunoprecipitated with anti-caveolin-1, anti-PAR-1, and anti-EPCR antibodies, separated on SDS-PAGE and immunoblotted with different pairs of the same antibodies.
SDS-PAGE, immunoblotting, and coimmunoprecipitation of lipid raft/caveolae preparations derived from EA.hy926 cells with and without treatment with PC-S195A. (A) After SDS-PAGE of membrane fractions prepared by discontinuous sucrose gradient ultracentrifugation, they were immunoblotted with antibodies directed to PAR-1, EPCR, and caveolin-1. (B) The same as (A) except that the fractions were derived from EA.hy926 cells treated with 50 nmol/L PC-S195A. (C) Total cellular proteins from nontreated and PC-S195A-treated EA.hy926 cells were immunoprecipitated with anti-caveolin-1, anti-PAR-1, and anti-EPCR antibodies, separated on SDS-PAGE and immunoblotted with different pairs of the same antibodies.
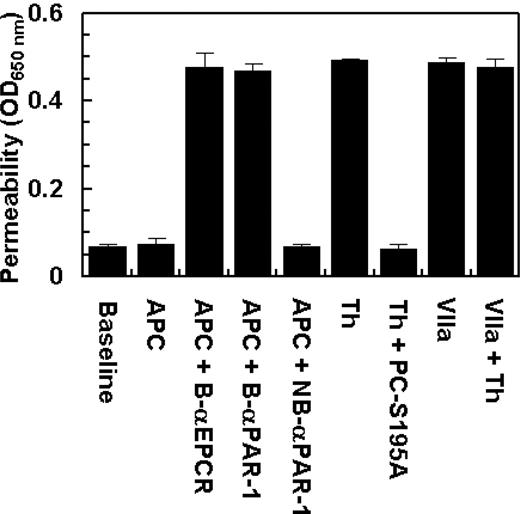
Two recent studies have demonstrated that EPCR also acts as a cellular receptor for factor VIIa on endothelium, exhibiting an affinity comparable with that of APC/protein C.33,34 However, factor VIIa was unable to recruit PAR-1 to a protective pathway because, unlike protein C, the treatment of endothelial cells with factor VIIa failed to prevent the enhanced permeability induced by thrombin (Figure 6). These results suggest that the protective effect of the EPCR occupancy is specific for APC/protein C in endothelial cells. The data in Figure 6 also show that the prior incubation of endothelial cells with blocking anti-EPCR and anti-PAR-1 antibodies, but not with the nonblocking anti-PAR-1 antibody, eliminate the protective activity of APC.
The permeability barrier-enhancing effect of thrombin is reversed by the ligand occupancy of EPCR with PC-S195A, but not with factor VIIa. EA.hy926 cells were incubated with APC (10 nmol/L) and thrombin (2 nmol/L) in the absence and presence of either PC-S195A or factor VIIa (50 nmol/L) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. B-αEPCR is blocking anti-EPCR (clone RCR-252), B-αPAR-1 (H-111) is blocking anti-PAR-1, and NB-αPAR-1 (S-19) is nonblocking anti-PAR-1, which have been incubated with endothelial cells for 30 minutes before treatment with APC. Baseline represents the permeability of untreated control cells not stimulated with thrombin. Error bars represent SD.
The permeability barrier-enhancing effect of thrombin is reversed by the ligand occupancy of EPCR with PC-S195A, but not with factor VIIa. EA.hy926 cells were incubated with APC (10 nmol/L) and thrombin (2 nmol/L) in the absence and presence of either PC-S195A or factor VIIa (50 nmol/L) for 3 hours before inducing permeability with 5 nmol/L thrombin for 10 minutes. B-αEPCR is blocking anti-EPCR (clone RCR-252), B-αPAR-1 (H-111) is blocking anti-PAR-1, and NB-αPAR-1 (S-19) is nonblocking anti-PAR-1, which have been incubated with endothelial cells for 30 minutes before treatment with APC. Baseline represents the permeability of untreated control cells not stimulated with thrombin. Error bars represent SD.
Discussion
We have demonstrated here that the occupancy of EPCR by protein C switches the specificity of PAR-1-dependent signaling by thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Further support for this proposal was provided by the observation that the thrombin agonist peptide TRAP also increased the permeability barrier in endothelial cells, but PC-S195A reversed the hyperpermeability effect of PAR-1 signaling by TRAP, clearly indicating that the occupancy of EPCR recruits PAR-1 to a protective pathway. The observation that PC-Gla/meizothrombin and thrombin plus PC-S195A both elicited a protective response in both apoptosis and permeability assays is also in line with the overall conclusion of this study.
The protective signaling activity of thrombin in the presence of PC-S195A was dependent on the concentration of the protease, exhibiting an optimal effect at approximately 2 nmol/L thrombin and thereafter decreasing and reversing to a barrier-disruptive effect at 10 nmol/L thrombin. To determine whether this biphasic effect of thrombin is mediated through the cleavage of PAR-1, we incubated endothelial cells with 2 nmol/L thrombin in the presence of PC-S195A for 10 minutes and then treated cells with the function-blocking anti-PAR-1 antibody followed by incubation of cells with 10 nmol/L thrombin. However, blocking PAR-1 did not block the enhanced permeability in endothelial cells, suggesting that the hyperpermeability effect of the high concentration of thrombin may not be mediated via the cleavage of PAR-1 (data were not presented). Further support for this hypothesis was provided by the observation that the hyperpermeability effect of TRAP in the absence of PC-S195A and its protective effect in the presence of the mutant zymogen were both monophasic, reaching saturation at approximately 10 μmol/L TRAP. Thus, we hypothesize that a higher concentration of thrombin may elicit a barrier-disruptive response in endothelial cells via the activation of other PARs. In addition to PAR-1, HUVECs are known to express PAR-2 and PAR-4.29 Thrombin is not known to cleave PAR-2, and the function-blocking anti-PAR-2 antibody further ruled out a role for the thrombin cleavage of this receptor in endothelial cells. However, the knockdown of PAR-4 expression by siRNA eliminated the hyperpermeability effect of the high concentration of thrombin in the presence of PC-S195A, suggesting that when EPCR is occupied by its ligand, the thrombin cleavage of PAR-1 elicits only a protective response. A similar enhanced permeability in HUVECs has been observed in response to supraphysiologic concentrations of APC (above 100 nmol/L).17 The siRNA knockdown of PAR-4 also eliminated the hyperpermeability effect of a high concentration of APC, suggesting that this effect is also mediated though the cleavage of PAR-4 in cultured endothelial cells.
PAR-1, as a 7-transmembrane G protein coupled receptor, can signal through interaction with different members of the G protein subfamilies, including Gi, Gq, and G12/13.18,32 It has been hypothesized that thrombin disrupts endothelial barrier function through PAR-1 coupled to Gq and/or G12/13,35 but APC may signal through the cleavage of PAR-1 coupled to Gi.18 Among these G proteins, only the signaling function of Gi is sensitive to PTX.31 The observation that the PAR-1-dependent permeability-enhancing effect of thrombin was insensitive to PTX but the protective response of APC and thrombin plus PC-S195A were both sensitive to PTX suggested that the ligand occupancy of EPCR recruits PAR-1 to a protective pathway by coupling the receptor to Gi protein in the lipid rafts of endothelial cells. Thus, thrombin cleavage of PAR-1 on vascular endothelial cells would be protective as long as they express EPCR and there is sufficient amount of ligand to occupy the receptor. The mechanism by which protein C binding to EPCR changes the G protein coupling specificity of PAR-1 is not known. However, EPCR seems to be associated with caveolin-1, and the occupancy of EPCR by its ligand seems to result in dissociation and/or migration of EPCR out of the caveolar compartment. The EPCR dissociation from caveolae somehow couples PAR-1 to Gi protein, thereby switching the specificity of the receptor signaling by thrombin from a barrier-disruptive to a barrier-protective pathway. The EPCR-dependent switch in the specificity of PAR-1 signaling was unique for protein C and not recapitulated by factor VIIa, although factor VIIa can bind to EPCR with a high affinity.33,34 The basis for this observation is not clear, but the 32 Ω-loop residues of the Gla-domain of protein C, which are critical for interaction with EPCR, are known to be entirely conserved in factor VIIa, thereby accounting for the similar affinity of both proteases for the receptor.33 Interactions outside of the Ω-loop, which must be specific for protein C, seem to be required for the EPCR-dependent protective signaling. The findings of the current study are consistent with the report that factor VIIa-bound EPCR does not elicit cell signaling by activation of PARs.34
APC has been approved by the FDA as a drug for treating severe sepsis.13 Whether the beneficial effect of APC is mediated through the activation of PAR-1 is not settled.36,37 An EPCR and PAR-1 dependent protective activity for APC has been observed in mouse models of ischemic stroke,12,38 supporting the hypothesis that the beneficial effect of APC in severe sepsis may also be mediated through the same mechanism.37 However, other investigators disagree with this hypothesis, arguing that PAR-1 activation by APC cannot contribute to its beneficial effect in severe sepsis.15,36 The controversy stems primarily from the observation that thrombin exhibits a proinflammatory effect in HUVECs, and has 3 to 4 orders of magnitude higher catalytic efficiency than APC toward PAR-1.15 Thus, it has been justifiably contended that thrombin would cleave all available cell surface PAR-1, leaving no opportunity for APC to function through the cleavage of the same receptor.15,36 Our observation that EPCR recruits PAR-1 to a protective signaling pathway in endothelial cells supports this contention and explains the basis for the paradoxical effects of PAR-1 cleavage by either thrombin or APC, as observed in the cultured endothelial cells. Our data suggest that, in endothelial cells expressing EPCR, thrombin would also invoke an anti-inflammatory response when protein C is present. Thus, the EPCR occupancy by protein C is the missing link in the cross-talk between the 2 receptors that has hindered a better understanding of the PAR-1 cleavage-dependent signaling by thrombin and APC in cultured endothelial cells. Assuming that the results obtained in HUVECs are relevant to endothelial cells in vivo, for which there is some evidence,39 thrombin would primarily be responsible for the cleavage of the EPCR-dependent Gi-protein-coupled PAR-1 in intact endothelial cells. This is in contrast to the traditional view that thrombin elicits inflammatory responses in endothelial cells such as in pulmonary edema. Noting that the protein C concentration in plasma is high enough to yield at least 50% occupancy of EPCR on the endothelial cells, the findings of this study predict that thrombin would play a protective role through the EPCR-dependent activation of PAR-1. These findings further suggest that a complete inhibition of thrombin activity by anticoagulants during an inflammatory disorder such as sepsis may adversely affect the course of the disease. The down-regulation of EPCR expression of endothelial cells by endotoxin and proinflammatory cytokines in sepsis may also abolish the protective role of thrombin. Hence, the type of proinflammatory properties that have been attributed to thrombin in cultured vein endothelial cells may not reflect the true function of the protease under physiologic conditions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Audrey Rezaie for proofreading the manuscript.
This work was supported by National Heart, Lung, and Blood Institute, National Institutes of Health grants HL68571 and HL62565 (to A.R.R.).
National Institutes of Health
Authorship
Contribution: J.-S.B. performed research, L.Y. constructed the mutant constructs, C.M. purified recombinant proteins, and A.R.R. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alireza R. Rezaie, Ph.D., Department of Biochemistry and Molecular Biology, St. Louis University School of Medicine, 1402 S. Grand Blvd., St. Louis, MO 63104; e-mail: rezaiear@slu.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal