Abstract
A majority of tissue factor (TF) on cell surfaces exists in a cryptic form (ie, coagulation function inactive) but retains its functionality in cell signaling. Recent studies have suggested that cryptic TF contains unpaired cysteine thiols and that activation involves the formation of the disulfide bond Cys186-Cys 209 and that protein disulfide isomerase (PDI) regulates TF coagulant and signaling activities by targeting this disulfide bond. This study was carried out to investigate the validity of this novel concept. Although treatment of MDA 231 tumor cells, fibroblasts, and stimulated endothelial cells with the oxidizing agent HgCl2 markedly increased the cell-surface TF coagulant activity, the increase is associated with increased anionic phospholipids at the cell surface. Annexin V, which binds to anionic phospholipids, attenuated the increased TF coagulant activity. It is noteworthy that treatment of cells with reducing agents also increased the cell surface TF activity. No evidence was found for either detectable expression of PDI at the cell surface or association of TF with PDI. Furthermore, reduction of PDI with the gene silencing had no effect on either TF coagulant or cell signaling functions. Overall, the present data undermine the recently proposed hypothesis that PDI-mediated disulfide exchange plays a role in regulating TF procoagulant and cell signaling functions.
Introduction
Tissue factor (TF) is a plasma membrane glycoprotein that activates coagulation factor VIIa (VIIa) allosterically when it binds to it. The formation of TF-VIIa complexes on cell surfaces triggers both the coagulation cascade1,2 and cell signaling.3,4 Although TF is not typically expressed in cells that come in contact with blood, monocytes and endothelial cells can both express TF in response to pathologic stimuli.5-7 TF is constitutively expressed in many extravascular cells, including fibroblasts and pericytes found in and surrounding blood vessel walls, epithelial cells, and tumor cells.8-10 Several studies have indicated that only a small fraction of the TF found on the cell surface is actually functionally active in coagulation, whereas the majority is nonfunctional (cryptic).11,12 However, it is unclear, at present, how the TF that is functionally active in coagulation differs from the cryptic form or what mechanisms are involved in its activation. Earlier studies from our laboratory13,14 and others15-17 have suggested that whether TF is active or cryptic depends primarily on the availability of anionic phospholipids in the vicinity of TF on the outer cell surface membrane, because increasing the cell surface anionic phospholipids by a variety of stimuli resulted in an increase in the active TF population. However, the mechanism through which the increased anionic phospholipids convert cryptic TF to active TF is not entirely clear.12 In addition to anionic phospholipids, self-association of TF18 and the association of TF with cholesterol and lipid rafts19-21 were also shown to affect TF procoagulant activity
Recent studies suggest that the cryptic form of TF contains unpaired cysteine thiols at Cys186 and Cys209 and that activation of TF involves the formation of the Cys186-Cys209 disulfide bond.22 This novel hypothesis is based on observations that ablation of the disulfide bond by mutating both cysteines to serine or alanine severely impaired TF procoagulant activity23,24 and that treatment of cells with HgCl2, which oxidizes dithiols to disulfides, increased TF activity at cell surfaces.22,25 In a parallel publication, the same group of investigators24 also suggested that protein disulfide isomerase (PDI) plays a critical role in the disulfide isomerization of TF. These studies hypothesized that PDI disables coagulation by targeting the Cys186-Cys209 disulfide bond. It is noteworthy that the Cys186-Cys209 disulfide bond is not required for TF-VIIa-induced PAR2-mediated cell signaling.24 Based on these data, it has been suggested that disulfide exchange mediated by PDI serves as the regulatory mechanism that switches TF-VIIa from coagulation to cell signaling.24,26 Although these data are tantalizingly interesting and novel, they are somewhat circumstantial and fail to provide unequivocally convincing evidence that disulfide switching alone and not other potential changes, such as exposure of anionic phospholipids, at the cell surface is responsible for the activation of TF associated with the treatment of cells with thiol-oxidizing agents. Moreover, PDI is predominantly localized in ER, and in the absence of a traditional membrane attachment site, it would seem probable that, at best, only traces of PDI would be present on cell surfaces.27 Thus, the hypothesis that disulfide isomerization plays a critical role in modulating TF coagulant and cell signaling functions and that such a mechanism could function as a general regulatory mechanism controlling TF function on various cell types remains equivocal.
The data presented here show that exposure of cells to an oxidizing agent results in increased cell surface TF coagulant activity but that this could stem from increased anionic phospholipids at the cell surface rather than from PDI-mediated disulfide bond formation in TF. No evidence was found for the presence of PDI at cell surfaces or for PDI association with TF. Attenuation of PDI expression had no effect on either the coagulant or signaling functions of TF on cell surfaces. These data raise a valid question whether disulfide switching by PDI plays any regulatory role in controlling the TF coagulant activity and signaling functions.
Materials and methods
Reagents
Recombinant human FVIIa was obtained from Novo Nordisk A/S (Maaloev, Denmark). Factor X and other coagulation factors were either from Enzyme Research Laboratories (South Bend, IN) or Haematological Technologies (Essex Junction, VT). Monospecific polyclonal antibodies against human TF were prepared as described previously.28 TF monoclonal antibody (10H10) was kindly provided by Wolfram Ruf (Scripps Research Institute, La Jolla, CA). Polyclonal anti-PDI antibody was obtained from David Essex (University of Texas Health Science Center at San Antonio) and monoclonal anti-PDI (RL77) was from Affinity Bioreagents (Golden, CO). Recombinant human PDI was purchased from BioVision (Mountain View, CA). Sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate (NHS-SS-biotin) was from Pierce (Rockford, IL) and 3-(N-maleimidylpropionyl)-biocytin (MPB) was from Invitrogen (Carlsbad, CA). HgCl2, ionomycin, bacitracin, and other reagents were from Sigma (St Louis, MO). Bacitracin was repurified by gel filtration chromatography29 or ultrafiltration using 3000 Da cut-off filter. Secondary antibodies conjugated to fluorescein isothiocyanate (FITC), Oregon green or Rhodamine red were obtained from Invitrogen.
Cell culture
MDA-MB-231 breast carcinoma cells and human lung fibroblasts (WI-38) were obtained from American Type Culture Collection (Manassas, VA) and cultured in Dulbecco modified Eagle medium supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS). Primary human umbilical vein endothelial cells (HUVEC) were obtained from Lonza Walkersville (Walkersville, MD) and cultured in EBM-2 basal media supplemented with growth supplements (Lonza Walkersville) and 5% FBS. Endothelial cells from passages between 3 and 10 were used in the present studies. All cell types were cultured at 37°C and 5% CO2 in a humidified incubator. Monolayers were washed twice with buffer A (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 0.15 M NaCl, 4 mM KCl, and 11 mM glucose, pH 7.5) before they were used in experiments.
125I-FVIIa binding to cells
TF and prothrombinase activity assays
TF procoagulant activity on intact cells was determined by incubating confluent monolayers with enzyme FVIIa (10 nM) and substrate factor X (175 nM) in buffer B (buffer A containing 5 mM CaCl2 and 1 mg/mL bovine serum albumin) at 37°C. Unless otherwise specified, an aliquot was removed at a specific time point (usually at 2 minutes) into stopping buffer (Tris-buffered saline containing 1 mg/mL bovine serum albumin and 10 mMethylenediaminetetraacetic acid [EDTA]), and the amount of factor Xa generated was measured in a chromogenic assay as described previously.31 To determine prothrombinase activity, monolayers were incubated with FVa (10 nM), factor Xa (1 nM), and substrate prothrombin (1.4 μM). At the end of a 2-minute activation period, an aliquot was removed into stopping buffer, and the thrombin generated was measured by a chromogenic assay using Chromozym TH, as described previously.32
Generation of stable PDI knockout tumor cells
PDI-specific and control nonspecific short hairpin RNA (shRNA) constructs based on pSilencer2.1-U6hygo (Ambion, Austin, TX) were kindly provided by Drs Wu Ou and Jonathan Silver (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). A day after seeding the cells, the cells in a 6-well plate were transfected with 5 μg of plasmid(s) in 10 μl of Lipofectamine 2000 (Invitrogen) diluted in 250 μL of Opti-MEM (Invitrogen). The stable cell lines were selected 24 hours after transfection by subculturing in a medium containing 0.4 mg/mL hygromycin B (A.G. Scientific, San Diego, CA). After 2 weeks in the selection medium, the stable cell lines were maintained in complete medium containing 0.2 mg/mL hygromycin. We tested 4 PDI shRNA constructs. shRNA1 (5′-GAGTGTCTGACTATGAC-3′, base pairs [bp] 818-836) and shRNA3 (5′-GATCCTGTTCATCTTCATC 3′, bp 884-902) inhibited the PDI expression maximally in MDA-MB-231 cells. Therefore, cells expressing PDI shRNA1 or shRNA3 were used in the present studies.
FACS analysis
Fluorescence-activated cell sorting (FACS) analysis was performed essentially as described previously33 with slight modifications. In brief, monolayers cultured in T-25 flasks or 6-well plates were washed with phosphate-buffered saline (PBS) and cells were detached from the dish by incubating them with 0.5 mM EDTA (versene solution) for 4 minutes at 37°C. After 2 washes with PBS to remove any residual EDTA, cells were fixed in 2% paraformaldehyde for 30 minutes, washed with PBS, and then permeabilized with 0.1% Triton X-100 in PBS for 5 minutes. Intact and permeabilized cells were placed in blocking buffer (5% goat serum in PBS) for 30 minutes. The cells were washed once with PBS and incubated with the requisite primary antibody (10 μg/mL) in the blocking buffer for 60 minutes at 4°C. 2 additional washes were performed to remove any residual antibody and then the cells were incubated with FITC-conjugated secondary antibody for 30 minutes at 4°C in the dark. Finally, the cells were washed, refixed in 2% paraformaldehyde, and analyzed using a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer.
Cell surface biotinylation and labeling of free thiols
Cell surface biotinylation was performed essentially as described previously34 with slight modifications. In brief, monolayers cultured in T-25 flasks were washed twice with PBS+ (PBS containing 0.25 mM MgSO4 and 0.7 mM CaCl2) and incubated in 0.5 mg/mL NHS-SS-biotin (Pierce) in PBS+ for 30 minutes on ice. The biotinylation reaction was terminated by removing the biotin and washing the monolayers twice with PBS, followed by a 5-minute incubation with 50 mM glycine on ice. To label free thiols, monolayers were incubated with 1 mM MPB for 5 minutes in HEPES buffer containing 13 mM CaCl2. The cells were washed twice with the HEPES buffer, and the residual unreacted MPB was quenched with 200 μM glutathione for 30 minutes at room temperature.
Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting were performed essentially as described earlier.34 Biotin-labeled cells were solubilized in the HEPES buffer containing 25 mM octylglucopyranoside or 1% Triton X-100 supplemented with cocktail of protease inhibitors, and the cell lysates were incubated overnight at 4°C with Affi-Gel-15 (Bio-Rad Laboratories, Hercules, CA) coupled with anti-TF IgG or streptavidin-agarose beads. After the removal of the unbound material, the beads were washed 3 times, and the bound material was eluted with 50 μL of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Eluted samples were subjected to SDS-PAGE, followed by immunoblot analysis with anti-biotin, anti-TF, or anti-PDI antibodies.
Immunofluorescence microscopy
Immunofluorescence microscopy and the subsequent imaging were performed essentially as described in our recent publications.33,34 The fixed cells, either nonpermeabilized or permeabilized with 0.1% Triton X-100, were stained with antibodies against TF or PDI, followed by Oregon green or Rhodamine red-conjugated secondary antibodies. The immunostained cells were viewed using a Nikon Eclipse TE2000-S (Yokohama, Japan) microscope, and images were acquired of a field of view at 2-μm z-axis increments using an UltraVIEW LCI confocal system (PerkinElmer Life and Analytical Sciences, Waltham, MA) equipped with a digital charge-coupled device camera (Ultra Pix; Hamamatsu Photonics, Hamamatsu City, Japan) with a resolution of 1344 × 1024 × 12. PerkinElmer's ImagingSuite (version 5.2) Acquisition & Processing Software was used for the acquisition of images and determining the colocalization (overlap of the green and the red fluorescence).
Data collection
All experiments were repeated 2 to 6 times. Data shown in the figures represent mean (± SEM) or representative Western or Northern blots.
Results
Effect of thiol-oxidizing and -reducing reagents on TF activity
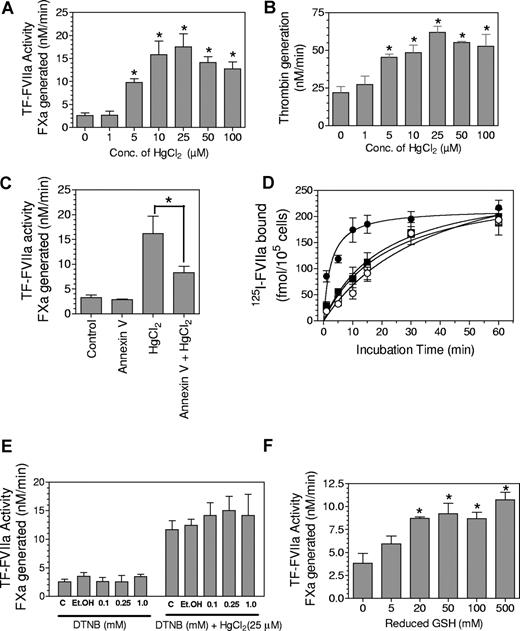
Increased cell surface TF activity in HL-60 cells after treatment with thiol-oxidizing agent, HgCl2, was used as supporting evidence for the theory that the TF allosteric disulfide was reduced in the cryptic form and that the formation of the disulfide bond converts cryptic TF to TF that is functionally active in coagulation.22 Consistent with these earlier published data,22 treatment of MDA-MB-231 cells with various concentrations of HgCl2 (1-100 μM) increased the cell surface TF activity, in a concentration-dependent manner, by 4- to 7-fold (Figure 1A). To investigate the potential effect of HgCl2 treatment on availability of anionic phospholipids at the cell surface, which would profoundly influence TF procoagulant activity,11,12,35 we measured the effect of HgCl2 treatment on prothrombinase activity. As shown in Figure 1B, HgCl2 treatment also markedly increased the cell surface prothrombinase activity. Although the relative increase in the prothrombinase activity after the HgCl2 treatment was somewhat lower than that noted with TF activity, a 3-fold increase in prothrombinase activity is highly significant. These data suggest that HgCl2 treatment increased the exposure of anionic phospholipids at the cell surface. Next, to determine whether the increased availability of anionic phospholipids on the cell surface after the HgCl2 treatment might have contributed to the increased TF activity, the HgCl2-treated MDA-MB-231 cells were exposed to annexin V, a phosphatidylserine binding protein that serves to reduce effectively the availability of anionic phospholipids, before the addition of FVIIa and substrate factor X to measure TF activity. Annexin V markedly decreased the HgCl2-mediated increased TF activity (Figure 1C). A characteristic difference between cryptic TF and active TF is the rate at which FVIIa binds to TF.13 Exposure of anionic phospholipids on the outer cell surface, which is known to facilitate the transition of cryptic TF to active TF, has been shown to increase the rate of FVIIa association to TF.14 Consistent with our hypothesis that HgCl2 treatment enhances anionic phospholipids at the cell surface, 125I-FVIIa bound more rapidly to MDA 231 cells that were treated with HgCl2 than to control cells (Figure 1D). However, as expected, at the end of a 1-hour incubation, a similar amount of 125I-FVIIa was associated with both HgCl2-treated and control cells. More importantly, annexin V blocked the faster association of FVIIa to TF in cells treated with HgCl2 but had no significant effect on FVIIa binding to cryptic TF. These data suggest that activation of TF associated with HgCl2 treatment could be explained simply by an increased exposure of phosphatidylserine on the plasma membrane. However, these data alone do not eliminate a potential role of HgCl2-mediated TF allosteric disulfide bond in conversion of cryptic TF to active TF.
Increased cell surface TF coagulant activity associated with HgCl2 treatment could be explained by increased anionic phospholipids at the cell surface. (A) MDA 231 cells were treated with various concentrations (Conc.) of HgCl2 for 15 minutes. After 15 minutes, HgCl2 was removed, and cells were washed once before adding FVIIa (10 nM) and factor X (FX; 175 nM). Cell surface TF-FVIIa coagulant activity was measured as its ability to activate FX. (B) MDA 231 cells were treated with HgCl2 as described in (A), and cell surface prothrombinase activity was measured by adding FXa (1 nM), FVa (10 nM), and prothrombin (1.4 μM) and then measuring the rate of thrombin generation. (C) MDA 231 cells were exposed to control vehicle or annexin V (200 nM) before they were treated with HgCl2 (25 μM) for 15 minutes, and cell surface TF activity was measured as described in panel A. (D) MDA 231 cells were treated with a control vehicle for 15 minutes (○); annexin V (200 nM) for 15 minutes (□); a control vehicle for 15 minutes followed by HgCl2 (50 μM) for 10 minutes (•); and annexin V (200 nM) for 15 minutes followed by HgCl2 (50 μM) for 10 minutes (■). After the removal of HgCl2, monolayers were incubated with 125I-FVIIa (10 nM) (annexin V was added along 125I-FVIIa to the cells that were preincubated with annexin V). 125I-FVIIa bound to cells at various times was determined as described in an earlier publication.30 (E) MDA 231 cells were pretreated with control buffer or control vehicle (Et.OH) or various concentrations of DTNB for 15 minutes before they were exposed to control vehicle or HgCl2 (25 μM) for 15 minutes. Cell surface TF activity was determined as described in panel A. (F) MDA 231 cells were exposed to various concentrations of reduced glutathione for 15 minutes before their cell surface TF activity was determined as in panel A. (A-F) Data are expressed as mean plus or minus SEM (n = 3). *denotes the value significantly (P < 0.05) differs from the control value.
Increased cell surface TF coagulant activity associated with HgCl2 treatment could be explained by increased anionic phospholipids at the cell surface. (A) MDA 231 cells were treated with various concentrations (Conc.) of HgCl2 for 15 minutes. After 15 minutes, HgCl2 was removed, and cells were washed once before adding FVIIa (10 nM) and factor X (FX; 175 nM). Cell surface TF-FVIIa coagulant activity was measured as its ability to activate FX. (B) MDA 231 cells were treated with HgCl2 as described in (A), and cell surface prothrombinase activity was measured by adding FXa (1 nM), FVa (10 nM), and prothrombin (1.4 μM) and then measuring the rate of thrombin generation. (C) MDA 231 cells were exposed to control vehicle or annexin V (200 nM) before they were treated with HgCl2 (25 μM) for 15 minutes, and cell surface TF activity was measured as described in panel A. (D) MDA 231 cells were treated with a control vehicle for 15 minutes (○); annexin V (200 nM) for 15 minutes (□); a control vehicle for 15 minutes followed by HgCl2 (50 μM) for 10 minutes (•); and annexin V (200 nM) for 15 minutes followed by HgCl2 (50 μM) for 10 minutes (■). After the removal of HgCl2, monolayers were incubated with 125I-FVIIa (10 nM) (annexin V was added along 125I-FVIIa to the cells that were preincubated with annexin V). 125I-FVIIa bound to cells at various times was determined as described in an earlier publication.30 (E) MDA 231 cells were pretreated with control buffer or control vehicle (Et.OH) or various concentrations of DTNB for 15 minutes before they were exposed to control vehicle or HgCl2 (25 μM) for 15 minutes. Cell surface TF activity was determined as described in panel A. (F) MDA 231 cells were exposed to various concentrations of reduced glutathione for 15 minutes before their cell surface TF activity was determined as in panel A. (A-F) Data are expressed as mean plus or minus SEM (n = 3). *denotes the value significantly (P < 0.05) differs from the control value.
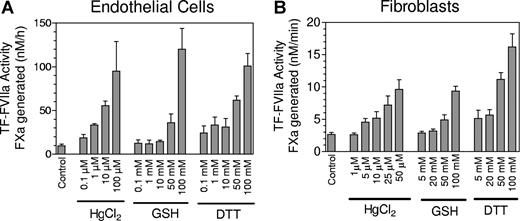
To investigate the possibility that cryptic TF has free thiols and the HgCl2 treatment facilitated the formation of disulfide bond, we compared the labeling of TF in control and HgCl2-treated cells with membrane-impermeant biotin-linked MPB, which specifically alkylates thiols. We were unable to detect any incorporation of MPB into TF in either control or HgCl2-treated MDA 231 cells (data not shown). It is pertinent to note here that earlier studies also failed to show incorporation of MPB into TF in HL-6022 and keratinocytes.24 To address the question of whether TF disulfide bond formation is essential for increased TF activity associated with HgCl2, we pretreated the cells with 5,5′-dithio-bis(2-nitronezoic acid; DTNB), a sulfhydryl reagent that reacts with thiol groups and thus blocks disulfide switching, before they were exposed to HgCl2. Blocking the thiol group with DTNB had no effect on HgCl2-mediated increase in cell surface TF activity (Figure 1E). If TF activation involved the formation of allosteric disulfide bond and the disulfide bond reduced form represents cryptic TF, then one would predict that reducing the disulfide bond with a reducing agent should diminish the TF activity at the cell surface. To test this, MDA-MB-231 cells were exposed to various concentrations of reduced glutathione (GSH), a cell-impermeant thiol-reducing agent. It is interesting to note that GSH treatment, rather than decreasing cell surface TF activity, actually enhanced the activity (Figure 1F). It is important to note that these observations are not unique to MDA-MB-231 cells, but are also true in other cell model systems, such as fibroblasts and endothelial cells (Figure 2). Moreover, we observed similar results in cells treated with dithiothreitol (DTT), a strong reducing agent widely used to quantitatively reduce disulfide bonds and maintain monothiols in a reduced state. Overall, these data raise serious doubt of the validity of the recently proposed hypothesis that the Cys186-Cys209 disulfide bond is reduced in the cryptic form of TF and the activation involves formation of the disulfide bond.22,24
Effect of thiol-oxidizing and -reducing agents on cell surface tissue factor activity of stimulated endothelial cells and fibroblasts. (A) Monolayers of HUVEC were stimulated with tumor necrosis factor α (TNF-α) (20 ng/mL) + IL-1β (20 ng/mL) for 6 hours at 37°C to induce TF expression. Stimulated HUVEC were treated with various concentrations of HgCl2, reduced glutathione, or DTT for 5 minutes, and the oxidizing/reducing agent was removed and cells were washed twice before adding FVIIa (10 nM) and factor X (175 nM) to measure cell surface TF activity as the rate of factor Xa generated in a chromogenic assay. (B) Same as panel A except fibroblasts were used in place of stimulated endothelial cells. Data are expressed as mean plus or minus SEM (n = 3).
Effect of thiol-oxidizing and -reducing agents on cell surface tissue factor activity of stimulated endothelial cells and fibroblasts. (A) Monolayers of HUVEC were stimulated with tumor necrosis factor α (TNF-α) (20 ng/mL) + IL-1β (20 ng/mL) for 6 hours at 37°C to induce TF expression. Stimulated HUVEC were treated with various concentrations of HgCl2, reduced glutathione, or DTT for 5 minutes, and the oxidizing/reducing agent was removed and cells were washed twice before adding FVIIa (10 nM) and factor X (175 nM) to measure cell surface TF activity as the rate of factor Xa generated in a chromogenic assay. (B) Same as panel A except fibroblasts were used in place of stimulated endothelial cells. Data are expressed as mean plus or minus SEM (n = 3).
In addition to examining the effect of HgCl2 on TF coagulant activity, we also investigated the effect of HgCl2 on TF-FVIIa-induced cell signaling. HgCl2 treatment inhibited, in a dose-dependent manner, TF-FVIIa-induced CCN1 and IL-8 gene expression. However, the inhibitory effect seems to be nonspecific because HgCl2 treatment was also found to inhibit PAR1 agonist peptide (AP), PAR2 AP, and thrombin-induced gene expression in MDA 231 cells (data not shown).
No evidence for the presence of cell surface PDI or its association with TF
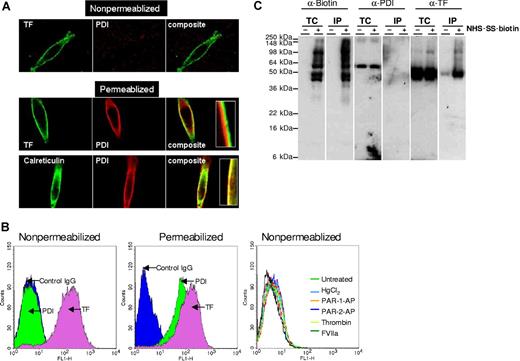
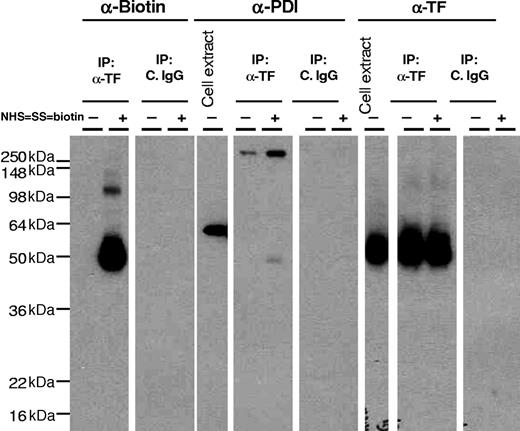
In addition to hypothesizing that the surface-accessible Cys186-Cys209 disulfide bond of TF is critical for coagulation, it has been suggested that the disulfide bond reduced cryptic form of TF retains cell signaling activity,24 and that PDI, a protein disulfide isomerase, switches TF from coagulation to cell signaling.24 To examine whether such a mechanism was evident in our cell model systems, we first investigated whether MDA-MB-231 cells express PDI on the cell surface and, if so, whether it associates with TF. Immunofluorescence confocal microscopy studies with both monoclonal and polyclonal PDI antibodies revealed that MDA 231 cells express abundant PDI, but the PDI is exclusively localized inside the cell, because the antibodies brightly stained permeabilized cells but no immunofluorescence staining was detectable with nonpermeabilized cells (Figure 3A). In contrast to PDI antibodies, anti-TF antibodies intensely stained the cell surfaces of both nonpermeabilized and permeabilized cells. Similar data were obtained with 2 other cell types that we examined (ie, fibroblasts and stimulated endothelial cells; data not shown). It is interesting to note that intracellular PDI is prominently localized beneath the plasma membrane in the endoplasmic reticulum and not with TF (Figure 3A insets). This prompted us to examine whether the PDI would be externalized in response to proteases or the oxidizing agent, HgCl2, and we found that treatment of MDA 231 cells with thrombin, Xa, plasmin, VIIa (10 nM each), PAR1 or PAR2 peptide agonists (50 μM), or HgCl2 (50 μM) did not cause the externalization of PDI to the cell surface (data not shown). Consistent with immunofluorescence confocal microscopy data, no differences were found in fluorescence of nonpermeabilized cells stained with control IgG or PDI antibodies in FACS analysis (Figure 3B). In contrast, as expected, marked fluorescence shift was noted with PDI antibodies in permeabilized cells. As observed with confocal microscopy, no PDI was detected in FACS analysis of nonpermeabilized cells even after the cells were exposed to various stimuli (ie, HgCl2, thrombin, VIIa, or PAR activation peptides; Figure 3B). To strengthen the conclusion that there was no PDI at the cell surface, we performed additional studies in which cell surface proteins were biotinylated with cell-impermeant NHS-SS-biotin, and biotinylated proteins were absorbed on streptavidin beads, and the eluate was probed for the presence of PDI by immunoblotting. PDI antibodies stained prominently a band that corresponds to a molecular mass of PDI (64 kDa) in total cell extracts but not in the eluate of streptavidin beads (Figure 3C). In additional studies, cell extracts were immunoprecipitated with anti-TF antibodies, and the immunoprecipitates were probed for the presence of PDI by immunoblotting. No PDI was detectable in cell extracts that were immunoprecipitated with anti-TF antibodies (Figure 4). These data not only confirm the lack of PDI at the cell surface but also indicate that intracellular PDI does not associate with TF. Similar data were obtained with fibroblasts (data not shown).
PDI was localized intracellularly and not at the cell surface. (A) Cellular localization of PDI by immunofluorescence confocal microscopy. Nonpermeabilized and permeabilized MDA-MB-231 cells were immunostained with rabbit anti-human PDI or monoclonal anti-human PDI, monoclonal anti-human TF (10H10), or rabbit anti-calreticulin antibody, followed by Oregon Green–labeled anti-rabbit and Rhodamine red-conjugated anti-mouse antibodies as secondary reporter antibodies. (B) Nonpermeabilized and permeabilized MDA-MB-231 cells were stained with anti-PDI IgG or anti-TF immunoglobulin G (IgG), followed by FITC-conjugated secondary antibodies. In the right panel, MDA 231 cells were treated with HgCl2 (25 μM), PAR-1 or PAR-2 agonist peptides (50 μM), thrombin, or FVIIa (10 nM) for 30 minutes before the cells were stained with PDI antibodies. The cells were analyzed by flow cytometry. (C) MDA-MB-231 cells were labeled with cell-impermeant NHS-SS-biotin or a control vehicle. Total cell extracts and cell extracts subjected to immunoprecipitation with streptavidin-agarose were immunoblotted with anti-biotin, anti-PDI, or anti-TF antibodies. TC indicates total cell extract; IP, immunoprecipitated; and kDa, kilodaltons.
PDI was localized intracellularly and not at the cell surface. (A) Cellular localization of PDI by immunofluorescence confocal microscopy. Nonpermeabilized and permeabilized MDA-MB-231 cells were immunostained with rabbit anti-human PDI or monoclonal anti-human PDI, monoclonal anti-human TF (10H10), or rabbit anti-calreticulin antibody, followed by Oregon Green–labeled anti-rabbit and Rhodamine red-conjugated anti-mouse antibodies as secondary reporter antibodies. (B) Nonpermeabilized and permeabilized MDA-MB-231 cells were stained with anti-PDI IgG or anti-TF immunoglobulin G (IgG), followed by FITC-conjugated secondary antibodies. In the right panel, MDA 231 cells were treated with HgCl2 (25 μM), PAR-1 or PAR-2 agonist peptides (50 μM), thrombin, or FVIIa (10 nM) for 30 minutes before the cells were stained with PDI antibodies. The cells were analyzed by flow cytometry. (C) MDA-MB-231 cells were labeled with cell-impermeant NHS-SS-biotin or a control vehicle. Total cell extracts and cell extracts subjected to immunoprecipitation with streptavidin-agarose were immunoblotted with anti-biotin, anti-PDI, or anti-TF antibodies. TC indicates total cell extract; IP, immunoprecipitated; and kDa, kilodaltons.
No evidence that PDI associates with tissue factor. MDA-MB-231 cells were labeled with cell-impermeant NHS-SS-biotin or a control vehicle. Total cell extracts and cell extracts subjected to immunoprecipitation with anti-TF IgG or control IgG were immunoblotted with anti-biotin, anti-PDI, or anti-TF antibodies.
No evidence that PDI associates with tissue factor. MDA-MB-231 cells were labeled with cell-impermeant NHS-SS-biotin or a control vehicle. Total cell extracts and cell extracts subjected to immunoprecipitation with anti-TF IgG or control IgG were immunoblotted with anti-biotin, anti-PDI, or anti-TF antibodies.
PDI regulated neither TF coagulant nor cell signaling activity
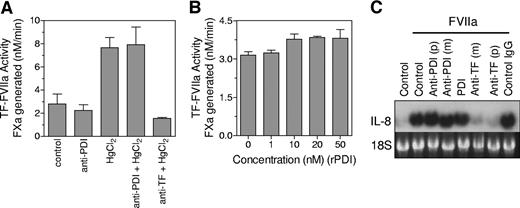
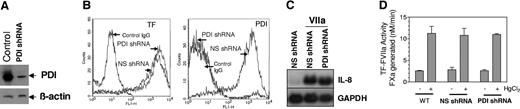
It has been suggested that PDI mediates the activating effect of HgCl2, and PDI-mediated disulfide isomerization of TF acts as a regulatory switch between TF-VIIa cell signaling and coagulant activity.24 Our data presented here do not fit with this hypothesis. However, undetectable amounts of PDI may be present on cell surfaces of MDA-MB-231 and the other cell types that we examined, and this PDI could possibly regulate TF coagulant and cell signaling functions. Therefore, we performed additional studies to investigate the effect of PDI on TF coagulant and cell-signaling activities. First, we investigated the effect of anti-PDI antibodies on HgCl2-mediated increased TF coagulant activity. Anti-PDI antibodies failed to diminish the effect of HgCl2 in increasing TF activity (Figure 5A). In additional studies, we examined the effect of exogenously added recombinant PDI on TF coagulant activity and found that recombinant protein disulfide isomerase (rPDI) had no effect on TF activity (Figure 5B). Similarly PDI antibodies or exogenously added rPDI exhibited no effect on TF-VIIa-induced IL-8 gene expression (Figure 5C). In contrast, TF antibodies fully attenuated TF-VIIa signaling. In additional studies, we used bacitracin, which is widely used to block cell-surface PDI function. Bacitracin inhibited HgCl2-mediated increase in TF coagulant activity in MDA 231 cells and stimulated endothelial cells (data not shown). Bacitracin also inhibited TF-VIIa signaling in MDA 231 cells as reported previously with keratinocytes (data not shown).24 However, these data were difficult to interpret because bacitracin preparations, despite repurification to eliminate known protease contaminants,29 either degraded or inhibited FVIIa's enzymatic activity. Therefore, we tried a better approach and used small interfering RNA (siRNA) technology to knock down PDI expression. Because transient expression of PDI-specific siRNA resulted in only a partial inhibition of PDI expression, we stably transfected MDA 231 cells with PDI-specific shRNA. Immunoblot and FACS analysis of cells transfected with PDI shRNA construct showed that PDI expression was markedly reduced (Figure 6A,B). Silencing PDI did not alter the level of TF expression at the cell surface (Figure 6B). More significantly, it had no measurable effect on the HgCl2-mediated increase in TF activity (Figure 6D). Similar to that observed with TF coagulant activity, PDI silencing had no significant effect of TF-VIIa-induced cell signaling (Figure 6C).
PDI antibodies or exogenously added PDI failed to alter TF-FVIIa coagulant and cell signaling activities. (A). MDA-MB-231 cells were incubated with control IgG, anti-PDI IgG (100 μg/mL), or anti-TF IgG (50 μg/mL) for 30 minutes and then treated with control vehicle or HgCl2 (25 μM) for 15 minutes. TF-FVIIa coagulant activity was determined as described in Figure 1 legend. (B) MDA-MB-231 cells were incubated with control vehicle or various concentrations of recombinant PDI for 30 minutes before TF-FVIIa coagulant activity was measured. (C) MDA-MB-231 cells were incubated with control IgG, anti-PDI IgG, recombinant PDI (10 nM), or anti-TF IgG for 30 minutes and then treated with FVIIa (10 nM) for 1 hour. TF-FVIIa-induced IL-8 gene expression was determined by Northern blot analysis. p denotes polyclonal antibodies (100 μg/mL) and m, monoclonal antibodies (RL77 for PDI and 10H10 for TF, 10 μg/mL). (A,B) Data are expressed as mean plus or minus SEM (n = 3).
PDI antibodies or exogenously added PDI failed to alter TF-FVIIa coagulant and cell signaling activities. (A). MDA-MB-231 cells were incubated with control IgG, anti-PDI IgG (100 μg/mL), or anti-TF IgG (50 μg/mL) for 30 minutes and then treated with control vehicle or HgCl2 (25 μM) for 15 minutes. TF-FVIIa coagulant activity was determined as described in Figure 1 legend. (B) MDA-MB-231 cells were incubated with control vehicle or various concentrations of recombinant PDI for 30 minutes before TF-FVIIa coagulant activity was measured. (C) MDA-MB-231 cells were incubated with control IgG, anti-PDI IgG, recombinant PDI (10 nM), or anti-TF IgG for 30 minutes and then treated with FVIIa (10 nM) for 1 hour. TF-FVIIa-induced IL-8 gene expression was determined by Northern blot analysis. p denotes polyclonal antibodies (100 μg/mL) and m, monoclonal antibodies (RL77 for PDI and 10H10 for TF, 10 μg/mL). (A,B) Data are expressed as mean plus or minus SEM (n = 3).
PDI silencing had no effect on TF-FVIIa coagulant and cell-signaling functions. (A) Immunoblot analysis of MDA-MB-231 cell extracts that were stably transfected with PDI shRNA or a nonspecific shRNA. Blots were probed with anti-PDI or anti-β-actin antibodies. (B) FACS analysis of MDA-MB-231 cells transfected with nonspecific shRNA and PDI-specific shRNA for TF (left) and PDI (right) expression, respectively. For PDI staining, cells were permeabilized with 0.1% Triton X-100, whereas nonpermeabilized cells were used to stain cell surface TF expression. (C) MDA-MB-231 cells stably transfected with PDI-specific shRNA or nonspecific shRNA control vector were stimulated with FVIIa (10 nM) for 60 minutes and TF-FVIIa-induced IL-8 expression was determined by Northern blot analysis. NS shRNA indicates nonspecific shRNA; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (D) Wild-type (WT) MDA 231 cells or MDA 231 cells stably transfected with nonspecific or PDI-specific shRNA were treated with control vehicle or HgCl2, and the cell surface TF-FVIIa coagulant activity was determined as described in Figure 1. Data are expressed as mean plus or minus SEM (n = 3).
PDI silencing had no effect on TF-FVIIa coagulant and cell-signaling functions. (A) Immunoblot analysis of MDA-MB-231 cell extracts that were stably transfected with PDI shRNA or a nonspecific shRNA. Blots were probed with anti-PDI or anti-β-actin antibodies. (B) FACS analysis of MDA-MB-231 cells transfected with nonspecific shRNA and PDI-specific shRNA for TF (left) and PDI (right) expression, respectively. For PDI staining, cells were permeabilized with 0.1% Triton X-100, whereas nonpermeabilized cells were used to stain cell surface TF expression. (C) MDA-MB-231 cells stably transfected with PDI-specific shRNA or nonspecific shRNA control vector were stimulated with FVIIa (10 nM) for 60 minutes and TF-FVIIa-induced IL-8 expression was determined by Northern blot analysis. NS shRNA indicates nonspecific shRNA; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (D) Wild-type (WT) MDA 231 cells or MDA 231 cells stably transfected with nonspecific or PDI-specific shRNA were treated with control vehicle or HgCl2, and the cell surface TF-FVIIa coagulant activity was determined as described in Figure 1. Data are expressed as mean plus or minus SEM (n = 3).
Discussion
Unperturbed cells, in general, express little TF coagulant activity even though many express TF constitutively on their cell surfaces to which FVII and FVIIa can readily bind.11,12 This nonfunctional TF is often referred to as cryptic TF. Perturbation of cells with various stimuli—for example, calcium ionophores, oxidizing agents, or those that induce apoptosis or cell lysis—transforms cryptic TF to active TF.12 Although most evidence suggests that an increase in the exposure of anionic phospholipids at the cell surface is responsible for this functional transformation of TF,12,14,15,17,36 several reports have suggested that alternative mechanisms, possibly phospholipid-independent, may be responsible for this process.17-20,22 It has been hypothesized that TF with the unpaired cysteine thiols constitutes the cryptic form of TF and activation involves the formation of the Cys186-Cys209 disulfide bond, which might be required to maintain the conformation needed for productive binding of substrates factors IX and X.22 Furthermore, it has been proposed that cryptic TF retains the signaling functions and that PDI controls the disulfide bond formation, thus regulating TF function between coagulation and signaling.24 Although this proposed mechanism has been stated to play an important role in regulating TF activity post-translationally,22,24 the data presented herein fail to support the above concept unequivocally and raise serious reservations on the importance and validity of the proposed mechanism.22,24
The primary evidence for the postulate that activation of TF involves the disulfide bond formation of Cys186-Cys209 comes from 2 observations. First, alanine substitution for Cys186 or Cys209 in the extracellular domain of TF markedly decreased TF-VIIa activation of factor X24 ; second, the thiol-oxidizing agent HgCl2, which can oxidize dithiols to disulfides, increased the activity of endogenously expressed TF on cell surfaces.22 Although substitution of alanine for Cys186 or Cys209 would result in breaking of the disulfide bond, there is no direct evidence that lack of disulfide bond and not other subtle changes associated with the alanine substitution at these positions is responsible for the loss of TF-VIIa coagulant activity. In this regard, it is important to note that TF-VIIa signaling seemed normal with C209A, but no signaling was detectable with the C186A TF mutant.24 If the alanine substitution for either Cys186 or Cys209 results in breakage of the disulfide with no other conformational changes, then both the mutants should have behaved similarly (ie, retained the signaling function). Therefore, it is distinctly possible that alanine substitutions at Cys186 and Cys209 impaired TF coagulant function independent of the disulfide bond breakage.
Consistent with the earlier data in HL-60 cells,22 treatment of tumor cells, fibroblasts, or stimulated HUVEC with the oxidizing agent HgCl2 markedly increased TF-VIIa activation of factor X. A characteristic difference between cryptic TF and active TF is the rate to which VIIa binds; that is, there is a slower rate of VIIa binding to cryptic TF and a much faster rate of VIIa binding to active TF. In agreement with this notion, activation of TF after HgCl2 treatment increased the rate of VIIa binding to the cell surface TF. However, increased phosphatidylserine at the cell surface and not the disulfide bond formation is responsible for the increased TF activity. This conclusion is supported by the following observations: (1) HgCl2 treatment increased the cell surface prothrombinase activity, which depends on PS levels at the cell surface. (2) Annexin V, a phosphatidylserine binding protein, not only inhibited the HgCl2-mediated increased TF-VIIa activation of factor X but also blocked the increased rate of VIIa binding to TF. (3) DTNB, a sulfhydryl agent that reacts with thiol groups and therefore prevents disulfide bond formation, failed to attenuate HgCl2-induced TF activation. (4) Treatment of cells with glutathione, a cell-impermeant reducing agent or DTT, a strong thiol-stabilizing reducing agent, also increased the cell surface TF-VIIa activity. Here, it may be pertinent to note that the increased TF activity associated with these treatments was also inhibited by annexin V (data not shown). As reported in the earlier study with HL-60 cells,22 we were unable to detect the incorporation of biotin linked MPB, the agent that specifically alkylates thiols, in TF on cell surfaces of MDA 231 cells and fibroblasts. Therefore, we were unable to confirm the status of free thiols and disulfide bonds in TF on control cells and cells treated with HgCl2.
Association of TF with PDI and increased TF coagulant activity after partial inhibition of PDI by siRNA in keratinocytes was cited as the evidence for the contention that PDI regulates TF function by targeting the disulfide bond in the extracellular domain of TF.24 PDI is a multifunctional enzyme that can catalyze thio-disulfide oxidation, reduction, and isomerization.37 Although PDI is an ER protein, traces of PDI have been found on the surface of variety of cells.27 Thus, in theory, it is possible that PDI could regulate disulfide isomerization of TF at the cell surface and switch TF from coagulation to cell signaling or vice versa based on local oxidative environment. However, analysis of PDI localization by a variety of techniques showed no evidence for the presence of PDI at the cell surface in our cell model systems (ie, MDA 231 tumor cells, fibroblasts and HUVEC). All of them contained PDI in intracellular compartments, particularly in the ER compartment. Stimulation of cells with VIIa, thrombin and other agonists failed to relocalize PDI to the cell surface. Thus, it is not surprising that we found no evidence for an association of TF with PDI at the cell surface. We found no evidence that TF associates with PDI intracellularly, although a substantial fraction of TF resides intracellularly. Even if there were an undetectable amount of PDI present on cell surfaces in these cell types and this PDI associated with TF, the PDI levels would be far below the stoichiometric levels of TF and would have no significant consequences on the status of the disulfide bond of TF. This raises a question about the feasibility of disulfide exchange mediated by PDI acting as a regulatory mechanism that controls TF activity and switching TF between coagulation and signaling. Our observation that inhibition of PDI by PDI-neutralizing antibodies or gene silencing had no effect on either TF coagulant or signaling function adds further weight to this question. It had been reported earlier that PDI was required for the activating effect of Hg2+. However, our data clearly show that complete knockout of PDI failed to diminish the activating effect of Hg2+, indicating that this effect is independent of PDI. This supports our conclusion that the increased anionic phospholipid and not the disulfide bond formation is responsible for the increased TF activity associated with Hg2+ treatment. It is interesting to note here that while the present article was in preparation, Versteeg and Ruf38 reported that the coagulant function of soluble TF was enhanced by purified PDI, and this enhancing effect of PDI was independent of its oxidoreductase activity. PDI had no effect on the procoagulant activity of soluble TF in the presence of phospholipids or full-length relipidated TF. Although our present studies were not aimed at examining the chaperone function of PDI, it may be pertinent to point out that recombinant PDI had no effect on the procoagulant activity of TF on cell surfaces.
Finally, the ability of TF monoclonal antibody (mAB) 10H10 to inhibit TF-VIIa signaling and not the coagulation activation was used as one piece of evidence for the presence of 2 distinct pools of TF that differ conformationally, the cryptic form of TF being the one capable of functioning in cell signaling. Although this observation is consistent with the underlying hypothesis that TF on cell surface exists in 2 pools, cryptic and coagulant active TF, and they could possibly differ in their conformation, these data do not constitute evidence that mAB 10H10 exclusively binds to cryptic TF or that the difference between cryptic and coagulant active TF is the disulfide bond. Here, it may be pertinent to point out that in our earlier binding studies, in which we quantitatively determined the number of TF sites on cell surfaces in fibroblasts,30 tumor cells,39 and other cell types (unpublished data) using radiolabeled VIIa or mAB 10H10, our data indicated a comparable number TF sites or a slightly higher number when using mAB 10H10. This suggests that mAB 10H10, as with VIIa, binds to both cryptic and coagulant active TF. The ability of mAB 10H10 to inhibit TF-VIIa signaling and not the coagulant activation could be explained simply by its ability to interfere with TF-VIIa interaction with PAR2. Unlike TF-VIIa activation of factors IX and X, TF-VIIa activation of PAR2 could be largely independent of negatively charged phospholipids. This could explain how cryptic TF retains the cell signaling function but not coagulant function.
Overall, our data leads inexorably to the conclusion that PDI plays no role in HgCl2-mediated TF activation and puts in question the validity of the recently proposed mechanism that PDI-dependent disulfide isomerization plays a critical role in regulating different functions of TF.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants HL58869 and HL65550. S.K.M. was a recipient of a postdoctoral fellowship award from the American Heart Association, Texas Affiliate.
We thank Marriam Kaleemuddin (University of Texas Health Center at Tyler) for technical assistance and Dr Mark Atkinson (University of Texas Health Center at Tyler) for critical reading of the manuscript. We are thankful to Dr Wolfram Ruf (Scripps Research Institute, La Jolla, CA) and Dr David Essex (University of Texas Health Science Center at San Antonio) for providing TF monoclonal antibodies and polyclonal PDI antibodies, respectively.
National Institutes of Health
Authorship
Contribution: U.R.P. initiated the research, performed VIIa binding and TF signaling studies, generated PDI knockout cells, and contributed to experimental design, data interpretation, and the preparation of the manuscript. S.G. performed and analyzed TF activation studies, and performed FACS analysis. S.K.M. performed and analyzed biotinylation, FACS, and confocal microscopy studies. L.V.M.R. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Usha R. Pendurthi or L. Vijaya Mohan Rao, Biomedical Research, University of Texas Health Science Center at Tyler, 11937 US Hwy 271, Tyler, TX 75708; e-mail: usha.pendurthi@uthct.edu or vijay.rao@uthct.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal