Abstract
E-selectin plays critical roles in tethering leukocytes to endothelial cells (ECs). We studied the role of E-selectin in endothelial progenitor cell (EPC) homing and vasculogenesis. After ischemia, the expression of E-selectin on ECs peaked 6 to 12 hours and returned to baseline at 24 hours, whereas the level of soluble E-selectin (sE-selectin) in serum increased over 24 hours and remained high at day 7. Mouse bone marrow–derived EPCs expressed not only E-selectin but also its ligand. Homing of circulating EPCs to ischemic limb was significantly impaired in E-selectin knock-out mice, as well as wild-type mice pretreated with blocking antibody against E-selectin, which was rescued by local sE-selectin injection. Mechanism for this is that sE-selectin stimulated not only ECs to express ICAM-1, but also EPCs to secrete interleukin-8 (IL-8), leading to enhanced migration and incorporation to ECs capillary formation. In therapeutic aspect, local treatment with sE-selectin enhanced efficacy of EPC transplantation for vasculogenesis and salvage of ischemic limb. Conversely, when E-selectin was knocked down by E-selectin small interfering RNA, blood flow recovery after EPC transplantation was significantly impaired. But this impaired vasculogenesis was rescued by sE-selectin. In conclusion, these data demonstrate E-selectin is a pivotal molecule for EPCs' homing to ischemic limb and vasculogenesis.
Introduction
E-selectin is an inducible cell-adhesion molecule on endothelial cells (ECs), which mediates the binding of the neutrophils and functions as a calcium-dependent lectin.1-3 It consists of 5 components: an amino-terminal “C type” lectin domain critical for ligand interaction, an epidermal growth factor–like domain, 6 complement regulatory repeats, a single transmembrane domain, and a cytoplasmic carboxyl-terminal tail.4,5 E-selectin mediates adhesive interactions of circulating leukocytes with the vascular endothelium during inflammatory conditions such as rheumatoid arthritis and atherosclerosis.6,7 It also plays a role in the homing of hematopoietic stem cells, and its constitutive expression on ECs of hematopoietic tissue is essential in the initial step of the homing process.8,9 In addition, Koch et al have reported that soluble E-selectin (sE-selectin), which is thought to be a cleavage form of membrane-bound E-selectin and therefore lacks the trans-membrane and cytoplasmic domains, induces angiogenesis in the rat cornea and stimulates chemotaxis and tube formation of human dermal microvascular ECs through Src- and phosphatidylinositol 3-kinase–mediated pathways.10,11
Endothelial progenitor cells (EPCs) have the potential to proliferate and to differentiate into mature ECs.12 Recent studies in animal and humans suggest the ability of EPCs to home to the areas with reduced oxygen supply and to induce vasculogenesis and angiogenesis.13,14
Because the number of circulating EPCs may limit the ultimate magnitude of therapeutic angiogenesis, strategy of ex vivo expansion of EPCs harvested from the patient's circulating blood has been tried and proven to be effective.15 Similarly, increased recruitment and specific lodging of EPCs into ischemic tissues may be another target.16 Therefore, the elucidation of molecules involved in the homing mechanism of EPCs seems important.
In this study, we investigated the changes of E-selectin expressions in hindlimb ischemic model and assessed the role of E-selectin for the circulating EPCs to home to ischemic tissue in wild-type (WT) and E-selectin knock-out mice (E-sel−/−). Furthermore, we evaluated the impact of E-selectin knock down by siRNA technique on blood flow recovery to ischemic limb. Finally, we tested whether the impaired vasculogenesis by siRNA would be rescued by local treatment of sE-selectin.
Materials and methods
Sequential change of E-selectin expression in various tissues and serum in mice
All procedures were approved by the Institutional Review Board of Seoul National University and were performed in accordance with the Institutional Animal Care and Use Committee of Seoul National University Hospital. To induce muscle ischemia, 6-week-old C57BL6/J (WT) mice (Biogenomics, Korea) were anesthetized with ketamine 100 mg/kg plus xylazine 10 mg/kg intraperitoneally, and a unilateral femoral artery was removed. Surgery to induce hindlimb ischemia was performed as previously described.17 Mice were killed at predetermined times after surgery (0 hour, 6 hours, 12 hours, 24 hours, and 7 days) by administering an overdose of sodium pentobarbital. Please see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) for more information.
Characterization of EPCs
Please see Document S1 for more information.
In vitro knock down of E-selectin using siRNA and analysis of its effect on EPC differentiation
For E-selectin small interfering RNA (siRNA) transfection, bone marrow mononuclear cells (BMMNCs; 2 × 105 cells) were plated per well of a 6-well plates in 2 mL antibiotic-free EGM-2 medium (Clonetics, San Diego, CA) and incubated for 18 hours. For 6 replicas, 480 pmol siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) and 48 μL siRNA transfection reagent were mixed in 6 mL siRNA transfection medium according to the manufacturer's instructions. The cells were incubated in transfection mixture for 6 hours. The transfected BMMNCs were then cultured in 2 mL antibiotic-free EGM-2 medium and incubated for 42 hours. Surface antigens were analyzed by FACS (fluorescence-activated cell-sorter scanner) as previously described.15 We used primary antibodies against CD31, CD34, CD44, Sca-1, and Flk-1 (eBioscience, San Diego, CA), E-selectin, FucT-VII (BD Pharmingen, San Diego, CA). We also used same-species, same-isotype, irrelevant antibodies (R&D Systems, Minneapolis, MN).
Adhesion of EPCs to E-selectin–coated culture dish
Please see Document S1 for more information.
Assessment of E-selectin-dependent eGFP-EPC homing to ischemic hindlimb in WT or E-sel−/− mice
To demonstrate the necessity of E-selectin for EPC homing to ischemic limb, we used 2 strategies to block E-selectin expression: one was blocking anti–E-selectin antibody in WT mice, and the other was E-sel−/− mice. After unilateral femoral artery excision in WT mice and age-matched B6, 129S2-Seletm1Hyn/J (E-sel−/−) mice (Jackson Laboratory, Bar Harbor, ME), a million EPCs, which were harvested from eGFP-transgenic mice and cultured, were administered systemically by cardiac puncture using an insulin syringe with 27-guage needle. In WT mice, 10 μg of anti–E-selectin antibody or control antibody were administered to 4 mice with hindlimb ischemia 30 minutes prior to cell transplantation intravenously. In E-sel−/− mice, 10 μg sE-selectin or bovine serum albumin (BSA) were intramuscularly administered 30 minutes before cell transplantation. To evaluate EPC incorporation into the vasculature in ischemic muscles, mice in each group were harvested at day 7, and the calf muscles of ischemic limbs were prepared for fluorescent imaging. ECs were identified by immunofluorescent staining for BS-1 lectin (TRITC conjugate, Sigma, St Louis, MO). Five random fields were selected from each slice, and dual-positive cells (green and red) were counted as incorporated EPCs.
Effect of sE-selectin on ECs expression of adhesion molecule in vitro and in vivo
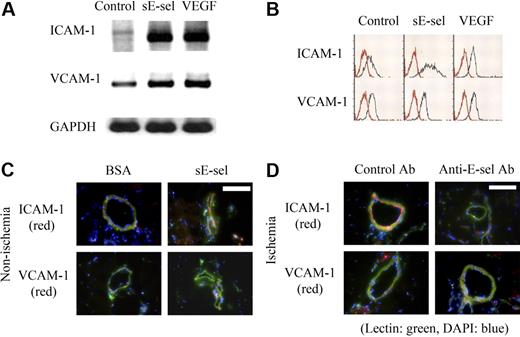
Mouse ECs were starved for 6 hours with 0.1% fetal bovine serum (FBS) in endothelial basal medium (EBM) medium. Cells were then stimulated with sE-selectin (4 μg/mL) and vascular endothelial growth factor (VEGF) (100 ng/mL) in the presence of 0.1% FBS for 1 hour. To assess the change of surface adhesion molecule, we performed reverse transcriptase–polymerase chain reaction (RT-PCR) and FACS analysis as described previously.14 We used monoclonal fluorescein isothiocyanate (FITC)–conjugated anti–ICAM-1 (R&D Systems) and monoclonal FITC-conjugated anti–VCAM-1 (R&D Systems).
To evaluate the effect of sE-selectin on the expression of ICAM-1 and VCAM-1 in ischemic tissue, sE-selectin 10 μg or BSA were injected into muscle in normal mice, and E-selectin monoclonal antibody 10 μg or control antibody were injected into ischemic muscle in hindlimb ischemic mice. After 24 hours, the mice were killed, and we performed immunofluorescent staining using anti-mouse ICAM-1, VCAM-1, and BS-1 lectin antibodies as primary antibodies, and FITC (green, for BS-1 lectin)–conjugated or phycoerythrin (PE; red, for ICAM-1/VCAM-1)–conjugated antibodies as secondary antibodies.
Effect of sE-selectin on EPCs migration and tube formation on matrigel through interleukin-8
Please see Document S1 for more information.
In vivo knock down of E-selectin using administration of high-volume E-selectin siRNA
E-selectin siRNAs were delivered in vivo using a modified “hydrodynamic transfection method,”18 by which 100 to 500 pmol siRNA dissolved in 0.8 to 1 mL transfection media was rapidly injected into jugular or femoral vein through a 27-gauge needle over 20 to 40 seconds. Control mice were injected with an equal volume of normal saline or control siRNA. To verify the effects of siRNA, we made hindlimb ischemia to induce the expression of E-selectin. Mice were killed 12 hours after surgery, and total RNA was isolated from murine organs (nonischemic muscle, ischemic muscle, heart, liver, and spleen). Real-time RT-PCR was done to detect the expression of E-selectin as described above.
Functional assessment of the murine ischemic hindlimb model
In an experiment to demonstrate that sE-selectin local treatment increases blood flow recovery to ischemic limb, WT mice received a local intramuscular injection of 10 μg sE-selectin or BSA in the center of the lower calf muscle, just after operative excision of one femoral artery and 30 minutes prior to an intra-cardiac systemic injection of a million of culture-expanded EPCs from eGFP transgenic mice. Four mice in each group were harvested at day 7 for quantitative analysis of incorporated EPCs. Laser Doppler perfusion image (LDPI) analyzer was used to record serial blood flow measurements over the course of 3 weeks after operation. At day 21, the outcomes of ischemic limb were assessed as 3 categories: “limb salvage,” “tip necrosis,” or “auto-amputation.”19
In an experiment to demonstrate that E-selectin knock down impairs blood flow recovery to ischemic limb and that such impairment can be rescued by local treatment of sE-selectin, we used 3 groups: control siRNA-treated mice with BSA injection, E-selectin siRNA-treated mice with BSA injection, and E-selectin siRNA-treated mice with sE-selectin injection.
Image acquisition
Images were acquired using an Olympus IX2 inverted fluorescence microscope (Olympus, Tokyo, Japan) equipped with an Olympus DP50 CF CCD camera with LCPlan FI 10×/0.30 numeric aperture (NA) PhC and 40×/0.60 NA Ph2 objectives and analySIS 5.0 software (Olympus Soft Imaging Solutions, Münster, Germany). Images were assembled using Adobe Photoshop CS2 software version 9.0 (Adobe Systems, San Jose, CA).
Statistical analysis
All data are presented as mean plus or minus SD. Intergroup comparisons were performed by Mann-Whitney test or Kruskal-Wallis test. Probability values of P less than .05 were interpreted to denote statistical significance. All calculations were performed using SPSS 12.0.
Results
Membrane-bound E-selectin in vessels and soluble E-selectin in serum increased after ischemia induction in a mouse hindlimb ischemia model
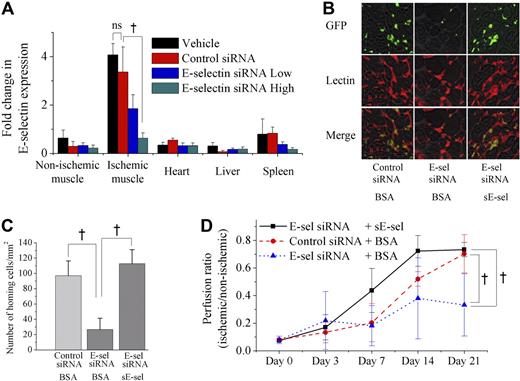
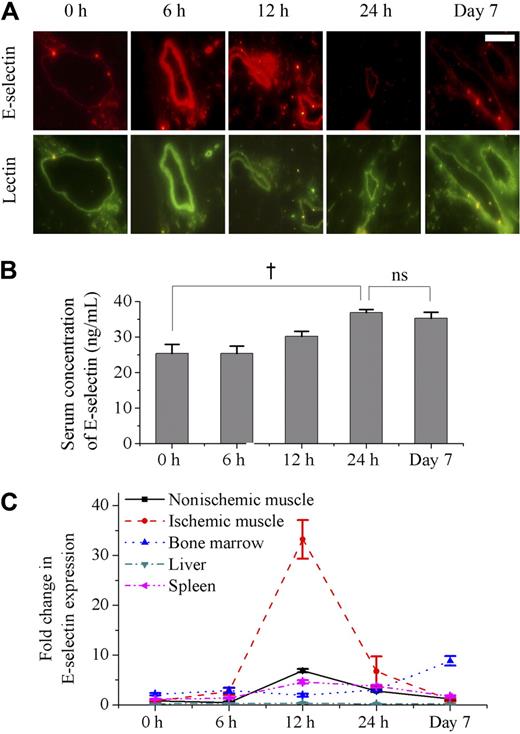
To identify the changes of E-selectin in response to ischemia, the expression of mE-selectin and sE-selectin were measured by an immunofluorescence examination of nonischemic and ischemic muscles and ELISA of serum, respectively. Constitutive mE-selectin expression was low in nonischemic muscle (0 hour). After ischemia, its expression peaked between 6 and 12 hours and returned to baseline levels by 24 hours. The expression of mE-selectin 7 days after ischemia was the same as that of nonischemia (Figure 1A). In addition, mE-selectin was expressed only on small- to medium-sized vessels (0.05-0.1 mm) and not found on the capillaries. These findings were similar to our previous report, where we showed the expression pattern of various adhesion molecules in ischemic tissue at 24 hours after ischemia.14 As for sE-selectin, it increased gradually in serum after ischemia and peaked at 24 hours. In contrast to mE-selectin, sE-selectin level was maintained up to 7 days after ischemia (Figure 1B)
Sequential changes of mE-selectin in vessels, sE-selectin in serum, and mRNA levels for E-selectin in various tissues after induction of limb ischemia in mice. (A) Immunofluorescent staining of mE-selectin (red) and murine EC (BS-1 lectin, green) in muscle after ischemia (scale bar is 200 μm). The expression level of mE-selectin was low in vessels of nonischemic muscle (0 hour). However, it peaked between 6 and 12 hours after ischemia and returned to baseline level by 24 hours. It showed no change at day 7. (B) ELISA analysis showed the changes of sE-selectin concentration in serum after ischemia. sE-selectin increased gradually over 24 hours. It was still high at 7 days (†P < .05; n = 4). ns indicates not significant. (C) Real-time RT-PCR demonstrated the comparison of E-selectin mRNA expression in various tissues after ischemia (n = 4). The level of mRNA for E-selectin in ischemic muscle showed a remarkably high peak around 12 hours after induction of ischemia, whereas nonischemic muscle showed a mild increase at 12 hours. In bone marrow, it was maintained at a high level at day 7. On the other hand, it had been low throughout 7 days in other tissues such as liver and spleen. Error bars in panels B and C represent SD.
Sequential changes of mE-selectin in vessels, sE-selectin in serum, and mRNA levels for E-selectin in various tissues after induction of limb ischemia in mice. (A) Immunofluorescent staining of mE-selectin (red) and murine EC (BS-1 lectin, green) in muscle after ischemia (scale bar is 200 μm). The expression level of mE-selectin was low in vessels of nonischemic muscle (0 hour). However, it peaked between 6 and 12 hours after ischemia and returned to baseline level by 24 hours. It showed no change at day 7. (B) ELISA analysis showed the changes of sE-selectin concentration in serum after ischemia. sE-selectin increased gradually over 24 hours. It was still high at 7 days (†P < .05; n = 4). ns indicates not significant. (C) Real-time RT-PCR demonstrated the comparison of E-selectin mRNA expression in various tissues after ischemia (n = 4). The level of mRNA for E-selectin in ischemic muscle showed a remarkably high peak around 12 hours after induction of ischemia, whereas nonischemic muscle showed a mild increase at 12 hours. In bone marrow, it was maintained at a high level at day 7. On the other hand, it had been low throughout 7 days in other tissues such as liver and spleen. Error bars in panels B and C represent SD.
Since the source of the highly maintained sE-selectin could be from other tissues besides the ischemic muscle, we tried to quantify the expression of E-selectin in various tissues using real-time RT-PCR. As expected, the E-selectin mRNA expression showed a sharp peak in the ischemic muscle in contrast to a mild rise in the nonischemic muscle at 12 hours after induction of hindlimb ischemia (Figure 1C). Interestingly, in the bone marrow, although the increase in E-selectin mRNA expression was less dramatic than that of the ischemic muscle, the level of E-selectin expression was maintained at a significantly increased level at 1 week. In other tissues, such as the liver and spleen, the change was only minimal.
BM-derived EPCs express both E-selectin and its ligands, but EPC differentiation was not affected by E-selectin blockade
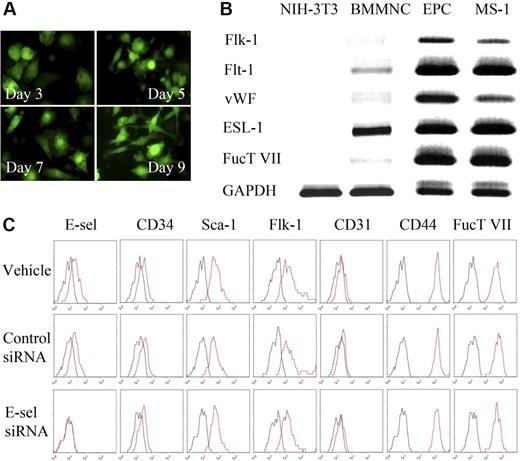
Next, we examined whether BM-derived EPCs contain potential ligands capable of interaction with E-selectin. BMMNCs were cultured in an EPC-culture condition for 9 days, when they became spindle shaped (Figure 2A) and expressed mRNAs of Flt-1, Flk-1, and VWF, as shown by RT-PCR (Figure 2B), which are characteristics of EPCs. These cells were used as EPCs for the experiments. A functional E-selectin ligand requires the addition of a fucose residue in a α1,3 linkage to α2,3-sialyllactosamine precursor, a reaction catalyzed by FucT-VII.20 EPCs cultured from mouse bone marrow also expressed the mRNA of ESL-1 and FucT-VII as shown by RT-PCR (Figure 2B). The expression of FucT-VII in EPCs suggests the possibility that ESL or its variants expressed on EPCs is most likely a fucosylated, functional ligand capable of binding to E-selectin expressed on activated ECs. The expression of endothelial surface markers such as flk-1 and CD31, as well as stem cell markers CD34 and Sca-1, along with E-selectin and E-selectin ligands CD4421 and FucT VII, was also confirmed by flow cytometry (Figure 2C, first row).
Characterization of murine bone marrow–derived EPCs and the effect of E-selectin on EPC differentiation. (A) Sequential changes of cultured BM-derived cells from eGFP-transgenic mice. Round, floating cells had changed into spindle-like, attaching cells as times went by. (B) RT-PCR analysis showing RNA expression profiles of EC-specific markers and functional E-selectin ligands in fibroblasts (NIH-3T3), bone marrow mononuclear cells, EPCs, and EC (MS-1) (ESL-1, E-selectin ligand-1; FucT VII, fucosyltransferase-VII). (C) Surface antigens on EPC by flow cytometry. Both E-selectin and E-selectin ligands (CD44 and FucT VII), in addition to progenitor cell or EC makers, were expressed on EPCs. E-selectin expression on EPCs was totally suppressed by E-selectin siRNA, but not by control siRNA, demonstrating the reliability of both E-selectin and control siRNA action. But the expression of other EC-specific markers and progenitor cell markers, or EPC differentiation was affected little by E-selectin siRNA.
Characterization of murine bone marrow–derived EPCs and the effect of E-selectin on EPC differentiation. (A) Sequential changes of cultured BM-derived cells from eGFP-transgenic mice. Round, floating cells had changed into spindle-like, attaching cells as times went by. (B) RT-PCR analysis showing RNA expression profiles of EC-specific markers and functional E-selectin ligands in fibroblasts (NIH-3T3), bone marrow mononuclear cells, EPCs, and EC (MS-1) (ESL-1, E-selectin ligand-1; FucT VII, fucosyltransferase-VII). (C) Surface antigens on EPC by flow cytometry. Both E-selectin and E-selectin ligands (CD44 and FucT VII), in addition to progenitor cell or EC makers, were expressed on EPCs. E-selectin expression on EPCs was totally suppressed by E-selectin siRNA, but not by control siRNA, demonstrating the reliability of both E-selectin and control siRNA action. But the expression of other EC-specific markers and progenitor cell markers, or EPC differentiation was affected little by E-selectin siRNA.
To examine whether E-selectin played a significant role in EPC differentiation, we used siRNA to knock down E-selectin expression. E-selectin expression was totally suppressed by E-selectin siRNA, but not by control siRNA (Figure 2C, second and third rows). However, the expression of various surface markers was not affected by E-selectin siRNA, suggesting that although EPCs express E-selectin ligand on its surface, E-selectin does not directly affect EPC differentiation.
E-sel−/− mice showed impairment in EPCs homing to ischemic limb, which was rescued by local sE-selectin treatment
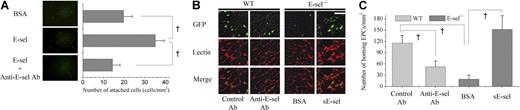
To demonstrate that EPCs actually bind with E-selectin for adhesion, we performed an in vitro adhesion assay. Significantly more EPCs adhered to gelatin plates adsorbed with E-selectin than plates adsorbed with BSA (Figure 3A; 35 ± 4 vs 14 ± 3 cells per high-power field (HPF), P < .05), and this was significantly reversed when plates were pretreated with an E-selectin blocking antibody (19 ± 4 cells/HPF), suggesting that EPCs have functional E-selectin ligands, which mediate the adhesion of EPCs.
The modulation of EPC homing to ischemic tissue by sE-selectin. (A) Representative figure showing more EPCs attached to E-selectin–adsorbed gelatin plate (E-sel) than BSA-adsorbed plate (BSA, control protein). Pretreatment with blocking antibody against E-selectin (anti–E-selectin Ab) significantly attenuated EPC attachment to the E-selectin–adsorbed plate. Quantitative analysis of attached EPCs (†P < .05; n = 4, respectively; scale bar, 500 μm). (B) Representative fluorescent images of muscle 1 week after ischemia (scale bar, 100 μm). One million EPCs cultured from eGFP transgenic mice (green) were “systemically administered” to WT and E-sel−/− mice after induction of ischemic hindlimb. Before EPC transplantation, either control antibody or anti–E-selectin antibody was injected into WT mice intravenously. In E-sel−/− mice, BSA or sE-selectin (sE-sel) was locally injected into the ischemic muscle. BS-1 lectin staining (red) was used for visualizing ECs. Dual-positive cells were the differentiated ECs from donor EPCs. They were less observed in anti–E-selectin antibody group of WT mice than in control antibody group. In E-sel−/− mice, sE-selectin treatment increased dual-positive cells compared with BSA treatment. (C) Quantitative analysis of incorporated EPCs to ischemic limb. The number of EPCs homing to ischemic limb decreased by blocking E-selectin in WT mice (†P < .05; n = 4, respectively) and increased by injecting sE-selectin in E-sel−/− mice (†P < .05; n = 4, respectively). Error bars in panels A and C represent SD.
The modulation of EPC homing to ischemic tissue by sE-selectin. (A) Representative figure showing more EPCs attached to E-selectin–adsorbed gelatin plate (E-sel) than BSA-adsorbed plate (BSA, control protein). Pretreatment with blocking antibody against E-selectin (anti–E-selectin Ab) significantly attenuated EPC attachment to the E-selectin–adsorbed plate. Quantitative analysis of attached EPCs (†P < .05; n = 4, respectively; scale bar, 500 μm). (B) Representative fluorescent images of muscle 1 week after ischemia (scale bar, 100 μm). One million EPCs cultured from eGFP transgenic mice (green) were “systemically administered” to WT and E-sel−/− mice after induction of ischemic hindlimb. Before EPC transplantation, either control antibody or anti–E-selectin antibody was injected into WT mice intravenously. In E-sel−/− mice, BSA or sE-selectin (sE-sel) was locally injected into the ischemic muscle. BS-1 lectin staining (red) was used for visualizing ECs. Dual-positive cells were the differentiated ECs from donor EPCs. They were less observed in anti–E-selectin antibody group of WT mice than in control antibody group. In E-sel−/− mice, sE-selectin treatment increased dual-positive cells compared with BSA treatment. (C) Quantitative analysis of incorporated EPCs to ischemic limb. The number of EPCs homing to ischemic limb decreased by blocking E-selectin in WT mice (†P < .05; n = 4, respectively) and increased by injecting sE-selectin in E-sel−/− mice (†P < .05; n = 4, respectively). Error bars in panels A and C represent SD.
From these initial findings, we hypothesized that E-selectin could play an important role in the homing of circulating EPCs to the ischemic limb and thus induction of new vessel formation. EPCs are well known to preferentially home to ischemic rather than normal limb. First, we performed in vivo experiments to test whether E-selectin is necessary for circulating EPC to home to ischemic limb. EPCs were ex vivo expanded from bone marrow mononuclear cells of eGFP transgenic mice and systemically administered to WT mice with ischemic hindlimb. The number of administered EPCs that homed to ischemic limb significantly decreased when E-selectin was blocked compared with that when E-selectin was not blocked (Figure 3B,C; control Ab vs anti–E-selectin Ab = 115 ± 20 vs 52 ± 15 cells/mm2; P < .05; n = 4, respectively).
Second, to confirm these observations, we induced limb ischemia in E-sel−/− mice. In E-sel−/− mice, the homing of systemically administered eGFP-EPCs to the ischemic limb was markedly decreased compared with WT mice (Figure 3B,C; WT mice vs E-sel−/− mice = 115 ± 20 vs 19 ± 12 cells/mm2; P < .05; n = 4, respectively). The decreased homing of EPCs to the ischemic limb in E-sel−/− mice, however, was rescued when sE-selectin was injected into ischemic limb (Figure 3B,C; E-sel−/− mice + BSA vs E-sel−/− mice + sE-selectin = 19 ± 12 vs 152 ± 37 cells/mm2, P < .05; n = 4, respectively), suggesting that not only mE-selectin but also sE-selectin may play an important role in the recruitment of EPCs and be sufficient to induce EPC homing.
Mechanism 1: effect of sE-selectin on ECs adhesion molecule
To examine the mechanism by which sE-selectin rescued the poor homing rate of EPCs to ischemic limb in E-sel−/− mice, we investigated the effects of sE-selectin on ECs and EPCs. First we examined the effect of sE-selectin on ECs. RT-PCR and FACS analysis showed that sE-selectin increased the expression of ICAM-1 markedly, but VCAM-1 mildly in ECs, which are related to EPC homing in ischemic muscle14,22 (Figure 4A,B). The magnitude of increase by stimulation of sE-selectin was greater in ICAM-1 than VCAM-1. VEGF, an inducer of these adhesion molecules,23 was used as positive control (Figure 4A,B). The magnitude of increase by VEGF stimulation was also greater in ICAM-1 than VCAM-1, which is the same as sE-selectin stimulation in terms of magnitude and trend.
The induction of adhesion molecules on ECs by sE-selectin. (A) RT-PCR analysis of ICAM-1 and VCAM-1 expression on ECs after stimulation with sE-selectin (sE-sel). (B) FACS analysis of ICAM-1 and VCAM-1 protein expression on ECs. sE-selectin increased both mRNA and protein expressions of ICAM-1 remarkably and VCAM-1 mildly in ECs. VEGF was used as positive control. (C,D) Immunofluorescent images of ICAM-1 and VCAM-1 in nonischemic (C) and ischemic hindlimb (D). The expression of ICAM-1 (red) on endothelium (green) was induced by injecting sE-selectin into nonischemic muscle (C, scale bar, 200 μm). Overexpressed ICAM-1 by ischemia was suppressed when E-selectin was neutralized by injecting anti–E-selectin antibody into ischemic muscle (D, scale bar, 200 μm). The expression of VCAM-1 was low in all conditions. DAPI (blue) staining was performed to visualize the nucleus.
The induction of adhesion molecules on ECs by sE-selectin. (A) RT-PCR analysis of ICAM-1 and VCAM-1 expression on ECs after stimulation with sE-selectin (sE-sel). (B) FACS analysis of ICAM-1 and VCAM-1 protein expression on ECs. sE-selectin increased both mRNA and protein expressions of ICAM-1 remarkably and VCAM-1 mildly in ECs. VEGF was used as positive control. (C,D) Immunofluorescent images of ICAM-1 and VCAM-1 in nonischemic (C) and ischemic hindlimb (D). The expression of ICAM-1 (red) on endothelium (green) was induced by injecting sE-selectin into nonischemic muscle (C, scale bar, 200 μm). Overexpressed ICAM-1 by ischemia was suppressed when E-selectin was neutralized by injecting anti–E-selectin antibody into ischemic muscle (D, scale bar, 200 μm). The expression of VCAM-1 was low in all conditions. DAPI (blue) staining was performed to visualize the nucleus.
To confirm these in vitro observations, we performed in vivo experiments. When sE-selectin was injected into the nonischemic hindlimb, ICAM-1 expression increased markedly (Figure 4C), suggesting that sE-selectin is sufficient to induce ICAM-1 expression. Conversely, the increased ICAM-1 expression induced by ischemia was suppressed when E-selectin was neutralized by injecting anti–E-selectin antibody into the ischemic muscle (Figure 4D), suggesting that sE-selectin is necessary for ICAM-1 expression. We did not see much difference in the expression of VCAM-1 in immunofluorescent examination of the muscle tissues.
Mechanism 2: effect of sE-selectin on EPCs migration and incorporation to ECs capillary formation
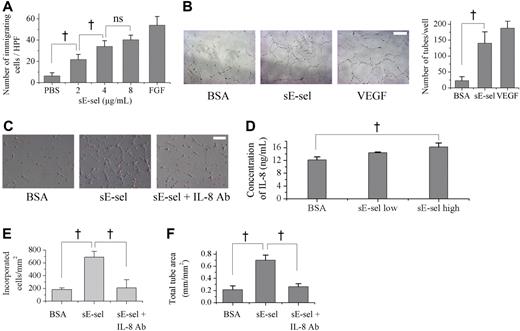
As another possible underlying mechanism, we hypothesized that sE-selectin may not only stimulate ECs but also modulate EPCs, which have functional E-selectin ligands on their surface. Thus, we examined the effect of sE-selectin on several cell biologic functions of EPC. First, the effect of sE-selectin on EPC chemotaxis, an integral component of new vessel formation, was evaluated in 10-well modified Boyden chambers. sE-selectin induced a dose-dependent increase in EPC migration, reaching significance at 2 μg/mL to 4 μg/mL (Figure 5A; negative control vs 2 μg/mL, 2 μg/mL vs 4 μg/mL, P < .05; 4 μg/mL vs 8 μg/mL sE-selectin, P = .08), compared with negative control PBS. FGF was used as positive control.
The chemotaxis and tube formation of EPCs by sE-selectin. (A) Modified Boyden chamber assay showed that sE-selectin (sE-sel) enhanced chemotaxis of EPC dose-dependently. PBS and FGF were used as negative and positive control, respectively (†P < .05; ns, P = .08; n = 4). (B) Capillary tube formation on matrigel (scale bar, 50 μm). sE-selectin induced the capillary tube formation of EPC as much as VEGF, positive control in contrast to BSA, negative control (BSA vs sE-selectin = 22.5 ± 12.6 vs 140.0 ± 35.6; †P < .05; n = 4). (C) Representative figure of EPCs, incubated on the ECM (scale bar, 50 μm). EPCs that were labeled with DiI (red) and HUVEC were cocultured. (D) Concentration of interleukin-8 (IL-8) in supernatant measured by ELISA. EPCs secreted a higher level of IL-8 when they were treated with sE-selectin (†P < .05; n = 4). (E, F) More incorporation of EPCs to ECs (E) and more capillary tube formation of EPCs (F) were observed when sE-selectin was added to culture media. Both effects were attenuated by blocking IL-8. (†P < .05; n = 4). Error bars in panels A, B, D-F represent SD.
The chemotaxis and tube formation of EPCs by sE-selectin. (A) Modified Boyden chamber assay showed that sE-selectin (sE-sel) enhanced chemotaxis of EPC dose-dependently. PBS and FGF were used as negative and positive control, respectively (†P < .05; ns, P = .08; n = 4). (B) Capillary tube formation on matrigel (scale bar, 50 μm). sE-selectin induced the capillary tube formation of EPC as much as VEGF, positive control in contrast to BSA, negative control (BSA vs sE-selectin = 22.5 ± 12.6 vs 140.0 ± 35.6; †P < .05; n = 4). (C) Representative figure of EPCs, incubated on the ECM (scale bar, 50 μm). EPCs that were labeled with DiI (red) and HUVEC were cocultured. (D) Concentration of interleukin-8 (IL-8) in supernatant measured by ELISA. EPCs secreted a higher level of IL-8 when they were treated with sE-selectin (†P < .05; n = 4). (E, F) More incorporation of EPCs to ECs (E) and more capillary tube formation of EPCs (F) were observed when sE-selectin was added to culture media. Both effects were attenuated by blocking IL-8. (†P < .05; n = 4). Error bars in panels A, B, D-F represent SD.
Second, Matrigel tube formation assay was performed to investigate the ability of EPCs to form EC networks reminiscent of tubules. EPCs seldom formed tubules in BSA but, when exposed to sE-selectin, they formed capillary networks by themselves, similar to the phenomenon observed when cell were treated with VEGF (Figure 5B; number of tubes per well: BSA versus sE-selectin = 22.5 ± 12.6 vs 140.0 ± 35.6, P < .05).
Third, we found that sE-selectin increased incorporation of EPCs to ECs when we cocultured EPCs with human umbilical vein endothelial cells (HUVECs; Figure 5C).
Finally, to investigate the mechanism how sE-selectin enhanced EPCs functions such as capillary tube formation or incorporation to ECs, several cytokines in the supernatant of sE-selectin–treated EPCs were measured. The concentration of IL-8 was significantly higher in the supernatant of EPC treated with sE-selectin than with BSA (Figure 5D; BSA vs sE-selectin high = 12.2 ± 0.9 vs 16.2 ± 1.2 ng/mL; P < .05; n = 4) in contrast to the other cytokines (IL-2, 4, 5, 10, 12, 13, GM-CSF, IFN-gamma, TNF-alpha, VEGF), which were not affected by sE-selectin treatment.
To clarify that IL-8 is an effector molecule that transmits the effect of sE-selectin on EPCs, we used neutralizing antibody against IL-8. The increased incorporation and capillary tube formation of EPCs after sE-selectin treatment were attenuated when IL-8 blocking antibody was added to the culture media (Figure 5C,E,F) (incorporated cells, BSA vs E-sel vs E-sel + IL-8 Ab = 183 ± 28 vs 688 ± 91 vs 211 ± 127 cells/mm2; total tube area, 0.21 ± 0.06 vs 0.70 ± 0.08 vs 0.26 ± 0.05 mm/mm2).
Taken together, sE-selectin may not only up-regulate ICAM-1 on ECs, but also increase EPC chemotaxis, capillary tube formation, and incorporation to ECs through IL-8. These effects may thus provide an adequate local milieu for EPC homing and rescue the decreased homing of EPCs observed in E-sel−/− mice as demonstrated in Figure 3B.
Local treatment with sE-selectin enhances the efficacy of EPC transplantation to increase vasculogenesis in ischemic limb
Based on the data that E-selectin is necessary for EPC homing and that sE-selectin modulates both ECs and EPCs to rescue the poor EPCs' homing observed in E-sel−/− mice, we next investigated the effect of sE-selectin to enhance vasculogenesis in ischemic limb. To test whether local injection of sE-selectin to ischemic limb could enhance recruitment of transplanted GFP-positive EPCs from the systemic circulation to ischemic limb in WT mice, we quantified incorporation of transplanted EPCs into the microvasculature of ischemic limbs. At day 7 after treatment, histological examination showed that sE-selectin pretreatment increased EPC homing in the ischemic muscle (Figure 6A). The number of EPCs that homed to ischemic limb was markedly increased by sE-selectin, but not by BSA (Figure 6B; 109 ± 24 vs 155 ± 15; P < .05). Serial measurements of hindlimb perfusion by LDPI were performed at days 0, 3, 7, 14, and 21 in WT mice, which received local intramuscular administration of either sE-selectin or BSA 30 minutes before systemic EPC transplantation. LDPI showed profound improvement in perfusion recovery of the ischemic limb at day 14 and 21 after induction of limb ischemia in sE-selectin group (Figure 6C; perfusion ratio of ischemic limb to nonischemic limb: day 14, 0.68 ± 0.06 vs 0.46 ± 0.08; day 21, 0.79 ± 0.10 vs 0.59 ± 0.08; P < .05; n = 9, respectively). Limb salvage was more frequently observed in the sE-selectin group (55.5%, 5 of 9) than the control group (33.3%, 3 of 9; Figure 6D).
Local treatment with sE-selectin enhanced homing of circulating EPCs and vasculogenesis in ischemic hindlimb of WT mice. A million EPCs cultured from bone marrow of eGFP transgenic mice (green) were systemically transplanted into WT mice after induction of ischemic hindlimb. Before EPC transplantation, either BSA or sE-selectin (sE-sel) was injected into ischemic muscle. (A) Representative fluorescent image of muscle one week after ischemia (scale bar, 100 μm). Transplanted EPCs were identified in tissue sections by green fluorescence, and the ECs were stained by BS-1 lectin (red) in the same tissue sections. (B) Quantitative analysis of incorporated EPCs. EPC homing was enhanced to ischemic muscle where sE-selectin was injected compared with BSA (BSA vs sE-selectin = 109 ± 23 vs 154 ± 14 cells/mm2; †P < .05; n = 4). (C) Computer-assisted quantitative analysis of hindlimb perfusion showed a significantly improved blood perfusion ratio of ischemic/nonischemic limb in the group of mice injected with sE-selectin than BSA at day 14 and 21 (†P < .05; n = 9, respectively). (D) Local injection of sE-selectin rescued ischemic limb more than BSA. Administration of sE-selectin resulted in a lower rate of limb loss (amputation) compared with BSA (sE-selectin vs BSA, 11.1% [1 of 9] vs 33.3% [3 of 9]). The rate of complete limb salvage was also higher in sE-selectin (55.5% [5 of 9]) than BSA (33.3% [3 of 9]). Error bars in panels B and C represent SD.
Local treatment with sE-selectin enhanced homing of circulating EPCs and vasculogenesis in ischemic hindlimb of WT mice. A million EPCs cultured from bone marrow of eGFP transgenic mice (green) were systemically transplanted into WT mice after induction of ischemic hindlimb. Before EPC transplantation, either BSA or sE-selectin (sE-sel) was injected into ischemic muscle. (A) Representative fluorescent image of muscle one week after ischemia (scale bar, 100 μm). Transplanted EPCs were identified in tissue sections by green fluorescence, and the ECs were stained by BS-1 lectin (red) in the same tissue sections. (B) Quantitative analysis of incorporated EPCs. EPC homing was enhanced to ischemic muscle where sE-selectin was injected compared with BSA (BSA vs sE-selectin = 109 ± 23 vs 154 ± 14 cells/mm2; †P < .05; n = 4). (C) Computer-assisted quantitative analysis of hindlimb perfusion showed a significantly improved blood perfusion ratio of ischemic/nonischemic limb in the group of mice injected with sE-selectin than BSA at day 14 and 21 (†P < .05; n = 9, respectively). (D) Local injection of sE-selectin rescued ischemic limb more than BSA. Administration of sE-selectin resulted in a lower rate of limb loss (amputation) compared with BSA (sE-selectin vs BSA, 11.1% [1 of 9] vs 33.3% [3 of 9]). The rate of complete limb salvage was also higher in sE-selectin (55.5% [5 of 9]) than BSA (33.3% [3 of 9]). Error bars in panels B and C represent SD.
Knock down of E-selectin by siRNA reduces the efficacy of EPC transplantation for vasculogenesis in ischemic limb, which was rescued by treatment with sE-selectin
As we observed that sE-selectin potentiated the therapeutic efficacy of EPC transplantation to increase vasculogenesis in ischemic limb, we next examined whether blocking of E-selectin may inhibit the efficacy of EPCs transplantation for ischemic limb and then whether this inhibition would be rescued by local treatment of sE-selectin. First, to block E-selectin expression and thus “knock down” its function, E-selectin siRNA was delivered in vivo using a modified “hydrodynamic transfection method.”18 Real-time RT-PCR analysis showed that the expression of E-selectin in various tissues after ischemia was significantly suppressed by administration of E-selectin siRNA, especially in ischemic muscle, but not by vehicle or control siRNA (Figure 7A; P < .05; n = 4). Then, 106 EPCs cultured from eGFP transgenic mice (green) were transplanted into E-selectin siRNA-treated, in other words, E-selectin knocked down mice, after induction of hindlimb ischemia. The number of EPCs homing to ischemic limb was markedly decreased by E-selectin siRNA but not by control siRNA (Figure 7B,C; 97 ± 19 vs 27 ± 15 cells/mm2; P < .05; n = 4, respectively). LDPI showed that knock down of E-selectin (blue triangle) significantly attenuated blood flow recovery to ischemic limb compared with the control (red circle) (Figure 7D; E-selectin siRNA-treated group vs control siRNA-treated group at day 21 = 0.33 ± 0.23 vs 0.70 ± 0.13; P < .05; n = 7, respectively). To confirm that the impaired vasculogenesis by E-selectin siRNA would be rescued by local treatment of sE-selectin, we injected sE-selectin into ischemic hindlimb before EPC transplantation in E-selectin siRNA-treated animals. sE-selectin treatment rescued the impaired EPCs homing in E-selectin siRNA-treated mice (27 ± 15 vs 113 ± 18 cells/mm2; P < .05; n = 4, respectively) (Figure 7B,C). Furthermore, administration of sE-selectin (black square) was able to rescue the impaired blood flow recovery of the ischemic limb in E-selectin knock down animals (blue triangle) (Figure 7D; sE-selectin/E-selectin-siRNA-treated group vs BSA/E-selectin-siRNA-treated group at day 21 = 0.73 ± 0.05 vs 0.33 ± 0.23; P < .05; n = 7, respectively).
Knock down of E-selectin by in vivo administration of E-selectin siRNA impaired vasculogenesis of ischemic hindlimb, which was rescued by sE-selectin treatment. (A) Real-time RT-PCR showed that E-selectin siRNA significantly attenuated the expression of E-selectin in various tissues after ischemia, especially in ischemic muscle, when the mice were treated with E-selectin siRNA (†P < .05; n = 4). (B-D) A million EPCs cultured from bone marrow of eGFP transgenic mice (green) were transplanted into siRNA-treated mice after induction of ischemic hindlimb. Before EPC transplantation, either BSA or sE-selectin (sE-sel) was injected into ischemic muscle. (B) Representative fluorescent images of muscle 1 week after ischemia (scale bar, 100 μm). BS-1 lectin staining (red) was used for visualizing ECs. (C) Quantitative analysis of incorporated EPCs to ischemic limb. The number of homing EPCs to ischemic limb was markedly decreased by E-selectin siRNA, but not by control siRNA (†P < .05). Injecting sE-selectin recovered EPC homing in E-selectin siRNA-treated mice (†P < .05). (D) LDPI analysis of siRNA-treated mice. Compared with control animals treated with control siRNA + BSA (red circle), the animals treated with E-selectin siRNA + BSA (blue triangle) showed significantly attenuated blood flow recovery to ischemic limb. But administration of sE-selectin (black square) was able to rescue the impaired blood flow recovery to ischemic limb in E-selectin siRNA-treated animals (blue triangle) (†P < .05; n = 7, respectively). Error bars in panels A, C, and D represent SD.
Knock down of E-selectin by in vivo administration of E-selectin siRNA impaired vasculogenesis of ischemic hindlimb, which was rescued by sE-selectin treatment. (A) Real-time RT-PCR showed that E-selectin siRNA significantly attenuated the expression of E-selectin in various tissues after ischemia, especially in ischemic muscle, when the mice were treated with E-selectin siRNA (†P < .05; n = 4). (B-D) A million EPCs cultured from bone marrow of eGFP transgenic mice (green) were transplanted into siRNA-treated mice after induction of ischemic hindlimb. Before EPC transplantation, either BSA or sE-selectin (sE-sel) was injected into ischemic muscle. (B) Representative fluorescent images of muscle 1 week after ischemia (scale bar, 100 μm). BS-1 lectin staining (red) was used for visualizing ECs. (C) Quantitative analysis of incorporated EPCs to ischemic limb. The number of homing EPCs to ischemic limb was markedly decreased by E-selectin siRNA, but not by control siRNA (†P < .05). Injecting sE-selectin recovered EPC homing in E-selectin siRNA-treated mice (†P < .05). (D) LDPI analysis of siRNA-treated mice. Compared with control animals treated with control siRNA + BSA (red circle), the animals treated with E-selectin siRNA + BSA (blue triangle) showed significantly attenuated blood flow recovery to ischemic limb. But administration of sE-selectin (black square) was able to rescue the impaired blood flow recovery to ischemic limb in E-selectin siRNA-treated animals (blue triangle) (†P < .05; n = 7, respectively). Error bars in panels A, C, and D represent SD.
Discussion
In this study, we have shown that (1) EPCs have a functional ligand capable of interaction with E-selectin, (2) mE-selectin is expressed in ischemic tissue at an early time point and falls back down to baseline levels at 24 hours, while sE-selectin is increased up to 24 hours in the serum, (3) E-selectin is necessary for the up-regulation of ICAM-1 even under the ischemic condition and thus plays an important role in the recruitment of circulating EPCs into ischemic tissues, and (4) sE-selectin not only significantly augments vasculogenesis by EPCs in WT mice, but also rescues E-sel−/− mice or E-selectin knock down mice from attenuated new vessel formation through increased homing of EPCs.
Expression of mE-selectin and sE-selectin in an ischemic disease model
EC adhesion molecules have dual forms in vivo. One is a cell surface (bound) form, and the other is a soluble (unbound) form. Membrane form of cell adhesion molecules such as E-selectin, P-selectin, and L-selectin are reported to be involved in leukocyte rolling.7 Levels of soluble adhesion molecules have been postulated to be useful predictors of cardiovascular events in healthy populations and various settings of diseases, even though their pathological role remains uncertain.24 In a previous study,25 TNF-α up-regulated mE-selectin in ECs with maximum expression at 6 hours, which returned to baseline levels by 24 hours. sE-selectin, when shed into the microcirculation, increased from 12 hours through 24 hours.
In the present study, after the induction of ischemia the expression of mE-selectin peaked at 6 to 12 hours in small- to medium-sized vessels in ischemic muscle and returned to baseline level after 24 hours. This was similar to our previous report,14 where mE-selectin was only scantly expressed on small- to medium-sized vessels 1 day after ischemia. In contrast, the level of sE-selectin increased gradually and was maintained at a significantly increased level up to 7 days after ischemia. The reason or mechanism of the differential level of local and systemic E-selectin is unclear. Wyble et al have suggested that sE-selectin may originate from mE-selectin through shedding.25 The early increase within 1 day of sE-selectin could be explained by the significant increase in mE-selectin in the ischemic limb, which could be shed into the microcirculation, and the slight time lag between mE-selectin and sE-selectin supports this. The high level at day 7, however, suggested that other sources of sE-selectin may exist that respond to ischemia, considering the short half life of sE-selectin in vivo.26 Further experiments showed that the BM showed a gradual but significant increase in E-selectin expression up to 7 days after hindlimb ischemia, while the change in E-selectin expression was minimal in the liver or spleen, suggesting that the bone marrow could have been the source for the maintained level of sE-selectin at day 7. However, there may be other mechanisms involved, such as nonviable cell fragments,27 separate gene transcription,28 and alternative gene splicing,28-30 which have not been extensively studied. We believe this topic needs further investigations and experiments to more accurately dissect the relationship between mE-selectin and sE-selectin.
From these initial data and considering the fact that EPCs home to ischemic tissue for several days, we could not explain the homing of EPCs solely by the transient increase in mE-selectin, and thus, we hypothesized that sE-selectin could also play a role in this process.
The interaction between E-selectin and E-selectin ligand in differentiation and homing of EPC
The exact identity of E-selectin receptors remains to be fully understood. Selectin receptors require a protein or lipid core with multiple O- or N-linked oligosaccharide chains that contain the tetrasaccharide Neu NAc α2-3 Gal β1-4 (Fuc α1-3) Glc N Ac (sialyl-Lewis X) or a related structure.31 Several glycoproteins have been proposed as E-selectin ligands including PSGL-1,32 a glycoform of CD44 expressed on human HPCs,21 L-selectin,33 and in the mouse, E-selectin ligand-1 (ESL-1).34 In the present study, we found that EPCs and MS-1(mouse endothelial cell line) expressed ESL-1 and FucT-VII, suggesting that E-selectin(membrane-bound or soluble)–ESL-1 binding could affect the interaction between EPCs and vascular ECs and play a significant role in the homing of EPCs to ischemic muscle.
As shown in Figure 3B, when E-selectin was blocked by neutralizing antibody, the recruitment of EPCs into ischemic tissue decreased, suggesting that E-selectin may be important for EPC homing. However, since E-selectin blocking antibody blocks both types of E-selectin (membrane and soluble), and E-sel−/− mice have both types of E-selectin knocked out (data not shown), the role of sE-selectin could not be selectively studied. Therefore, we tested whether the injection of only sE-selectin could rescue the poor homing of EPCs in E-sel−/−. Interestingly, homing of EPCs recovered when sE-selectin was injected in ischemic limb, suggesting that sE-selectin is sufficient to induce EPC homing into ischemic tissue where mE-selectin does not exist.
Since EPCs contained both E-selectin and E-selectin ligand, we investigated whether modulation of E-selectin affects EPC differentiation. When bone marrow mononuclear cells were treated with E-selectin siRNA to almost completely knock out its function, we obtained EPCs at 7 to 10 days of culture, with similar characteristics to nontreated cells (Figure 2C), suggesting the binding between E-selectin and E-selectin ligand do not affect EPC differentiation.
Novel roles of sE-selectin in neovascularization
Koch et al showed in a rat cornea model using human umbilical vein ECs and human dermal microvascular ECs that sE-selectin is a potent angiogenic mediator.10,11 To further study the role of sE-selectin on EPC homing and new vessel formation, we analyzed multiple effects of sE-selectin on ECs and EPCs.
First, sE-selectin induced the expression of adhesion molecules in ECs, which increased the contact between ECs and EPCs. As shown in Figure 4, sE-selectin markedly increased the expression of ICAM-1 and very mildly increased VCAM-1. However, since ICAM-1 and VCAM-1 could be influenced by various cytokines that respond to ischemia, we were not sure whether these adhesion molecules were under the regulation of E-selectin. Therefore we showed that in nonischemic muscles, where ICAM-1 and VCAM-1 expression is extremely low, sE-selectin treatment resulted in augmentation of ICAM expression. Conversely, we also showed that even under ischemia, when E-selectin was blocked using blocking antibodies, the expression of ICAM-1 dropped dramatically. We believe that although the expression of ICAM-1 and VCAM-1 is controlled by multiple mechanisms and regulated by various cytokines, E-selectin is at least one of the partial regulators of these adhesion molecules, especially ICAM-1. Second, sE-selectin was a chemotactic factor for EPCs. As shown in Figure 5A, sE-selectin induced dose-dependent migration of EPCs toward sE-selectin. This suggests that EPCs can easily migrate into ischemic tissue if the concentration of sE-selectin is increased in the tissue. Third, sE-selectin augmented matrigel tube formation of EPCs. As shown in Figure 5B,C, more capillary tubes were observed in sE-selectin group. This suggests that E-selectin, which is known as cell adhesion molecule, may be another potent mediator of angiogenesis like VEGF,35 SDF-1,36 and G-CSF.37 Fourth, sE-selectin enhanced incorporation of EPCs into ECs and increased the secretion of IL-8 from EPCs. IL-8 is another well-known mediator of angiogenesis.38,39 On the basis of these in vitro data, we assumed that sE-selectin might play an important role in the EPC homing process.
In addition, we investigated whether sE-selectin would increase EPC homing and improve neovascularization in vivo in a hindlimb ischemia model. First we confirmed the importance of E-selectin in EPC homing and neovascularization in E-selectin siRNA-treated mice model where E-selectin was knocked down. EPC treatment for ischemic hindlimb was not effective when the E-selectin expression was suppressed. This phenomenon was significantly rescued by the treatment of sE-selectin, suggesting that E-selectin is necessary for significantly meaningful EPC homing and that sE-selectin injection is sufficient to induce EPC homing where E-selectin is absent or knocked down. Furthermore, to study a possible therapeutic role of sE-selectin, we injected sE-selectin into WT mice with hindlimb ischemia. This resulted in a significant increase in the recruitment of transplanted EPCs, and recovered blood flow in ischemic tissue compared with injection of control protein and thus led to greater salvage of ischemic hindlimb. These data suggest that local injection of soluble E-selectin could be a therapeutic modality in ischemic disease. To the best of our knowledge, this is the first experimental proof that sE-selectin has an important role in the recruitment of EPCs and neovasculogenesis. However, certain issues need to be addressed before sE-selectin can be considered more seriously as a therapeutic agent, such as its effect on atherosclerosis. Additional experiments using atherosclerotic animal models may shed light on this concern.
In conclusion, mE-selectin is an initial key adhesion molecule for EPC trafficking to ischemic limb. sE-selectin shed from mE-selectin is also an important molecule not only because it induces a second adhesion molecule such as ICAM-1, but also because it may directly induce mobilization or homing of circulating EPCs to ischemic tissue. Therefore, modulating the concentration of sE-selectin in target tissue could be a promising way to increase homing of EPCs and enhance neovascularization in the ischemic organ.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the program of National Research Laboratory for Cardiovascular Stem Cell, Stem Cell Research Center (SC 3150), Republic of Korea.
Authorship
Contribution: I.-Y.O. designed the research and analyzed data. C.-H.Y. designed the research and wrote the paper. J.H., J.-H.K., T.-Y.K., and C.-S.L. performed research. K.-W.P., I.-H.C., B.-H.O., and Y.-B.P. analyzed data. H.-S.K. designed the research and analyzed data.
I.-Y.O. and C.-H.Y. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hyo-Soo Kim or Young-Bae Park, Department of Internal Medicine, Seoul National University College of Medicine, 28 Yongon-dong Chongno-gu, Seoul 110–744, Korea; e-mail: hyosoo@snu.ac.kr.

![Figure 6. Local treatment with sE-selectin enhanced homing of circulating EPCs and vasculogenesis in ischemic hindlimb of WT mice. A million EPCs cultured from bone marrow of eGFP transgenic mice (green) were systemically transplanted into WT mice after induction of ischemic hindlimb. Before EPC transplantation, either BSA or sE-selectin (sE-sel) was injected into ischemic muscle. (A) Representative fluorescent image of muscle one week after ischemia (scale bar, 100 μm). Transplanted EPCs were identified in tissue sections by green fluorescence, and the ECs were stained by BS-1 lectin (red) in the same tissue sections. (B) Quantitative analysis of incorporated EPCs. EPC homing was enhanced to ischemic muscle where sE-selectin was injected compared with BSA (BSA vs sE-selectin = 109 ± 23 vs 154 ± 14 cells/mm2; †P < .05; n = 4). (C) Computer-assisted quantitative analysis of hindlimb perfusion showed a significantly improved blood perfusion ratio of ischemic/nonischemic limb in the group of mice injected with sE-selectin than BSA at day 14 and 21 (†P < .05; n = 9, respectively). (D) Local injection of sE-selectin rescued ischemic limb more than BSA. Administration of sE-selectin resulted in a lower rate of limb loss (amputation) compared with BSA (sE-selectin vs BSA, 11.1% [1 of 9] vs 33.3% [3 of 9]). The rate of complete limb salvage was also higher in sE-selectin (55.5% [5 of 9]) than BSA (33.3% [3 of 9]). Error bars in panels B and C represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/12/10.1182_blood-2006-10-048991/4/m_zh80230709870006.jpeg?Expires=1765898577&Signature=ZwmWBa69RG-Wz6rUY6yzcortL10ut~xD0KA6vqVwS-P~VWJiJ~RlLVW4Lkb2IzIsCAjdNhqgS4OYyAKXmHww-kLlXSZ94cFNEIIwF~TBf1eSRTQzkBYHobuFL-qvyJvGDY~yjesHZ0OGkBFpIBrTkjQAlOY-OkZev9OyN6M-iW6Dfbo4g1ZzkfbUSkyrSfBUfhnXgIJlYb~vqz0Hr3Vf9vp5qiaXKhZzbkZUEV42yLgbKdKFRSP8F~covSpsx-Zoj39OferHbum-tdOCa-ILMAiuJTjyrZlrfftxlrL5Q-6kgmZtWlphEByoHNm4FgdRxBzgESw1MWiUFxczBiBagQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)