Abstract
A major disadvantage of cord blood (CB) hematopoietic stem cell transplant is delayed engraftment. Cell dose has been identified as the key variable limiting neutrophil and platelet reconstitution. This has lead to the development of culture systems promoting the ex-vivo expansion of CB hematopoietic stem and progenitor cells (HSC/HPC). Typically such systems involve suspension cultures with cytokine supplementation, where the selected media supplies differing essential nutrients. Physiologically, HSCs are regulated by their interactions with the osteoblastic stem cell niche, a region characterized by low oxygen tension. We therefore investigated the effects of oxygen tension as well as differing medium composition on the ex-vivo expansion of CB HSC/HPC.
METHODS: Replilcate cultures of fresh CB CD34+ (3,000 cells/mL) cells were established at 5% (hypoxia) or 20% O2 (normoxia) in αMEM medium supplemented with 20% fetal bovine serum (FBS) or in CellGro serum-free medium. 100ng/mL stem cell factor (SCF), Fms-like tyrosine kinase 3 ligand (Flt3-L), thrombopoietin (Tpo) and granulocyte- colony stimulating factor (G-CSF) were added to each medium. The cellular output was evaluated after 7 and 14 days by counting total nucleated cells (TNC) and flow cytometric analysis measuring HSC/HPC (CD34), myeloid (CD11b), and megakaryocytic (CD41) cell progeny.
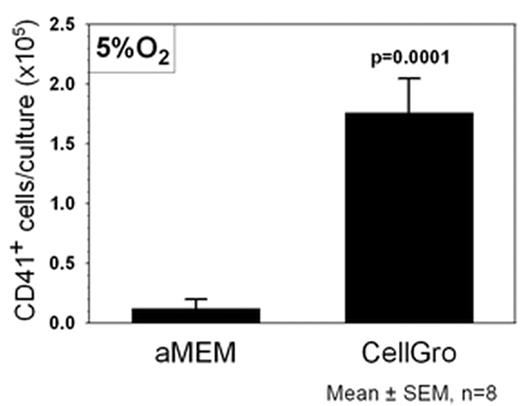
RESULTS: Cultures established under hypoxic conditions demonstrated a consistent increase in TNC (range 1.15 to 2.27-fold; N=8, p= 0.02) compared to those grown in normoxia. In addition, six of eight CB CD34+ samples showed equivalent or greater TNC production in CellGro versus αMEM/FBS. In accord with this, cultures initiated in CellGro at 5% O2 demonstrated a nearly 2-fold higher incidence (10.1% vs. 5.5%) and content (2.5 ± 0.5 x104vs. 1.3 + 0.3 x104, p=0.001) of CD34+ cells at day 7 than in αMEM/FBS. However the most striking difference between the two culture media was their capacity to support megakaryocyte differentiation in 5% O2. At day 14, a mean of 4.3% of cells cultured in CellGro expressed CD41 corresponding to a mean of 1.8± 0.3 x105 CD41+ cells/culture compared to only 0.12 ± 0.08 x105 CD41 cells (0.03% of cells) in αMEM/FBS cultures (p =0.0001). This reveals a 15-fold difference in megakaryocytic cell production. These data demonstrate that significant increases in TNC, CD34+ and CD41+ fractions can be gained by modifying oxygen tension and medium composition for ex-vivo expansion of CB progenitors. The increase in megakaryocytic cells may be of particular importance in ameliorating bleeding complications and the need for extensive platelet transfusions as a consequence of thrombocytopenia following CB transplant.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal