Abstract
The MPL (W515L and W515K) mutations have been detected in granulocytes of patients suffering from certain types of primitive myelofibrosis (PMF). It is still unknown whether this molecular event is also present in lymphoid cells and therefore potentially at the hematopoietic stem cell (HSC) level. Toward this goal, we conducted MPL genotyping of mature myeloid and lymphoid cells and of lymphoid/myeloid progenitors isolated from PMF patients carrying the W515 mutations. We detected both MPL mutations in granulocytes, monocytes, and platelets as well as natural killer (NK) cells but not in T cells. B/NK/myeloid and/or NK/myeloid CD34+CD38−-derived clones were found to carry the mutations. Long-term reconstitution of MPL W515 CD34+ cells in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice was successful for as long as 12 weeks after transplantation, indicating that MPL W515 mutations were present in HSCs. Moreover, the 2 MPL mutations induced a spontaneous megakaryocytic growth in culture with an overall normal response to thrombopoietin (TPO). In contrast, erythroid progenitors remained EPO dependent. These results demonstrate that in PMF, the MPL W515L or K mutation induces a spontaneous megakaryocyte (MK) differentiation and occurs in a multipotent HSCs.
Introduction
The BCR-ABL–negative chronic myeloproliferative disorders (MPDs) include polycythemia vera (PV), essential thrombocytosis (ET), and primitive myelofibrosis (PMF).1 These disorders are hematologic malignancies characterized by a clonal proliferation of one or several myeloid lineages.2 PV, ET, PMF, and chronic myeloid leukemia (CML), defined as the classical MPDs, are considered to arise from the transformation of a multipotent hematopoietic stem cell.3-6 However, the stem cell origin of the malignant clone had been demonstrated only in CML by the detection of either the characteristic t(9;22) translocation or the BCR/ABL transcript in all hematopoietic lineages.7 More recently, detection of the JAK2 V617F mutation in a lympho/myeloid progenitor has been shown in some PV and PMF.8 The JAK2 V617F mutation is found in almost all patients with PV, in 35% to 70% with ET, and in 50% with PMF.9-13 Recently, we have detected the JAK2 V617F mutation in B and natural killer (NK) cells in approximately half of the PMF patients and in a minority of PV patients. Using the fetal thymus organ culture (FTOC) and B/NK/myeloid assays, we also have demonstrated that immature lympho/myeloid progenitors from PV and PMF carry the JAK2 V617F.8
In 2006, 2 novel MPL somatic mutations (MPL W515L and MPL W515K) have been discovered in 5% and 1%, respectively, of JAK2 V617F–negative PMFs. The MPL W515L mutation confers to hematopoietic cells a cytokine-independent proliferation capacity, and results in a constitutive activation of JAK-STAT signaling.14 The MPL W515K was later identified by sequencing granulocytes from 1182 MPD patients, but its precise effects on signaling are presently unknown.15 W515 is the key amino acid located in a unique amphipathic domain that prevents spontaneous activation of MPL.16 The substitution of W515 to an alanine or a serine has been shown to induce a factor-independent growth of the Ba/F3 cell line.14,16 Therefore, it is expected that other amino acid changes in 515, such as the W515K, may induce a spontaneous MPL signaling.
The growth factor–independent cellular proliferation of the megakaryocytic lineage was suggested to be based on the spontaneous homodimerization of MPL.17-22 The viral oncogenic form of MPL, v-MPL, is characterized by the deletion of its extracellular domain. Its homodimerization results from the fusion of the truncated MPL sequence with the virus envelope.18,23 Furthermore, introduction of a cysteine residue at codon 368 leads to a covalent disulfide-bonded homodimerization19 and ligand-independent receptor activation. Similarly, the forced dimerization of MPL using monoclonal antibodies or the FK506-binding protein FKPB12 induces factor-independent proliferation.24 W515 seems to play an important role in the prevention of basal MPL homodimerization. Indeed, the amphipathic domain of MPL, more precisely W515, prevents 2 receptors from coming close to each other in the absence of thrombopoietin (TPO).16 In in vivo models, transplantation of murine hematopoietic stem cells transduced with v-MPL,18 W515L,14 or MPL devoid of its amphipathic domain (delta 5 TPO-R; S. Constantinescu, Ludwig Institute for Cancer Research, Brussels, Belgium, oral communication, May 2006) led to an acute lethal myeloproliferative disease. Because transcription is regulated by a ubiquitous promoter in these different models, the truncated or mutated MPL is expressed in all hematopoietic cell lineages. In human MPDs, the expression of MPL is relatively restricted,25 in contrast with the wide expression of JAK2. MPL is mainly expressed in the megakaryocytic/platelet lineage, but also in the stem cell and early hematopoietic progenitor compartment.25 In agreement with this expression pattern, it has been shown that TPO/MPL plays a major role both in the regulation of hematopoietic stem cells and in platelet production.26-29
To better understand the pathogenesis induced by the MPL W515L and W515K mutations, it is important to determine at which level of the hematopoietic differentiation these mutations occur. In this study, we have investigated whether they occurred at the myeloid, the lympho/myeloid, or the stem-cell compartment level and if it had any consequences in response to TPO.
Patients, materials, and methods
Patients and samples
The study was approved by the Local Research Ethics Committee from the St Louis, Henri Mondor, and La Pitié-Salpitrière hospitals and informed consent was obtained from each patient in accordance with the Declaration of Helsinki. Baseline characteristics and diagnostic information were collected prospectively, and clinical diagnoses were defined according to standard criteria. Patient diagnosis was defined according to the Italian criteria for PMF.30 Only patients presenting either a JAK2 V617F mutation or a MPL W515L/K mutation in the granulocytic compartment were enrolled in this study. Normal cytapheresis products from granulocyte colony-stimulating factor (G-CSF)–mobilized patients were used as controls, after informed consent.
Cell purification
Platelets were purified from blood samples using the platelet-rich plasma (PRP) technique. Granulocytes were obtained using a dextran (Sigma, St Louis, MO) basic method followed with a Ficoll separation and red blood cell lysis. Monocytes, and NK, B, and T cells were purified from peripheral blood mononuclear cells (PBMCs) by the Miltenyi (Paris, France) immunomagnetic bead technique according to the manufacturer's protocol. To obtain highly purified cell populations, immunomagnetic bead–isolated cells were labeled with anti–CD3-FITC, anti–CD19-PE, anti–CD14-PE-CY7 (Becton Dickinson, Le Pont de Claix, France), and anti–CD56-APC (Beckman Coulter, Roissy, France) antibodies and sorted using a FACS-DIVA cell sorter (Becton Dickinson). Isotype-matched antibodies were used as controls. Peripheral blood CD34+ cells were separated after Ficoll-metrizoate gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway) by the same immunomagnetic procedure (Miltenyi). The CD34+CD38− and CD34+CD38+ cell populations were subsequently isolated after the staining of immunomagnetically purified CD34+ cells with CD34-PeCy5 and CD38-FITC antibodies (Immunotech, Marseille-Lumigny, France) and flow cytometric cell sorting.
Assessment of CFU-GM and BFU-E differentiation: methyl assays
After CD34+ cell purification, methylcellulose cultures were performed as previously described.31 Briefly, cultures were performed with SCF and IL-3 cytokines to determine spontaneous erythroid cell growth, and with either a combination of SCF, IL-3, and G-CSF or a combination of SCF, IL-3, and EPO to obtain colony-forming unit–granulocyte-macrophage (CFU-GM) and burst-forming unit–erythroid (BFU-E) colonies, respectively. Colonies were counted, and picked at day 14. DNA was isolated from 5 to 50 colonies lysed in 0.2 mg/mL proteinase K (Invitrogen, Cergy-Pontoise, France) buffer containing 0.2% Tween20 (Sigma-Aldrich, St Quentin Fallavier, France) and subjected to genotyping.
Assessment of simultaneous B, NK, and granulo-monocytic differentiation
After the enrichment step described in “Cell purification,” the CD34+CD38− cell suspension was sorted at one cell per well in 96-well plates using the FACS-DIVA cell sorter (Becton Dickinson). According to the setup of the cell sorter, this procedure discards all doublets and cell aggregates. The exclusion of wells containing more than one cell was performed by a careful microscopic examination of each individual well 2 hours after sorting. Single CD34+CD38− cells were incubated on a confluent layer of MS-5 cells in RPMI medium supplemented with 10% human serum, 5% fetal calf serum (FCS), and a combination of 7 cytokines: 10 ng/mL IL-3 (generous gift from Novartis, Basel, Switzerland), 50 ng/mL SCF, 50 ng/mL FLT3-L (generous gifts from Immunex, Seattle WA), 10 ng/mL TPO (generous gift from Kyrin Laboratories, Tokyo, Japan), 20 ng/mL IL-7, 10 ng/mL IL15 (Peprotech, London, United Kingdom), and 5 ng/mL IL-2 (generous gift from Chiron laboratories, France). Wells with significant cell proliferation were collected after 4 to 6 weeks, and cell phenotype was determined by flow cytometry in half of the cell suspension using anti–CD15-FITC, anti–CD19-PE (Becton Dickinson), and anti–CD56-APC (Beckman Coulter) antibodies. The remaining cells were pelleted for genotyping analysis.
Assessment of long-term reconstitution in NOD/SCID mice
CD34+ cells from MPL W515L (550 000) or MPL W515K (660 000) PMF patients were intravenously injected into 2- to 8-week-old nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Prior to transplantation, mice received a preparative regimen consisting in 3.5 Gy irradiation followed with a single intraperitoneal injection of 75 μg anti-CD122 antibody generated from the TM-β1 hybridoma cell line. At 3, 6, 12, and 15 weeks after transplantation, bone marrow samples were aspirated from the right femurs. An immunophenotypic analysis was performed as previously described,32 and cells were seeded in methylcellulose for human colony assays. Each harvested human colony was genotyped to assess the presence of WT, W515L, and W515K MPL mutations. At 15 weeks after transplantation, mice were killed and secondary transplantation was performed. At 3 and 6 weeks after secondary transplantation, phenotypic analysis was performed.
Assessment of TPO dose response in MPL W515–mutated or JAK2 V617F CD34+ cell fraction
CD34+ cells were sorted as single cells into 96-well Terazaki plates containing 20 μL Iscoves modified Dulbecco medium (Invitrogen) supplemented with 250 U/mL penicillin, 250 μg/mL streptomycin, 2 mM glutamine (Invitrogen), 76 μM alphamonothioglycerol (Sigma), 1.5% deionized bovine serum albumin (BSA, Cohn fraction V; Sigma), 10 μL insulin-transferrin-selenium (Invitrogen), and a mixture of sonicated lipids (20 μL/mL). Various concentrations ranging from 0 to 100 ng/mL of a truncated form of recombinant human TPO (kindly provided by Kirin Brewery, Tokyo, Japan) were added to the serum-deprived medium. Two 96-well plates were used for each TPO concentration per patient sample (n = 3 for MPL-mutated PMF, n = 3 for JAK2 V617F PMF, and n = 3 for normal control cytapheresis products). Cells were cultured for 10 days at 37°C in a fully humidified atmosphere containing 5% CO2, fixed, and counted in situ at a × 100 magnification by the same manipulator. Previous studies have shown that only CD41+ cells develop under these conditions.27 The term “megakaryocyte clone” was used as a generic name for all megakaryocyte entities growing from single sorted cells.27,28 Such entities included colonies, generally defined as clones with 2 or more megakaryocytes, as well as 1 megakaryocyte clone.
Nucleic acid extraction
DNA and RNA were extracted from the different cell fractions using the RNA + reagent (Quantum; Appligene, Illkirch, France) and the Qiagen DNA extraction kit (Qiagen, Hilden, Germany), respectively.
Genotyping
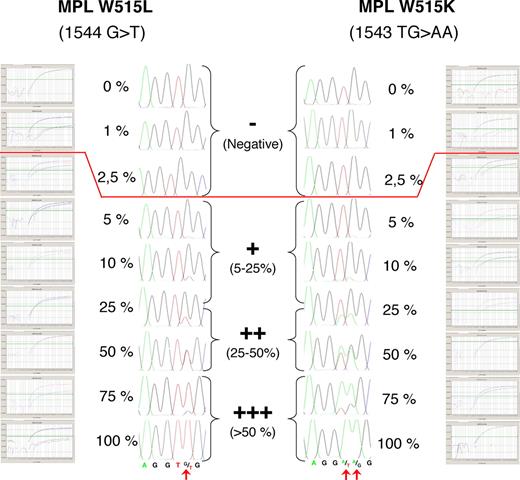
Direct sequencing was performed on the different cell fractions on DNA or RNA for platelet samples. For platelet RNA analysis, after reverse transcription, the cDNA (1 to 6 μL) served as a template in the polymerase chain reaction (PCR). Samples were subjected to 35 cycles of amplification under the following conditions: denaturing at 94°C for 30 seconds, annealing at 58°C for 2 minutes, extension at 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. The resulting PCR products were purified on a Qiagen column and subjected to DNA sequencing as previously reported on an ABI PRISM 3100 Genetic Analyser (Applied Biosystems, Foster City, CA). Semiquantitative results were expressed as follows: +++ for samples with more than 50% of mutated allele; ++ for samples with mutated allele ranging from 25% to 50%; + for samples with 5% to 25% of mutated allele; and − for samples with no detectable MPL W515 mutation. Alternatively, the mutational status was determined by a real-time PCR single nucleotide polymorphism (SNP) detection system with fluorescent competitive probes using an ABI 7500 analyzer (Applied Biosystems). To ensure the semiquantitative estimation of the MPL W515/total MPL ratio, the DNA from a plasmid harboring the mutated MPL W515 L or K was used and diluted at various proportions in normal DNA control. Each dilution was tested by sequencing and real-time PCR methods (Figure 1). Sequencing semiquantitative results were obtained using peak height analysis. Quantitative results were obtained by the real-time PCR technique using the ΔCt method as previously described for JAK2 V617F.8
Semiquantitative estimation of the MPL 1544 G>T and MPL 1543 TG>AA/total MPL ratio using sequencing and real-time PCR assay. One hundred percent mutated DNA was mixed in various proportions with 100% normal control DNA. The sequence traces and real-time PCR amplification plots indicating mutated (W515L or W515K) and wild-type MPL amplification plots for each dilution are shown, demonstrating the correlation of the techniques. The sensitivity of the sequencing approach was 5% to 10%, whereas the sensitivity of the real-time PCR assay was approximately 2% to 3% of mutated allele. However, because the purity of the lymphoid cell populations studied in this work was 98%, results lower than 2% of mutated allele were arbitrarily considered as negative because 2% of mutated myeloid cells could contaminate the samples. Semiquantitative results were expressed as shown in the center part of the figure.
Semiquantitative estimation of the MPL 1544 G>T and MPL 1543 TG>AA/total MPL ratio using sequencing and real-time PCR assay. One hundred percent mutated DNA was mixed in various proportions with 100% normal control DNA. The sequence traces and real-time PCR amplification plots indicating mutated (W515L or W515K) and wild-type MPL amplification plots for each dilution are shown, demonstrating the correlation of the techniques. The sensitivity of the sequencing approach was 5% to 10%, whereas the sensitivity of the real-time PCR assay was approximately 2% to 3% of mutated allele. However, because the purity of the lymphoid cell populations studied in this work was 98%, results lower than 2% of mutated allele were arbitrarily considered as negative because 2% of mutated myeloid cells could contaminate the samples. Semiquantitative results were expressed as shown in the center part of the figure.
Results
Sensitivity of genotyping for MPL W515 detection
To ensure the semiquantitative estimation of the MPL W515 mutation/total MPL ratio, a DNA plasmid carrying the MPL W515L mutation was diluted in various proportions with a normal DNA control. Each dilution was tested by sequencing. Semiquantitative results were obtained using peak integration. The sensitivity threshold of the technique was 5% to 10% of mutated DNA with a lower concentration of mutated allele giving a wild-type (WT) pattern of sequencing. In parallel, the same dilutions were used for MPL W515L and MPL W515K samples and subjected to SNP analysis. Sensitivity of SNP analysis was 2% to 3% (Figure 1).
MPL W515 mutations are present in myeloid, NK, and B cells but are not present in T lymphocytes from PMF patients
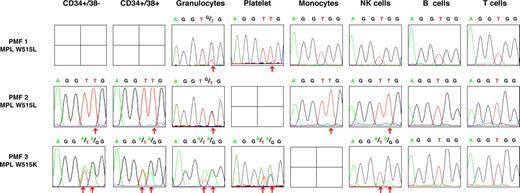
Sequence analysis showed the presence of the MPL W515 mutations in the peripheral blood myeloid lineages, including granulocytes (DNA) and platelets (RNA) isolated from 3 and 2 PMF samples, respectively, and in the monocyte compartment in 1 of 2 patients. In all cases, the normal allele was detected with the mutated peak ranging from 100% to 30% of the normal peak. This suggested the presence of a mixture of WT and mutated cells (Figure 2; Table 1). To study the presence of the MPL W515 mutations in peripheral blood lymphocytes, T, B, and NK cells were purified by combined immunomagnetic and flow cytometric methods on the basis of the tandem CD3 and CD19, and CD56 expressions, respectively. Cell purity was more than 98%. The mutated MPL W515 peak was similar in NK and myeloid cells. Sequence analysis revealed the lack of the mutation in T and B cells for the 3 patients, but the mutations were found in NK cells for 2 of 3 patients. To confirm these results, B-and T-cell fractions were subjected to SNP analysis to increase sensitivity (sensitivity 2% to 3%). Using this approach, we found that the B-cell but not the T-cell fraction harbored the MPL mutations (data not shown).
Detection of MPL 1544 G>T and MPL 1543 TG>AA (MPL W515) mutations in peripheral blood cells from PMF patients. Peripheral cells from MPL-mutated PMF patients were isolated using standard density, immunomagnetic and flow cytometric methods for further DNA extraction, and sequence analysis. The MPL 1544 G>T and MPL 1543 TG>AA mutations were detected in some CD34+ cells, granulocytes, and platelets cells from 2 of the PMF patients. The CD34+/38− cells, CD34+/38+ cells, granulocytes, platelets, monocytes, NK cells, and T- and B-cell sequence traces from 3 PMF patients are shown. Red arrows indicate the presence of a mutant peak.
Detection of MPL 1544 G>T and MPL 1543 TG>AA (MPL W515) mutations in peripheral blood cells from PMF patients. Peripheral cells from MPL-mutated PMF patients were isolated using standard density, immunomagnetic and flow cytometric methods for further DNA extraction, and sequence analysis. The MPL 1544 G>T and MPL 1543 TG>AA mutations were detected in some CD34+ cells, granulocytes, and platelets cells from 2 of the PMF patients. The CD34+/38− cells, CD34+/38+ cells, granulocytes, platelets, monocytes, NK cells, and T- and B-cell sequence traces from 3 PMF patients are shown. Red arrows indicate the presence of a mutant peak.
MPL 1544 G>T and MPL 1543 TG>AA (W515) genotyping of blood fractionated cell populations
| . | Peripheral progenitors cells . | Peripheral myeloid cells . | Peripheral lymphoid cells . | |||||
|---|---|---|---|---|---|---|---|---|
| 34+/38− . | 34+/38+ . | Gr . | Pl . | Mo . | NK . | B . | T . | |
| PMF 1 MPL W515L | ND | ND | + | +++ | − | − | − | − |
| PMF 2 MPL W515L | +++ | +++ | ++ | ND | +++ | +++ | − | − |
| PMF 3 MPL W515K | ++ | ++ | ++ | ++ | ND | ++ | − | − |
| . | Peripheral progenitors cells . | Peripheral myeloid cells . | Peripheral lymphoid cells . | |||||
|---|---|---|---|---|---|---|---|---|
| 34+/38− . | 34+/38+ . | Gr . | Pl . | Mo . | NK . | B . | T . | |
| PMF 1 MPL W515L | ND | ND | + | +++ | − | − | − | − |
| PMF 2 MPL W515L | +++ | +++ | ++ | ND | +++ | +++ | − | − |
| PMF 3 MPL W515K | ++ | ++ | ++ | ++ | ND | ++ | − | − |
Gr indicates granulocytes; Pl, platelets, Mo, monocytes, NK, natural killer cells, B, lymphocyte B cells, T, lymphocyte T cells; ND, no data; +, MPL W515 5% to 25%; ++, MPL W515 25% to 50%; +++, MPL W515 over 50%; and −, absence of the MPL W515 mutation.
Frequencies and genotyping of CD34+ and CD34+ CD38− cells
We studied the presence of the MPL W515 mutations in the more immature CD34+CD38+ and CD34+CD38− cells, purified at up to 98% from blood, as described in “Patients, materials, and methods.” No statistical difference in the frequencies of these different CD34 subpopulations was observed between normal or PMF samples whatever their MPL W515 or JAK2 V617F status was (Table 1). Sequence analysis revealed the presence of the mutation in both CD34+ subpopulations in the 2 MPL W515 analyzed patients with an equal expression in CD34+CD38− and CD34+CD38+ cell populations.
MPL W515 mutations are not associated with spontaneous erythroid cell growth in vitro
CD34+ cells were plated in methylcellulose as described in “Patients, materials, and methods.” Cells were cultured at a density of 2000 cells/mL in presence of optimal concentrations of either SCF and IL-3 (to grow endogenous erythroid colonies [EECs]); SCF, IL-3, and Epo (total erythroid cell growth); or SCF, IL-3, and G-CSF (total granulocytic cell growth). Three samples from MPL-mutated PMF and 6 from JAK2-mutated patients were studied. In all JAK2 V617F cases, spontaneous erythroid growth was observed. In the 3 MPL-mutated samples, there was no evident spontaneous erythroid growth. However, erythroid progenitors grown in the presence of Epo carried the MPL mutation. Cloning efficiencies were also compared. Despite an interindividual heterogeneity, we did not find statistical differences in the cloning efficiency of erythroid and granulocytic progenitors between MPL-mutated and JAK2-mutated samples. The cloning efficiencies seemed to be lower for the erythroid progenitors in MPL-mutated samples despite that there was a nonstatistically significant decreasing trend in the number of erythroid progenitors from MPL W515–mutated PMF samples (n = 3) in comparison with JAK2 V617F–only PMF samples (n = 6; P = .14; Table 2).
Number of myeloid progenitors derived from PMF-purified CD34+ in methylcellulose medium
| . | EECs . | Total BFU-Es . | Total CFU-GMs . |
|---|---|---|---|
| PMF1 MPL W515L | 0 | 6 | 82 |
| PMF2 MPL W515L | 0 | 2 | 14 |
| PMF3 MPL W515K | 0 | 55 | 185 |
| Mean PMF MPL W515 | 0 | 21 | 71 |
| PMF4 JAK2 V617F | 9 | 84 | 136 |
| PMF5 JAK2 V617F | 28 | 144 | 198 |
| PMF6 JAK2 V617F | 3 | 21 | 17 |
| PMF7 JAK2 V617F | 17 | 17 | 37 |
| PMF8 JAK2 V617F | 3 | 11 | 10 |
| PMF9 JAK2 V617F | 10 | 40 | 150 |
| Mean PMF JAK2 V617F | 12 | 53 | 91 |
| P | .015 | .141 | .387 |
| . | EECs . | Total BFU-Es . | Total CFU-GMs . |
|---|---|---|---|
| PMF1 MPL W515L | 0 | 6 | 82 |
| PMF2 MPL W515L | 0 | 2 | 14 |
| PMF3 MPL W515K | 0 | 55 | 185 |
| Mean PMF MPL W515 | 0 | 21 | 71 |
| PMF4 JAK2 V617F | 9 | 84 | 136 |
| PMF5 JAK2 V617F | 28 | 144 | 198 |
| PMF6 JAK2 V617F | 3 | 21 | 17 |
| PMF7 JAK2 V617F | 17 | 17 | 37 |
| PMF8 JAK2 V617F | 3 | 11 | 10 |
| PMF9 JAK2 V617F | 10 | 40 | 150 |
| Mean PMF JAK2 V617F | 12 | 53 | 91 |
| P | .015 | .141 | .387 |
Peripheral blood CD34+ cells from PMF patients was seeded in methylcellulose in the presence or absence of Epo or G-CSF. Erythroid and granulocytic colonies were picked on day 14 and genotyped by SNP analysis.
EECs indicates endogenous erythroid colonies; BFU-Es, burst-forming units–erythroid; and CFU-GMs, colony-forming units–granulocyte-macrophage.
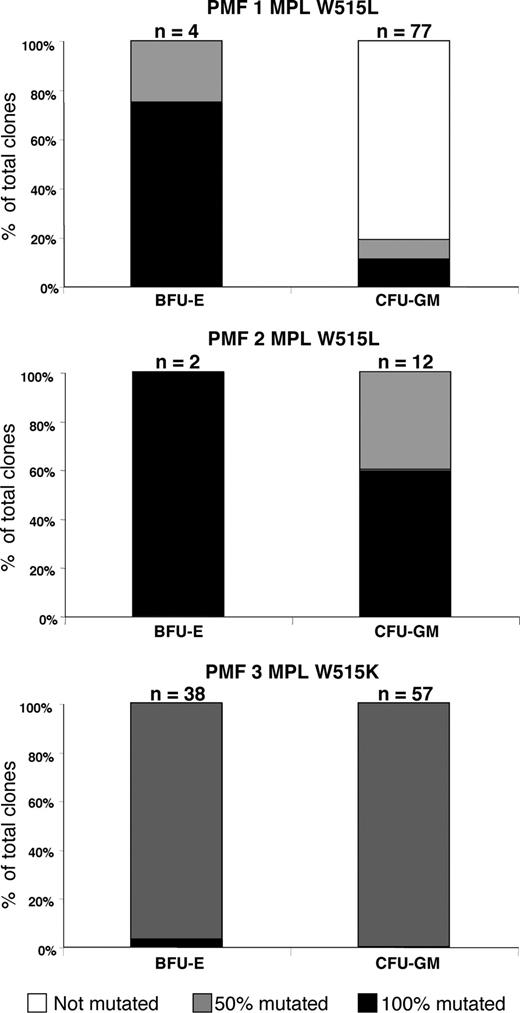
Heterogeneity in the MPL mutation status of BFU-Es and CFU-GMs in 3 PMF patients
We then analyzed the genotypes of single colonies obtained from methylcellulose assays. Briefly, 2 to 77 proliferating clones from each type (BFU-Es or CFU-GMs) were analyzed for each MPL-mutated patient (Figure 3). Lack of PCR product occurred in 6 of 196 samples for MPL-mutated PMF clones. The 3 analyzed PMF patients harbored different patterns of MPL W515 mutation/WT in their different clones. The first patient presented a 50% mutation ratio in all but one analyzed clone, the second patient had a mixed pattern of 100% and 50% ratio in the different clones without detectable normal clones, and the third patient exhibited a more heterogeneous pattern with the presence of nonmutated, and 50% and 100% ratio clones. For the erythroid compartment, all analyzed BFU-Es grown in culture carried the MPL mutations. Furthermore, in all 3 cases at least one BFU-E colony harbored a 100% ratio of mutation. In summary, the CFU-GM compartment was the most heterogeneous between among patients, with at least some clones carrying the MPL W515–mutated allele in all patients.
Clonal genotypic patterns of erythroid and granulocytic progenitors from PMF patients. Detection of the MPL W515 mutations by direct sequencing. Histograms showing the percentages of MPL W515 100% mutated (■), 50% mutated ( ), and MPL WT (□) colonies from 3 PMF patients. BFU-E indicates burst-forming unit–erythroid; CFU-GM, colony-forming unit–granulocyte-macrophage; and n, the numbers of analyzed clones for each histogram bars.
), and MPL WT (□) colonies from 3 PMF patients. BFU-E indicates burst-forming unit–erythroid; CFU-GM, colony-forming unit–granulocyte-macrophage; and n, the numbers of analyzed clones for each histogram bars.
Clonal genotypic patterns of erythroid and granulocytic progenitors from PMF patients. Detection of the MPL W515 mutations by direct sequencing. Histograms showing the percentages of MPL W515 100% mutated (■), 50% mutated ( ), and MPL WT (□) colonies from 3 PMF patients. BFU-E indicates burst-forming unit–erythroid; CFU-GM, colony-forming unit–granulocyte-macrophage; and n, the numbers of analyzed clones for each histogram bars.
), and MPL WT (□) colonies from 3 PMF patients. BFU-E indicates burst-forming unit–erythroid; CFU-GM, colony-forming unit–granulocyte-macrophage; and n, the numbers of analyzed clones for each histogram bars.
The MPL mutations are present at the level of B/NK/myeloid progenitors derived from PMF CD34+CD38− cells
To ascertain more precisely whether MPL mutations are present in lympho/myeloid progenitors, 350 CD34+CD38− cells from 2 patients, one with the MPL W515L (PMF 1) and the other with the MPL W515K (PMF 3) mutations, were purified. They were subsequently grown at one cell per well on the murine stromal MS-5 cell line in the presence of a combination of 7 cytokines (“Patients, materials, and methods”). The mean frequency of proliferating clones (more than 200 cells) at 4 and 6 weeks was 12% and 31.1%, respectively. These results were the same as the ones published previously, with normal cells and JAK2-mutated PMF (25% ± 4.3% for JAK2 V617F PMF) using the same technique.8 Myeloid, B, and NK potencies of each proliferating clone were analyzed by flow cytometry. First, there was no detectable difference in size, nor in the variety and the composition of the differentiated clones in vitro between controls (either normal cells or JAK2-mutated PMF samples). Although some heterogeneity between the 2 patient samples existed, similar percentages of myeloid, NK/myeloid, and B/NK/myeloid clones were obtained in patient and control samples (Figure 4A). Progenitor cells from patient samples were able to give birth to all blood cell types except pure B cells and B/NK. Clones with B/myeloid potency were rare, and less than 10% of B cells were identified in B/NK/myeloid clones from both patient and control samples.
Assessment and genotyping analysis of B/M/NK individual clones derived from PMF-purified CD34+CD38−. Analysis of the progeny of single CD34+CD38− cell from PMF blood cells. Myeloid cells correspond to CD15+ cells; B cells, to CD19+ cells; and NK cells, to CD56+ cells. (A) Genotype analysis of single CD34+CD38− cell culture–derived clones from 2 PMF patients with respect to their monopotent B, NK, myeloid (M), bipotent B/M, B/NK, M/NK, or tripotent B/NK/M immunophenotypic characterization (clone type). (B) Immunophenotypic analysis of B/NK/myeloid clones from this PMF patient. The isotype control scattergrams are shown in the upper panel. The B, NK, and myeloid potentials were assessed by the presence of CD19+, CD56+, and CD15+cells, respectively, after 5 weeks of B, NK, and myeloid differentiation culture condition. The sequence traces of the PMF tripotent clone (MPL 1544 G>T [top] and MPL 1543 TG>AA [bottom]). Red arrows indicate the presence of a mutant peak.
Assessment and genotyping analysis of B/M/NK individual clones derived from PMF-purified CD34+CD38−. Analysis of the progeny of single CD34+CD38− cell from PMF blood cells. Myeloid cells correspond to CD15+ cells; B cells, to CD19+ cells; and NK cells, to CD56+ cells. (A) Genotype analysis of single CD34+CD38− cell culture–derived clones from 2 PMF patients with respect to their monopotent B, NK, myeloid (M), bipotent B/M, B/NK, M/NK, or tripotent B/NK/M immunophenotypic characterization (clone type). (B) Immunophenotypic analysis of B/NK/myeloid clones from this PMF patient. The isotype control scattergrams are shown in the upper panel. The B, NK, and myeloid potentials were assessed by the presence of CD19+, CD56+, and CD15+cells, respectively, after 5 weeks of B, NK, and myeloid differentiation culture condition. The sequence traces of the PMF tripotent clone (MPL 1544 G>T [top] and MPL 1543 TG>AA [bottom]). Red arrows indicate the presence of a mutant peak.
Proliferating clones were also sequenced (Figure 4B). Lack of PCR product occurred in 12 of 151 tested samples for PMF clones. The 2 PMF patients harbored different patterns with 50% to 100% mutation ratio in their different clones. All analyzed clones, except for 3 clones from the PMF 1 patient, were mutated at 100% (Figure 4B top), and the second patient had 100% mutated clones (Figure 4B bottom). Moreover, in one case, NK/myeloid and B/NK/myeloid clones were 100% mutated. When analyzing the mutational status with respect to the immunophenotype of the clones, we observed that both patients had clones with lympho/myeloid potencies carrying the mutation. In contrast, with JAK2 V617F–positive patients, no WT clone was detected in the lympho/myeloid progenitors.
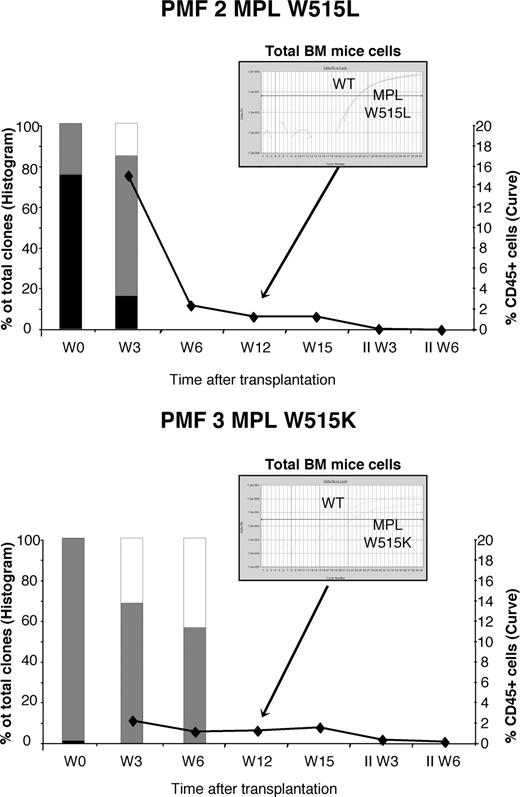
The MPL mutations are present in NOD/SCID long-term reconstitution cells from PMF CD34+ cells
To assess the presence of the MPL mutations in the hematopoietic stem cell (HSC) compartment, we repopulated immune-deficient mice with human MPL W515–mutated PMF CD34+ cells from 2 patients. Given that MPL signaling is important in HSC self-renewal,33 we hypothesized that the introduction of MPL mutations would increase their self-renewal properties. CD34+ cells (5-6.6 × 105) were transplanted in one preconditioned NOD/SCID per patient (PMF 2 and 3). At 3 weeks after transplantation, the degree of human chimerism was 2.1% and 15.2% for W515K and W515L cells, respectively, and decreased gradually at weeks 6, 12, and 15 (Figure 5). Phenotypic analysis of cells aspirated from mouse bone marrow transplanted with MPL W515L CD34+ cells showed the presence of human CD33+ cells but no human CD19+ cells 15 weeks after transplantation. At weeks 12 and 15, very low levels of human chimerism were obtained, so that cell cultures were not performed and aspirated bone marrow cells were subjected only to human SNP analysis. Using this approach, we still found human MPL-mutated cells at 12 weeks after transplantation, however we did not detect either W515K or W515L human cells in secondary recipients.
Long-term reconstitution in NOD/SCID mice with CD34+CD38− cells from MPL W515 PMF. Human MPL mutant PMF CD34 cells (5-6.6 × 105) were injected into NOD/SCID mice or harvested in methylcellulose assay to determine the frequency of 100% mutated (■), 50% mutated ( ), or unmutated (□) human myeloid colonies before or 3 and 6 weeks after transplantation. In parallel, chimerism of human cells was determined using CD45 expression by flow cytometry. Twelve weeks after transplantation, chimerism was too low to perform methyl assay but SNP analysis was performed. Results from 2 different MPL mutant reconstituted mice are shown.
), or unmutated (□) human myeloid colonies before or 3 and 6 weeks after transplantation. In parallel, chimerism of human cells was determined using CD45 expression by flow cytometry. Twelve weeks after transplantation, chimerism was too low to perform methyl assay but SNP analysis was performed. Results from 2 different MPL mutant reconstituted mice are shown.
Long-term reconstitution in NOD/SCID mice with CD34+CD38− cells from MPL W515 PMF. Human MPL mutant PMF CD34 cells (5-6.6 × 105) were injected into NOD/SCID mice or harvested in methylcellulose assay to determine the frequency of 100% mutated (■), 50% mutated ( ), or unmutated (□) human myeloid colonies before or 3 and 6 weeks after transplantation. In parallel, chimerism of human cells was determined using CD45 expression by flow cytometry. Twelve weeks after transplantation, chimerism was too low to perform methyl assay but SNP analysis was performed. Results from 2 different MPL mutant reconstituted mice are shown.
), or unmutated (□) human myeloid colonies before or 3 and 6 weeks after transplantation. In parallel, chimerism of human cells was determined using CD45 expression by flow cytometry. Twelve weeks after transplantation, chimerism was too low to perform methyl assay but SNP analysis was performed. Results from 2 different MPL mutant reconstituted mice are shown.
MPL W515 mutations are able to induce spontaneous megakaryocytic growth and increase cell proliferation rate from patient CD34+ cells in response to TPO
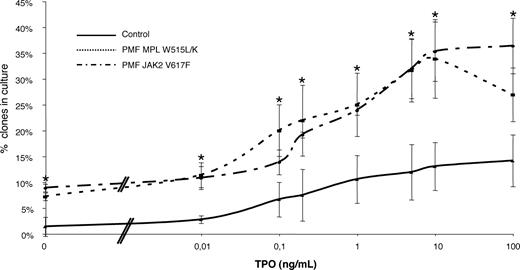
We assessed whether the spontaneous megakaryocytic growth observed in nondefined genetic status of PMF could be a result of the MPL mutations. We also tested whether MPL W515 or JAK2 V617F mutations could induce a hypersensitivity to TPO. To do so, we investigated at the clonal level the ability of patients with normal CD34+ cells to proliferate in absence or increasing doses of TPO in serum-free media. As seen before, in normal cells, single megakaryocytes were present in some wells to which no TPO had been added.34 Therefore, we counted the frequency of “spontaneously grown” megakaryocytes in MPL- and JAK2-mutated samples. Spontaneous growth was obtained in 9.16% (± 0.85%) of MPLW515 clones, 8.6% (± 0.52%) for JAK2 V617F, and 1.4% (± 2.42%) for normal CD34+ cells (Figure 6). The differences were statistically significant between pathological and normal samples (P < .01 for both) but not when comparing MPL mutants and JAK2 mutants (P = .66). This confirmed that JAK2-mutated PMF as well as MPL-mutated PMF present a spontaneous megakaryocytic growth.
TPO dose response of PMF MPL 1544 G>T and 1543 TG>AA and PMF JAK2 1849G>T (V617F) CD34+ cells compared with normal CD34+. CD34+ cells from PMF patients were cultured in Terazaki plate at 1 cell/well. For each TPO concentration (0, 0.01, 0.1, 0.2, 1, 5, 10, and 100 ng/mL), 120 cells were plated. Clones were counted at 10 days. The data represent the mean plus or minus SEM of 3 separate experiments performed in each group (PMF W515, n = 3; PMF JAK2 V617F, n = 3; and healthy controls, n = 3). *P < .05 compared with normal CD34+. The difference between PMF MPL W515L/K and PMF JAK2 V617F is not significant. The same TPO dose response was noticed for MPL W515L (n = 2) and MPL W515K (n = 1).
TPO dose response of PMF MPL 1544 G>T and 1543 TG>AA and PMF JAK2 1849G>T (V617F) CD34+ cells compared with normal CD34+. CD34+ cells from PMF patients were cultured in Terazaki plate at 1 cell/well. For each TPO concentration (0, 0.01, 0.1, 0.2, 1, 5, 10, and 100 ng/mL), 120 cells were plated. Clones were counted at 10 days. The data represent the mean plus or minus SEM of 3 separate experiments performed in each group (PMF W515, n = 3; PMF JAK2 V617F, n = 3; and healthy controls, n = 3). *P < .05 compared with normal CD34+. The difference between PMF MPL W515L/K and PMF JAK2 V617F is not significant. The same TPO dose response was noticed for MPL W515L (n = 2) and MPL W515K (n = 1).
A log dose-response curve for each clone (≥ 1 megakaryocyte) is shown in Figure 6. In 3 independent experiments, the mean plateau cloning efficiency (percentage of wells containing at least 1 megakaryocyte) was 33.9 (± 9.92), 36.4 (± 7.1), and 12.7 (± 6.31) for MPLW515, JAK2V617F, and normal mobilized CD34+ cells, respectively. A statistical difference was observed between pathological samples and normal cells (P < .01 for both) but not between the 2 different mutation types (P = .73), indicating that PMF samples, whatever their JAK2 or MPL status, have a higher megakaryocytic cloning efficiency than cells from cytapheresis product. The inflection point, that is to say the TPO concentration (TPO50) corresponding to the log TPO at half-plateau number of total clones, was 5.91 (± 0.72) pg/mL (mean ± 1 SEM; n = 9) and was identical in normal, JAK2 V617F PMF–, and MPL W515 PMF–mutated cells. This value is in agreement with a previous study of normal cells.35 Altogether, these results confirmed that MPL-mutated PMFs are able to develop spontaneous megakaryocytic growth but that the dose response to TPO is not altered in MPL-mutated as well as in JAK2-mutated samples.
Discussion
It has been well established that CML can arise from a multipotent HSC that contains the shortened chromosome 22 known as Philadelphia chromosome. Recently, we and others have demonstrated that in other MPDs such as the JAK2 V617F–positive disorders, the oncogenic process initiates at the level of a lympho/myeloid progenitor. With the discovery of MPL W515 mutations in some rare cases of MPDs, the question arose of whether the MPL mutants could be characterized as an HSC-based pathologic event. The work on MPL W515L retrovirus-transduced HSCs has already partly answered this question as the mice reconstituted with these cells developed a disease mimicking PMF, with first the onset of a myeloproliferative process followed with fibrosis in hematopoietic tissues. Interestingly, the presence of both JAK2 V617F and MPL W515 mutations have been described, though rarely, in some patients, suggesting that MPL W515 mutations could be only a secondary oncogenic event in such cases. Therefore, the involvement of the HSC compartment in humans carrying a MPL W515 mutation needed to be clearly demonstrated. In the above mouse model, the MPL W515L cDNA was transduced in a multipotent HSC capable of reconstituting irradiated mice, but the developed disorder was lethal in fewer than 6 months. On the contrary, the JAK2 V617F mouse model or other PMF-like models induced either by TPO high expression or GATA-1 low expression survived to their associated disease.36-42 Moreover, in humans carrying the MPL mutations, the disorders are long-term processes, questioning the possibility of the MPL mutations to occur at a stem cell level rather than at the level of a more mature megakaryocytic progenitor. Therefore, we hypothesized that the phenotype induced by the mutated MPL could be related to the precise stage at which the clonal process occurs. A multipotent stem-cell/progenitor involvement could result in a rapidly progressing lethal disorder, just like the one developed by the MPL W515L mouse model. If the mutation occurs in a common myeloid progenitor, the disease course could be more chronic. To test this hypothesis, we investigated the presence of the MPL mutations in the different hematopoietic compartments of PMF samples. We first analyzed the MPL status in the different circulating mature cell populations. As expected, the MPL mutations were found in all analyzed “myeloid” peripheral blood cells including monocytes, platelets, and neutrophils, demonstrating that these mutations occur at least in a common myeloid progenitor. Although we detected MPL mutations at the clonal CFU levels, MPL W515 mutations, in contrast to JAK2-mutated samples, were unable to induce “spontaneous” erythroid colony-forming units (EECs). These results are in accordance with previous works performed with constitutionally active forms of MPL and with the clinical presentation of MPL W515 PMF harboring a lower hemoglobin level than the JAK2-mutated PMF.43 Furthermore, we examined the ability of MPL W515 progenitors to give birth to a spontaneous megakaryocytic growth and to become hypersensitive to TPO. Results were compared either to JAK2 V617F–mutated PMF samples or to normal CD34+ cells. First, we observed that the ability of MPL W515–mutated PMF to induce spontaneous megakaryocytic growth was identical to that of JAK2-mutated PMF. Second, the cloning efficiencies of megakaryocytic progenitors was 4-fold greater in PMF samples, whatever their JAK2 or MPL status, than the one observed in normal samples at the plateau concentration of TPO. This is in accordance with Ciurea et al results.44 We cannot definitively rule out that the CD34+ compartment of PMF samples could be richer in megakaryocytic progenitors. However, the frequencies of CD34+CD41+ cells (ie, megakaryocytic progenitors) in MPL-mutated samples, JAK2 V617F samples, and normal cytapheresis products were not statistically different (data not shown). These data suggest that megakaryocyte (MK) hyperplasia may be due to the greater capacity of CD34+ PMF cells to generate MKs. Another explanation for these findings could be that the mechanism at the basis of all the genetic lesions induced in PMF (JAK2 V617F, MPL W515 mutations, and other undefined mutations) could be a commitment to the megakaryocytic compartment of other progenitors. In favor of this hypothesis is the decreasing trend in erythroid progenitor counts in these PMF samples (Table 2).
When we analyzed the lymphoid compartments, we found the mutation only in the NK and to a very low level in the B cells but not in the peripheral T cells. However, Pardanani et al recently reported that MPL W515K mutation was present in the T-cell compartment in one patient.45 If the mutation occurred at a stem cell level, we hypothesized that, just like previously demonstrated for the JAK2 V617F MPDs, the mutation would have to be present in more immature lympho/myeloid progenitors. Therefore, there was a need to directly analyze the stem cell/progenitor compartment. The first approach we used was to study immature stem cells based on differentiation membrane antigens. Analysis of the MPL status in CD34+ cells and in the CD34+CD38− cell population, which is enriched in lympho/myeloid progenitors, was performed. The MPL mutations were detected in both cell fractions from PMF samples when the mutation was present in granulocytes. We performed a clonal analysis in culture conditions supporting myeloid, B-, and NK cell differentiation, to analyze in detail the lympho/myeloid potency of these CD34+CD38− cells. We found al least one mutated clone able to give birth to myeloid, B, and NK differentiation and numerous clones with a myeloid and NK or myeloid and B-cell differentiation, confirming our findings obtained in peripheral blood samples. This clearly demonstrates that the disease is driven by a lympho/myeloid progenitor. To clarify whether these mutations occurred in more immature stem cells, NOD/SCID mice repopulation assays were performed. We successfully brought back together the human hematopoiesis in this animal model 12 weeks after transplantation. As assessed by the colony-forming cell (CFC) analysis, mice had lower amounts of MPL W515 clones at 3 and 6 weeks after reconstitution. Therefore the second reconstitution was not possible. This differs from JAK2-mutated PMF CD34+ cells that were able to reconstitute NOD/SCID mice with an increase in JAK2-mutated CFCs over time (weeks 3 and 6; C.J., unpublished data, May 2007).
Taken together, these results show some differences with the JAK2-mutated MPDs. First, JAK2-mutated MPDs have a spontaneous erythroid growth that cannot be detected in MPL-mutated MPDs. Second, the JAK2 mutation using a direct sequencing approach was found in peripheral B and NK cells in PMF and in 1 of 6 cases in T cells with a 5% sensitivity technique.8 Third, we found the mutation in long-term reconstituted mice. Differences between JAK2 V617F PMF and MPL W515 PMF are also noticeable in retroviral mouse models.42 Therefore, one can hypothesize that the clonal processes involved in JAK2 V617F and MPL W515 MPDs are different.
Another finding in this work was the fact that MPL mutations, just like the JAK2 mutation, can vary between 100% and 50% in CFCs from a given patient. Indeed, it is unclear whether this 100% mutated state is a consequence of a mitotic recombination just like was demonstrated in JAK2 V617F PV and PMF or if the MPL normal allele is lost. However, our results clearly demonstrate that the 100% mutation is present at the stem cell level just like the JAK2 mutation.
To date, whether the MPL W515 mutations are capable to expand the HSC compartment remains unknown; however, our results on NOD/SCID mice are not in favor of this hypothesis. In CML, it has been shown that during the chronic phase the BCR-ABL fusion protein gives a proliferative advantage only to progenitors.46 For JAK2 V617F mutants, the amplification process also seems to give a proliferative advantage to progenitors.31 Based on these findings with MPDs and oncogenic events, and in agreement with our experiments in NOD/SCID mice demonstrating that the human chimerism decreased over time, we can hypothesize that MPL W515 mutations also induce a proliferative advantage to the progenitors rather than HSCs. However, MPL is a key cytokine receptor playing a major physiological role in stem cell amplification. Under physiological conditions, MPL expression is restricted to the stem cell and megakaryocyte compartment. Then, it is probable that MPL W515 PMFs are different from JAK2-mutated PMFs in their pathophysiologies. Moreover, our results indicate that abnormal MPL functions could be toxic in nonphysiological compartments or could force the engagement toward the myeloid and megakaryocytic compartments.
Since the presence of a clonal mutation in peripheral blood leukocytes depends not only on the cellular level at which the clonal event has occurred but also on the proliferative advantage given by the oncogene to the stem cell progeny, we hypothesized that clonal processes of JAK2 mutation and MPL mutation were different, with MPL mutants favoring the proliferative advantage of the HSCs. Whether this event solely modifies the constitutive activity of MPL leading to the acquisition of new stem cell functions remains to be determined.
In conclusion, this study provides the first evidence that the MPL W515 mutations in PMF drive a lympho/myeloid stem/progenitor cell and that the phenotype of the disease is probably related to the proliferative advantage essentially toward the myeloid series, therefore leading to a pure myeloproliferative rather than a lympho/myeloid proliferative disorder. Defining the precise signaling defect induced by the MPL W515 mutations may allow to better determine the pathogenesis of PMF. However, an activating MPL mutation has previously been observed in familial ET without fibrosis (MPL S505N mutation),20 and so we cannot definitively exclude that additional genetic or epigenetic events may occur during the disease progression in the MPL W515–mutated PMF.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from INSERM and La Ligue Nationale contre le Cancer (équipe labellisée 2004 and 2007). R.C. is supported by the Research Ministry.
The authors are grateful to Caroline Marty and Caroline Lefebvre for helpful discussions and improving the English usage.
Authorship
Contribution: R.C., C.J., C.T., R.B., S.G., J.P.L.C., I.G., F.F., and F.M. performed cellular experiments and genotyping analysis; S.G., W.V., and K.M. contributed to the recruitment of the patients; F.L. and Y.L. performed cell sorting; W.V. and S.G. designed the study, analyzed the data, and wrote the paper. C.J. and C.T. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stéphane Giraudier, INSERM U790, Institut Gustave Roussy, PR1, 39 rue Camille Desmoulins, 94805 Villejuif, France; e-mail:sgiraudi@igr.fr or stephane.giraudier@hmn.aphp.fr.

![Figure 4. Assessment and genotyping analysis of B/M/NK individual clones derived from PMF-purified CD34+CD38−. Analysis of the progeny of single CD34+CD38− cell from PMF blood cells. Myeloid cells correspond to CD15+ cells; B cells, to CD19+ cells; and NK cells, to CD56+ cells. (A) Genotype analysis of single CD34+CD38− cell culture–derived clones from 2 PMF patients with respect to their monopotent B, NK, myeloid (M), bipotent B/M, B/NK, M/NK, or tripotent B/NK/M immunophenotypic characterization (clone type). (B) Immunophenotypic analysis of B/NK/myeloid clones from this PMF patient. The isotype control scattergrams are shown in the upper panel. The B, NK, and myeloid potentials were assessed by the presence of CD19+, CD56+, and CD15+cells, respectively, after 5 weeks of B, NK, and myeloid differentiation culture condition. The sequence traces of the PMF tripotent clone (MPL 1544 G>T [top] and MPL 1543 TG>AA [bottom]). Red arrows indicate the presence of a mutant peak.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/10/10.1182_blood-2007-05-089003/6/m_zh80240709990004.jpeg?Expires=1767709138&Signature=RX9aMOiqa8xSV-JMm8N8ncspxQOoo~OBkrz3MBUKgy5FR9b0YUl5IGK-Gw~DDX7ylryXNqza0EmZxU20bwfYHrcG-X81uWZsTYMrZOUwLuWg9JuhFP-89fzVJj~RGMYYnm6PkvSbfdM-i6PpcPzdBp6ywNA~kNqAXoOL0IRvnU6P7dcmJjjoHB53xMfG-Oy7DTG714sw0zwPn4lZly4gY8zNFLqBCA2hHQdzFBel6IsJtqzAoiAZ6EGVJirUnTbtup1AMiiLiq4wYDBTe7wUqhd7hIGXSCS-HI8xP4D4AOISUctS98VVN-GCBy4YgNRZXq4wqX9WiAUph6YdmMXgLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)