Abstract
The 8p11 myeloproliferative syndrome (EMS) is an aggressive, atypical stem cell myeloproliferative disorder associated with chromosome translocations that disrupt and constitutively activate FGFR1 by fusion to diverse partner genes. To explore the possibility of targeted therapy for EMS, we have investigated the use of TKI258, a multitargeted receptor tyrosine kinase inhibitor with activity against FGFR, VEGFR, PDGFR, FLT3, and KIT that is currently being assessed for the treatment of a variety of malignancies in phase 1 clinical studies. The viability of Ba/F3 cells transformed to IL3 independence by ZNF198-FGFR1 or BCR-FGFR1 was specifically inhibited by TKI258 with IC50 values of 150 nM and 90 nM, respectively. Inhibition was accompanied by dose-dependent inhibition of phosphorylation of each fusion gene, ERK, and STAT5. TKI258 also specifically inhibited proliferation and survival of the FGFR1OP2-FGFR1–positive KG1 and KG1A cell lines, resulting in increased levels of apoptosis. Primary cells from EMS patients showed significant, dose-dependent responses in liquid culture and in methylcellulose colony assays compared with controls. This work provides evidence that targeted therapy may be beneficial for patients with EMS.
Introduction
The 8p11 myeloproliferative syndrome (EMS), also known as stem cell leukemia/lymphoma syndrome, is an atypical stem cell myeloproliferative disorder typically characterized by eosinophilia and in many cases an associated T- or B-cell lymphoma.1 EMS is caused by constitutive activation of the fibroblast growth factor receptor 1 (FGFR1) by fusion to unrelated partner genes, the most common being ZNF198. Other FGFR1 translocation partner genes are BCR, TRIM24 (TIF1), MYO18A, CEP110, FGFR1OP (FOP), FGFR1OP2, and LOC113386 (HERV-K). The partner protein promotes dimerization and ligand-independent activation of the FGFR1 kinase in a manner analogous to BCR-ABL. The disease is aggressive and usually progresses to acute myeloid leukemia (AML) usually within 1 or 2 years of diagnosis. Currently, stem cell transplantation remains the only curative treatment.1
The development of small molecules with inhibitory activity against activated tyrosine kinases has revolutionized therapy for patients with appropriate molecular targets, most notably the BCR-ABL fusion in chronic myeloid leukemia (CML). Treatment of CML patients with the ABL inhibitor imatinib induces durable, complete hematologic and cytogenetic remissions in the majority of patients and is likely to substantially improve survival.2,3 Imatinib also induces durable responses for myeloproliferative disorders (MPDs) with PDGFRA and PDGFRB fusion genes.4-6
These spectacular successes suggest that other conditions characterized by activated tyrosine kinases, such as EMS, may also be treatable by targeted therapy. However imatinib is not active against FGFR1 and therefore alternative compounds are needed. Previous in vitro studies have demonstrated activity of the tyrosine kinase inhibitors SU5402,7 PD173074,7 and PKC412,8 against Ba/F3 cells transformed with the ZNF198-FGFR1 fusion gene, but none of these compounds is in general clinical use.
TKI258 (formerly CHIR258) (4-amino-5-fluor-3-[5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]quinolin-2(1H)-one, MW = 392.4) is an ATP-competitive inhibitor with activity against class III or IV receptor tyrosine kinases, which includes FGFR, VEGFR, PDGFR, FLT3, and KIT.9-12 In vitro studies have demonstrated activity of TKI258 against FGFR3 in t(4;14)-positive multiple myeloma, and this compound is currently being assessed for the treatment of diverse malignancies. Here we report in vitro studies that demonstrate that TKI258 has significant activity against ZNF198-FGFR1– and BCR-FGFR1–transformed Ba/F3 cells, the KG1 and KG1A AML cell lines expressing the FGFR1OP2-FGFR1 fusion gene,13 and primary material from patients with FGFR1 translocations.
Materials and methods
Cell lines
The IL3-independent Ba/F3 clones containing pcDNA3.1/ZNF198-FGFR1 (Ba/F3-ZF) or pcDNA3.1/BCR-FGFR1 (Ba/F3-BF) constructs have been described elsewhere.14,15 Ba/F3-ZF, Ba/F3-BF, KG1,16 KG1A,17 and HEL18 cell lines were grown in RPMI-1640 supplemented with 10% serum. A Ba/F3 clone containing the pcDNA3.1 vector alone was grown in RPMI-1640 medium supplemented with 10% serum and 10% WEHI3B-conditioned medium as a source of IL3.
Patient material
In vitro response to TKI258 was investigated in cryopreserved cells from 5 patients with 4 different FGFR1 fusion genes (Table 1), all of which were confirmed by reverse-transcription–polymerase chain reaction (RT-PCR). As controls, we used samples from healthy individuals and from 3 patients with FGFR1-rearrangement–negative MPDs (one case each of JAK2 V617F–positive polycythemia vera, chronic eosinophilic leukemia, and unclassified myeloproliferative disorder). The study was approved by the Internal Review Board of South Wiltshire Regional Ethics Committee and informed consent was obtained in accordance with the Declaration of Helsinki.
Details of patients with FGFR1 translocations
| Case . | Translocation . | Fusion gene . | Age, y . | Sex . | Disease . |
|---|---|---|---|---|---|
| 1 | t(8;13)(p11;q12) | ZNF198-FGFR1 | 50 | M | EMS |
| 2 | t(6;8)(q27;p11) | FGFR1OP-FGFR1 | 44 | F | Atypical MPD with eosinophilia |
| 3 | t(6;8)(q27;p11) | FGFR1OP-FGFR1 | 45 | F | EMS (evolved from polycythemia vera) |
| 4 | t(8;17)(p11;q23) | MYO18A-FGFR1 | 74 | F | MDS/MPD |
| 5 | t(8;22)(p11;q11) | BCR-FGFR1 | 74 | F | BCR-ABL-negative CML |
| Case . | Translocation . | Fusion gene . | Age, y . | Sex . | Disease . |
|---|---|---|---|---|---|
| 1 | t(8;13)(p11;q12) | ZNF198-FGFR1 | 50 | M | EMS |
| 2 | t(6;8)(q27;p11) | FGFR1OP-FGFR1 | 44 | F | Atypical MPD with eosinophilia |
| 3 | t(6;8)(q27;p11) | FGFR1OP-FGFR1 | 45 | F | EMS (evolved from polycythemia vera) |
| 4 | t(8;17)(p11;q23) | MYO18A-FGFR1 | 74 | F | MDS/MPD |
| 5 | t(8;22)(p11;q11) | BCR-FGFR1 | 74 | F | BCR-ABL-negative CML |
MDS indicates myelodysplastic syndrome.
Proliferation and survival assays
Each cell line was seeded at 2 × 104/mL in 96-well plates (100 μL per well) with and without TKI258. TKI258 concentrations ranged from 10 nM to 10 μM with half-log concentration increments. Proliferation was measured at day 0 without inhibitor and after 48 hours by CellTitre 96 One Solution Cell Proliferation MTS assay (Promega, Madison, WI); absorbance was measured on a MRX Microplate Reader (Thermo Labsystems, Waltham, MA). Each experiment was repeated twice. To calculate IC50 values, the MTS readings were plotted as a percentage of the control (without inhibitor) and analyzed using Graphpad Prism 4 software (GraphPad Software, San Diego, CA).
Western blotting
Cells were serum-starved and exposed to TKI258 for 2 to 4 hours or, for Ba/F3-BCR-FGFR1, exposed to inhibitor overnight with serum. Cells were lysed in Triton-X lysis buffer (20 mM Tris-Cl [pH 7.8], 1% Triton-X, 150 mM NaCl, 10% glycerol) containing Complete Mini Protease Inhibitor Cocktail (Roche, Basel Switzerland) and 1 mM sodium vanadate (Sigma Aldrich, St Louis, MO). Equal volumes of lysate were electrophoresed on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. Detection was performed with antibodies against FGFR1 (antibody sc-121; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-FGFR (Tyr 653/654; Cell Signaling Technology; Beverly, MA), STAT5 (Cell Signaling Technology), phosph-STAT5 (Tyr694; Cell Signaling Technology), ERK (antibody sc-94; Santa Cruz Biotechnology), and phospho-ERK (antibody sc-7383; Santa Cruz Biotechnology). Signals were detected with enhanced chemiluminescence (ECL) Plus Western Blotting Detection Reagents (Amersham Biosciences, Little Chalfont, United Kingdom).
Determination of response of primary cells to TKI258
Responses were determined using liquid culture and colony assays as described.19 Briefly, for the liquid culture response assay, cells were seeded at 2 × 106 to 5 × 106 in 1-mL volumes in 24-well plates in duplicate at 0, 20, and 100 nM TKI258. Cell number was counted manually twice weekly and medium was changed weekly.
For colony assays, peripheral blood or bone marrow mononuclear cells were seeded at 2 × 105 cells/mL in 1-mL volumes in 3-cm dishes in Methocult, with cytokines but without erythropoietin (Stem Cell Technologies, Vancouver, BC) in triplicate at 0, 20, and 100 nM TKI258 or in Human Methylcellulose Base Media without cytokines (R&D Systems, Minneapolis, MN). Colonies of greater than 50 cells were counted on day 7, and of greater than 100 cells on day 14. To compare inhibition between samples, response was calculated as the mean proportional reduction in colony numbers at days 7 and 14 relative to the number of colonies grown without TKI258.19 For example, a response of 0.66 indicates that the number of colonies in a treated culture was 0.66 times the number seen in untreated cultures from the same patient.
Fluorescence in situ hybridization
Colonies for fluorescence in situ hybridization (FISH) were either plucked into phosphate-buffered saline (PBS) and cytospun onto slides, dried briefly, and fixed and stored in 3:1 methanol–acetic acid at −20°C, or were plucked into 3:1 methanol–acetic acid fixative, stored at −20°C until required, then washed in fixative and dropped onto slides. FISH was performed using standard methods as previously described20 except that cells were refixed with 1% paraformaldehyde before hybridization. Clones flanking FGFR1 (5′ FGFR1 PAC RP1–224C10; 3′ FGFR1 PAC RP1–162N14) were obtained from the Sanger Institute (Hinxton, United Kingdom).
Apoptosis assays
Caspase-3 activity was measured using the Ac-DEVD-pNA substrate in a colorimetric assay as previously described.21 Cells were exposed to inhibitor for 48 hours. Lysates were prepared in 50 mM HEPES, pH 7.4, 0.1% CHAPS, 1 mM DTT, 0.1 mM EDTA, 0.1% Triton X-100, and protein concentration was measured by the Bradford method (BioRad Laboratories, Hercules, CA). To each reaction, 100 μg protein in 73 μL lysis buffer, 25 μL 4 × reaction buffer (200 mM HEPES, pH 7.4, 0.4% CHAPS, 40 mM DTT, 4 mM EDTA, 400 mM NaCl, 40% glycerol) and 2 μL 10-mM Ac-DEVD-pNA (BioMol, Plymouth Meeting, PA) were added. Reactions were incubated at 37°C and the increase in absorbance over a 3-hour period was measured on an MRX Microplate Reader (Thermo Labsystems). In addition, TdT-mediated dUTP nick end-labeling (TUNEL) assay was performed with the DeadEnd Colorimetric TUNEL System (Promega) according to the manufacturer's instructions following 48 hours of exposure to inhibitor.
Results
TKI258 inhibits proliferation and survival of ZNF198-FGFR1– and BCR-FGFR1–transformed Ba/F3 cells
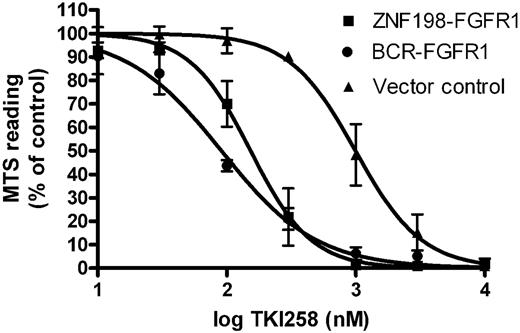
ZNF198-FGFR1 and other EMS-associated FGFR1 fusion genes have been shown previously to encode chimeric proteins with constitutive tyrosine kinase activity that can transform Ba/F3 cells to IL3 independence and induce a fatal MPD in mice.14,15,22,23 To determine whether TKI258 can inhibit this transforming activity, we compared the growth of Ba/F3-ZF cells, Ba/F3-BF cells, and control Ba/F3-pcDNA3.1 cells with increasing concentrations of TKI258. Reduced proliferation and survival were seen for both Ba/F3-ZF and Ba/F3-BF with cellular IC50 values for Ba/F3-ZF, Ba/F3-BF, and Ba/F3-pcDNA3.1 after 48 hours of exposure to TKI258 of 150 nM, 90 nM, and 1000 nM, respectively (Figure 1).
TKI258 inhibits proliferation and survival of Ba/F3 cells transformed to IL3 independence by ZNF198-FGFR1 or BCR-FGFR1. Cells (■ Ba/F3-ZNF198-FGFR1 and ● Ba/F3-BCR-FGFR1 grown in the absence of IL3; ▴ Ba/F3-pcDNA3.1 grown in the presence of IL3) were exposed to TKI258 for 48 hours. Values are means plus or minus SE of 3 independent experiments.
TKI258 inhibits proliferation and survival of Ba/F3 cells transformed to IL3 independence by ZNF198-FGFR1 or BCR-FGFR1. Cells (■ Ba/F3-ZNF198-FGFR1 and ● Ba/F3-BCR-FGFR1 grown in the absence of IL3; ▴ Ba/F3-pcDNA3.1 grown in the presence of IL3) were exposed to TKI258 for 48 hours. Values are means plus or minus SE of 3 independent experiments.
TKI258 treatment results in dose-dependent reduction in phosphorylation of ZNF198-FGFR1, BCR-FGFR1, and downstream signaling molecules
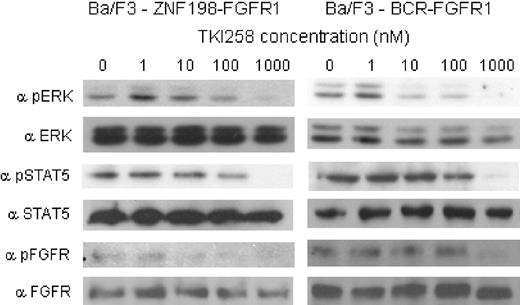
Western blotting was used to examine the effect of TKI258 treatment on phosphorylation of ZNF198-FGFR1, BCR-FGFR1, and the downstream signaling molecules STAT5 and ERK. After exposure to TKI258, all molecules showed a dose-dependent reduction in phosphorylation with increasing concentrations of TKI258 (Figure 2).
TKI258 treatment induces dose-dependent inhibition of phosphorylation of ZNF198-FGFR1, BCR-FGFR1, ERK, and STAT5. Phosphorylation of ERK, STAT5, and the BCR-FGFR1 and ZNF198-FGFR1 fusion proteins after exposure to TKI258 was assessed by Western blotting.
TKI258 treatment induces dose-dependent inhibition of phosphorylation of ZNF198-FGFR1, BCR-FGFR1, ERK, and STAT5. Phosphorylation of ERK, STAT5, and the BCR-FGFR1 and ZNF198-FGFR1 fusion proteins after exposure to TKI258 was assessed by Western blotting.
TKI258 inhibits proliferation and survival and induces apoptosis in the KG1 and KG1A cell lines
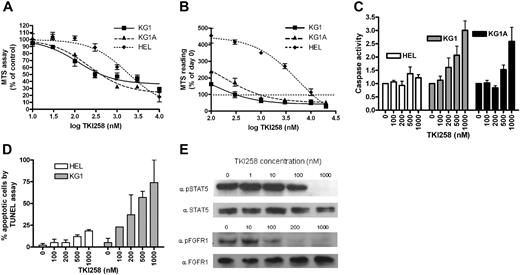
The KG1 cell line and its derivative KG1A have recently been shown to harbor the FGFR1OP2-FGFR1 fusion gene.13 We therefore examined the inhibitory effect of TKI258 on KG1 and KG1A compared with the erythroleukemia cell line HEL, which harbors the JAK2 V617F mutation and would not be expected to be responsive to TKI258. A cell viability assay demonstrated reduced survival of both KG1 and KG1A compared with HEL (Figure 3A). Cellular IC50 values for KG1, KG1A, and HEL after 48 hours of exposure to TKI258 were 180, 180, and 1500 nM, respectively. Analysis of the data as a percentage of the day-0 reading (Figure 3B) demonstrated that cell death (ie, at inhibitor concentrations where the curve falls below 100%) in the KG1 and KG1A cell lines occurred at 1-to 1.5-log concentration values below that of HEL. Caspase activation, a marker of apoptosis, was elevated in KG1 and KG1A at 200 to 500 nM TKI258 compared with HEL, which showed a minimal increase in caspase activation even at 1000 nM TKI258 (Figure 3C). Increased apoptosis of KG1 cells compared with HEL cells was also confirmed using the TUNEL assay (Figure 3D).
TKI258 inhibits proliferation and survival of KG1 and KG1A and induces apoptosis. TKI258 treatment results in (A) reduced viability and (B) increased cell death (where the curves fall below 100% of day-0 reading) in KG1 and KG1A cell lines assessed at 48 hours, in contrast to the nonresponsive HEL cell line. Values are means plus or minus SE of 3 independent experiments. (C) TKI258 treatment resulted in a dose-dependent increase in apoptosis, assessed by a colorimetric caspase assay, in KG1 and KG1A compared with HEL. Values are means plus or minus SD of 3 separate experiments and are normalized to 1.0 at 0 nM TKI258. (D) Increased apoptosis in KG1 cells was confirmed by the TUNEL assay. Values are means plus or minus SD of 2 separate experiments. (E) Reduced phosphorylation of the FGFR1OP2-FGFR1 fusion protein and STAT5 after TKI258 treatment.
TKI258 inhibits proliferation and survival of KG1 and KG1A and induces apoptosis. TKI258 treatment results in (A) reduced viability and (B) increased cell death (where the curves fall below 100% of day-0 reading) in KG1 and KG1A cell lines assessed at 48 hours, in contrast to the nonresponsive HEL cell line. Values are means plus or minus SE of 3 independent experiments. (C) TKI258 treatment resulted in a dose-dependent increase in apoptosis, assessed by a colorimetric caspase assay, in KG1 and KG1A compared with HEL. Values are means plus or minus SD of 3 separate experiments and are normalized to 1.0 at 0 nM TKI258. (D) Increased apoptosis in KG1 cells was confirmed by the TUNEL assay. Values are means plus or minus SD of 2 separate experiments. (E) Reduced phosphorylation of the FGFR1OP2-FGFR1 fusion protein and STAT5 after TKI258 treatment.
In addition, a dose-dependent reduction in phosphorylation of FGFR1OP2-FGFR1 and STAT5 was seen in KG1 cells (Figure 3E). Interestingly, we were unable to detect constitutive phosphorylation of ERK in serum-starved cells (results not shown), a finding also described by others.24 ERK is, however, phosphorylated in response to serum and is therefore phosphorylated by a pathway independent of FGFR1OP2-FGFR1.
TKI258 inhibits survival and proliferation of primary cells from EMS patients
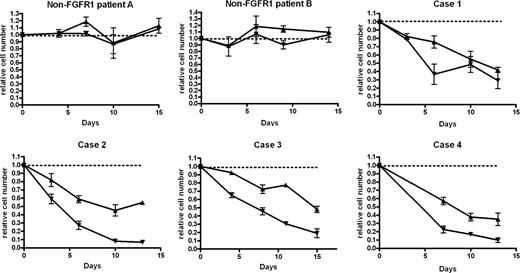
To determine whether TKI258 is active against primary EMS cells, we examined the effects of this compound in liquid culture and colony assays. In liquid culture assays using cells from 3 control MPD patients without FGFR1 translocations (of whom only 2 are shown in Figure 4), no difference was seen in the number of cells surviving in the presence of absence of TKI258. In contrast, all 4 patients with FGFR1 translocations showed clear reductions in relative cell numbers in cultures containing both 20 and 100 nM TKI258 compared with cultures without inhibitor (Figure 4). Furthermore, a dose-dependent effect was apparent with a greater reduction for all 4 patients tested with 100 nM TKI258 compared with 20 nM.
TKI258 treatment results in a dose-dependent reduction in cell survival in 4 EMS patients but not in 2 MPD patients without FGFR1 rearrangements. Cell counts (mean ± SE) are plotted relative to untreated control (dotted line at 1.0) at each time point for 20 nM (▴) and 100 nM (▾) TKI258-treated cell cultures.
TKI258 treatment results in a dose-dependent reduction in cell survival in 4 EMS patients but not in 2 MPD patients without FGFR1 rearrangements. Cell counts (mean ± SE) are plotted relative to untreated control (dotted line at 1.0) at each time point for 20 nM (▴) and 100 nM (▾) TKI258-treated cell cultures.
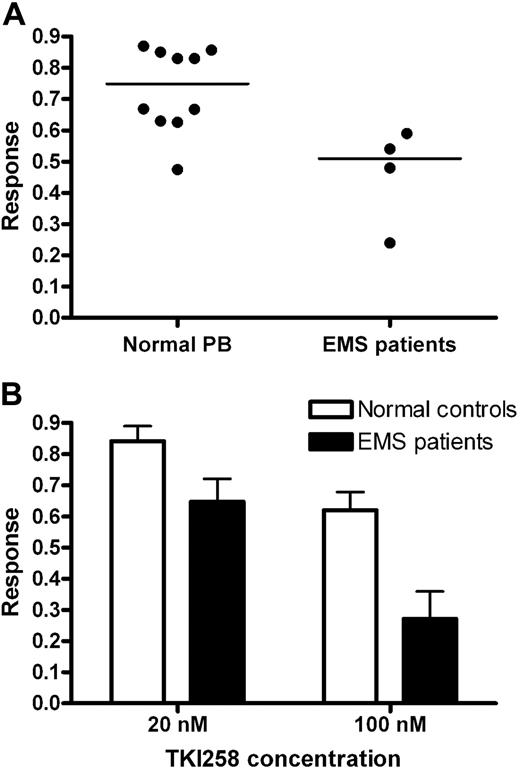
Colony growth from 10 normal peripheral blood samples was moderately inhibited by TKI258, with a mean response of 0.75 (range of 0.47–0.87). No obvious differences were observed between colonies from healthy individuals from treated and untreated cultures. For the 4 patients (cases 1-3, 5) for whom sufficient material was available, the median response was significantly lower at 0.51 (range, 0.24-0.58; P = .02, Mann-Whitney; Figure 5A). The degree of colony reduction was dependent on the dose of TKI258: for the healthy controls, responses at 20 nM and 100 nM were 0.87 and 0.66, respectively (Figure 3). For the 4 patients, the mean responses were 0.65 and 0.27 (P = .08 and P = .01 for 20 nM and 100 nM compared with controls, respectively, Mann-Whitney; Figure 5B). All colonies had a morphologic appearance indicative of colony-forming units granulocyte-macrophage (CFU-GM), however case 1 also grew erythroid burst-forming units (BFU-Es). Interestingly, the CFU-GMs in this case were unaffected by TKI258 (response = 1.04), but the BFU-Es were strongly inhibited (response = 0.13) with no growth at all at 100 nm.
Hematopoietic colonies from EMS patients show a greater, dose-dependent response to TKI258 than normal peripheral blood cells. (A) Overall and median responses in methylcellulose colony assays of 10 normal peripheral blood samples and EMS cases 1, 2, 3, and 5 (P = .02). (B) Dose response in colony reduction (± SD). A greater differential response was seen at 100 nM than 20 nM compared with healthy controls (P = .01 and P = .08, respectively).
Hematopoietic colonies from EMS patients show a greater, dose-dependent response to TKI258 than normal peripheral blood cells. (A) Overall and median responses in methylcellulose colony assays of 10 normal peripheral blood samples and EMS cases 1, 2, 3, and 5 (P = .02). (B) Dose response in colony reduction (± SD). A greater differential response was seen at 100 nM than 20 nM compared with healthy controls (P = .01 and P = .08, respectively).
Colony FISH analysis was performed to determine whether TKI258 selectively reduced the number of FGFR1-rearranged cells. Collectively for the 4 patients there was no difference between the proportion of colonies that showed split FGFR1 signals before treatment (106 of 119; 89%) compared with those after treatment (41 of 47; 87%). For cases 2 and 5, all colonies before and after treatment had FGFR1 split signals. Patients 1 and 3 had 18% and 38% unrearranged colonies, respectively, prior to treatment but there was no significant change after incubation with TKI258.
Discussion
Significant advances have been made in the use of small molecule inhibitors for the treatment of some malignancies with aberrant tyrosine kinase activation, for example, the use of imatinib for the treatment of BCR-ABL–positive CML and MPDs with translocations involving PDGFRA or PDGFRB. The development of inhibitors of the FGFR tyrosine kinases for the treatment of patients with t(4;14)-positive multiple myeloma and MPDs in which there is involvement of FGFR3 and FGFR1, respectively, has, however, made less progress; although there is evidence from in vitro and mouse model data that targeting FGFRs will be of therapeutic value. FGFR3 is up-regulated as a result of the t(4;14) translocation in approximately 15% of myeloma patients and inhibition has been demonstrated in t(4;14)-positive cell line models and/or mouse xenograft models with the FGFR inhibitors SU5402,7,25 PD173074,7 PKC412,26 and TKI258.9,12 As a result of this work, TKI258 is currently being assessed in clinical trials for the treatment of myeloma and other malignancies.
For MPDs with translocations involving FGFR1, inhibition has been demonstrated with SU5402,7 PD173074,7 and PKC4128 using transformed Ba/F3 cell lines and with PKC412 in a mouse xenograft model.8 Treatment of a single ZNF198-FGFR1–positive EMS patient with progressing disease with PKC412 for 6 months resulted in a partial hematologic response but no cytogenetic response, and there is clearly a need to examine other compounds with activity against FGFR1. We have therefore investigated the inhibitory activity of TKI258 in Ba/F3-ZNF198-FGFR1 and BCR-FGFR1 cell line models, in the FGFR1OP2-FGFR1–positive cell lines KG1 and KG1A, and in primary cells from EMS patients with FGFR1 translocations.
In the Ba/F3-ZNF198-FGFR1 and BCR-FGFR1 cell lines, treatment with TKI258 resulted in the inhibition of cell proliferation and survival accompanied by an inhibition of phosphorylation of the respective fusion proteins, ERK and STAT5. These results are thus similar to those previously demonstrated for the inhibitors SU5402, PD173074, and PKC412 in similar Ba/F3 cell line experiments.7,8 The AML KG1 cell line, and its derivative KG1A, has provided the first example of a patient-derived cell line with an FGFR1 fusion gene. We have demonstrated activity of TKI258 on proliferation, survival, and apoptosis in both cell lines with cellular IC50 values similar to those obtained in the Ba/F3-ZNF198-FGFR1 cell line. This suggests that transformed EMS still depends on the constitutive activity of FGFR1 fusions; this is similar to transformed CML, which still depends on BCR-ABL.
Importantly, we have also demonstrated for the first time that targeting FGFR1 selectively inhibits the growth and proliferation of primary cells from EMS patients. In a liquid culture assay, cells from all 4 EMS patients tested showed substantially reduced, dose-dependent survival over 2 weeks compared with 3 MPD patients without FGFR1 translocations, who showed no differential cell survival with and without TKI258. Using methylcellulose assays, we showed a significant, dose-dependent decrease in colony numbers grown in the presence of TKI258 compared with controls. However more detailed examination of the colony results failed to show clear evidence of selection for normal cells. The reasons for this are unclear and may relate to the fact that EMS is an aggressive disease, however for cases 2 and 5 only FGFR1-rearrangement–positive colonies were seen in the pretreatment cultures, and it is possible that the proportion of normal clonogenic cells was simply so low that no differential effect could be observed. Similar findings have been described for CML CFU-GMs treated with imatinib.27 Interestingly, case 1 grew spontaneous BFU-Es that were strongly inhibited by TKI-258. This is consistent with reports indicating that, on occasion, patients with FGFR1 fusions present with polycythemia vera before progressing to EMS.28
EMS is an aggressive disease with a median time to transformation of 6 to 9 months and with approximately 10% of patients presenting with acute leukemia.1 In this study, we have demonstrated the effectiveness of FGFR1 fusion inhibition in both cell lines and primary cells, suggesting that targeted therapy is likely to be beneficial for patients with EMS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Leukemia Research Fund UK.
Authorship
Contribution: A.C. and F.H.G. performed the laboratory analysis. All authors contributed to the design of the study and the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: N. C. P. Cross, Wessex Regional Genetics Laboratory, Salisbury District Hospital, Salisbury SP2 8BJ, United Kingdom; e-mail:ncpc@soton.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal