Because the polycomb group gene BMI1 regulates the proliferation of both normal and leukemic stem cells, we examined whether BMI1 expression was associated with disease progression in chronic myeloid leukemia (CML). Levels of BMI1 RNA were significantly higher in patients with advanced-phase than in patients with chronic-phase CML in both CD34+ cells (P = .006) and total peripheral-blood mononuclear cells (P < .001). E2F1, a transcription factor regulating BMI1, was up-regulated in CML compared with controls (P = .001). In a cohort of 64 CML patients, the level of BMI1 at diagnosis correlated with time to transformation to blast crisis, and the combination of low BMI1 and high proteinase-3 expression was associated in multivariate analysis with an improved overall survival (P = .001). We conclude that BMI1 may be a biomarker for the intrinsic heterogeneity of CML, and its measurement at diagnosis can help predict overall survival and thus contribute to better therapeutic decisions.
Introduction
Despite a consistent molecular abnormality, the BCR-ABL oncogene, chronic myeloid leukemia (CML) exhibits marked heterogeneity in prognosis, and various attempts have been made to determine prognosis for individual patients at the time of diagnosis in chronic phase (CP).1 For example, the Sokal and Hasford prognostic scores derived from study of patients treated predominantly with busulfan and interferon-α, respectively, have proved moderately useful in predicting the duration of survival for individual patients,2 and recent clinical experience suggests that this heterogeneity is still reflected in response to therapy with imatinib.3
The polycomb group (PcG) gene BMI1 plays an essential role in regulating the proliferative activity of both normal and leukemic stem cells.4,5 BMI1 is a transcriptional repressor probably restricted to stem cells and progenitors. Coexpression of BMI1 and other proteins from the PcG, such as EZH2, confers a higher degree of malignancy.6 BMI1 overexpression was described in several types of cancer, including hematologic neoplasms.7,,–10 We therefore measured BMI1 expression in CML to discover whether it might be involved in pathogenesis and might serve as a biomarker to predict disease aggressiveness and progression from CP to more advanced phases.
Patients, materials, and methods
Patients and controls
Two independent cohorts of CML patients were studied: (1) patients in CP whose nucleated cells were collected by leukapheresis and cryopreserved within 3 months of diagnosis (before start of treatment) and for whom complete follow-up was available (n = 64)11 ; (2) patients with cryopreserved cells collected at CP or blast crisis (BC). Informed consent for the use of these cells for research was obtained in accordance with the Declaration of Helsinki and with approval from the Hammersmith and Queen Charlotte's and Chelsea Research Ethics Committee Institutional Review Board. Diagnosis of CML and disease staging was based on clinical parameters and morphology of blood and bone marrow.11 Peripheral-blood mononuclear cells (PBMCs) from healthy donors, granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral-blood stem cells (PBSCs) from non-CML patients, and bone marrow from healthy donors (StemCell, London, United Kingdom) were also obtained by informed consent and were used as controls.
Q-RT/PCR amplification
PBMCs from cryopreserved material were isolated by density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway). CD34+ cells were selected by binding to immunomagnetic beads (MiniMACS; Miltenyi Biotech, Bergisch-Gerbach, Germany). Total RNA was extracted using the Qiagen RNeasy kit (Qiagen, Crawley, United Kingdom), treated with DNase I (Invitrogen, Paisley, United Kingdom) to eliminate genomic DNA, and reverse transcribed into cDNA according to standard methods. Expression of BMI1, E2F1, and GAPDH was assessed by quantitative real-time reverse-transcription and polymerase chain reaction (Q-RT/PCR) using the Applied Biosystems 7300/7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). All Q-RT/PCR reactions were performed in 25-μL volume.11 GAPDH expression was used as the endogenous cDNA quality control. The ABI Assays-on-demand TaqMan probe-and-primer reagents for BMI1, E2F1, and GAPDH were used according to the manufacturer's instructions.
Statistical methods
Groups were compared using the Mann-Whitney test for continuous data and Fisher exact test for categoric data. Survival curves were calculated using the Kaplan-Meier method, and groups were compared using the log-rank test. Patients were divided into groups using Q-RT/PCR values delineated by the median. Genes or parameters identified from the univariate analysis with P values of less than .20 (P < .20) were entered into a Cox regression analysis, and a forward and backward stepping procedure was used to find the best model to predict survival. All quoted P values are from 2-sided tests with values less than .05 considered significant.
Results and discussion
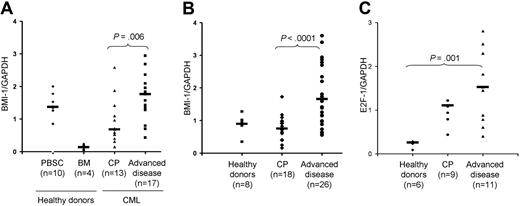
BMI1 expression levels in CD34+ cells were significantly lower in CP (n = 13) than in more advanced stages (accelerated phase and BC, n = 17) of CML (P = .006; Figure 1A). The same significant difference also held true when BMI1 expression was compared in unfractionated CML-derived PBMCs (P < .001; Figure 1B). In 8 patients for whom both CD34+ cells and PBMCs were available, a paired comparison disclosed a trend toward a significant correlation between BMI1 expression in the 2 cell populations (P = .05; Pearson correlation R = 0.7). Of note, BMI1 expression in bone marrow-derived CD34+ stem cells from healthy donors was significantly lower compared with CML patients (P = .003; Figure 1A). In order to gain insights into the mechanisms underlying BMI1 up-regulation in CML, we also assessed the expression of E2F1, a transcription factor that controls various genetic programs including cell-cycle progression and apoptosis13 and that was shown to directly regulate BMI1 activity.14 We found that PBMCs from CML patients (all disease stages) displayed significantly higher levels of E2F1 compared with healthy controls (P = .001; Figure 1C).
BMI1 expression in CML as assessed by Q-RT/PCR. (A) BMI1 expression in CD34+ immunomagnetically selected hematopoietic progenitors from CML patients at diagnosis in chronic phase (CP) compared with patients in more advanced disease stage (acceleration phase and blast crisis). The definition of CP and advanced phases (accelerated phase and blast crisis) was based on previously established criteria2,11,12 : CP, less than 10% blasts; accelerated phase 10% to 30% blasts or less than 10% blasts with clonal evolution; and blast crisis, more than 30% blasts. Bone marrow (BM)-derived CD34+ cells from healthy donors and G-CSF-mobilized CD34+ stem cells (PBSCs) from non-CML donors were used as controls. As previously described, G-CSF-mobilized CD34+ PBSCs express high levels of BMI1 compared with nonstimulated normal cells.10 (B) BMI1 expression in total unfractionated PBMCs from CML patients at diagnosis in CP compared with patients in more advanced disease stage (accelerated phase and blast crisis). Total PBMCs from healthy donors were used as controls. (C) E2F1 expression in total unfractionated PBMCs from CML patients at diagnosis in CP compared with patients in more advanced disease stage (accelerated phase and blast crisis). Total PBMCs from healthy donors were used as controls. Horizontal bars denote the medians. Values of genes represent the Q-RT/PCR expression as a ratio of the gene of interest to the GAPDH control gene. For establishment of the Q-RT/PCR assay, the Jurkat and HeLa cell lines were used as positive controls for BMI1 and E2F1 expression, respectively, with a standard curve being generated for the amplification of logarithmic dilutions (10−1 to 10−5) of their cDNAs. An average of the duplicates of each data point was taken and plotted against the cycle threshold (Ct). The technical variability between duplicate samples in our RT/PCR assays has been established for a number of different genes as less than 1.3-fold at the 95% level of confidence (data not shown).
BMI1 expression in CML as assessed by Q-RT/PCR. (A) BMI1 expression in CD34+ immunomagnetically selected hematopoietic progenitors from CML patients at diagnosis in chronic phase (CP) compared with patients in more advanced disease stage (acceleration phase and blast crisis). The definition of CP and advanced phases (accelerated phase and blast crisis) was based on previously established criteria2,11,12 : CP, less than 10% blasts; accelerated phase 10% to 30% blasts or less than 10% blasts with clonal evolution; and blast crisis, more than 30% blasts. Bone marrow (BM)-derived CD34+ cells from healthy donors and G-CSF-mobilized CD34+ stem cells (PBSCs) from non-CML donors were used as controls. As previously described, G-CSF-mobilized CD34+ PBSCs express high levels of BMI1 compared with nonstimulated normal cells.10 (B) BMI1 expression in total unfractionated PBMCs from CML patients at diagnosis in CP compared with patients in more advanced disease stage (accelerated phase and blast crisis). Total PBMCs from healthy donors were used as controls. (C) E2F1 expression in total unfractionated PBMCs from CML patients at diagnosis in CP compared with patients in more advanced disease stage (accelerated phase and blast crisis). Total PBMCs from healthy donors were used as controls. Horizontal bars denote the medians. Values of genes represent the Q-RT/PCR expression as a ratio of the gene of interest to the GAPDH control gene. For establishment of the Q-RT/PCR assay, the Jurkat and HeLa cell lines were used as positive controls for BMI1 and E2F1 expression, respectively, with a standard curve being generated for the amplification of logarithmic dilutions (10−1 to 10−5) of their cDNAs. An average of the duplicates of each data point was taken and plotted against the cycle threshold (Ct). The technical variability between duplicate samples in our RT/PCR assays has been established for a number of different genes as less than 1.3-fold at the 95% level of confidence (data not shown).
We have previously shown that the combination of CD7, proteinase-3 (PR3), and elastase-2 (ELA2) expression levels at diagnosis can reflect the intrinsic molecular heterogeneity of CML in CP, especially duration of CP. This was observed in patients with an “aggressive disease” who develop BC early after diagnosis (< 3 years), as opposed to patients with an “indolent disease” whose BC occurs more than 7 years after diagnosis.11 We therefore measured BMI1 expression in CD34+ cells from a cohort of 64 CP CML patients (for details see Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article). We found a significant difference in BMI1 levels between patients with an “indolent” or an “intermediate” (patients surviving between 3 and 7 years without developing BC) clinical pattern compared with those who had an “aggressive” clinical evolution (P = .01 for comparing the 3 groups, Kruskal-Wallis test; Figure 2A). Patients displaying a low BMI1 expression level at diagnosis had significantly longer survival than other patients (P = .005; Figure 2B). When BMI1 was included in a Cox multivariate survival analysis model (together with the previously established prognostic markers CD7, ELA2, and PR3 and other relevant demographic and clinical parameters; Table S1), the combination of low BMI1 and high PR3 expression levels was found to be a strong independent marker associated with significantly longer overall survival (P = .001; RR = 0.20, 95% confidence interval [CI]; 0.08-0.54; Figure 2C).
BMI1 expression and probabilities of overall survival. (A) BMI1 expression in CD34+ immunomagnetically selected hematopoietic progenitors from diagnosis in a cohort of 64 CP CML patients, showing different disease evolution patterns: patients who developed blast crisis (BC) within 3 years of diagnosis were defined as having “aggressive disease” (n = 17), whereas those who survived for longer than 7 years prior to the onset of BC were defined as having “indolent disease” (n = 23). Patients who survived between 3 and 7 years without developing BC were categorized as having “intermediate disease” (n = 24). There was a significant difference in BMI1 expression among the 3 groups (P = .01 when comparing all 3 groups), between patients with “intermediate” and “aggressive” disease (P = .01), and between “indolent” and “aggressive” disease (P = .01) but not between “indolent” versus “intermediate” disease (P = not significant [NS]). The median age at diagnosis of the selected patients was 45.7 years (range, 17.6-68.3 years). The male-female ratio was 1.8:1 (41 males, 23 females). The majority of patients were diagnosed in the preimatinib era. (B) Overall survival according to BMI1 expression as assessed by Q-RT/PCR in the whole cohort of the above 64 patients. The median gene expression level is used to segregate the patients into a “low BMI1” group (BMI1 expression < median) and a “high BMI1” group (BMI1 expression > median). (C) Cox multivariate analysis yielded a model with the combination of low BMI1 and high proteinase-3 (PR3) expression as predictive of significantly improved survival. The median gene expression levels were used to segregate the patients into a “low BMI1-high PR3” group (BMI1 expression < median and PR3 > median; n = 21) and a “high BMI1-low PR3” group (BMI1 expression > median and PR3 < median; n = 43). Values of genes represent the Q-RT/PCR expression as a ratio of the gene of interest to the GAPDH control gene.
BMI1 expression and probabilities of overall survival. (A) BMI1 expression in CD34+ immunomagnetically selected hematopoietic progenitors from diagnosis in a cohort of 64 CP CML patients, showing different disease evolution patterns: patients who developed blast crisis (BC) within 3 years of diagnosis were defined as having “aggressive disease” (n = 17), whereas those who survived for longer than 7 years prior to the onset of BC were defined as having “indolent disease” (n = 23). Patients who survived between 3 and 7 years without developing BC were categorized as having “intermediate disease” (n = 24). There was a significant difference in BMI1 expression among the 3 groups (P = .01 when comparing all 3 groups), between patients with “intermediate” and “aggressive” disease (P = .01), and between “indolent” and “aggressive” disease (P = .01) but not between “indolent” versus “intermediate” disease (P = not significant [NS]). The median age at diagnosis of the selected patients was 45.7 years (range, 17.6-68.3 years). The male-female ratio was 1.8:1 (41 males, 23 females). The majority of patients were diagnosed in the preimatinib era. (B) Overall survival according to BMI1 expression as assessed by Q-RT/PCR in the whole cohort of the above 64 patients. The median gene expression level is used to segregate the patients into a “low BMI1” group (BMI1 expression < median) and a “high BMI1” group (BMI1 expression > median). (C) Cox multivariate analysis yielded a model with the combination of low BMI1 and high proteinase-3 (PR3) expression as predictive of significantly improved survival. The median gene expression levels were used to segregate the patients into a “low BMI1-high PR3” group (BMI1 expression < median and PR3 > median; n = 21) and a “high BMI1-low PR3” group (BMI1 expression > median and PR3 < median; n = 43). Values of genes represent the Q-RT/PCR expression as a ratio of the gene of interest to the GAPDH control gene.
Our observations suggest an important role for BMI1 in CML pathophysiology and prognosis. BMI1 is essential for the self-renewal of both hematopoietic and neuronal stem cells, as well as cancer stem cells.5,15,16 It has also been shown to cooperate with MYC in the generation of lymphomas in double-transgenic mice.17 Furthermore, BMI1 blocks senescence and immortalizes mouse embryo fibroblasts and, in combination with an activated H-RAS gene, leads to neoplastic transformation.18 These oncogenic functions depend in part on the ability of BMI1 to repress the INK4A locus, which encodes the tumor suppressor proteins p16Ink4a and p14Arf.19 All of these pathways are known to be involved in the proliferation of BCR-ABL-positive cells,20 suggesting that overexpression of BMI1 acts in conjunction with its related partner genes in the genesis and transformation of CML in a manner analogous to its role in other malignancies.
Although our data do not provide a complete picture of the mechanisms involved in BMI1 up-regulation in CML, they show that these probably involve the E2F1 gene, which we also found to be overexpressed in CML. Thus, E2F1 (1) directly regulates BMI1,14 (2) has its activity controlled by the retinoblastoma-cyclin pathway,21 and, (3) via this pathway, defines a route from Bcr-Abl to MYC transcription, which is required for Bcr-Abl transformation.22 This implies that genetic alterations impairing E2F1, BMI1, and their downstream targets may render hematopoietic cells refractory to the induction of differentiation, as previously demonstrated in myeloid cell line models,23 and are thereby likely to play a major role in the progression and aggressiveness of CML. Moreover, induction of BMI1 would change the composition of the PcG complex to favor proliferation over cell-cycle arrest, because the relative amounts of BMI1 in the complex determine its biochemical and biologic functions.24 The identification of BMI1-cooperative factors in CML will surely help define it as a bona fide cancer stem cell inducer.
From the clinical standpoint, our findings demonstrate that BMI1 can serve as a novel molecular marker to predict prognosis in CML, particularly in conjunction with the expression level of immune-related proteins such as PR3.11,25 An interesting and useful aspect of our study, from a practical point of view, was the indication that the expression of BMI1 in CD34+ cells tends to parallel that found in total PBMCs, as these provide a more easily obtainable and less expensive biologic material in which a rapid Q-RT/PCR prognostication test can be done at diagnosis of the disease. Despite their great success, it is still unclear whether tyrosine kinase inhibitors can cure CML. Therefore, the prospective screening for BMI1 expression in combination with other molecular markers12 can help refine CML disease staging and prognosis toward optimizing therapeutic interventions, including perhaps BMI1-targeted inhibitors.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff of the Stem Cell Laboratory at the Hammersmith Hospital (London, United Kingdom) for help with identification of stored cells; Daniela Passos, Tamara Law, Maria Makri, and Chee Han Lim for excellent technical assistance; and B. Gaugler (Institut National de la Santé et de la Recherche Médicale UMR 599, Marseille, France) and John M. Goldman (Imperial College, London) for helpful discussions and critical reading of the manuscript.
This work was supported by grants from the British Council/‘Institut National du Cancer (INCa)' and the ‘Fondation de France' Paris, France (M.M.) and the Hammersmith Hospital Trust Research Committee, London, United Kingdom (J.V.M.).
Authorship
Contribution: M.M. conceived and designed the study, collected patient data, processed samples, performed experiments, analyzed data, performed bibliographic search, and wrote and revised the report; A.S.M.Y. conceived and designed the study, processed samples, and performed experiments; R.M.S. performed the statistical analysis and helped to write the report; J.F.A. provided clinical care and recorded clinical data; and J.V.M. conceived and designed the study, supervised its execution, and helped write and revise the report. The corresponding author (J.V.M.) had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junia V. Melo, Department of Hematology, Imperial College London, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK; e-mail: j.melo@imperial.ac.uk.

![Figure 2. BMI1 expression and probabilities of overall survival. (A) BMI1 expression in CD34+ immunomagnetically selected hematopoietic progenitors from diagnosis in a cohort of 64 CP CML patients, showing different disease evolution patterns: patients who developed blast crisis (BC) within 3 years of diagnosis were defined as having “aggressive disease” (n = 17), whereas those who survived for longer than 7 years prior to the onset of BC were defined as having “indolent disease” (n = 23). Patients who survived between 3 and 7 years without developing BC were categorized as having “intermediate disease” (n = 24). There was a significant difference in BMI1 expression among the 3 groups (P = .01 when comparing all 3 groups), between patients with “intermediate” and “aggressive” disease (P = .01), and between “indolent” and “aggressive” disease (P = .01) but not between “indolent” versus “intermediate” disease (P = not significant [NS]). The median age at diagnosis of the selected patients was 45.7 years (range, 17.6-68.3 years). The male-female ratio was 1.8:1 (41 males, 23 females). The majority of patients were diagnosed in the preimatinib era. (B) Overall survival according to BMI1 expression as assessed by Q-RT/PCR in the whole cohort of the above 64 patients. The median gene expression level is used to segregate the patients into a “low BMI1” group (BMI1 expression < median) and a “high BMI1” group (BMI1 expression > median). (C) Cox multivariate analysis yielded a model with the combination of low BMI1 and high proteinase-3 (PR3) expression as predictive of significantly improved survival. The median gene expression levels were used to segregate the patients into a “low BMI1-high PR3” group (BMI1 expression < median and PR3 > median; n = 21) and a “high BMI1-low PR3” group (BMI1 expression > median and PR3 < median; n = 43). Values of genes represent the Q-RT/PCR expression as a ratio of the gene of interest to the GAPDH control gene.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-12-065599/2/m_zh80130703230002.jpeg?Expires=1769618405&Signature=PejJ1wt1hBaLprp3HnvcLOJoDDBjaMLTW-Z-xcC7C5PTCY7oDYMO0F3NAoFLU0zTA04Jjkw5p1Kd6TRhPyyW9-oU8Qmfnkck~0u5RbmSOMkuYflinZXF9SCQK45uvX0p6aOT98gXWYfJfXdzOqR4j1CzSzIhO9QNdcFzKdY6~ZRIuqv614pWaZPQkxKWK84rYMGhXLob7X-c~nrOn8DLfX7Y2g7UDfZljP8aAEx83L6zPr3bGCb6UHt3loRUhPDWNZUxL64P9SirqMIDTyRae5ee6KBQR7PONzho9-bTA3vy0JH5FNQGm-P94rsMTVMli5He~x59LiqHfh~QfAFH1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal