Previous studies on apoptosis defects in acute lymphoblastic leukemia (ALL) have focused on chemotherapy-induced, primarily mitochondrial death pathways. Yet, immunologic surveillance mechanisms including sensitization to apoptotic signals mediated via the death receptor CD95 might contribute to leukemic control. Here, we show that primary B-cell precursor ALL cells from children escape from receptor-dependent cell death in 2 ways: Resting ALL blasts are protected from receptor-mediated apoptosis due to the absence of CD95 surface expression. However, even though CD40 ligation results in up-regulation of CD95, ALL blasts, unlike normal B cells, remain resistant to apoptosis. We show that this apoptosis resistance involves the selective up-regulation of the short isoforms of the caspase-8 inhibitor c-FLIP acting directly at the CD95 receptor level. Treatment with cycloheximide during CD40 activation prevents up-regulation of those c-FLIP isoforms and sensitizes ALL cells toward CD95-mediated apoptosis. We therefore propose that induction of the short c-FLIP isoforms inhibits the onset of CD95-induced apoptosis in primary CD40-stimulated ALL cells despite high CD95 expression.
Introduction
Acute lymphoblastic leukemia (ALL) in children is a malignant disease with a good overall prognosis. Treatment failure in those patients who suffer relapse has to a large part been attributed to drug resistance and defects in apoptosis. Consequently, investigations on the deregulation of apoptosis in ALL have primarily focused on chemotherapy-induced pathways.1,2 Yet immunologic surveillance also contributes to long-term survival.3,4 The capacity of mature normal B cells to respond to CD40 ligation by up-regulation of the CD95 (Fas/APO1) death receptor is critical for their susceptibility to immunologic control.5,–7 Primary B-cell precursor ALL blasts (BCP-ALLs) lack CD95 expression and are resistant to CD95-mediated cell death.8 Although BCP-ALL blasts express CD40 to variable degrees, there is no information on CD40-dependent modulation of blast sensitivity to apoptotic signals.9
Death receptor–mediated proapoptotic signals are transmitted via the so-called death-inducing signaling complex (DISC) formed upon oligomerization of CD95 by interaction with its ligand.10 The oligomerized death domains (DDs) of the receptor associate with the cytoplasmic adapter molecule FADD with subsequent recruitment of procaspase-8 (FLICE) into the DISC. The apoptotic cascade is then initiated by autocatalytic cleavage of procaspase-8 and release of the active enzyme into the cytoplasm. The FLICE inhibitory protein (c-FLIP) can bind to the DISC and interfere with receptor-induced apoptosis.11 c-FLIP occurs in 3 different isoforms as a result of alternative splicing: 2 short isoforms, c-FLIPShort (27 kDa) and c-FLIPRaji (26 kDa), and a long form c-FLIPLong (55 kDa).12,13 While the short isoforms, thereafter called c-FLIPS and c-FLIPR, comprise only 2 death effector domains (DEDs) critical for interaction with the DISC, the long isoform contains an additional catalytically inactive C-terminal caspaselike domain. Depending on its expression level, c-FLIPL can serve as a dual regulator with both proapoptotic and antiapoptotic function.14,15 Thus, it has been shown in adult T-cell leukemia that it is the equilibrium between FLIP and caspase-8 that regulates susceptibility to CD95-mediated apoptosis.16 On the other hand, the short isoforms c-FLIPS+R play an established role as inhibitors of apoptosis preventing cleavage and activation of procaspases at the DISC.17
In normal mature B cells, most components of the DISC are constitutively expressed, while c-FLIP is tightly regulated.18 To evaluate the significance of receptor-induced apoptosis in primary BCP-ALL, we thus examined expression levels of the death receptor CD95 as well as blast sensitization to CD95-mediated apoptosis upon CD40 activation. Here we show that up-regulation of c-FLIPS+R via CD40 confers resistance of BCP-ALL cells toward CD95-mediated apoptosis.
Materials and methods
The ALL samples were obtained from CoALL study patients. The respective protocols were approved by the ethics committee (IRB) in Hamburg as well as the local ethics committee (IRB) in Duesseldorf including the conductance of respective research projects. In accordance with the Declaration of Helsinki, informed consent was obtained from parents or legal guardians of the patients at the time of enrollment.
Induction of apoptosis in primary B cells and ALL blasts
After ethics committee approval and receipt of informed consent, normal and malignant B cells were isolated from the mononuclear cell fraction of peripheral blood or bone marrow by standard Ficoll gradient-centrifugation. All BCP-ALL samples used exhibited a CD19/10 double-positive preB-ALL or c-ALL phenotype and contained more than 80% leukemic blasts. No proB-ALL samples were included. In normal B cells, more than 80% purity was achieved by magnetic activated cell sorting using CD19 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany).19 CD95 receptor expression was assessed by flow cytometry using CD95 PE-coupled antibody (Becton Dickinson, San Jose, CA). For activation, BCP-ALL blasts or normal B cells were cultured for 72 hours on wild-type or transduced murine fibroblasts expressing human CD40 ligand (CD40L). After cross-linking of 106 cells with 1 μg/mL anti-CD95 (CH-11; Immunotech, Marseille, France), CD95-specific apoptosis was determined by flow cytometry after staining with annexin V–FITC and propidium iodide for 10 minutes according to the manufacturer's protocol (Immunotech). Specific apoptosis was calculated as [experimental apoptosis (%) − spontaneous apoptosis(%)]/[100% − spontaneous apoptosis (%)] × 100.
Western blot analysis
Postnuclear supernatant equivalents of 106 cells were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose membranes as described.20 The following primary antibodies were used: anti–c-FLIP mAb NF6, anti–caspase-8 (Alexis Biochemicals, San Diego, CA), and anti-FADD (BD Transduction Laboratories, San Jose, CA). Horseradish peroxidase–coupled isotype-specific secondary antibodies (Southern Biotech, Birmingham, AL) were used in combination with the chemiluminescence-based system (Chemicon, Temecula, CA) for detection.
Caspase activity assay
Induction of caspase-8–like and caspase-3/7–like activity was measured by a luminescent substrate (Caspase-Glo) assay according to manufacturer's instructions (Promega, Madison, WI). After CD95 cross-linking for 4 hours and 16 hours, cell lysates were incubated with the specific luminescent substrates, which upon caspase cleavage and in conjunction with luciferase result in light emission that was measured in a luminometer.
Statistics
Expression levels and specific apoptosis rates in different cell types were compared by Mann-Whitney test. All statistical analyses were performed with the SPSS program version 12.0 (Chicago, IL). A P value of less than .05 was considered significant.
Results and discussion
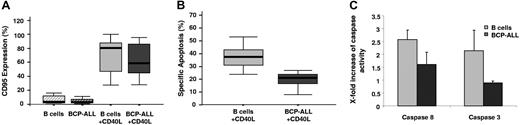
In order to investigate the sensitivity of primary B-cell precursor blasts (BCP-ALLs) toward death receptor–mediated apoptosis, we first assessed CD95 expression by flow cytometry. On primary BCP-ALL blasts and normal peripheral B cells, constitutive expression of CD95 was negligible. After activation via CD40, a similar up-regulation of CD95 cell surface expression was observed in both cell types (P = .68) (Figure 1A). Yet, while levels of CD95 expression were comparable in CD40-activated BCP blasts and normal B cells, receptor-specific apoptosis was significantly lower in BCP-ALL (P = .001) (Figure 1A-B). In keeping with this observation, induction of initiator caspase-8– and effector caspases-3/7–like activity remained unchanged in CD40-stimulated blasts upon CD95 cross-linking, while caspase activity increased in activated normal B cells (Figure 1C).
Up-regulation of CD95 expression in BCP-ALL blasts after activation via CD40 does not translate into sensitization to apoptosis. (A) Expression of the death receptor CD95 was analyzed by flow cytometry (median; range) in normal peripheral B cells (n = 8) and BCP-ALL blasts (n = 15) after 72-hour culture on nonmodified feeder cells or feeder cells transgenically expressing CD40L. (B) For assessment of receptor-mediated cell death, cells were also removed from feeder cells and stimulated for apoptosis induction with an agonistic CD95 monoclonal antibody (mAb) for 16 hours. Apoptosis was measured by flow cytometry using annexin V and propidium iodide staining (median; range). The increase in spontaneous apoptosis in the absence of anti-CD95 did not exceed 5% regardless of CD40 activation. Despite equivalent up-regulation of CD95 in CD40-activated normal B cells and BCP-ALLs, induction of specific apoptosis is significantly lower in ALL blasts (P = .001). (C) Caspase-8– and caspase-3/7–like proteolytic activities were examined in CD40-activated normal peripheral B cells (n = 4) and BCP-ALL blasts (n = 4) in a luminescent substrate assay after cross-linking with anti-CD95 mAb for 4 hours (caspase-8) and 16 hours (caspase-3/7). The assay was performed in triplicates of 2.5 × 104 cells/well for caspase-8 and 1 × 104 cells/well for caspase-3/7. The increase in CD95-induced caspase activity in normal B cells and BCP-ALL blasts after activation via CD40 is shown. The increase is presented as the ratio of CD40-activated over resting B cells and blasts (mean ± SEM).
Up-regulation of CD95 expression in BCP-ALL blasts after activation via CD40 does not translate into sensitization to apoptosis. (A) Expression of the death receptor CD95 was analyzed by flow cytometry (median; range) in normal peripheral B cells (n = 8) and BCP-ALL blasts (n = 15) after 72-hour culture on nonmodified feeder cells or feeder cells transgenically expressing CD40L. (B) For assessment of receptor-mediated cell death, cells were also removed from feeder cells and stimulated for apoptosis induction with an agonistic CD95 monoclonal antibody (mAb) for 16 hours. Apoptosis was measured by flow cytometry using annexin V and propidium iodide staining (median; range). The increase in spontaneous apoptosis in the absence of anti-CD95 did not exceed 5% regardless of CD40 activation. Despite equivalent up-regulation of CD95 in CD40-activated normal B cells and BCP-ALLs, induction of specific apoptosis is significantly lower in ALL blasts (P = .001). (C) Caspase-8– and caspase-3/7–like proteolytic activities were examined in CD40-activated normal peripheral B cells (n = 4) and BCP-ALL blasts (n = 4) in a luminescent substrate assay after cross-linking with anti-CD95 mAb for 4 hours (caspase-8) and 16 hours (caspase-3/7). The assay was performed in triplicates of 2.5 × 104 cells/well for caspase-8 and 1 × 104 cells/well for caspase-3/7. The increase in CD95-induced caspase activity in normal B cells and BCP-ALL blasts after activation via CD40 is shown. The increase is presented as the ratio of CD40-activated over resting B cells and blasts (mean ± SEM).
As inhibition of caspase-8 in CD40-stimulated blasts suggests a proximal block in the CD95 pathway, molecules participating in formation of the DISC were assessed by Western blot analysis. FADD, procaspase-8, and the long isoform of c-FLIP were constitutively expressed in ALL as well as in normal B cells, and expression remained largely unchanged upon CD40 stimulation (Figure 2A). In contrast, the antiapoptotic short isoforms c-FLIPS and c-FLIPR were barely detectable in both nonactivated cell types. Of interest, in ALL blasts, pronounced up-regulation of c-FLIPS+R was observed after ligation of CD40, while in normal B cells expression of the short isoform c-FLIPS remained weak and c-FLIPR was absent (Figure 2A). Specific up-regulation of the antiapoptotic isoforms c-FLIPS+R in CD40-activated ALL blasts with c-FLIPS+R exceeding c-FLIPL expression was subsequently confirmed in 5 individual patients by quantitative densitometry (Figure 2B).
Pronounced up-regulation of the short isoforms of c-FLIP in CD40-activated ALL blasts mediates resistance to death receptor–dependent apoptosis. (A) For assessment of expression levels of molecules that form the death-inducing signaling complex (DISC), cell lysates of resting and CD40-activated normal B cells and ALL blasts were analyzed by Western blotting. (B) The relative expression of c-FLIPS+R and c-FLIPL proteins in CD40-activated normal B cells and ALL blasts was measured by densitometry using Image Java software program (NIH, Bethesda, MD). Results of samples from 5 individual patients and 4 healthy B-cell controls are represented as the mean ± SEM of FLIPS+R/FLIPL peak area ratio. (C-D) CD40-activated ALL cells were treated with anti-CD95 mAb in the absence or presence of 0.1 μg/mL cycloheximide (CHX). After 48 hours, expression of the long and short isoforms of c-FLIP was examined by Western blot analysis (C), and CD95-dependent apoptosis was measured by flow cytometry (D). Values represent mean percentage of specific apoptosis (± SEM; n = 7 patients) (t test ± CD40L, P = .004; t test CD40L ± CHX, P = .01).
Pronounced up-regulation of the short isoforms of c-FLIP in CD40-activated ALL blasts mediates resistance to death receptor–dependent apoptosis. (A) For assessment of expression levels of molecules that form the death-inducing signaling complex (DISC), cell lysates of resting and CD40-activated normal B cells and ALL blasts were analyzed by Western blotting. (B) The relative expression of c-FLIPS+R and c-FLIPL proteins in CD40-activated normal B cells and ALL blasts was measured by densitometry using Image Java software program (NIH, Bethesda, MD). Results of samples from 5 individual patients and 4 healthy B-cell controls are represented as the mean ± SEM of FLIPS+R/FLIPL peak area ratio. (C-D) CD40-activated ALL cells were treated with anti-CD95 mAb in the absence or presence of 0.1 μg/mL cycloheximide (CHX). After 48 hours, expression of the long and short isoforms of c-FLIP was examined by Western blot analysis (C), and CD95-dependent apoptosis was measured by flow cytometry (D). Values represent mean percentage of specific apoptosis (± SEM; n = 7 patients) (t test ± CD40L, P = .004; t test CD40L ± CHX, P = .01).
To further delineate the role of c-FLIPS+R expression in resistance of BCP-ALL blasts toward CD95-mediated apoptosis, protein synthesis was inhibited by addition of cycloheximide. Indeed, cycloheximide prevented up-regulation of both short isoforms of c-FLIP in CD40-activated blasts, particularly c-FLIPR (Figure 2C), and sensitized BCP-ALL blasts to CD95-mediated cell death after CD40 ligation (Figure 2D). This demonstrated that modulation of c-FLIPS+R expression was closely associated with resistance and sensitivity to CD95-mediated apoptosis in primary BCP-ALL.
Thus, BCP-ALL cells can escape from receptor-dependent cell death in 2 ways: Resting ALL blasts are shielded against elimination by receptor-mediated apoptosis via the absence of CD95 surface expression. Upon activation by CD40L, blasts then evade programmed cell death by overexpression of c-FLIPS+R counteracting concomitant up-regulation of CD95. c-FLIP isoforms play a critical role in resistance to death receptor–mediated apoptosis in a variety of human cancers including B-cell–derived malignancies such as Hodgkin and B-cell lymphomas21,22 with high levels of c-FLIP associated with drug resistance, escape from cytotoxic effector mechanism, and poor clinical outcome.23,–25 In adult T-ALL, blasts are protected from CD95-mediated apoptosis by human T-lymphotropic virus (HTLV) 1-Tax.26 Here we describe for the first time that in BCP-ALL, strong and selective expression of the 2 short isoforms of c-FLIP facilitates resistance to receptor-mediated apoptosis. c-FLIPS+R remain stably expressed in ALL cells for 6 days after CD40 activation (data not shown). This is clearly different from mature normal and malignant B cells where up-regulation of c-FLIP after CD40 activation is transient.18,27 Activated tonsillar B cells are thus only temporarily protected from CD95-mediated cell death. Similarly in chronic lymphocytic leukemia (CLL), CD40L induces a shift in expression levels of the antiapoptotic and proapoptotic molecules of the DISC with initial protection but subsequent sensitization of CD40-stimulated CLL cells to receptor-dependent cell death.28,29 Moreover, this is the first report on inducible protein expression of the short isoform c-FLIPR in primary leukemic blasts of the B-cell lineage. With CD40-induced expression of c-FLIPS+R, we thus delineate a novel receptor-associated antiapoptotic mechanism in BCP-ALL complementing the inhibitors of the mitochondrial apoptosis pathways.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was sponsored by an intramural grant of the Medical Faculty of the University of Duesseldorf.
We thank A. Kauff and D. Scholtyssik for excellent technical assistance.
Authorship
Contribution: A.T. and I.S. designed and performed research, analyzed data, and wrote the paper; M.S. and L.G. performed research and analyzed data; U.G. performed research; G.E.J.-S. provided vital materials and wrote the paper; K.S.-O. wrote the paper; D.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anja Troeger, Clinic for Pediatric Oncology, Hematology and Immunology, University Hospital, Moorenstr. 5, 40225 Duesseldorf, Germany; e-mail: troeger@med.uni-duesseldorf.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal