Activated tyrosine kinases have been frequently implicated in the pathogenesis of cancer, including acute myeloid leukemia (AML), and are validated targets for therapeutic intervention with small-molecule kinase inhibitors. To identify novel activated tyrosine kinases in AML, we used a discovery platform consisting of immunoaffinity profiling coupled to mass spectrometry that identifies large numbers of tyrosine-phosphorylated proteins, including active kinases. This method revealed the presence of an activated colony-stimulating factor 1 receptor (CSF1R) kinase in the acute megakaryoblastic leukemia (AMKL) cell line MKPL-1. Further studies using siRNA and a small-molecule inhibitor showed that CSF1R is essential for the growth and survival of MKPL-1 cells. DNA sequence analysis of cDNA generated by 5′RACE from CSF1R coding sequences identified a novel fusion of the RNA binding motif 6 (RBM6) gene to CSF1R gene generated presumably by a t(3;5)(p21;q33) translocation. Expression of the RBM6-CSF1R fusion protein conferred interleukin-3 (IL-3)–independent growth in BaF3 cells, and induces a myeloid proliferative disease (MPD) with features of megakaryoblastic leukemia in a murine transplant model. These findings identify a novel potential therapeutic target in leukemogenesis, and demonstrate the utility of phosphoproteomic strategies for discovery of tyrosine kinase alleles.
Introduction
There are more than 90 annotated protein tyrosine kinases (PTKs) in the human genome that are important regulators of intracellular signal transduction pathways mediating cellular proliferation, survival, and development.1 The activity of these kinases is normally tightly regulated, and constitutive activation of tyrosine kinases by acquired somatic mutation contributes to oncogenic transformation in many cancers including leukemia.
Acute myeloid leukemia (AML) is a genetically and phenotypically heterogeneous disease, characterized by uncontrolled proliferation of clonal hematopoietic progenitor cells and impaired hematopoietic differentiation leading to granulocytopenia, anemia, or thrombocytopenia.2 Many AML subtypes are characterized by mutations that activate signal transduction pathways, resulting in enhanced proliferation and/or survival of hematopoietic progenitors. Examples include activating mutations in RAS family members, in the receptor tyrosine kinases (C-KIT and FLT3), loss of function of NF-1, and activating mutations in the hematopoietic phosphatase PTPN11. Taken together, these mutations account for as many as 50% of cases of AML,3 and are mutually exclusive in individual cases of AML, suggesting functional redundancy.
There is evidence for activation of STAT5, a downstream effector of constitutively activated tyrosine kinases such as FLT3 and KIT, in approximately 70% of cases of AML.4,5 However, mutations in FLT3 or KIT account for only approximately 30% and 5% of cases of AML, respectively. Thus a significant proportion of AML cases has activation of STAT5, but no known upstream effector. We hypothesized that the upstream activators of STAT5 in some of these patients are activated tyrosine kinases.6 Despite the importance of activated tyrosine kinases in hematologic malignancies, their identification can be difficult due in part to the lack of functionally based experimental approaches that efficiently recognize unregulated tyrosine kinases.
To facilitate the identification of tyrosine kinases responsible for STAT5 phosphorylation in AML, we developed a strategy based on immunoaffinity purification of tyrosine phosphorylated peptides followed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) based identification.7 Using this phosphoproteomic approach, we identified several activated tyrosine kinases in an acute megakaryoblastic leukemia (AMKL) cell line MKPL-1 with constitutively activated STAT5 that lacks FLT3 or KIT mutations. Subsequent analysis using a small-molecule inhibitor and siRNA-induced down-regulation, as well as 5′RACE and DNA sequencing experiments enabled the identification of a novel fusion gene, encoding an in-frame fusion of the RNA-binding motif 6 (RBM6) protein to colony-stimulating factor 1 receptor (CSF1R). This is the first report of chromosomal translocation involving the CSF1R gene and provides new insights into signaling pathways and therapeutic targets of AMKL.
Materials and methods
Cell culture
K562 cells were obtained from American Type Culture Collection (Manassas, VA). BaF3, GDM-1, and CMK cells were obtained from the German National Resource Center for Biological Material (Braunschweig, Germany). NKM-1 cells were from the Japan Health Science Research Resources Bank (Tokyo, Japan). BaF3 cells were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 1.0 ng/mL IL-3. Other cell lines were grown in RPMI-1640 with 10% FBS. 293T cells were grown in DMEM with 10% fetal calf serum. Production of retroviral supernatant and transduction has been described previously.8 BaF3 cells were transduced with retroviral supernatant containing MSCV-Neo, MSCV-Neo/CSF1R, MSCV-Neo/RBM6-CSF1R, MSCV-Neo/CSF1R (truncation) vectors, and were selected for G418 (1 mg/mL). IL-3–independent growth was accessed by plating transduced BaF3 cells in IL-3–free medium, after the cells were washed 3 times in PBS. For Western blotting, cells were treated for 2 hours with 10 μM imatinib before lysis. For dose-response curves, cells were incubated for 48 hours in the presence of imatinib, and the number of viable cells was determined with the CellTiter 96 AQueous One solution cell proliferation assay (Promega, Madison, WI). IC50 was calculated with the use of OriginPro 6.1 software (OriginLab, Northampton, MA). The percentage of apoptotic cells at 48 hours was determined by flow cytometric analysis of cleaved caspase-3 (Cell Signaling Technology, Danvers, MA). For ploidy analysis, bone marrow cells were cultured 4 days in RPMI-1640 with 10% FBS, 10 ng/mL thrombopoietin (TPO), and 10 ng/mL stem cell factor (SCF).
FLT3 and KIT mutational analysis
Phosphopeptide immunoprecipitation and analysis by LC-MS/MS mass spectrometry
Phosphopeptides were prepared using PhosphoScan Kit (Cell Signaling Technology). Briefly, approximately 2 × 108 cells were lysed in urea buffer; trypsin-digested lysates were purified by Sep-pak C18 column. Then, lyophilized peptides were redissolved and immunoaffinity purified with pY-100 antibody. pTyr-containing peptides were concentrated on reverse-phase microtips. LC-MS/MS analysis was performed as previously described.11
siRNA and siRNA transfection
CSF1R and C-KIT SMARTpool siRNA duplexes (proprietary target sequences) were purchased from Dharmacon Research (Lafayette, CO). A nonspecific SMARTpool siRNA was used as a control. Cells were transfected with the siRNA via electroporation.11
Rapid amplification of complementary DNA ends
RNeasy Mini Kit (Qiagen, Valencia, CA) was used to extract RNA from human leukemia cell lines. DNA was extracted with the use of DNeasy Tissue Kit (Qiagen). Rapid amplification of cDNA ends was performed with the use of 5′ RACE system (Invitrogen, Carlsbad, CA) with primers CSF1R-P1 for cDNA synthesis and CSF1R-P2 and CSF1R-P3 for a nested polymerase chain reaction (PCR) reaction.
PCR assay
For reverse-transcription (RT)–PCR, first-strand cDNA was synthesized from 2.5 μg total RNA with the use of SuperScript III first-strand synthesis system (Invitrogen) with oligo (dT)20. Then, the RBM6-CSF1R fusion gene was amplified with the use of primer pairs RBM6-F1 and CSF1R-P3. The reciprocal fusion was detected with the use of primer pairs CSF1R-F and RBM6-R. Wild-type RBM6 and CSF1R were amplified with the use of primer pairs RBM6-F1 and RBM6-R and CSF1R-F and CSF1R-P3, respectively. For genomic PCR, amplification of the fusion gene was performed with the use of Platinum Taq DNA polymerase high fidelity (Invitrogen) with primer pairs gRBM6-F1 and gCSF1R-R1, or gRBM6-F1 and gCSF1R-R2.
Constructs
The open-reading frame of the RBM6-CSF1R fusion gene was amplified by PCR from cDNA of MKPL-1 cells with the use of Platinum Taq DNA polymerase high fidelity (Invitrogen) and primer pairs RBM6-Fc1 and CSF1R-Rc. This PCR product was cloned in the retroviral vector MSCV-Neo or MSCV-GFP. Construct with deletion mutation was obtained by PCR from RBM6-CSF1R clone with primer pairs RBM6-Fc2 and CSF1R-Rc. Wild-type CSF1R construct was kindly provided by Dr Joan Brugge (Harvard Medical School, Boston, MA).
Primers
The following primers were used: RBM6-F1: 5′GACCTGCTAACAGAACTGGACCTT; RBM6-R: 5′CTCTGAAGTCAACAGCGTGAGCAT; CSF1R-F: 5′ATCGAGAGCTATGAGGGCAACAGT; CSF1R-P1: 5′CCTTCCTTCGCAGAAAGTTGAGCA; CSF1R-P2: 5′AAAGTTGAGCAGGTCGCCATAGCA; CSF1R-P3: 5′AGCAACAGTACTCCGTGATGACCA; gRBM6-F1: 5′TGTAAAAGGAGGGCCAGTTCCTCGTGTTCTGAAATGGGAGCA; gCSF1R-R1: 5′AACAGCTATGACCATGCAAACTGCAGGTTGTTCC; gCSF1R-R2: 5′AACAGCTATGACCATGATCTTCACAGCCACCTTCAGGACA; GAPDH-F: 5′TGGAAATCCCATCACCATCT; GAPDH-R: 5′GTCTTCTGGGTGGCAGTGAT; RBM6-Fc1: 5′CGGAATTCGATAAAAAGAGATGTGGGGGGAT; RBM6-Fc2: 5′CGGAATTCACCATGAAGTGGGAGTTCCCCCGGA; and CSF1R-Rc: 5′CGGAATTCCGTCAACTCCTCAGCAGAACT.
Western blotting
Cells were lysed in 1 × cell lysis buffer (Cell Signaling Technology) supplemented with Protease Arrest (G Biosciences, St Louis, MO) and separated by electrophoresis. Transferrin receptor antibody was from Abcam (Cambridge, MA). All other antibodies and reagents for immunoblotting were from Cell Signaling Technology.
Cell staining, antibodies, and flow cytometry
Staining for flow cytometry was performed in PBS 1 × with 2% FBS. All antibodies were obtained from BD Pharmingen (San Diego, CA), except the CD42b antibody obtained from Emfret (Eibelstadt, Germany). Total splenocytes were used and analysis was gated on live cells using forward and side scatter. For ploidy analysis, cells were first stained with CD41 or control antibody, followed by staining with APC-conjugated secondary antibody. Cells were then incubated 30 minutes in a 0.1% sodium citrate solution containing 50 μg/mL of RNAseA and 50 μg/mL propidium iodide. Analysis of ploidy was performed on approximately 2 × 104 CD41+ cells. Acquisition of the data were performed on a FACSCalibur (BD Biosciences, San Jose, CA) and analyzed with CellQuest or FlowJo software. Hematoxylin and eosin stainings and von Willebrand factor immunochemistry (Dakocytomation, Glostrup, Denmark) on paraffin-embedded tissue sections were performed by the Brigham and Women's Hospital Histo-Pathology Core Facility (Boston, MA). For colony-forming assays, primary mouse bone marrow or spleen cells were collected from 5-month-old recipients and spleen cells were subsequently treated with red blood cell lysis buffer (Puregene; Gentra systems, Minneapolis, MN). For myeloid colony assays, 20 000 cells were plated in MethoCult 3434 (StemCell Technologies, Vancouver, BC) and colonies were scored after 7 days. For megakaryocyte colony assays, 220 000 cells were mixed with MegaCult-C (StemCell Technologies), containing Tpo, IL-11, IL-3, IL-6 and plated onto two double-chamber culture slides, and colonies were stained for acetylcholinesterase and enumerated after 8 days following the manufacturer's recommendations. Images of histologic slides were obtained on a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) equipped with SPOT RT color digital camera (model 2.1.1; Diagnostic Instruments, Sterling Heights, MI). The microscope was equipped with a 10 × /22 NA ocular lens. Low-power images (× 100) were obtained with a 10 × /0.25 objective lens. High-power images (× 1000) were obtained with a 100 × /1.4 objective lens with oil (Trak 300; Richard Allan Scientific, Kalamazoo, MI). Images were analyzed using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). Survival curves and Student t tests were performed using the program Prism (GraphPad, www.graphpad.com).
BaF3 xenograft, bone marrow transplantation, and animal analysis
BALB/c nude mice (6 weeks old) were injected subcutaneously with 1 × 107 parental BaF3 cells or BaF3 cells expressing RBM6-CSF1R or truncated CSF1R. Mice were monitored daily for tumor formation and size and were killed when tumors reached approximately 2 cm × 2 cm.
For bone marrow transplantation, viral supernatants were obtained as described before.8 Briefly, 8- to 10-week-old C57BL/6 or Balb/C donor mice were injected with 5-FU (Sigma, St Louis, MO) 5 days before bone marrow collection. On day 0, primary bone marrow cells were obtained from femurs and tibiae and cultured overnight in RPMI 1640 supplemented with 10% FBS and IL3, IL6, SCF after lysis of red blood cells. Cells were mixed with identical titer viral supernatants 2 times on day 1 and day 2 and spinfected for 1.5 hours at 1800 g each time. After the second spinfection, 1 × 106 of total cells was injected in the tail veins of lethally irradiated C57BL/6 or Balb/C recipients. Eye-bleeds were performed using EDTA-coated capillary tubes and blood counts were performed within 30 minutes on a HemaVet HV950FS (Drew Scientific, Dallas, TX). Percentage of GFP+ nucleated cells in the blood was evaluated 1 month after transplantation, and the following values were obtained: C57/Bl6 MIG: 81%–88%, C57/Bl6 RBM6-CSF1R: 41%–58%, C57/Bl6 CSF1R(trunc): 23%–52%, Balb/C MIG: 64%–77%, Balb/C RBM6-CSF1R: 38%–46%, Balb/C CSF1R(trunc): 49%–60%. Approval for the use of animals in this study was granted by the Children's Hospital Boston Animal Care and Use Committee.
Results
Identification of activated tyrosine kinases in MKPL-1 cells by LC-MS/MS mass spectrometry
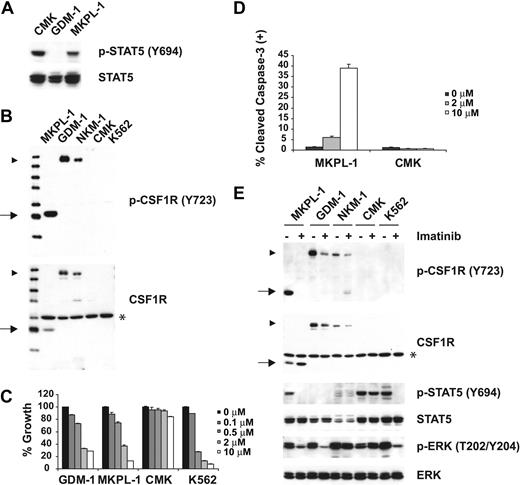
To identify novel activated tyrosine kinases that confer constitutive phosphorylation of STAT5, we first screened more than 40 human AML cell lines for evidence of STAT5 activation by immunoblot analysis using a phospho-STAT5–specific antibody. As we reported previously, JAK3A572V confers phosphorylation of STAT5 in CMK cells.11 In addition, STAT5 was constitutively tyrosine phosphorylated in the acute megakaryoblastic leukemia (AMKL) cell line MKPL-1 (Figure 1A) that was established from the bone marrow of an adult patient with acute megakaryoblastic leukemia. MKPL-1 cells have a complex cytogenetic profile with 92 chromosomes, express GpIIb/IIIa, and exhibit features of megakaryoblasts.12 Denaturing high-performance liquid chromatography (HPLC) analysis showed that MKPL-1 cells did not harbor activating mutations in FLT3 or KIT (data not shown). To identify the tyrosine kinase responsible for the constitutive tyrosine phosphorylation of STAT5 in MKPL-1 cells, whole-cell lysates were digested with trypsin, phosphopeptides were immunoprecipitated with phosphotyrosine antibody (pY-100), and resultant immunoprecipitates were analyzed by LC-MS/MS mass spectrometry. We identified 766 nonredundant phosphotyrosine-containing peptides corresponding to 673 phosphotyrosine sites in 483 tyrosine phosphorylated proteins, most of which have not been previously reported (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Among tyrosine phosphorylated kinases, several are not detected by MS analysis in other leukemia cell lines (T.G., unpublished data, 2006), including CSF1R, JAK2, and JAK3 (Table 1).
Colony-stimulating factor 1 receptor (CSF1R) is tyrosine phosphorylated in MKPL-1 cells. (A) Immunoblot analysis of whole-cell lysates revealed constitutive tyrosine phosphorylation of STAT5 in the MKPL-1 cell line. Detection of phospho-STAT5 is shown in the upper panel and total STAT5 is shown below. (B) Detection of a truncated form of CSF1R in MKPL-1 cells by Western blot. ▶ indicates full-length CSF1R; *, a nonspecific band; and →, a truncated form of CSF1R. (C) Treatment of MKPL-1 cells with increasing concentrations of imatinib resulted in a decrease in proliferation. (D) Treatment with imatinib increased apoptosis of MKPL-1 cells but not CMK cells as measured by cleaved caspase-3 staining. (E) Incubation with imatinib resulted in decreased phosphorylation of a truncated form of CSF1R in MKPL-1 cells, accompanied by decreased phosphorylation of STAT5 and ERK.
Colony-stimulating factor 1 receptor (CSF1R) is tyrosine phosphorylated in MKPL-1 cells. (A) Immunoblot analysis of whole-cell lysates revealed constitutive tyrosine phosphorylation of STAT5 in the MKPL-1 cell line. Detection of phospho-STAT5 is shown in the upper panel and total STAT5 is shown below. (B) Detection of a truncated form of CSF1R in MKPL-1 cells by Western blot. ▶ indicates full-length CSF1R; *, a nonspecific band; and →, a truncated form of CSF1R. (C) Treatment of MKPL-1 cells with increasing concentrations of imatinib resulted in a decrease in proliferation. (D) Treatment with imatinib increased apoptosis of MKPL-1 cells but not CMK cells as measured by cleaved caspase-3 staining. (E) Incubation with imatinib resulted in decreased phosphorylation of a truncated form of CSF1R in MKPL-1 cells, accompanied by decreased phosphorylation of STAT5 and ERK.
Phosphorylated tyrosine kinases identified by LC-MS/MS in MKPL-1 cells
| Name . | Accession . | Site . | Peptides . |
|---|---|---|---|
| Protein kinase, Tyr (receptor) | |||
| KAEAMLGPSLSPGQDPEGGVDyK | |||
| RKAEAMLGPSLSPGQDPEGGVDyK | |||
| KAEAMLGPSLSPGQDPEGGVDyKNIHLEK | |||
| CSFR | P07333 | 699* | KAEAMLGPSLSPGQDPEGGVDyKNIHLEKK |
| EphB4 | P54760 | 987 | SQAKPGTPGGTGGPAPQy |
| InsR | P06213 | 1185* | |
| InsR iso2 | P06213–2 | 1173* | |
| IGF1R | P08069 | 1161* | DIyETDYyRK |
| InsR | P06213 | 1189* | |
| InsR iso2 | P06213–2 | 1177* | DIYETDyYR |
| IGF1R | P08069 | 1165* | DIYETDyYRK |
| InsR | P06213 | 1190* | |
| InsR iso2 | P06213–2 | 1178* | |
| IGF1R | P08069 | 1166* | DIyETDYyRK |
| Kit | P10721 | 721* | ESSCSDSTNEyMDMKPGVSyVVPTK |
| Kit | P10721 | 730 | ESSCSDSTNEyMDMKPGVSyVVPTK |
| Kit | P10721 | 936* | QISESTNHIySNLANCSPNR |
| Mer | Q12866 | 749* | KIySGDYyR |
| KIySGDYYR | |||
| KIySGDyYR | |||
| Mer | Q12866 | 753* | KIYSGDyYR |
| KIySGDyYR | |||
| Mer | Q12866 | 754* | KIySGDYyR |
| TIE1 | P35590 | 1027 | WMAIESLNySVYTTK |
| KIySGDYyR | |||
| KIySGDYYR | |||
| Tyro3 | Q06418 | 681 | KIySGDyYR |
| KIYSGDyYR | |||
| Tyro3 | Q06418 | 685 | KIySGDyYR |
| Tyro3 | Q06418 | 686 | KIySGDYyR |
| Protein kinase, Tyr (nonreceptor) | |||
| Ack | Q07912 | 518* | KPTyDPVSEDQDPLSSDFKR |
| Ack | Q07912 | 857* | KVSSTHyYLLPERPSYLER |
| Ack | Q07912 | 858* | VSSTHYyLLPERPSYLER |
| VVALyDYMPMNANDLQLR | |||
| Btk | Q06187 | 222* | KVVALyDYMPMNANDLQLR |
| Btk | Q06187 | 224* | VVALYDyMPMNANDLQLR |
| Btk | Q06187 | 343 | HYVVCSTPQSQyYLAEK |
| Btk | Q06187 | 360* | HLFSTIPELINyHQHNSAGLISR |
| Btk | Q06187 | 550* | YVLDDEyTSSVGSKFPVR |
| Fer | P16591 | 714* | QEDGGVySSSGLK |
| Fgr | P09769 | 209 | KLDMGGYyITTR |
| Fyn | P06241 | 212 | |
| Yes | P07947 | 221 | KLDNGGyYITTR |
| Fyn | P06241 | 419* | |
| Yes | P07947 | 425* | |
| Src | P12931 | 418* | LIEDNEyTAR |
| Hck | P08631 | 208* | TLDNGGFyISPR |
| Jak2 | O60674 | 570* | REVGDyGQLHETEVLLK |
| Jak3 | P52333 | 785* | DLNSLISSDyELLSDPTPGALAPR |
| Lck | P06239 | 393* | LIEDNEyTAR |
| P07948 | 193 | SLDNGGYyISPR | |
| Lyn | P07948 | 305 | LyAVVTR |
| Lyn | P07948 | 472* | VENCPDELyDIMK |
| Lyn | P07948 | 396* | VIEDNEyTAR |
| Hck | P08631 | 410* | VIEDNEyTAREGAK |
| Pyk2 | Q14289 | 579* | YIEDEDyYK |
| Syk | P43405 | 323* | QESTVSFNPyEPELAPWAADKGPQR |
| Tyk2 | P29597 | 292* | LLAQAEGEPCyIR |
| Name . | Accession . | Site . | Peptides . |
|---|---|---|---|
| Protein kinase, Tyr (receptor) | |||
| KAEAMLGPSLSPGQDPEGGVDyK | |||
| RKAEAMLGPSLSPGQDPEGGVDyK | |||
| KAEAMLGPSLSPGQDPEGGVDyKNIHLEK | |||
| CSFR | P07333 | 699* | KAEAMLGPSLSPGQDPEGGVDyKNIHLEKK |
| EphB4 | P54760 | 987 | SQAKPGTPGGTGGPAPQy |
| InsR | P06213 | 1185* | |
| InsR iso2 | P06213–2 | 1173* | |
| IGF1R | P08069 | 1161* | DIyETDYyRK |
| InsR | P06213 | 1189* | |
| InsR iso2 | P06213–2 | 1177* | DIYETDyYR |
| IGF1R | P08069 | 1165* | DIYETDyYRK |
| InsR | P06213 | 1190* | |
| InsR iso2 | P06213–2 | 1178* | |
| IGF1R | P08069 | 1166* | DIyETDYyRK |
| Kit | P10721 | 721* | ESSCSDSTNEyMDMKPGVSyVVPTK |
| Kit | P10721 | 730 | ESSCSDSTNEyMDMKPGVSyVVPTK |
| Kit | P10721 | 936* | QISESTNHIySNLANCSPNR |
| Mer | Q12866 | 749* | KIySGDYyR |
| KIySGDYYR | |||
| KIySGDyYR | |||
| Mer | Q12866 | 753* | KIYSGDyYR |
| KIySGDyYR | |||
| Mer | Q12866 | 754* | KIySGDYyR |
| TIE1 | P35590 | 1027 | WMAIESLNySVYTTK |
| KIySGDYyR | |||
| KIySGDYYR | |||
| Tyro3 | Q06418 | 681 | KIySGDyYR |
| KIYSGDyYR | |||
| Tyro3 | Q06418 | 685 | KIySGDyYR |
| Tyro3 | Q06418 | 686 | KIySGDYyR |
| Protein kinase, Tyr (nonreceptor) | |||
| Ack | Q07912 | 518* | KPTyDPVSEDQDPLSSDFKR |
| Ack | Q07912 | 857* | KVSSTHyYLLPERPSYLER |
| Ack | Q07912 | 858* | VSSTHYyLLPERPSYLER |
| VVALyDYMPMNANDLQLR | |||
| Btk | Q06187 | 222* | KVVALyDYMPMNANDLQLR |
| Btk | Q06187 | 224* | VVALYDyMPMNANDLQLR |
| Btk | Q06187 | 343 | HYVVCSTPQSQyYLAEK |
| Btk | Q06187 | 360* | HLFSTIPELINyHQHNSAGLISR |
| Btk | Q06187 | 550* | YVLDDEyTSSVGSKFPVR |
| Fer | P16591 | 714* | QEDGGVySSSGLK |
| Fgr | P09769 | 209 | KLDMGGYyITTR |
| Fyn | P06241 | 212 | |
| Yes | P07947 | 221 | KLDNGGyYITTR |
| Fyn | P06241 | 419* | |
| Yes | P07947 | 425* | |
| Src | P12931 | 418* | LIEDNEyTAR |
| Hck | P08631 | 208* | TLDNGGFyISPR |
| Jak2 | O60674 | 570* | REVGDyGQLHETEVLLK |
| Jak3 | P52333 | 785* | DLNSLISSDyELLSDPTPGALAPR |
| Lck | P06239 | 393* | LIEDNEyTAR |
| P07948 | 193 | SLDNGGYyISPR | |
| Lyn | P07948 | 305 | LyAVVTR |
| Lyn | P07948 | 472* | VENCPDELyDIMK |
| Lyn | P07948 | 396* | VIEDNEyTAR |
| Hck | P08631 | 410* | VIEDNEyTAREGAK |
| Pyk2 | Q14289 | 579* | YIEDEDyYK |
| Syk | P43405 | 323* | QESTVSFNPyEPELAPWAADKGPQR |
| Tyk2 | P29597 | 292* | LLAQAEGEPCyIR |
y in peptide indicates phosphorylated tyrosine residue.
Known phosphorylation site.
Constitutive activation of CSF1R is required for growth and survival of MKPL-1 cells
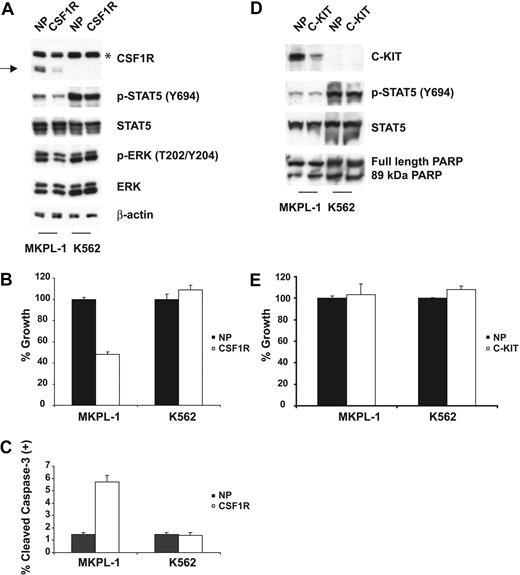
MKPL-1 cells express full-length JAK2 and JAK3 protein, but DNA sequencing analysis did not detect any mutations in their respective kinase domains (data not shown). The kinase domain of CSF1R was also wild type by DNA sequence analysis. However, a truncated form of CSF1R was detected by Western blot analysis (Figure 1B). Because CSF1R has been recently identified as a target of imatinib,13,14 we tested the effect of imatinib on the growth of MKPL-1 cells (Figure 1C). MKPL-1 cells were inhibited by imatinib with an IC50 of 1.26 μM, which is similar to the IC50 of imatinib for CSF1R.13 In addition, the GDM-1 cell line, which exhibited constitutively phosphorylated, full-length CSF1R, had a similar IC50 to that of MKPL-1 (Figure 1C,E). Although C-KIT is also phosphorylated in the MKPL-1 cell line by MS analysis, several lines of evidence ruled out the role of C-KIT in the proliferation and survival of MKPL-1 cells. First, DNA sequencing analysis did not detect any mutations of C-KIT in MKPL-1 cells. Second, if wild-type C-KIT promotes proliferation and survival in MKPL-1 cells, we would expect an IC50 of 0.1 μM for imatinib. Finally, siRNA against C-KIT did not affect proliferation and survival of MKPL-1 cells (Figure 2D-E). These data supported the hypothesis that CSF1R was the target of imatinib in MKPL-1 cells.
Effects of CSF1R and C-KIT siRNA on MKPL-1 cells. (A) siRNA-induced silencing of CSF1R was assessed by immunoblot of whole-cell lysates 72 hours after siRNA transfection. Down-regulation of CSF1R resulted in decreased phosphorylation of STAT5 and ERK. NP and CSF1R stand for siControl nontargeting siRNA pool and CSF1R SMARTpool siRNA, respectively. * indicates a nonspecific band; →, a truncated form of CSF1R. (B) Electroporation with CSF1R siRNA into MKPL-1 cells resulted in a decrease of proliferation as measured. Mean value plus SD of experiments performed in duplicate is represented. (C) Induction of apoptosis. Percentage of total population is indicated. (D) siRNA-induced silencing of C-KIT was assessed by immunoblot of whole-cell lysates 72 hours after siRNA transfection. Down-regulation of C-KIT did not affect phosphorylation of STAT5. In addition, treatment of MKPL-1 cells with C-KIT siRNA did not affect its apoptosis, as assessed by total and cleaved PARP. NP and C-KIT indicate siControl nontargeting siRNA pool and C-KIT SMARTpool siRNA, respectively. (E) Electroporation with C-KIT siRNA into MKPL-1 cells did not affect proliferation of MKPL-1 cells. Mean value plus SD of experiments performed in duplicate is represented.
Effects of CSF1R and C-KIT siRNA on MKPL-1 cells. (A) siRNA-induced silencing of CSF1R was assessed by immunoblot of whole-cell lysates 72 hours after siRNA transfection. Down-regulation of CSF1R resulted in decreased phosphorylation of STAT5 and ERK. NP and CSF1R stand for siControl nontargeting siRNA pool and CSF1R SMARTpool siRNA, respectively. * indicates a nonspecific band; →, a truncated form of CSF1R. (B) Electroporation with CSF1R siRNA into MKPL-1 cells resulted in a decrease of proliferation as measured. Mean value plus SD of experiments performed in duplicate is represented. (C) Induction of apoptosis. Percentage of total population is indicated. (D) siRNA-induced silencing of C-KIT was assessed by immunoblot of whole-cell lysates 72 hours after siRNA transfection. Down-regulation of C-KIT did not affect phosphorylation of STAT5. In addition, treatment of MKPL-1 cells with C-KIT siRNA did not affect its apoptosis, as assessed by total and cleaved PARP. NP and C-KIT indicate siControl nontargeting siRNA pool and C-KIT SMARTpool siRNA, respectively. (E) Electroporation with C-KIT siRNA into MKPL-1 cells did not affect proliferation of MKPL-1 cells. Mean value plus SD of experiments performed in duplicate is represented.
Consistent with this hypothesis, phosphorylation of the truncated form of CSF1R, as well as downstream effectors such as STAT5 and ERK, was inhibited by imatinib (Figure 1E). Treatment of MKPL-1 cells with imatinib induced apoptosis, but not in CMK cells.
To confirm these observations, the expression of CSF1R in MKPL-1 cells was inhibited by transduction of a CSF1R-targeted siRNA. Immunoblot analysis showed that expression of CSF1R was specifically and significantly reduced at 72 hours after transfection of the siRNA into MKPL-1 cells (Figure 2A). Decrease in CSF1R expression was accompanied by a decrease in the phosphorylation of STAT5 and ERK. Furthermore, siRNA down-regulation of CSF1R inhibited cell proliferation (Figure 2B), and induced apoptosis as measured by a cleaved caspase-3 flow cytometric assay (Figure 2C). Taken together, these results indicate that an apparently truncated form of CSF1R is essential for proliferation and survival of MKPL-1 cells.
Identification of the RBM6-CSF1R gene
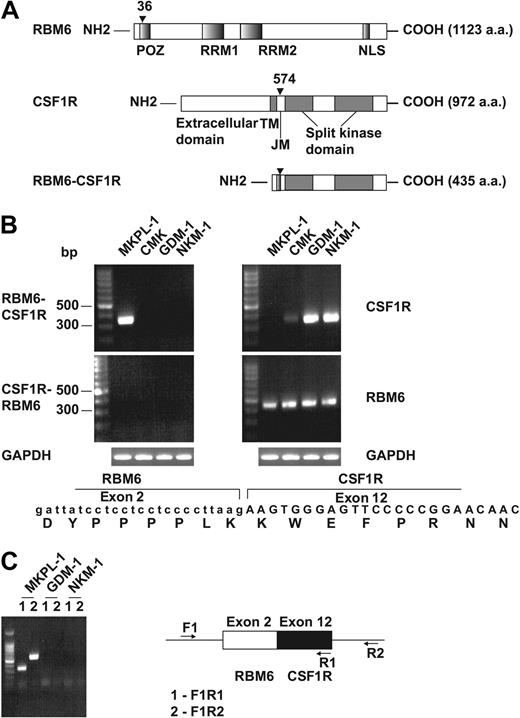
To elucidate the molecular mechanisms leading to truncation and activation of CSF1R in MKPL-1 cells, we performed 5′ rapid amplification of cDNA ends (5′ RACE) using the sequence encoding the kinase domain of CSF1R. Sequence analysis of the resulting product showed that the kinase domain of CSF1R was fused in-frame to the RNA-binding motif 6 (RBM6) gene. RBM6 (G16/DEF-3/NYLU12) is differentially expressed and undergoes alternative splicing in adult tissues, including those of hematopoietic origin, and encodes a protein of unknown function.15,–17 The RBM6-CSF1R fusion protein combines the amino terminal 36 amino acids of RBM6 with the carboxy terminal 399 amino acids of CSF1R (Figure 3A). RBM6 is localized to chromosome 3p21.3, whereas CSF1R is on chromosome 5q33. Thus, we infer that the fusion gene was created by a t(3;5)(p21;q33) translocation. However, conventional cytogenetic analysis did not reveal this translocation,12 thus we speculate there might be a cryptic translocation in this cell line. The fusion of RBM6 and CSF1R was confirmed by reverse-transcriptase–PCR on RNA and PCR on DNA from MKPL-1 cells (Figure 3B-C). Expression of the reciprocal CSF1R-RBM6 fusion gene was not detected. Moreover, the MKPL-1 cell line did not express wild-type CSF1R, but did express wild-type RBM6 (Figure 3B), suggesting that the RBM6-CSF1R fusion gene was expressed as a consequence of juxtaposition of the RMB6 promoter with sequences encoding the CSF1R gene.
Detection of the fusion gene formed by the genes for RNA binding motif 6 (RBM6) and colony stimulating factor 1 receptor (CSF1R). (A) Schematic diagram shows the RBM6, CSF1R, and RBM6-CSF1R proteins. The position of the breakpoint is indicated by arrowhead. POZ indicates poxvirus and zinc finger domain; RRM1 and RRM2, RNA recognition motif 1 and 2, respectively; TM, transmembrane; JM, juxtamembrane; and NLS, nuclear localization signal. (B) RT-PCR reaction indicated a fusion of RBM6 to CSF1R in RNA from MKPL-1 cells. GAPDH was used as a control. The sequence from junction of the fusion gene is listed at the bottom. (C) Genomic PCR reaction detected the RBM6-CSF1R fusion gene in DNA.
Detection of the fusion gene formed by the genes for RNA binding motif 6 (RBM6) and colony stimulating factor 1 receptor (CSF1R). (A) Schematic diagram shows the RBM6, CSF1R, and RBM6-CSF1R proteins. The position of the breakpoint is indicated by arrowhead. POZ indicates poxvirus and zinc finger domain; RRM1 and RRM2, RNA recognition motif 1 and 2, respectively; TM, transmembrane; JM, juxtamembrane; and NLS, nuclear localization signal. (B) RT-PCR reaction indicated a fusion of RBM6 to CSF1R in RNA from MKPL-1 cells. GAPDH was used as a control. The sequence from junction of the fusion gene is listed at the bottom. (C) Genomic PCR reaction detected the RBM6-CSF1R fusion gene in DNA.
RBM6-CSF1R transforms BaF3 cells both in vitro and in vivo
Retroviral transduction of RBM6-CSF1R transformed Ba/F3 cells to factor-independent growth (Figure 4A-B). RBM6-CSF1R was constitutively tyrosine phosphorylated, as well as its known downstream effector, STAT5 (Figure 4C). Deletion of the RBM6 moiety (amino acids 1-36) did not abrogate this phenotype, indicating that the RBM6 domain was not essential for activation of the chimeric kinase. Interestingly, wild-type CSF1R did not transform BaF3 cells, and it failed to phosphorylate STAT5 (Figure 4B-C). Treatment with imatinib at concentrations comparable with those that inhibit MKPL-1 cells abolished the growth of BaF3 cells expressing RBM6-CSF1R or N-terminal truncation of CSF1R (Figure 4D). These data confirm that RMB6-CSF1R is inhibited by imatinib, and is the most likely target of imatinib in MKPL1 cells.
Transformation, inhibition, and signaling properties of the fusion tyrosine kinase formed by RNA binding motif 6 (RBM6) and colony-stimulating factor 1 receptor (CSF1R). (A) The retroviral constructs used in the study. MSCV indicates murine stem cell virus; Neo, Neomycin; and LTR, long terminal repeat. (B) BaF3 cells retrovirally transduced with these constructs were grown in the absence of IL-3 after selecting with 1 mg/mL G418 for 7 days. (C) Detection of RBM6-CSF1R and its truncation in BaF3 cells after 3 hours of IL-3 withdrawal, accompanied by phosphorylation of STAT5. ▶ indicates full-length CSF1R; *, nonspecific band; ▶▶, cytoplasmic domain of CSF1R; and →, RBM6-CSF1R and CSF1R (trunc). (D) Dose-response graph of imatinib for BaF3 cells expressing RBM6-CSF1R and its N-terminal truncation. BaF3/BCR-ABL, FLT3-ITD, as well as MKPL-1 were used as controls. (E) BaF3 cells established as in panel B were injected subcutaneously into Balb/cnu/nu mice. Mice were monitored daily for tumor formation and size and were killed when tumors reached 2 cm × 2 cm.
Transformation, inhibition, and signaling properties of the fusion tyrosine kinase formed by RNA binding motif 6 (RBM6) and colony-stimulating factor 1 receptor (CSF1R). (A) The retroviral constructs used in the study. MSCV indicates murine stem cell virus; Neo, Neomycin; and LTR, long terminal repeat. (B) BaF3 cells retrovirally transduced with these constructs were grown in the absence of IL-3 after selecting with 1 mg/mL G418 for 7 days. (C) Detection of RBM6-CSF1R and its truncation in BaF3 cells after 3 hours of IL-3 withdrawal, accompanied by phosphorylation of STAT5. ▶ indicates full-length CSF1R; *, nonspecific band; ▶▶, cytoplasmic domain of CSF1R; and →, RBM6-CSF1R and CSF1R (trunc). (D) Dose-response graph of imatinib for BaF3 cells expressing RBM6-CSF1R and its N-terminal truncation. BaF3/BCR-ABL, FLT3-ITD, as well as MKPL-1 were used as controls. (E) BaF3 cells established as in panel B were injected subcutaneously into Balb/cnu/nu mice. Mice were monitored daily for tumor formation and size and were killed when tumors reached 2 cm × 2 cm.
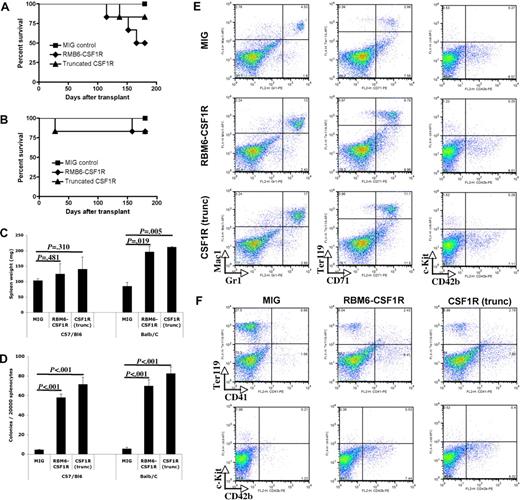
To assess the in vivo oncogenic potential of RBM6-CSF1R, we injected BALB/c nude mice with parental BaF3 cells as well as BaF3 cells expressing RBM6-CSF1R or truncated CSF1R. Daily monitoring of tumor size and formation more than a 40-day period showed an absence of tumor formation in mice injected with parental BaF3 cells, whereas all mice injected with BaF3 cells expressing RBM6-CSF1R or truncated CSF1R developed tumors. These data indicate that both CSF1R alleles have oncogenic potential in vivo (Figure 4E). Consistent with in vitro data indicating that the RBM6 portion of the translocation is dispensable for transformation, mice injected with truncated CSF1R-expressing cells showed equivalent kinetics and incidence of tumor formation as mice injected with cells expressing full-length RBM6-CSF1R.
RBM6-CSF1R induces myeloproliferative disease in a mouse bone marrow transplant model
To further assess the transforming potential of the RBM6-CSF1R fusion and N-terminal truncation of CSF1R in primary hematopoietic progenitors, bone marrow cells derived from 5-FU–treated C57Bl/6 or Balb/C mice were transduced with retrovirus containing the respective CSF1R alleles and transplanted into lethally irradiated syngenic recipients. Several animals succumbed to disease with a latency of approximately 5 months (Figure 5A-B) and full analysis of a cohort of animals was performed 150 days after transplantation. Compared with empty vector control recipients, RBM6-CSF1R or CSF1R(trunc) recipients showed significantly enlarged spleens in the Balb/C background but not in the C57Bl/6 background (Figure 5C). Histopathological analysis showed evidence of extramedullary hematopoiesis with expansion of the red pulp in the spleen as well as small infiltrates of mature myeloid cells in the liver (Figure S1) suggestive of a myeloproliferative disease (MPD).
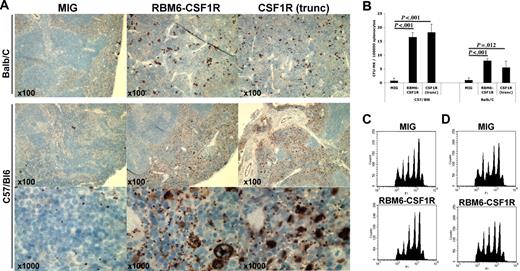
RBM6-CSF1R and truncated CSF1R induce a myeloproliferative disease in a murine bone marrow transplant model. (A) Kaplan-Meyer survival curves of C57/Bl6 transplant recipients. (B) Kaplan-Meyer survival curves of Balb/C transplant recipients. (C) Spleen weight at 150 days after transplantation. Mean ± SD is shown (for each group, n = 3). (D) Splenocytes were plated in methylcellulose cultures containing IL-3, IL-6, SCF, EPO, and myeloid colonies were scored after 7 days. Experiment was performed in triplicate and mean ± SD is shown. (E) Flow cytometric analysis of Balb/C recipients total splenocytes. Experiment was performed in duplicate and a representative experiment is shown. (F) Flow cytometric analysis of C57/Bl6 recipients total splenocytes. Experiment was performed in duplicate and a representative experiment is shown.
RBM6-CSF1R and truncated CSF1R induce a myeloproliferative disease in a murine bone marrow transplant model. (A) Kaplan-Meyer survival curves of C57/Bl6 transplant recipients. (B) Kaplan-Meyer survival curves of Balb/C transplant recipients. (C) Spleen weight at 150 days after transplantation. Mean ± SD is shown (for each group, n = 3). (D) Splenocytes were plated in methylcellulose cultures containing IL-3, IL-6, SCF, EPO, and myeloid colonies were scored after 7 days. Experiment was performed in triplicate and mean ± SD is shown. (E) Flow cytometric analysis of Balb/C recipients total splenocytes. Experiment was performed in duplicate and a representative experiment is shown. (F) Flow cytometric analysis of C57/Bl6 recipients total splenocytes. Experiment was performed in duplicate and a representative experiment is shown.
To further investigate the finding of extramedullary hematopoiesis in the spleen, splenocytes were plated in methylcellulose culture supplemented with IL3, IL6, SCF, and EPO. An increased number of myeloid colonies was observed in both backgrounds (Figure 5D) when splenocytes from RBM6-CSF1R or CSF1R(trunc) were compared with those from empty control recipients. Flow cytometric analysis of splenocytes revealed that the percentage of Mac1+Gr1+ cells was increased in both backgrounds in both RBM6-CSF1R and CSF1R(trunc) recipients compared with MIG control recipients (Figure 5E and data not shown). Abnormalities of the erythroid lineage were more pronounced in the Balb/C background with an increased percentage of CD71+Ter119+ and Cd71+Ter119− immature erythroid cells (Figure 5E). In contrast, abnormalities of the megakaryocyte lineage were more striking in the C57Bl/6 background with increased number of CD41+ and CD42b+ cells in both RBM6-CSF1R and CSF1R(trunc) recipients compared with empty control recipients (Figure 5D-E).
We were particularly interested in the megakaryocyte phenotype given the discovery of this allele in an AMKL cell line. To further characterize the megakaryocytic phenotype, we performed immunohistochemistry on spleen sections using the von Willebrand factor (VWF) antibody. In both murine backgrounds, VWF staining was more prominent in both RBM6-CSF1R and CSF1R(trunc) recipients compared with empty vector control recipients. However, an increase in both platelets and maturing megakaryocytes was evident in the C57Bl/6 background, whereas Balb/C recipients showed an increase in maturing megakaryocytes (Figure 6A). To assess immature megakaryocyte progenitors, splenocytes were plated in collagen-based culture supplemented with TPO, IL-11, IL-3, and IL-6. The colony-forming unit–megakaryocyte (CFU-Mk) potential was increased in both backgrounds for both RBM6-CSF1R and CSF1R(trunc) recipients compared with control recipients. Finally, analysis of megakaryocytes obtained from ex vivo culture of bone marrow cells in presence of TPO and SCF showed that ploidy profile of megakaryocytes was similar in all recipients in both backgrounds (Figure 6B-C). Histopathological analysis of animals that succumbed to the disease showed similar features, but had more prominent infiltration of the spleen and liver (Figure S2) and had marked leukocytosis composed of mature myeloid (Mac1+Gr1+) cells (data not shown). Interestingly, these animals also showed megakaryocyte hyperplasia and a moderate reticulin fibrosis in the spleen (Figure S2). Of note, 2 animals (one C57Bl/6 RBM6-CSF1R recipient and one Balb/C RBM6-CSF1R recipient) developed GFP-negative T-cell leukemia likely derived from recipient cells (data not shown). Together these results indicate that animals that received a transplant of RBM6-CSF1R– and CSF1R(trunc)-expressing bone marrow cells develop a myeloproliferative disease. In addition, although normal platelet levels in the blood (Figure S3) and normal ex vivo megakaryocyte maturation were observed, megakaryocyte hyperplasia was observed in the spleen of both C57Bl/6 and Balb/C recipients, and increased number of platelets was observed specifically in the spleen of C57Bl/6 recipients.
The RBM6-CSF1R–induced megakaryocyte phenotype is mouse-strain specific. (A) Immunohistochemistry on spleen section using von Willebrand factor antibody. Note that both megakaryocyte and platelet numbers are increased in the C57/Bl6 background, whereas only megakaryocytes numbers are increased in the Balb/C background. (B) Splenocytes were plated in collagen-based media containing TPO, IL-11, IL-3, and IL-6. Slides were stained for acetylcholinesterase and colonies were enumerated. Experiment was performed at least in triplicate and mean ± SD is represented. CFU-MK indicates colony-forming unit–megakaryocyte. Bone marrow cells from C57/Bl6 (C) or Balb/C (D) recipients were cultured for 4 days in presence of TPO and SCF. Cells were then stained with propidium iodide (PI) and CD41+ cells were analyzed for ploidy content.
The RBM6-CSF1R–induced megakaryocyte phenotype is mouse-strain specific. (A) Immunohistochemistry on spleen section using von Willebrand factor antibody. Note that both megakaryocyte and platelet numbers are increased in the C57/Bl6 background, whereas only megakaryocytes numbers are increased in the Balb/C background. (B) Splenocytes were plated in collagen-based media containing TPO, IL-11, IL-3, and IL-6. Slides were stained for acetylcholinesterase and colonies were enumerated. Experiment was performed at least in triplicate and mean ± SD is represented. CFU-MK indicates colony-forming unit–megakaryocyte. Bone marrow cells from C57/Bl6 (C) or Balb/C (D) recipients were cultured for 4 days in presence of TPO and SCF. Cells were then stained with propidium iodide (PI) and CD41+ cells were analyzed for ploidy content.
Discussion
Acute megakaryoblastic leukemia is a subtype of AML associated with poor prognosis. It is characterized by complex karyotypes, and the clinical and morphologic diagnosis can be difficult due in part to low blast counts in the peripheral blood, severe bone marrow fibrosis that impairs acquisition of bone marrow aspirates for analysis, and lack of molecular markers of disease.18,19 To facilitate the rapid identification of the potential tyrosine kinases activated in leukemia cells, we used a phosphoproteomic approach that led to the identification of a novel fusion tyrosine kinase involving CSF1R in the acute megakaryoblastic leukemia cell line MKPL-1. This approach is a sensitive and reproducible functional strategy to identify activated protein kinases and their phosphorylated substrates without prior knowledge of the signaling networks.6,7,11,20,21 Furthermore, in the context where protein tyrosine kinases are known to play an important role in many human cancer genes,22,23 phosphoproteomic analysis provides a functional screening assay to rapidly identify constitutively activated tyrosine kinases regardless of the molecular mechanism of activation (genomic rearrangement, gain of function mutations, insertions/deletions, and gene amplification).
In the hematopoietic system, RBM6 expression is high in the thymus, lymph nodes, and peripheral blood leukocytes. RMB6 is down-regulated on differentiation into granulocytes, but is expressed in progenitor cells and macrophages.15 No function has been assigned to the RBM6 protein, but it is interesting that RBM15 (also known as OTT, a family member of RNA-binding motif proteins) undergoes recurrent translocation t(1;22)(p13;q13) to produce the OTT-MAL fusion that is exclusively associated with acute megakaryoblastic leukemia.24,25 Although OTT-MAL transcript was not detected in MKPL-1 cells (data not shown), we did observe tyrosine phosphorylation of RBM15 at multiple sites (tyrosine 251, 536, 547). The importance of these tyrosine phosphorylation sites remains to be determined.
Although allele-specific PCR has reportedly identified activating mutations in CSF1R,26,27 these data have not been reproduced.28 CSF1R expression has been reported in AML, mainly of the monocytic lineage.29,30 It is not expressed in the megakaryocyte lineage.31 Thus, the novel RMB6-CSF1R gene rearrangement constitutively activates the CSF1R kinase in the megakaryocyte lineage. Though wild-type CSF1R is a membrane protein, RBM6-CSF1R fusion protein is expressed exclusively in cytosol (Figure S4). It remains to be analyzed whether the cytosolic localization of kinase is important for its oncogenic properties. The CSF1R, C-KIT, FLT3, PDGFRalpha, and PDGFRbeta kinases belong to the class III receptor tyrosine kinase family. Juxtamembrane domain mutations have been observed in FLT3 and C-KIT, and are thought to relieve the repressive effect of this domain on protein tyrosine kinase activity. This in turn allows autophosphorylation of the kinase domain, and results in additional activating conformational changes enabling full kinase activation.32 The RBM6-CSF1R fusion protein retains the N-terminal 36 amino acids of RBM6, which includes part of the predicted POZ (poxvirus and zinc finger) domain (Figure 3A). POZ domains are involved in protein-protein interactions.33 However, deletion of the RBM6 moiety had no effect on transforming activity of the fusion protein, indicating that the POZ domain of RBM6 is dispensable for kinase activation. Interestingly, simply overexpression of wild-type CSF1R is not sufficient to fully activate CSF1R (Figure 4). This suggests that the truncation in CSF1R is crucial for its full activation. The breakpoint in CSF1R occurred in exon 12, which encodes the juxtamembrane domain. The RBM6-CSF1R fusion protein contains the intact kinase domain of CSF1R. This fusion is reminiscent of the FIP1L1-PDGFRA fusion gene, where all breakpoints in PDGFRA also occurred in exon 12 the juxtamembrane region between 2 conserved tryptophan residues, and disruption of PDGFRA at this locus is sufficient for kinase activation.34,35 In addition, a TEL-FLT3 fusion has been reported in which the JM domain of FLT3 is disrupted.36 Thus, the chromosomal break in the juxtamembrane domain may also alleviate the inhibitory conformation of CSF1R.
Expression of RMB6-CSF1R in a murine bone marrow transplant model induces myeloproliferative disease with a long latency that is generally nonfatal, and has several features of AMKL that include splenomegaly, fibrosis, megakaryocyte hyperplasia, and increase in megakaryocyte progenitors. Although no significant difference in platelet counts was apparent in the blood, there was increased platelet production in the spleen of C57/Bl6 recipients. It is possible that platelets are sequestrated in the enlarged spleen. Of note, this abnormality in platelet production is not observed in other bone marrow transplantation (BMT) models of activated tyrosine kinase found in AMKL cell lines including JAK2T875N or JAK3,A572V,11,20 although increased number of platelets in the spleen was observed in a BMT model with the MPLW515L mutant recently described in myeloproliferative disease.37 Together these results suggest that this phenotype of increased platelets in the spleen without thrombocytosis may be characteristic of RBM6-CSF1R effect and is influenced by host modifiers as it is observed only in the C57Bl/6 background. Interestingly, differences in phenotypes between mouse strains were also observed in other models of leukemogenesis using the retroviral transduction/bone marrow transplantation approach.38 Although the megakaryocyte lineage is affected, it is equally clear that there was not transformation to an AMKL phenotype with 6 months of follow-up, suggesting that AMKL transformation requires other cooperating mutations. Although MKPL-1 cells were not derived from a Down syndrome patient and did not show GATA-1s mutation (data not shown), GATA-1 as well as other members of the GATA family were phosphorylated on several tyrosine residues including some in the DNA-binding domain (Table S1). This could be specific to MKPL-1 cells as no tyrosine phosphorylation of GATA-1 was found in 2 other AMKL cell lines using a similar phospho-tyrosine peptides profiling approach.11,20 To the best of our knowledge, this is the first report of tyrosine phosphorylation of GATA-1. Although serine phosphorylation of GATA-1 has been reported, its role on GATA-1 function remains unclear.39,–41 These observations indicate that study of the functional relevance of GATA-1 tyrosine phosphorylation is warranted in AMKL.
Although the IC50 of imatinib for RBM6-CSF1R (IC50 = 1.42 μM) in MKPL-1 cells is higher than what is observed for ABL (IC50 = 0.25 μM) and C-KIT (IC50 = 0.1 μM), it is still within the therapeutic dose range.13 Thus, imatinib could be considered for patients expressing activated CSF1R. Moreover, the identification of activated CSF1R in AML patients presents a rationale to develop small-molecule inhibitors with increased potency and selectivity for CSF1R. Recently, GW2580, an orally bioavailable inhibitor of CSF1R, inhibited CSF-1 signaling in macrophage lineages and tumor cells both in vitro and in vivo.42 However, beyond the MKPL1 cell line, we did not detect RBM6-CSF1R fusion in a collection of more than 40 AML cell lines (data not shown). The incidence of AML patients carrying the RBM6-CSF1R translocation or truncation of CSF1R remains to be determined. The search for such patients is currently under way. Finally, aberrant expression of CSF1R has been observed in a spectrum of cancers including breast and ovarian cancer, and its expression stimulates tumor invasion.43 Thus, searching for activated CSF1R in solid tumors may be warranted.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grant R01 CA66996 and the Leukemia and Lymphoma Society (D.G.G.). T.M. is the recipient of a Special Fellow Award by the Leukemia and Lymphoma Society. D.G.G. is an investigator of the Howard Hughes Medical Institute.
The authors thank Julie Nardone, Randy Wetzel, Herbert Haack, Sandra A. Moore, Dana Cullen, and Allison Coburn for technical assistance.
National Institutes of Health
Authorship
Contribution: T.G., T.M., and J.W.T. designed and performed research, analyzed data, and wrote the paper; V.L.G., D.K.W., M.G.C., C.R., L.P., and K.L. performed research; J.R. designed research; M.D. and I.M. performed cultivation of the MKPL-1 cell line; D.G.G. and B.J.D. contributed vital new reagents and revised the paper; and R.D.P. designed research and revised the paper.
T.G., T.M., and J.W.T. contributed equally to this work.
Conflict-of-interest disclosure: T.G., V.L.G., C.R., L.P., K.L., J.R., and R.D.P. are employed by Cell Signaling Technology, Inc, whose product was studied in the present work. The other authors declare no competing financial interests.
Correspondence: Roberto D. Polakiewicz, Cell Signaling Technology, Inc, 3 Trask La, Danvers, MA 01923; e-mail: rpolakiewicz@cellsignal.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal