The proteasome inhibitor bortezomib may increase osteoblast-related markers in multiple myeloma (MM) patients; however, its potential osteoblastic stimulatory effect is not known. In this study, we show that bortezomib significantly induced a stimulatory effect on osteoblast markers in human mesenchymal cells without affecting the number of osteoblast progenitors in bone marrow cultures or the viability of mature osteoblasts. Consistently we found that bortezomib significantly increased the transcription factor Runx2/Cbfa1 activity in human osteoblast progenitors and osteoblasts without affecting nuclear and cytoplasmatic active β-catenin levels. Consequently a stimulatory effect of bortezomib on bone nodule formation was also demonstrated in osteoblast progenitors. These in vitro observations were confirmed in vivo by the finding of a significant increase in the number of osteoblastic cells × mm2 of bone tissue and in the number of Runx2/Cbfa1-positive osteoblastic cells that was observed in MM patients who responded to bortezomib. Our in vitro and in vivo observations support the hypothesis that a direct stimulatory effect on bone formation process could occur during bortezomib treatment.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by the high capacity to induce osteolytic bone lesions.1 Hystomorphometric studies showed that osteoblast formation and function are profoundly impaired in MM patients and critical in the bone lesion development.1,–3 Several mechanisms are involved in MM-induced osteoblast suppression1,4,7 including the production of Wnt inhibitors as DKK-1 or sFRP-25,6 or the impairment of osteoblast formation and differentiation through the block of the critical osteoblast transcription factor Runx2/Cbfa1.7 In turn, osteoblastic cells also regulate myeloma cell growth8,9 and the increase of bone formation in mice results in a reduction of tumoral burden.10
Recent data suggest that ubiquitin-proteasome pathway, which is the major cellular degradative system and therapeutic target in myeloma cells,11 also regulates osteoblast differentiation.12,–14 The ubiquitin-proteasome pathway can modulate the BMP-2 expression,12 which can induce osteoblast differentiation through the Wnt signaling13 and regulates the proteolytic degradation of the osteoblast transcription factor Runx2/Cbfa1.14 Recently, Garrett et al12 demonstrated that proteasome inhibitors as PS1 and epoximicin stimulate bone formation in neonatal murine calvarial bones and in vivo in mice.12 A strong correlation between the capacity of these compounds to inhibit proteasomal activity in osteoblasts and their bone-forming activity was also demonstrated.12 Preliminary observations obtained in MM patients treated with the proteasome inhibitor bortezomib show an increase of serum bone-specific alkaline phosphatase and other osteoblast related markers suggesting a potential osteoblast stimulatory effect of this drug.15,,–18 Currently it is not known whether the proteasome inhibitor bortezomib may have a direct effect on osteoblast differentiation and formation in vitro in human cultures and in vivo in MM patients.
Patients, materials, and methods
Drugs
Bortezomib was purchased from Janssen-Cilag (Milan, Italy). For in vitro studies, the drug was reconstituted in DMSO at a stock concentration of 50 mM, and this stock was diluted in medium just before use, so that the concentration of DMSO never exceeded 0.1%. The proteasome inhibitors MG-132 and MG-262 were purchased from BIOMOL International (Plymouth Meeting, PA; DBA srl, Milan, Italy).
Cells and cell culture conditions
Isolated human BM mononuclear cells were seeded in 6-well plates at the density of 106/well in 4 mL α-MEM medium with 15% of FCS, plus ascorbic acid (50 μg/mL) and dexamethasone (Dex) (10−8M) and incubated in the presence or absence of bortezomib (concentration ranging from 2 nM to 5 nM) for 2 to 3 weeks replacing the medium every 3 days. Colonies forming units–fibroblast (CFU-Fs) and CFU-osteoblasts (OBs) were evaluated by alkaline phosphatase and alizarin red (Sigma Aldrich, St Louis, MO) staining and quantified as previously described.7
Human trabecular SV-40–transfected osteoblasts (HOBITs) (Dr Riggs, Mayo Clinic, Rochester, MN) or osteoblast-like cells MG-63 (DSM, Braunschweig, Germany) or human BM osteoprogenitor cells (PreOBs) obtained from mesenchymal cells as previously described.7 Cells (2 × 106) were incubated in the presence or absence of bortezomib (2 nM to 5 nM) or PTH (1-34) (Phoenix Pharmaceutical, Belmont, CA) at 50 ng/mL or BMP-2 (Peprotech, London, United Kingdom) at 50 ng/mL or MG-132 (10-100 nM) or MG-262 (10 nM) for 24 to 48 hours. Cell viability and survival were assessed as previously described.7 In parallel experiments, confluent PreOBs were incubated in the presence or absence of bortezomib (2-5 nM) or BMP-2 (50 ng/mL) or PTH (1-34) (50 ng/mL) or MG-132 (100 nM) or MG-262 (10 nM) for 21 days in α-MEM medium with 15% of FCS plus ascorbic acid (50 μg/mL), Dex (10−8 M) and β-glycerophosphate 10 mM. At the end of culture period, the cells were fixed with 1:1 mixture (vol/vol) of 37% formaldehyde and ethanol for 5 minutes, washed 3 times with PBS, and stained for 10 minutes with a solution of 2% of alizarin red (Sigma Aldrich) at pH 4.2. Bone nodules were quantified by light microscopy as previously described.7 Images were visualized under an Eclipse TE 300 microscope with a digital DS-UI slight (Nikon Instruments, Florence, Italy). Image processing was performed with Adobe Photoshop 5.02.
RNA isolation and reverse transcriptase-polymerase chain reaction amplification
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis was performed as previously described.7 cDNAs were amplified by PCR using specific primer pairs for human Runx2/Cbfa1, collagen I, osteocalcin (OC), alkaline phosphatase (ALP), and β-catenin.7 PCR reactions were performed in a thermal cycler (MiniCycler; MyResearch, Watertown, MA) for 30 cycles.
Western blot analysis
Nuclear and cytosolic extracts were obtained using a commercial kit (Active Motif, Carlsbad, CA). Polyclonal anti–collagen I and anti-Runx2/Cbfa1 (Santa Cruz Biotechnology, Santa Cruz, CA) were used as primary Abs. β-Catenin protein expression was checked using anti–β-catenin mAb recognizing the active dephospho nuclear form (aa 27-37) (Alexis Biochemicals, San Diego, CA), or anti–β-catenin mAb recognizing the inactive phospho form (pSer45) (Sigma, St Louis, MO). As internal controls, anti–β-actin (Sigma) and anti–Histone H1 mAbs (Upstate, Lake Placid, NY) were used for cytosol lysates and nuclear ones, respectively. Immunoblot procedures were performed as previously described.7
Runx2/Cbfa1 binding activity
Runx2/Cbfa1 binding activity was assessed by an enzyme-linked immunosorbent assay (ELISA)–based assay as previously described19 (TransAM Runx2; Active Motif, Vinci Biochem, Florence, Italy) according to the manufacturer's procedures. Nuclear extracts of human cell lines Saos2 with or without wild-type (WT) or mutated (MT) consensus-specific oligonucleotides were tested as controls for Runx2 activity.
Patients and treatment schedule
MM patients (n = 21) included in the study were at least 18 years old (median age, 72 years; range, 58–86 years), stages I to III, had measurable relapse, had at least 2 lines of therapy and/or refractory myeloma, and had BM plasmacytosis more than 30%. Fourteen of 21 patients have osteolytic lesions and previously underwent bisphosphonate therapy with zoledronic acid. Other inclusion criteria were similar to those previously reported.20
Bortezomib (1.3 mg/m2) was administered in monotherapy by intravenous bolus on days 1, 4, 8, and 11 of a 21-day cycle for up to 8 cycles. Patients with a suboptimal response, defined as progressive disease after 2 cycles or no change after the first 4 cycles of bortezomib, could receive oral dexamethasone (20 mg) on the same day and the day after bortezomib treatment. Responses were categorized as previously reported.2 All patients provided written informed consent in accordance with the Declaration of Helsinki. Our study was approved by our local ethics committee, “Azienda Ospedaliero-Universitaria di Parma,” Parma, Italy.
ELISA
Soluble osteocalcin levels in cell-conditioned media were detected using an ELISA assay purchased from Nordic Biosciences Diagnostic (Herlev, Denmark). The serum C-terminal telopeptides of collagen I (CTX) were measured in the peripheral serum of MM patients using the Serum CrossLaps ELISA kit from Nordic Biosciences Diagnostic.
Immunohistochemistry
Immunohistochemical staining was performed on 3-μm-thick BM sections obtained from the 21 MM patients before and after 8 cycles of bortezomib treatment with goat anti-Runx2/Cbfa1 Ab (Santa Cruz Biotechnology) using the immunoperoxidase technique as previously described.7 Normal bones obtained from 5 subjects without hematologic or bone disease were used as control. The total trabecular area of each section was measured by a dedicated software analyzer (Image Pro Plus 4.5, Media Cybernetics, Silver Spring, MD). Osteoblastic cells were identified in BM biopsies using morphologic criteria, as previously described.2,3,7 Osteoid surface was evaluated in Goldner trichrome-stained section (Olympus 8 × 60 F5 microscope equipped with a Uplan Fi 100×/1.30 iris, WH 10 × 22; Olympus, Tokyo, Japan). An Olympus oil DP10 digital microscope camera system was used for image acquisition. Images were processed by Adobe Photoshop 5.02.
Results
Effect of bortezomib on the number of osteoblast progenitors in human BM culture and on mature osteoblasts
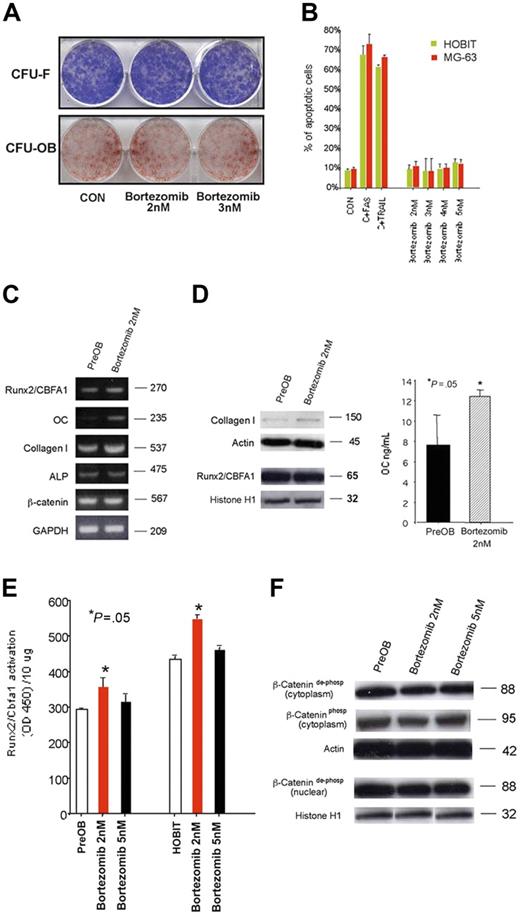
In human BM cultures, we found that bortezomib in a wide range of concentration did not significantly reduce the number of both early bone marrow (BM) osteoblast progenitors CFU-F and late CFU-OBs (Figure 1A). Similarly, we found that bortezomib did not inhibit the proliferation and survival (Figure 1B) of osteoblastic cell lines HOBIT and MG-63 at concentrations ranging from 2 nM to 5 nM that are known to be subapoptotic or apoptotic for human myeloma cells,21 indicating that long-term treatment with bortezomib did not show any toxic effect on osteoblast and the osteoblastogenesis process.
Effect of bortezomib on osteoblast progenitors and osteoblastic cells. Colony-forming units–fibroblast (CFU-Fs) and CFU-osteoblasts (OBs) were evaluated by alkaline phosphatase and alizarin red staining, respectively, in long-term human BM cell cultures incubated in the presence or absence of bortezomib at a concentration ranging from 2 nM to 5 nM (A). Human osteoblastic cells MG-63 or HOBITs (2 × 106) were incubated in the presence or absence of bortezomib (2 nM to 5 nM) for 48 hours. The number of dead and apoptotic osteoblastic cells was evaluated by a colorimetric method. Osteoblastic cells treated with TRAIL or stimulating anti-CD95 Ab (FAS) have been used as positive control (CON = control) (B). mRNA expression of the osteoblast-related markers was evaluated by RT-PCR in human PreOBs treated with bortezomib or vehicle for 24 hours (C). PreOBs were treated with bortezomib or vehicle for 48 hours; thereafter, collagen I and Runx2/Cbfa1 protein expression was evaluated by Western blot analysis in cell lysates and osteocalcin (OC) levels detected in conditioned media by ELISA. Graphs represent the mean OCs ± SD of 3 repeated experiments measured twice (D). The activity of the transcription factor Runx2/Cbfa1 was evaluated by an ELISA-based method in nuclear lysates of PreOBs and HOBITs treated for 48 hours with bortezomib (2-5 nM). Graphs represent the mean Runx2/Cbfa1 activation ± SD using 10 μg protein in 3 repeated experiments measured in triplicate (E). Both dephospho and phospho β-catenin were determined by Western blot in cytosolic or nuclear lysates of PreOBs treated with bortezomib (2-5 nM) or vehicle. Actin and histone H1 were used as internal controls (F).
Effect of bortezomib on osteoblast progenitors and osteoblastic cells. Colony-forming units–fibroblast (CFU-Fs) and CFU-osteoblasts (OBs) were evaluated by alkaline phosphatase and alizarin red staining, respectively, in long-term human BM cell cultures incubated in the presence or absence of bortezomib at a concentration ranging from 2 nM to 5 nM (A). Human osteoblastic cells MG-63 or HOBITs (2 × 106) were incubated in the presence or absence of bortezomib (2 nM to 5 nM) for 48 hours. The number of dead and apoptotic osteoblastic cells was evaluated by a colorimetric method. Osteoblastic cells treated with TRAIL or stimulating anti-CD95 Ab (FAS) have been used as positive control (CON = control) (B). mRNA expression of the osteoblast-related markers was evaluated by RT-PCR in human PreOBs treated with bortezomib or vehicle for 24 hours (C). PreOBs were treated with bortezomib or vehicle for 48 hours; thereafter, collagen I and Runx2/Cbfa1 protein expression was evaluated by Western blot analysis in cell lysates and osteocalcin (OC) levels detected in conditioned media by ELISA. Graphs represent the mean OCs ± SD of 3 repeated experiments measured twice (D). The activity of the transcription factor Runx2/Cbfa1 was evaluated by an ELISA-based method in nuclear lysates of PreOBs and HOBITs treated for 48 hours with bortezomib (2-5 nM). Graphs represent the mean Runx2/Cbfa1 activation ± SD using 10 μg protein in 3 repeated experiments measured in triplicate (E). Both dephospho and phospho β-catenin were determined by Western blot in cytosolic or nuclear lysates of PreOBs treated with bortezomib (2-5 nM) or vehicle. Actin and histone H1 were used as internal controls (F).
Effect of bortezomib on osteogenic differentiation of human osteoprogenitor cells
In short-term experiments, we found that 24-hour bortezomib (2-3 nM) treatment increased mRNA expression of the osteoblast markers collagen I and OC but not ALP, Runx2/Cbfa1, and β-catenin (Figure 1C) by human PreOBs. Collagen I production and OC secretion by PreOBs have been stimulated after 48 hours of bortezomib treatment without modifying Runx2/Cbfa1 protein level (Figure 1D). On the other hand, bortezomib did not have any effect on ALP activity in PreOBs (data not shown). Consistently with these observations, we found that bortezomib at low concentration significantly increased the activity of the transcription factor Runx2/Cbfa1 both in PreOBs and HOBITs (Figure 1E). On the other hand, bortezomib (2-5 nM) did not have any effect on active dephospho β-catenin cytosolic and nuclear levels or on inactive phosphorylated ones in PreOBs (Figure 1F) and HOBITs (data not shown), suggesting that bortezomib did not affect canonical Wnt signaling in these cells.
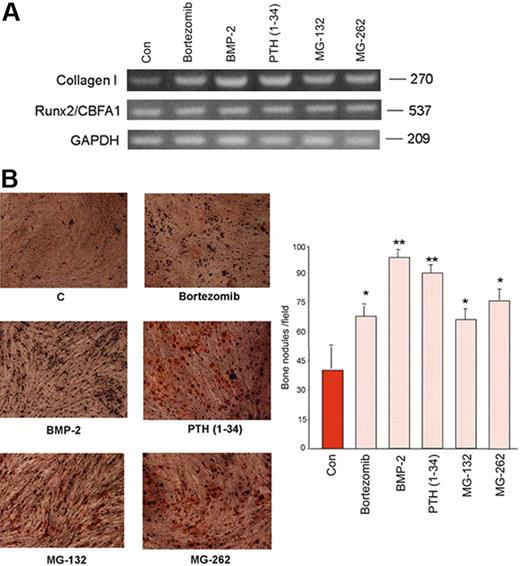
Thereafter, the effect of bortezomib was compared with those observed with BMP-2 and PTH (1-34) as well as with the specific proteasome inhibitors MG-132 and MG-262. A stimulatory effect of all these compounds was observed after short-term exposition on collagen I expression without affecting Runx2/CBFA1 mRNA expression (Figure 2A).
Effect of bortezomib on osteogenic differentiation of human mesenchymal cells. Confluent PreOB cells were treated with or without bortezomib (2 nM) or BMP-2 (50 ng/mL) or PTH (1-34) (50 ng/mL) or MG-132 (100 nM) or MG-262 (10 nM). After 24 hours, collagen I, Runx2/Cbfa1, and GAPDH mRNA expression was evaluated by RT-PCR (A). Adherent PreOB cells were incubated in osteogenic differentiating medium as described in “Patients, materials, and methods” in the presence or absence of bortezomib (2 nM) or BMP-2 (50 ng/mL) or PTH (1-34) (50 ng/mL) or MG-132 (100 nM) or MG-262 (10 nM) for 21 days, replacing medium with fresh one every 3 days. After the culture period, cells were fixed and stained for the presence of bone nodules by alizarin red. Graphs and bars represent the mean plus SD of bone nodules × field of 2 independent experiments performed in triplicate (*P < .05; **P < .01) (B). Images were obtained on a Nikon Eclipse TE 300 microscope at 10×/0.13 using a DS-U1 digital slight and at 4×/0.12 objective lens (Original magnification × 5).
Effect of bortezomib on osteogenic differentiation of human mesenchymal cells. Confluent PreOB cells were treated with or without bortezomib (2 nM) or BMP-2 (50 ng/mL) or PTH (1-34) (50 ng/mL) or MG-132 (100 nM) or MG-262 (10 nM). After 24 hours, collagen I, Runx2/Cbfa1, and GAPDH mRNA expression was evaluated by RT-PCR (A). Adherent PreOB cells were incubated in osteogenic differentiating medium as described in “Patients, materials, and methods” in the presence or absence of bortezomib (2 nM) or BMP-2 (50 ng/mL) or PTH (1-34) (50 ng/mL) or MG-132 (100 nM) or MG-262 (10 nM) for 21 days, replacing medium with fresh one every 3 days. After the culture period, cells were fixed and stained for the presence of bone nodules by alizarin red. Graphs and bars represent the mean plus SD of bone nodules × field of 2 independent experiments performed in triplicate (*P < .05; **P < .01) (B). Images were obtained on a Nikon Eclipse TE 300 microscope at 10×/0.13 using a DS-U1 digital slight and at 4×/0.12 objective lens (Original magnification × 5).
Then the potential effect of bortezomib on calcium deposition in adherent PreOBs has been evaluated in comparison with BMP-2, PTH, (1-34) MG-132, and MG-262. As shown in Figure 2, we found that bortezomib significantly increased bone nodule formation after 21 days of exposition. Both BMP-2 and PTH (1-34) had a more potent effect on calcium deposition by PreOBs, whereas MG-132 and MG-262 showed a similar stimulatory effect on bone nodule formation by PreOBs in comparison with bortezomib (Figure 2B).
Effect of bortezomib on osteoblastic cells, bone formation rate, and bone resorption in MM patients
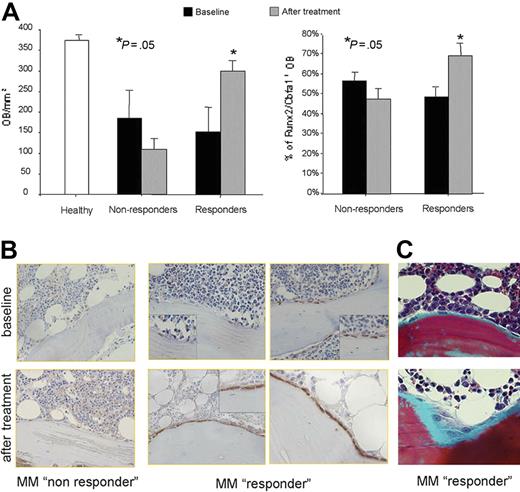
To put our in vitro observations in a clinical perspective, we evaluate the potential in vivo effect of bortezomib in a cohort of MM patients. A significant increase in the number of osteoblastic cells × mm2 of bone tissue was observed in MM patients responding to bortezomib treatment (complete remission [CR], near complete remission [nCR], and partial remission [PR]) but not in nonresponders (Figure 3A). However, the number of osteoblastic cells × mm2 of bone tissue in responder MM patients after therapy was still decreased compared with healthy bone obtained from the control group (Figure 3A). Finally, we found a significant increase in the number of Runx2/Cbfa1-positive osteoblastic cells in responder MM patients compared with nonresponders (Figure 3A-B). Consequently, an increase of osteoid surface was observed in the area with higher number of osteoblasts after bortezomib treatment in responders as shown for a representative patient (Figure 3B).
Effect of bortezomib on bone formation in MM patients. Histomorphometry and Runx2/Cbfa1 immunostaining performed on BM biopsies of 21 MM patients before and after 6 to 8 cycles of bortezomib in monotherapy according to the schedule described in the “Patients, materials, and methods.” The graphs and bars represent the mean plus SD of the number of osteoblasts (OBs)/mm2 and the percentage of Runx2/Cbfa1-positive OBs (A). Runx2/Cbfa1 immunostaining performed in a trabecula of 2 representative MM patients who responded or not to bortezomib treatment (original magnification, × 200; small insert, × 400) (B). Goldner trichrome–stained sections from a representative MM responder patient. (Microscope used was an Olympus B × 60 F5 equipped with a UPlan F: 100×/1.30 oil iris, WH10×/22. An Olympus DP10 digital microscope camera system was used for image acquisition (original magnification, × 200) (C).
Effect of bortezomib on bone formation in MM patients. Histomorphometry and Runx2/Cbfa1 immunostaining performed on BM biopsies of 21 MM patients before and after 6 to 8 cycles of bortezomib in monotherapy according to the schedule described in the “Patients, materials, and methods.” The graphs and bars represent the mean plus SD of the number of osteoblasts (OBs)/mm2 and the percentage of Runx2/Cbfa1-positive OBs (A). Runx2/Cbfa1 immunostaining performed in a trabecula of 2 representative MM patients who responded or not to bortezomib treatment (original magnification, × 200; small insert, × 400) (B). Goldner trichrome–stained sections from a representative MM responder patient. (Microscope used was an Olympus B × 60 F5 equipped with a UPlan F: 100×/1.30 oil iris, WH10×/22. An Olympus DP10 digital microscope camera system was used for image acquisition (original magnification, × 200) (C).
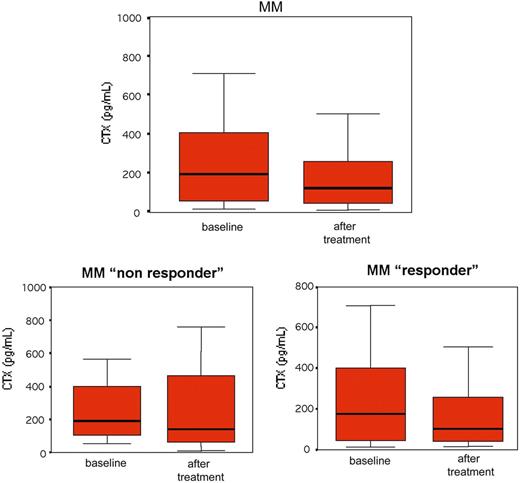
Osteoclast activity was also evaluated in our cohort of patients by the measure of serum CTX levels. A reduction of serum CTX was observed in MM patients treated with bortezomib even if it did not reach a statistical significance either in nonresponder or responder patients (Figure 4).
Serum CTX in MM patients before and after bortezomib. Serum CTX was measured by ELISA assay in 21 MM patients before and after bortezomib treatment. Box plots represent the median CTX serum levels with the 25th to 75th percentiles.
Serum CTX in MM patients before and after bortezomib. Serum CTX was measured by ELISA assay in 21 MM patients before and after bortezomib treatment. Box plots represent the median CTX serum levels with the 25th to 75th percentiles.
Discussion
Emerging data indicate that the proteasome inhibitors may have a critical role in the regulation of osteoblast proliferation and differentiation in murine system.12,–14 In this study, we show that bortezomib, the first member of this class of drugs approved for the clinical use, affects human osteoblast differentiation process both in vitro and in vivo.
It is known that human osteoblastogenesis is primarily associated with increased Runx2/Cbfa1 activity without a change in mRNA or protein level.22 The binding sites of Runx2/Cbfa1 have been identified in the major bone matrix genes as collagen I and OC and its activation induced the expression of these genes or activated their promoters in vitro.23 Our data suggest that bortezomib may induce osteoblast phenotype in osteoblast progenitors increasing Runx2/Cbfa1 activity without changing Runx2/Cbfa1 mRNA level and consequently stimulating the expression of late osteoblast markers as collagen I and OC rather than the early ones as ALP. On the other hand, we failed to find a stimulatory effect on the canonical Wnt signaling. Accordingly, it has been reported that Wnt/β-catenin signaling induces ALP expression, but has no effect on OC and collagen I expression by osteoprogenitor cells.13,24 The stimulatory effect of bortezomib on Runx2/Cbfa1 activity was observed in our system at concentrations as low as 2 nM, whereas higher doses of bortezomib did not show any effect. This behavior was not related to a toxic effect of bortezomib because we find that this drug did not induce apoptosis or inhibit proliferation of either osteoblast progenitors or osteoblasts at concentration ranging between 2 and 5 nM. Thus, on the basis of this in vitro evidence, we can suppose that low doses of bortezomib rather than higher ones could be more effective to stimulate osteoblastogenesis with a relevant clinical perspective in MM patients. A similar behavior on bone formation was previously reported with bisphosphonates that may increase osteoblastogenesis in vitro at low doses.25
The stimulatory effect of bortezomib on Runx2/Cbfa1 activity and the related osteoblast markers was further confirmed by the finding that bortezomib increased bone nodule formation by human PreOBs. A similar effect was observed using specific proteasome inhibitors as MG-132 and MG-262 indicating that the pro-osteogenic effect of bortezomib is due to its capacity to block proteasome activity in osteoblast and osteoblast progenitors.
In vitro observations were expanded and confirmed in vivo by the finding of a significant increase of the number of osteoblastic cells and Runx2/Cbfa1-positive cells in MM patients responding to bortezomib treatment. Based on our evidence, we can suppose that bortezomib may directly stimulate osteoblast differentiation. Alternatively, the rapid apoptosis of MM cells induced by bortezomib and their removal from the BM could result in the recovery of osteoblast differentiation and an increase in the number of osteoblastic cells as also demonstrated by the elevation of bone alkaline phosphatase levels in MM patients responding to bortezomib therapy.15,–17
To have a better overall picture of bone formation and bone destruction in our group of MM patients, we checked the levels of serum CTX, a marker of bone resorption that has been demonstrated useful to evaluate osteoclast activity in MM patients.26,27 In our cohort of MM patients, despite the reduction of serum CTX levels after bortezomib treatment, we failed to find a statistical significance. This observation could be due to either the low number of patients in our study or the lower serum CTX levels at the baseline compared with those reported in another study.28
In conclusion, our data indicate that bortezomib increases osteoblast differentiation in human mesenchymal cells without affecting the number of osteoblast progenitors and the viability of mature osteoblasts. In vivo and in vitro observations support the hypothesis that both direct and indirect effects on bone formation process could occur during bortezomib treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by an International Myeloma Foundation (IMF) Grant. We thank the “Associazione Italiana Contro le Leucemie” (AIL) Parma section for the support.
We thank Janssen-Cilag for the financial support for vital reagents used in the study.
We thank Dr Simona Colla and Dr Marina Magnani for critical revision of the paper.
Authorship
Contribution: N.G. designed the research, analyzed the data, and wrote the paper; F.M. performed all cell culture experiments and MM cell purification; S.T. performed molecular biology and array methodologies; M.L. performed immunohistochemistry; S.B. contributed to the characterization of patients; M.C. included patients in the study and performed bone biopsies; C.M. contributed to immunohistochemistry and histomorphometry; E.M. contributed to immunohistochemistry and histomorphometry; L.F. contributed to cell culture experiments; A.T. included patients in the study; V.R. provided vital reagents and contributed to the critical revision of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola Giuliani, Hematology and BMT Center, Department of Internal Medicine and Biomedical Science, University of Parma, via Gramsci 14, 43100 Parma, Italy; e-mail: nicola.giuliani@unipr.it; n_giuliani@yahoo.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal