Abstract
Decoy lymphotoxin β receptor (LTβR) has potent immune inhibitory activities and thus represents a promising biologic for the treatment of inflammation, autoimmune diseases, and graft-versus-host disease (GVHD). As this reagent interrupts multiple molecular interactions, including LTβ-LTβR and LIGHT-HVEM/LTβR, underlying molecular mechanisms have yet to be fully understood. In this study, we demonstrate that blockade of the LIGHT-HVEM pathway is sufficient to induce amelioration of GVHD in mouse models. Anti–host cytotoxic T lymphocyte (CTL) activity following in vivo transfer of allogeneic lymphocytes was completely abrogated when LIGHT- or HVEM-deficient (KO) T cells were used as donor cells. Accordingly, survival of the recipient mice following the transfer of allogeneic bone marrow cells plus LIGHT-KO or HVEM-KO T cells was significantly prolonged. In the absence of LIGHT-HVEM costimulation, alloreactive donor T cells undergo vigorous apoptosis while their proliferative potential remains intact. Furthermore, we prepared a neutralizing monoclonal antibody (mAb) specific to HVEM and showed that administration of anti–HVEM mAb profoundly ameliorated GVHD and led to complete hematopoietic chimerism with donor cells. Collectively, our results demonstrate an indispensable role of LIGHT-HVEM costimulation in the pathogenesis of GVHD and illustrate a novel target for selective immunotherapy in allogeneic bone marrow transplantation.
Introduction
The functional network of tumor necrosis factor (TNF) and TNF receptor superfamily members is composed of complex cross-talk between multiple ligands and multiple receptors, which regulate pleiotropic functions in the immune system.1 LIGHT, standing for homologous to lymphotoxins, exhibits inducible expression, and competes with herpes simplex virus glycoprotein D for herpesvirus entry mediator (HVEM), a receptor expressed by T lymphocytes, is a type II transmembrane glycoprotein belonging to the TNF ligand superfamily.2 LIGHT is expressed on immature dendritic cells (DCs) and activated T cells2,3 and interacts with 2 functional receptors, lymphotoxin-β receptor (LTβR) and HVEM.2 LIGHT interaction with LTβR triggers the production of proinflammatory mediators,4,5 up-regulates adhesion molecule expression,6 and induces apoptotic cell death in certain tumors.7 On the other hand, by signaling through HVEM, LIGHT costimulates T-cell activation.8 In vivo experiments demonstrated that transgenic expression of LIGHT leads to spontaneous progression of inflammatory autoimmunity such as Crohn disease,9–11 while genetic disruption of LIGHT results in impaired T-cell activation, particularly in CD8+ T cells,12–15 and renders mice less vulnerable to pathogenic inflammation, as shown in acute hepatitis models.16 Thus, LIGHT regulates multiple immune functions of innate and adaptive immunity through interactions with LTβR and HVEM.
There are numerous reports demonstrating therapeutic effects of decoy protein of LTβR in various immunologic diseases, including autoimmunity, inflammation, and transplantation,17,18 indicating that decoy LTβR could be a potential biologic for clinical immunotherapy, analogous to a decoy form of TNF-receptor.19 Prolonged administration of decoy LTβR, however, might become a double-edged sword since it abrogates the maintenance of DC and natural killer/natural killer T (NK/NKT) cells20,21 and inhibits the microstructure formation of lymphoid organs,22 thus disrupting immune homeostasis. Therefore, it is of great interest to discover novel approaches that separate the therapeutic effects of decoy LTβR from the potential adverse effects. While decoy LTβR interferes with 3 molecular interactions—LTβ-LTβR, LIGHT-LTβR, and LIGHT-HVEM—the antihomeostatic effects are largely dependent on LTβ-LTβR functions since the corresponding phenotypes are observed in LTβ- or LTβR-KO mice but not in LIGHT-KO mice.12–15,23–26 Elucidation of molecular mechanisms of decoy LTβR effects on immunologic diseases is highly significant.
Graft-versus-host disease (GVHD) is a major complication associated with allogeneic hematopoietic stem cell transplantation. Posttransplantation administration of immunosuppressants prevails as the current therapeutic choice for GVHD, but this treatment results in systemic immunosuppression that often leads to opportunistic pathogen infections and leukemic relapse.27,28 To overcome these issues, blockade of T-cell costimulatory signals is among the most sought after alternatives to immunosuppressants.27,28 In this regard, our previous findings have suggested a therapeutic potential of LIGHT costimulation, in which administration of LTβR-Ig, a decoy LTβR, inhibits alloreactive cytotoxic T lymphocyte (CTL) generation and prolongs the survival of GVHD mice.8 Combined therapy of LTβR-Ig and anti–CD40 ligand monoclonal antibody (mAb) further protects the recipient mice from GVHD by rendering alloreactive donor CTL anergic.29 However, the actual contribution of the LIGHT-HVEM costimulatory system to these findings remains elusive due to the antihomeostatic effects of decoy LTβR. It is possible that changes of DC function or cellular structure in lymphoid tissues could affect the intensity of adaptive immune responses. Thus, direct evidence indicating a pathogenic role of LIGHT-HVEM costimulation in GVHD needs to be elucidated.
In this study, by using mice genetically deficient of LIGHT or HVEM, we directly evaluate the notion that LIGHT-HVEM costimulatory signal plays an indispensable role in GVHD pathogenesis. Cellular mechanisms underlying this effect are also explored. Importantly, our study further demonstrates the therapeutic efficacy of antagonistic anti–HVEM mAb for GVHD, thus proposing a highly specific, clinically applicable strategy targeting the LIGHT-HVEM costimulatory pathway.
Materials and methods
Mice
Female C57BL/6J (B6, H-2b), BALB/c (H-2d), and F1 (B6 x DBA/2J) (BDF1; H-2bxd) mice were purchased from the National Cancer Institute (Frederick, MD). C3H.SW mice (C3.SW-H2b/SnJ) were purchased from The Jackson Laboratory (Bar Harbor, ME). B6-background LIGHT-KO mice were generated in our laboratory.13 HVEM-KO mice (H-2b) and 2C TCR transgenic mice were kindly provided by, respectively, Dr Wayne Hancock and Dr Larry Pease (Department of Immunology, Mayo Clinic College of Medicine, Rochester, MN). Age- and sex-matched 6- to 8-week-old mice were used for all experiments. All the animal experiments described in this manuscript were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Cell lines and antibodies
P815 mouse mastocytoma cells (DBA/2, H-2d) and EL4 mouse T-cell lymphoma cells (B6, H-2b) were purchased from the American Type Culture Collection (Rockville, MD). All cell lines were maintained in the complete medium under appropriate conditions. Anti–mouse HVEM mAbs (clone; LBH1) were generated in our laboratory by a standard technique, as described previously.30 Control hamster IgG was purchased from Rockland Immunochemicals (Gilbertsville, PA). Anti–2C TCR clonotypic mAb was purified from the supernatants of 1B2 hybridoma and further conjugated with phycoerythrin in our laboratory.
Mouse parent-to-F1 transfer GVHD model in nonirradiated hosts
In the nonirradiated parent-to-F1 GVHD model, 5 × 107 spleen cells isolated from wild-type (WT) B6 mice, LIGHT-KO mice, or HVEM-KO mice were transferred intravenously into BDF1 recipients on day 0. In some experiments, donor spleen cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) prior to transfer. In the mice transferred with WT B6 splenocytes, 100 μg anti–HVEM mAb or control hamster IgG was administered intraperitoneally on days 0, 3, and 6. The recipient mice were killed on the indicated days, and the spleen cells were analyzed by flow cytometry and chromium 51 (51Cr)–release assay. In the model employing 2C T cells, 1 × 107 spleen cells from 2C TCR-transgenic mice were mixed with 3 × 107 B6 spleen cells and then transferred intravenously into the BDF1 host on day 0. Recipient mice were subsequently administered 100 μg anti–HVEM mAb or control hamster IgG intraperitoneally on days 0 and 4. On day 7, recipient spleen cells were harvested and assessed for the presence of 2C T cells by flow cytometric analysis using 1B2 clonotypic mAb and anti–CD8 mAb.
Mouse GVHD models employing allogeneic BM transfer into irradiated recipients
Three models of GVHD induced by allogeneic bone marrow (BM) transplantation were employed in this study. First, BDF1 recipient mice, which were preconditioned with lethal irradiation (12 Gy), were injected intravenously with T cell–depleted B6 BM cells (5 × 106 cells) with or without B6 T cells (2-3 × 106 cells) isolated from either WT or LIGHT-KO mice. T-cell depletion from BM cells and T-cell isolation from spleen cells was performed by MACS systems using anti–Thy1.2 mAb-conjugated microbeads and pan–T cell isolation kits, respectively (Miltenyi Biotec, Auburn, CA). In mice transferred with WT B6 T cells, cohorts of mice were intraperitoneally administered 150 μg anti–HVEM mAb or control hamster IgG on days 0, 3, and 6. The survival of recipient mice was monitored daily.
In the second model, BALB/c mice were exposed to lethal irradiation (10 Gy) followed by intravenous transfer of T cell–depleted B6 BM cells (5 × 106 cells) with or without B6 T cells (1 × 106 cells) isolated from WT, LIGHT-KO, or HVEM-KO mice. In this fully major histocompatibility complex (MHC)–mismatched GVHD model, the survival and body weight change of recipient mice were monitored regularly.
The third GVHD model was induced by MHC-matched, minor histocompatibility antigen (miHA)–mismatched BM transfer. B6 mice were exposed to lethal irradiation (10 Gy) and subsequently injected intravenously with 4 × 106 T cell–depleted BM cells from C3H.SW mice (H-2b, Ly9.1+) with or without 3 × 107 C3H.SW spleen cells. B6 recipient mice injected with C3H.SW spleen cells were intraperitoneally administered either anti–HVEM mAb or control hamster IgG at 100 μg on days 0, 5, 10, 15, 20, and 25. Recipient mice were monitored for survival daily and evaluated for body weight and GVHD clinical score regularly. For scoring, 5 clinical parameters—weight loss, posture, activity, fur texture, and skin integrity (0-2 in each parameter, maximal score of 10)—were used, as previously described.31 In the recipient mice that survived long term, reconstitution of host lymphoid tissues by donor cells was assessed by flow cytometry using double staining with Ly9.1 and CD3 or B220. On day 60, cohorts of recipients were killed, and tissues from liver, skin, and intestine were harvested for pathological analysis by hematoxylin and eosin (H&E) staining. Tissue images were observed using an Olympus CH30 microscope (Olympus, Center Valley, PA) equipped with a 20×/0.40 numerical aperture (NA) or a 40×/0.65 NA objective lens. Images were acquired using an Olympus DP12 camera and associated image acquisition software, and were processed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA).
Assessment of division, apoptosis, and anti–host CTL activity of donor cells
In the GVHD model of parent-to-F1 transfer, division of donor T cells was assessed by CFSE intensity of H-2Kd-negative, CD4+, or CD8+ cells in the spleen at the indicated time points. Apoptosis of donor T cells was examined by Annexin V staining of H-2Kd-negative, CD4+, or CD8+ spleen cells at the indicated time points. Donor anti–host CTL activity was examined, as previously described.8 Briefly, recipient spleen cells were harvested 10 days after donor cell transfer and, without any in vitro manipulation, were examined for CTL activity against P815 (H-2d) or EL4 (H-2b) by standard 4-hour 51Cr-release assay.
Statistical analysis
For survival data, Kaplan-Meier survival curves were prepared using StatView 5.0 software (SAS Institute, Cary, NC), and statistical differences were analyzed using the log-rank (Mantel-Cox) test. P values less than .05 were considered significant.
Results
Indispensable role of donor T cell–derived LIGHT in GVHD pathogenesis
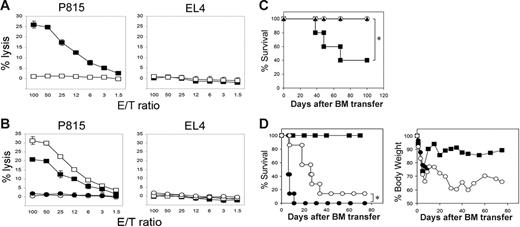
To selectively investigate LIGHT functions in GVHD, we first employed mice deficient in the Light gene (LIGHT-KO).13 As previously established, profound activity of donor anti–host MHC Ag (H-2d)–specific CTLs was generated 10 days after transfer of WT B6 mice splenocytes into BDF1 mice (Figure 1A). In sharp contrast, anti–host CTL activity was completely diminished when LIGHT-KO B6 spleen cells were transferred into BDF1 hosts, indicating a crucial role of donor-derived LIGHT in allo-CTL generation in vivo. Given that LIGHT is expressed and functions on both antigen-presenting cells (APCs) and activated T cells,2,3 we next examined which subset among transferred donor cells is responsible for the effects of LIGHT. To this end, donor cells composed of WT or LIGHT-KO T cells combined with WT or LIGHT-KO non–T cells were subsequently transferred into BDF1 mice. Anti–host CTL activity was completely abrogated when LIGHT-KO T cells were transferred, irrespective of genotypes of coinjected non–T cells (Figure 1B). In contrast, the mice transferred with WT T cells plus LIGHT-KO non–T cells showed a marginal decrease of CTL activity compared to those injected with WT T and non–T cells. These results strongly indicate that LIGHT associated with donor T cells, rather than APCs, plays an indispensable role in the generation of anti–host CTLs in vivo.
Compromised allo-CTL and GVHD generation in LIGHT-KO donor T cells. (A) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT (▪) or LIGHT-KO (□) B6 mice. Ten days later, spleen cells from the recipient mice were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b) tumor cells by 51Cr-release assay. (B) T cells (2 × 107 cells) and non–T cells (4 × 107 cells) purified from spleen cells of WT or LIGHT-KO B6 mice were mixed in the following combinations: WT T cells plus WT non–T cells (□), WT T cells plus LIGHT-KO non–T cells (▪), LIGHT-KO T cells plus WT non–T cells (○), LIGHT-KO T cells plus LIGHT-KO non–T cells (•). These combined cells were injected intravenously into BDF1 mice. Ten days later, anti–host CTL activity from recipient spleen cells were analyzed as in panel A. (A-B) Data representative of 3 independent experiments. (C) BDF1 mice (n = 5 in each group) were exposed to lethal-dose irradiation (12 Gy) followed by intravenous injection of 5 × 106 T cell–depleted B6 BM cells alone (○) or together with 2 × 106 B6 WT (▪) or LIGHT-KO (▴) T cells. The survival of recipient mice is shown. *P = .049. (D) BALB/c mice (n = 7 in each group) were exposed to lethal-dose irradiation (10 Gy) followed by intravenous injection of 5 × 106 T cell-depleted B6 BM cells alone (▪) or together with 1 × 106 B6 WT (•) or LIGHT-KO (○) T cells. Survival (left panel) and body weight changes (right panel) were monitored. *P = .0008. (C-D) Results are representative of 2 independently performed experiments.
Compromised allo-CTL and GVHD generation in LIGHT-KO donor T cells. (A) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT (▪) or LIGHT-KO (□) B6 mice. Ten days later, spleen cells from the recipient mice were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b) tumor cells by 51Cr-release assay. (B) T cells (2 × 107 cells) and non–T cells (4 × 107 cells) purified from spleen cells of WT or LIGHT-KO B6 mice were mixed in the following combinations: WT T cells plus WT non–T cells (□), WT T cells plus LIGHT-KO non–T cells (▪), LIGHT-KO T cells plus WT non–T cells (○), LIGHT-KO T cells plus LIGHT-KO non–T cells (•). These combined cells were injected intravenously into BDF1 mice. Ten days later, anti–host CTL activity from recipient spleen cells were analyzed as in panel A. (A-B) Data representative of 3 independent experiments. (C) BDF1 mice (n = 5 in each group) were exposed to lethal-dose irradiation (12 Gy) followed by intravenous injection of 5 × 106 T cell–depleted B6 BM cells alone (○) or together with 2 × 106 B6 WT (▪) or LIGHT-KO (▴) T cells. The survival of recipient mice is shown. *P = .049. (D) BALB/c mice (n = 7 in each group) were exposed to lethal-dose irradiation (10 Gy) followed by intravenous injection of 5 × 106 T cell-depleted B6 BM cells alone (▪) or together with 1 × 106 B6 WT (•) or LIGHT-KO (○) T cells. Survival (left panel) and body weight changes (right panel) were monitored. *P = .0008. (C-D) Results are representative of 2 independently performed experiments.
This notion was bolstered by GVHD models induced by allogeneic BM plus T-cell transfer to lethally irradiated recipient mice. First, BDF1 mice exposed to a lethal dose of irradiation were injected with T cell–depleted B6 BM cells, together with either WT or LIGHT-KO B6 T cells. Mice transferred with WT T cells underwent GVHD, and 60% of them died within 70 days, whereas all mice that underwent transfer with LIGHT-KO T cells survived indefinitely (Figure 1C). In the second model, fully MHC-mismatched BM transfer was employed as a condition of severe GVHD, in which lethally irradiated BALB/c mice were transferred with T cell–depleted B6 BM cells plus either WT or LIGHT-KO B6 T cells. Recipient mice transferred with WT T cells all died within 11 days of severe GVHD along with profound weight loss (Figure 1D). In contrast, transfer of LIGHT-KO T cells resulted in a significantly prolonged recipient survival along with a transient recovery of body weight following acute collapse by the irradiation and BM transfer. Together, these findings indicate an indispensable role of donor T cell–derived LIGHT in GVHD pathogenesis.
Impaired survival of LIGHT-deficient donor T cells
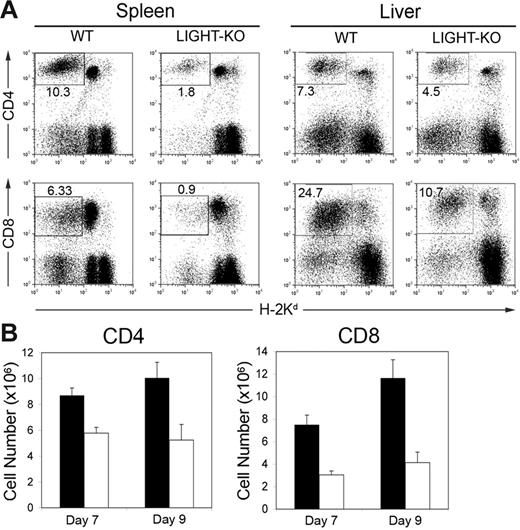
We next investigated the cellular mechanisms of the abrogated anti–host CTL activity in LIGHT-KO donor cells. We first monitored the fate of donor T cells following a transfer into BDF1 recipient mice. After transfer, the percentage and absolute number of LIGHT-KO donor T cells in the recipient spleen were significantly lower than those of WT donor T cells (Figure 2A-B). The decrease of LIGHT-KO donor T cells was more prominent in CD8+ T cells than CD4+ T cells. Donor T-cell decrease was observed in both hepatic and splenic lymphocytes, suggesting that changes of cellular distribution are not responsible for this finding.
Diminished LIGHT-KO donor T cells in BDF1 recipient mice. (A) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT or LIGHT-KO B6 mice. After 9 days, spleen and liver from the BDF1 recipients were harvested and stained with anti–H-2Kd mAb, together with either anti–CD4 or anti–CD8 mAb. Percentage of donor (H-2Kd-negative) CD4+ or CD8+ T cells in total spleen or liver lymphocytes is shown in the panels. (B) As in panel A, absolute numbers of donor T cells in the recipient spleen were examined at 7 and 9 days after donor cell transfer. Each column shows the number of WT donor T cells (▪) or LIGHT-KO donor T cells (□) as average ± SD. Representative data of 5 independently performed experiments are shown.
Diminished LIGHT-KO donor T cells in BDF1 recipient mice. (A) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT or LIGHT-KO B6 mice. After 9 days, spleen and liver from the BDF1 recipients were harvested and stained with anti–H-2Kd mAb, together with either anti–CD4 or anti–CD8 mAb. Percentage of donor (H-2Kd-negative) CD4+ or CD8+ T cells in total spleen or liver lymphocytes is shown in the panels. (B) As in panel A, absolute numbers of donor T cells in the recipient spleen were examined at 7 and 9 days after donor cell transfer. Each column shows the number of WT donor T cells (▪) or LIGHT-KO donor T cells (□) as average ± SD. Representative data of 5 independently performed experiments are shown.
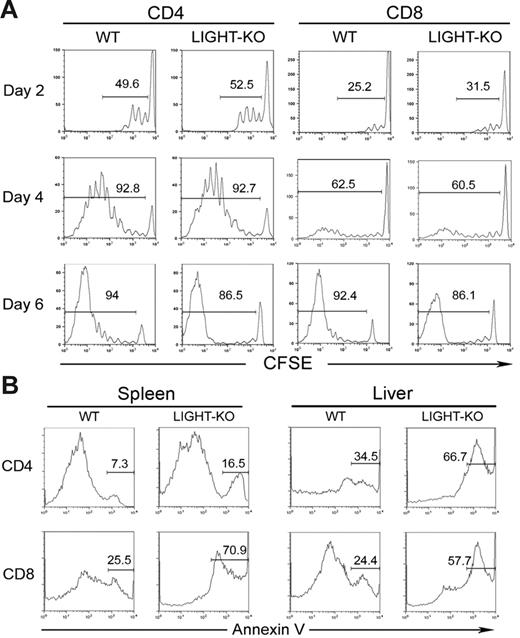
The decrease of LIGHT-KO donor T cells can be explained by 2 potential mechanisms, an impaired proliferation and an accelerated cell death. In order to address these possibilities, we first compared the expansion kinetics of WT and LIGHT-KO donor T cells in vivo. Two to 6 days after transfer, division of donor T cells labeled with CFSE was comparable between WT and LIGHT-KO cells in both CD4+ and CD8+ T cells (Figure 3A). This result indicates a dispensable role of LIGHT in driving the expansion of alloreactive T cells, thus denying the first possibility. Next, we evaluated apoptotic cell death in the transferred donor T cells. In both spleen and liver, the percentage of Annexin V–positive cells in LIGHT-KO donor T cells was significantly increased compared to those of WT T cells (Figure 3B). Taken together, our findings suggest that deficiency of LIGHT costimulation impairs survival of host-reactive donor T cells by rendering them vulnerable to activation-induced cell death.
Impaired survival, but competent proliferation, of LIGHT-KO donor T cells in BDF1 recipient mice. (A) BDF1 mice were injected intravenously with CFSE-labeled spleen cells (5 × 107 cells) from WT or LIGHT-KO B6 mice. Division of host-reactive donor T cells was analyzed 2, 4, and 6 days after cell transfer. CFSE intensity of donor CD4+ or CD8+ T cells, gated as H-2Kd-negative CD4+, or CD8+, is shown as a histogram. The percentage of donor T cells with more than one division is indicated in each panel. (B) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT or LIGHT-KO B6 mice. Seven days later, spleen cells and liver lymphocytes from the BDF1 recipients were harvested and stained with Annexin V, anti–H-2Kd mAb, and either anti–CD4 or anti–CD8 mAb. Annexin V staining of donor CD4+ or CD8+ T cells, gated as H-2Kd-negative, CD4+, or CD8+, is shown as a histogram. Percentage of Annexin V–positive cells in donor T cells is indicated in each panel. Data representative of 3 independent experiments are shown.
Impaired survival, but competent proliferation, of LIGHT-KO donor T cells in BDF1 recipient mice. (A) BDF1 mice were injected intravenously with CFSE-labeled spleen cells (5 × 107 cells) from WT or LIGHT-KO B6 mice. Division of host-reactive donor T cells was analyzed 2, 4, and 6 days after cell transfer. CFSE intensity of donor CD4+ or CD8+ T cells, gated as H-2Kd-negative CD4+, or CD8+, is shown as a histogram. The percentage of donor T cells with more than one division is indicated in each panel. (B) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT or LIGHT-KO B6 mice. Seven days later, spleen cells and liver lymphocytes from the BDF1 recipients were harvested and stained with Annexin V, anti–H-2Kd mAb, and either anti–CD4 or anti–CD8 mAb. Annexin V staining of donor CD4+ or CD8+ T cells, gated as H-2Kd-negative, CD4+, or CD8+, is shown as a histogram. Percentage of Annexin V–positive cells in donor T cells is indicated in each panel. Data representative of 3 independent experiments are shown.
Essential role of HVEM on donor T cells in their survival
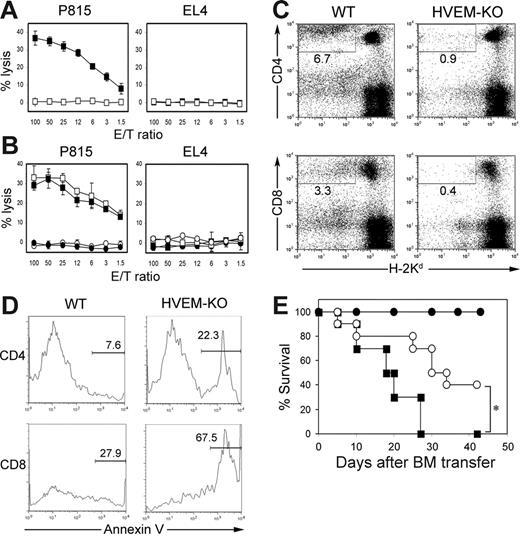
Among the 2 functional receptors of LIGHT, HVEM but not LTβR is expressed on T cells and suggested to be responsible for the T cell costimulatory effects of LIGHT.11 In order to directly address a role of HVEM in GVHD, we employed HVEM-KO lymphocytes as donor cells and transferred them into BDF1 recipient mice. No anti–host CTL activity was generated in the mice injected with HVEM-KO cells, in striking contrast to the ample CTL activity induced by a transfer of control lymphocytes (Figure 4A). Considering the expression and function of HVEM on broad immune populations, including DC, T, and B cells,32,33 we further dissected the functional role of HVEM on donor T and non–T cells using experiments similar to those described in Figure 1B. Anti–host CTL activity was completely abrogated when HVEM-KO T cells were transferred as donor cells, irrespective of the genotypes of cotransferred non–T cells, whereas a lack of HVEM on non–T cells did not hamper CTL generation when cotransferred with WT T cells (Figure 4B). These findings indicate that HVEM on donor T cells plays a crucial role in the generation of anti–host CTL in GVHD.
Indispensable role of HVEM in allo-CTL generation and donor T-cell survival. (A) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT (▪) or HVEM-KO (□) mice. Ten days later, spleen cells from the BDF1 mice were harvested and analyzed for the CTL activity against P815 and EL4, as shown in Figure 1. (B) T cells (2 × 107 cells) and non–T cells (4 × 107 cells) purified from spleen cells of WT or HVEM-KO mice were mixed as follows: WT T cells plus WT non–T cells (□), WT T cells plus HVEM-KO non–T cells (▪), HVEM-KO T cells plus WT non–T cells (○), HVEM-KO T cells plus HVEM-KO non–T cells (•). These combined cells were injected intravenously into BDF1 mice. Ten days later, anti–host CTL activity from recipient spleen cells were analyzed. (C-D) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT or HVEM-KO mice. Ten days later, spleens from the BDF1 recipients were harvested and stained with Annexin V and anti–H-2Kd mAb, along with either anti–CD4 or anti–CD8 mAb. (C) Percentage of donor (H-2Kd-negative) CD4+ or CD8+ T cells in total spleen lymphocytes is shown. (D) Percentage of Annexin V–positive cells in donor CD4+ or CD8+ T cells is shown. (A-D) Results shown in are representative of 3 independently performed experiments. (E) BALB/c mice (n = 10 in each group) were exposed to lethal-dose irradiation (10 Gy) followed by intravenous injection of 5 × 106 T cell–depleted B6 BM cells alone (•) or together with 1 × 106 B6 WT (▪) or HVEM-KO (○) T cells. The survival was monitored daily. *P = .005.
Indispensable role of HVEM in allo-CTL generation and donor T-cell survival. (A) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT (▪) or HVEM-KO (□) mice. Ten days later, spleen cells from the BDF1 mice were harvested and analyzed for the CTL activity against P815 and EL4, as shown in Figure 1. (B) T cells (2 × 107 cells) and non–T cells (4 × 107 cells) purified from spleen cells of WT or HVEM-KO mice were mixed as follows: WT T cells plus WT non–T cells (□), WT T cells plus HVEM-KO non–T cells (▪), HVEM-KO T cells plus WT non–T cells (○), HVEM-KO T cells plus HVEM-KO non–T cells (•). These combined cells were injected intravenously into BDF1 mice. Ten days later, anti–host CTL activity from recipient spleen cells were analyzed. (C-D) BDF1 mice were injected intravenously with 5 × 107 spleen cells from WT or HVEM-KO mice. Ten days later, spleens from the BDF1 recipients were harvested and stained with Annexin V and anti–H-2Kd mAb, along with either anti–CD4 or anti–CD8 mAb. (C) Percentage of donor (H-2Kd-negative) CD4+ or CD8+ T cells in total spleen lymphocytes is shown. (D) Percentage of Annexin V–positive cells in donor CD4+ or CD8+ T cells is shown. (A-D) Results shown in are representative of 3 independently performed experiments. (E) BALB/c mice (n = 10 in each group) were exposed to lethal-dose irradiation (10 Gy) followed by intravenous injection of 5 × 106 T cell–depleted B6 BM cells alone (•) or together with 1 × 106 B6 WT (▪) or HVEM-KO (○) T cells. The survival was monitored daily. *P = .005.
HVEM-KO donor T cells undergo massive apoptosis after transfer into the recipient mice and result in a significant decrease of surviving donor T cells (Figure 4C-D). These results concur with the findings in LIGHT-KO donor cells, suggesting that HVEM is a receptor responsible for LIGHT effects on donor T-cell survival. We also evaluated the severity of GVHD when HVEM-KO donor T cells are employed in the fully MHC-mismatched BM transfer model. Survival of recipient mice transferred with HVEM-KO cells was significantly prolonged compared to those injected with WT cells (Figure 4E), highlighting an essential role of donor-derived HVEM in GVHD pathogenesis.
Immunotherapy of GVHD by antagonistic anti–HVEM mAb
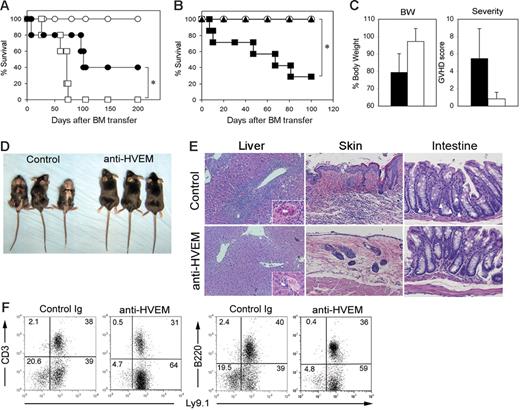
To validate the effects observed in LIGHT-KO or HVEM-KO mice and further extend our findings to the treatment of GVHD with a potential application in the clinical setting, we developed anti–HVEM mAbs interfering with LIGHT-HVEM interactions. One of these mAbs, designated LBH1, was used for further studies. LBH1 abrogates LIGHT-HVEM interactions while it does not deliver a costimulatory signal when used in immobilized form (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article), indicating that LBH1 is an antagonistic mAb. Therapeutic efficacy of LBH1 on GVHD was investigated by 2 allogeneic BM transfer models. First, lethally irradiated BDF1 mice were injected with T cell–depleted BM cells together with T cells from B6 mice and subsequently were treated with either LBH1 or control IgG. In this MHC-mismatched model, recipient mice treated with control IgG succumbed to GVHD by day 75, whereas 40% of the mice treated with LBH1 survived more than 200 days (Figure 5A). In the second model, GVHD was induced by MHC-matched, miHA-mismatched BM transfer. Lethally irradiated B6 mice were injected with T cell–depleted BM cells plus T cells from C3H.SW mice and were further treated with either control IgG or LBH1. In contrast to less than 30% survival in the recipient mice treated with control IgG, all the mice treated with LBH1 survived more than 100 days (Figure 5B). LBH1-treated mice showed significantly less body weight loss and improved systemic GVHD scores compared with those treated with control IgG (Figure 5C). After 60 days of BM transfer, control IgG-treated mice displayed a hunched posture and developed severe GVH skin lesions associated with alopecia, crusting, and erosion formation, whereas none of the mice treated with LBH1 exhibited these symptoms (Figure 5D). Histologic analysis revealed massive inflammatory cell infiltration of the portal tracts and bile duct injury in the livers of control IgG-treated mice but not those treated with LBH1 (Figure 5E). Skin of the control IgG-treated mice showed epidermal hyperplasia, thickening of the dermis, loss of hair follicles, and profound cellular infiltration, whereas LBH1 treatment prevented such changes. Further histologic evidence of GVHD was shown by the significant number of apoptotic cells in the intestinal crypt epithelium seen in recipient mice treated with control IgG but not LBH1. In flow cytometric analysis using Ly9.1, which is a cellular marker expressed on C3H.SW but not B6 mice, hematopoietic cells in the LBH1-treated mice were almost completely replaced by donor cells (Figure 5F), indicating an accelerated donor hematopoietic chimerism by this therapy. In addition, there was no anti–host CTL activity detected in these long-term surviving mice after LBH1 treatment (data not shown). Taken together, our results suggest that blockade of the LIGHT-HVEM pathway by antagonistic anti–HVEM mAb effectively ameliorates GVHD associated with allogeneic BM transplantation.
Treatment of mouse GVHD by antagonistic anti–HVEM mAb. (A) BDF1 mice (n = 5 in each group) were exposed to lethal-dose irradiation (12 Gy) followed by intravenous injection of 5 × 106 T cell–depleted B6 BM cells alone (○) or together with 3 × 106 B6 T cells. In the groups receiving T-cell transfer, the mice were treated intraperitoneally with 150 μg LBH1 (•) or control hamster IgG (□) on days 0, 3, and 6 after BM transfer. The survival of recipient mice was monitored daily. *P = .03. (B) B6 mice (n = 7 in each group) were exposed to lethal-dose irradiation (10 Gy) followed by intravenous injection of 4 × 106 T cell–depleted C3H.SW BM cells alone (○) or together with 3 × 107 C3H.SW spleen cells. In groups with spleen cell transfer, the mice were treated intraperitoneally with 100 μg LBH1 (▴) or control Ig (▪) on days 0, 5, 10, 15, 20, and 25. The survival of recipient mice was monitored daily. *P = .003. (C) On day 60 after BM transfer under the same conditions described for panel B, the percentage of body weight compared to the original (pre-BM transfer) and GVHD clinical scores of recipient mice treated with either LBH1 (open bar) or control IgG (filled bar) are shown as average ± SD. At the same time, gross appearance of recipient mice (D) and pathological analyses of liver, skin, and large intestine by H&E staining (magnification, 200 ×/400 × in liver and 200 × in others) (E) are shown. In the sections of colon, apoptotic epithelial cells are indicated by white arrowheads. (F) Spleen cells from the recipient mice surviving GVHD at least 40 days following injections of LBH1 or control Ig were examined for the expression of Ly9.1 and CD3 or B220 by flow cytometry. Percentages of cells in each quadrant are indicated. Representative data from 2 independent series of experiments are shown.
Treatment of mouse GVHD by antagonistic anti–HVEM mAb. (A) BDF1 mice (n = 5 in each group) were exposed to lethal-dose irradiation (12 Gy) followed by intravenous injection of 5 × 106 T cell–depleted B6 BM cells alone (○) or together with 3 × 106 B6 T cells. In the groups receiving T-cell transfer, the mice were treated intraperitoneally with 150 μg LBH1 (•) or control hamster IgG (□) on days 0, 3, and 6 after BM transfer. The survival of recipient mice was monitored daily. *P = .03. (B) B6 mice (n = 7 in each group) were exposed to lethal-dose irradiation (10 Gy) followed by intravenous injection of 4 × 106 T cell–depleted C3H.SW BM cells alone (○) or together with 3 × 107 C3H.SW spleen cells. In groups with spleen cell transfer, the mice were treated intraperitoneally with 100 μg LBH1 (▴) or control Ig (▪) on days 0, 5, 10, 15, 20, and 25. The survival of recipient mice was monitored daily. *P = .003. (C) On day 60 after BM transfer under the same conditions described for panel B, the percentage of body weight compared to the original (pre-BM transfer) and GVHD clinical scores of recipient mice treated with either LBH1 (open bar) or control IgG (filled bar) are shown as average ± SD. At the same time, gross appearance of recipient mice (D) and pathological analyses of liver, skin, and large intestine by H&E staining (magnification, 200 ×/400 × in liver and 200 × in others) (E) are shown. In the sections of colon, apoptotic epithelial cells are indicated by white arrowheads. (F) Spleen cells from the recipient mice surviving GVHD at least 40 days following injections of LBH1 or control Ig were examined for the expression of Ly9.1 and CD3 or B220 by flow cytometry. Percentages of cells in each quadrant are indicated. Representative data from 2 independent series of experiments are shown.
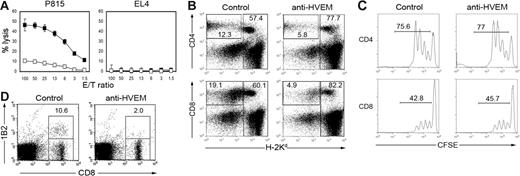
The B6 to BDF1 transfer model was used to investigate the immunologic mechanism of LBH1 therapy. Anti–host CTL activity was profoundly attenuated by the treatments with LBH1 (Figure 6A). The number of donor T cells was significantly decreased by LBH1 treatment without impairing their division kinetics (Figure 6B-C), suggesting that an analogous mechanism found in LIGHT-KO or HVEM-KO donor cells is operating. No significant decrease of the host immune population was detected (Figure 6B), indicating that the effects of LBH1 are not ascribed to the nonspecific depletion capacity of this mAb. Finally, by employing H-2Ld-reactive 2C TCR-transgenic T cells,29 we directly monitored the fate of host Ag-specific donor T cells after abrogation of the LIGHT-HVEM costimulatory system. LBH1 treatment of BDF1 recipient mice, which had been transferred with 2C T cells and WT B6 spleen cells, resulted in a significant reduction of 2C T cells in the recipient spleen (Figure 6D). This finding suggests that impaired survival of host Ag-specific donor T cells is responsible for the therapeutic effects of LIGHT-HVEM costimulatory blockade in GVHD.
Impaired survival of host-reactive donor T cells by anti–HVEM mAb treatment. (A) BDF1 mice were injected intravenously with 5 × 107 B6 spleen cells on day 0 and subsequently were treated with intraperitoneal administrations of 100 μg control hamster IgG (▪) or LBH1 (□) on days 0, 3, and 6. On day 10, spleen cells from the recipient mice were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b) by 51Cr-release assay. (B) Under the same conditions described for panel A, recipient spleen cells were stained on day 10 with anti–H-2Kd mAb, together with anti–CD4 or anti–CD8 mAb. Percentage of donor (H-2Kd-negative) CD4+ or CD8+ T cells as well as total host cells (H-2Kd-positive) are shown. (C) BDF1 mice were injected intravenously with CFSE-labeled B6 spleen cells (5 × 107 cells) followed by intraperitoneal injections of 100 μg control hamster IgG or LBH1 on days 0 and 3. CFSE dilution of donor (H-2Kd-negative) CD4+ or CD8+ T cells in the spleen was examined on day 4. (D) BDF1 recipient mice were injected intravenously with a mixture of spleen cells from 2C TCR-transgenic mice (1 × 107 cells) and WT B6 spleen cells (3 × 107 cells). On days 0 and 4, 100 μg control hamster IgG or LBH1 was administered intraperitoneally to the recipient mice. On day 7, the presence of 2C T cells in the host spleen was assessed by a staining with 1B2 clonotypic mAb and anti–CD8 mAb. Percentages of 2C T cells in CD8+ T cells are indicated. Representative results from 3 independent experiments are shown.
Impaired survival of host-reactive donor T cells by anti–HVEM mAb treatment. (A) BDF1 mice were injected intravenously with 5 × 107 B6 spleen cells on day 0 and subsequently were treated with intraperitoneal administrations of 100 μg control hamster IgG (▪) or LBH1 (□) on days 0, 3, and 6. On day 10, spleen cells from the recipient mice were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b) by 51Cr-release assay. (B) Under the same conditions described for panel A, recipient spleen cells were stained on day 10 with anti–H-2Kd mAb, together with anti–CD4 or anti–CD8 mAb. Percentage of donor (H-2Kd-negative) CD4+ or CD8+ T cells as well as total host cells (H-2Kd-positive) are shown. (C) BDF1 mice were injected intravenously with CFSE-labeled B6 spleen cells (5 × 107 cells) followed by intraperitoneal injections of 100 μg control hamster IgG or LBH1 on days 0 and 3. CFSE dilution of donor (H-2Kd-negative) CD4+ or CD8+ T cells in the spleen was examined on day 4. (D) BDF1 recipient mice were injected intravenously with a mixture of spleen cells from 2C TCR-transgenic mice (1 × 107 cells) and WT B6 spleen cells (3 × 107 cells). On days 0 and 4, 100 μg control hamster IgG or LBH1 was administered intraperitoneally to the recipient mice. On day 7, the presence of 2C T cells in the host spleen was assessed by a staining with 1B2 clonotypic mAb and anti–CD8 mAb. Percentages of 2C T cells in CD8+ T cells are indicated. Representative results from 3 independent experiments are shown.
Discussion
Although potential contributions of LIGHT-HVEM costimulatory pathway to GVHD pathogenesis have been suggested by the experiments using LTβR-Ig and HVEM-Ig decoy proteins,8,34,35 definitive evidence for the involvement of this pathway had not emerged. In this study, by using LIGHT-KO and HVEM-KO mice, we directly demonstrate an indispensable role for LIGHT-HVEM interaction in the promotion of host-reactive donor T-cell survival in GVHD. Without this costimulatory activation, anti–host T cells undergo progressive apoptosis, thus reducing the severity of GVHD. Importantly, we have developed an anti–HVEM antagonistic mAb capable of ameliorating GVHD in clinically relevant BM transfer models, thus proposing a novel strategy for GVHD treatment.
While most mechanistic details involving LIGHT costimulation have been elucidated using systems exogenously expressing LIGHT,9,10 the role of endogenous LIGHT-HVEM costimulation has not been fully defined at the cellular level. In the analyses of LIGHT-KO mice, multiple studies consistently recognize defective T-cell responses, particularly in CD8+ T cells.13–15 Regarding the underlying mechanisms, previous in vitro studies have proposed 2 distinct possibilities, an impaired proliferative capacity15 and a compromised survival without defects in cellular division.14 In the current study, our in vivo findings indicate that lack of endogenous costimulation of LIGHT-HVEM results in an increased apoptosis of alloreactive T cells without changing the kinetics of cellular divisions, agreeing with the latter possibility. These intriguing phenotypes of LIGHT-KO T cells are analogous to those observed in CD27-KO mice, in which influenza virus infection generates a reduced pool of antivirus effector T cells while sparing cell cycle activity.36,37 Furthermore, the mice deficient in 4-1BB ligand, OX40 ligand/OX40, or other prototypic costimulatory molecules in the TNF superfamily also demonstrate a predominant defect in T-cell survival during the late phase of activation and subtle or no changes in T-cell proliferation during the early activation phase.38–40 Conversely, deficiency of costimulatory molecules belonging to the Ig superfamily, such as CD28-KO and ICOS-KO mice, manifests an apparent defect in the early division of T cells in response to Ag stimulation.41–43 Thus, at the endogenous interaction levels, these 2 major costimulatory families seem to play nonredundant and perhaps complementary roles in the regulation of T-cell immunity. It is conceivable that distinct intracellular signaling pathways between TNF and Ig superfamily molecules are responsible for such nonoverlapping functions.
In our previous studies, combined administration of LTβR-Ig decoy protein and anti–CD40 ligand mAb inhibits GVHD by rendering anti–host CTL anergic.29 In the current study, however, our findings indicate that amelioration of GVHD by LIGHT-HVEM costimulatory blockade is ascribed to a progressive death of host-reactive donor T cells. These seemingly discordant observations could be explained by multifaceted effects of in vivo administration of LTβR-Ig. LTβR-Ig abrogates not only the LIGHT-HVEM costimulatory signal but also the LIGHT/LTβ-LTβR system, which regulates the homeostasis of DCs20,23 and NK/NKT cells,21,24 the microarchitecture of lymphoid organs,25,26 and the expression of inflammatory mediators.6 Furthermore, by cross-linking with Fc receptor in vivo, LTβR-Ig potentially delivers a reverse signal through LIGHT, costimulating T-cell responses by enhancing MAP kinase activation.44 In fact, preliminary experiments performing cotransfer of WT and LIGHT-KO B6 T cells into single BDF1 recipient mice show a preferential reduction of LIGHT-KO donor T cells (Figure S2), implying a role of LIGHT not merely as a ligand but also as a positive receptor in T-cell responses. Thus, findings in LTβR-Ig treatment do not necessarily reflect the outcomes of LIGHT-HVEM costimulatory blockade in GVHD. In contrast, our current approaches utilizing LIGHT-KO, HVEM-KO, and anti–HVEM blocking mAb rigorously address this point. Our studies thus give mechanistic insights into the previous findings on the therapeutic effects of LTβR-Ig in GVHD, such as MHC class II-disparate BM transplantation34 and skin GVHD induced by miHA-disparate BM transfer model.35
It has been suggested that the LIGHT-HVEM costimulatory signal can be delivered through T cell–T cell interaction when a TCR signal is provided in “trans” through immobilized anti–CD3 mAb.9 Cell-surface LIGHT expression on DC is diminished by their maturation3 while LIGHT expression on T cells is up-regulated by their activation,2 also implying that T cell–T cell interaction would dominate LIGHT-HVEM effects in the late phase of T-cell responses. In the current study using LIGHT-KO and HVEM-KO mice, we have elucidated indispensable roles of both LIGHT and HVEM on donor T cells in the survival of host-reactive T cells, also suggesting potential T cell–T cell interactions between LIGHT and HVEM on donor T cells. Our preliminary experiment indicated that host-derived LIGHT plays a minor role in the generation of anti–host CTL (Figure S3), also supporting this hypothesis. Thus, it is conceivable to expect that alloreactive donor T cells are primed by host or donor APCs, both essential for full-fledged development of GVHD,45,46 and simultaneously or subsequently costimulated by the LIGHT-HVEM interaction between donor T cells that can augment their survival. Interestingly, recent study indicated that OX40 ligand-OX40 signal through T cell–T cell interaction contributes to the survival of CD4+ T cells,47 suggesting a functional similarity in these pathways.
HVEM functions as not only the receptor for LIGHT costimulation but also the ligand for B- and T-lymphocyte attenuator (BTLA), which delivers inhibitory signals into T cells.48 A recent study using BTLA-deficient mice and an anti–BTLA neutralizing mAb revealed that HVEM-BTLA interactions inhibit allogeneic responses mediated by partial MHC-mismatch while stimulating those by full mismatch of MHC.49 Differential expression and functional capability of BTLA and PD-1 in different conditions may be a potential mechanism of these observations.49 In this regard, our anti–HVEM mAb employed in this study interferes with both LIGHT-HVEM and HVEM-BTLA interactions (Figure S1). Administration of this mAb resulted in a significant amelioration of GVHD induced by miHA disparity (Figure 5B-E). These findings may be interpreted as the results of LIGHT-HVEM costimulatory blockade, which overwhelms the effects of HVEM-BTLA blockade. Alternatively, a loss of LIGHT-HVEM costimulation might hinder the expression of BTLA, which is normally induced along with T-cell activation,50 so as to mask the effects of BTLA blockade. Interestingly, in contrast to inhibited allo-CTL activity and GVHD in HVEM-KO donor cells (Figure 4), HVEM-KO mice exhibit hyperreactive T-cell responses to the systemic administration of concanavalin A,51 suggesting that the LIGHT-HVEM-BTLA system governs T-cell immunity either positively or negatively, depending on the nature of the stimuli.
Although significant therapeutic effects of LTβR-Ig on various immunologic diseases have been demonstrated,17,18 it might lead to unwanted adverse effects caused by global abrogation of both LTβR and HVEM functions. In this regard, our current study focused on LIGHT-HVEM pathway and developed a novel antibody capable of ameliorating GVHD by selectively targeting this interaction while sparing LTβR functions. In addition, based on the previous reports that LIGHT-KO mice exhibit competent anti–virus immunity,14,52 therapeutic blockade of LIGHT-HVEM pathway will not accelerate opportunistic pathogen infections associated with the usage of nonspecific immunosuppressants in allogeneic hematopoietic stem cell transplantation. Thus, our current investigations open a new avenue for the treatment of hematologic disorders by regulating the LIGHT-HVEM costimulatory system.
Authorship
Contribution: Y.X. and K.T. participated in designing and performing the research and writing the manuscript. A.S.F., D.B.F, G.Z., S.J.F., S.A., and W.W.H. contributed vital new reagents. H.X. participated in performing the experiments. R.A.A. helped collect the data. L.C. analyzed data and wrote the paper. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare that they have no competing financial interests.
This study is in partial fulfillment of the requirement for the degree of Doctor of Philosophy for Y.X. at the Mayo Clinic College of Medicine.
Correspondence: Lieping Chen or Koji Tamada, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, 1550 Orleans St, CRB II, Rm 205, Baltimore, MD; lchen42@jhmi.edu or ktamada1@jhmi.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by National Institutes of Health grants CA106861 and CA85721 and by a Young Investigator Award from Alliance for Cancer Gene Therapy.
We thank Jennifer Osborne for manuscript editing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal