Abstract
Dendritic cells (DCs) are professional antigen-presenting cells that initiate the immune response by activating T lymphocytes. DCs express plasma membrane receptors for extracellular nucleotides named P2 receptors (P2Rs). Stimulation of P2Rs in these cells is known to cause chemotaxis, cytokine release, and cell death and to modulate LPS-dependent differentiation. Here we show that stimulation of the P2X7 receptor subtype (P2X7R) causes fast microvesicle shedding from DC plasma membrane. Vesicle release occurs from both immature and mature DCs; however, only vesicles from mature DCs, due to their previous exposure to LPS, contain IL-1β. Microvesicles, whether from immature or mature DCs, also contain caspase-1 and -3 and cathepsin D. They also express the P2X7R in addition to other P2Rs and known markers of immune cells such as major histocompatibility complex II (MHC II) and CD39. Activation of the P2X7R by extracellular ATP causes IL-1β release from the vesicle lumen. Previous studies demonstrated that high extracellular K+ inhibits IL-1β processing and release; here we show that high ionic strength reduces microvesicle shedding when compared with a low ionic strength medium but strongly increases microvesicle IL-1β loading.
Introduction
Dendritic cells (DCs) have become a focus of active investigation because of their role in immunity,1 autoimmunity,2 and cancer.3 DCs are the “true professional” antigen-presenting cells (APCs) and thus the most efficient initiators of primary immune responses.1,4 In peripheral tissues, immature DCs are specialized to capture and process antigens for major histocompatibility complex (MHC)–associated presentation. After antigen capture, DCs undergo a differentiation process known as “maturation.” This process is strongly enhanced by inflammatory mediators (eg, the microbial cell-wall component lipopolysaccharide [LPS]),1 neurotransmitters (eg, bradykinin),5 inflammatory cytokines (IL-1, GM-CSF, or TNFα),1 and Toll-like receptor ligands.6
Mature DCs have a reduced phagocytic and endocytic activity, a higher surface expression of costimulatory and MHC II molecules, an enhanced cytokine-secreting activity, and high ability to activate naive antigen-specific T cells.1,7 Mediators known to activate DCs have been classified into primary and feedback danger signals. Primary danger signals are derived from any injured or stressed cells, whereas feedback danger signals are released by the DCs themselves or by other cell types that have been stimulated by activated APCs.8
Nucleotides (ATP, UTP, ADP, UDP) are attracting interest as a new class of extracellular mediators, since they can be released into the extracellular milieu and activate a specific family of plasma membrane receptors named P2 (P2Rs). ATP is present within the cell cytosol at a concentration of up to 5 to 10 mM and is released by dying or injured cells or via as yet poorly defined nonlytic pathways.9,10 DCs express functional P2YR and P2XR11 and their function is modulated by the extracellular ATP concentration, thus ATP might act as an early danger signal for DCs.4,12,13
In DCs, ATP triggers increases in the intracellular calcium concentration ([Ca2+]i), cytoskeletal actin reorganization, and chemotaxis.14,15 Furthermore, chronic exposure to micromolar ATP concentrations induces a distorted maturation of human DCs impairing their capacity to initiate T-helper 1 (Th1) responses.16,17 On the contrary, acute stimulation with high levels of ATP triggers IL-1β and TNFα release and may even be cytotoxic.11
IL-1β is a key proinflammatory cytokine lacking a signal sequence needed for transfer through the classical endoplasmic reticulum–Golgi pathway and is released via an as yet unknown pathway.18 Interestingly, all cells capable of IL-1β release also express the P2X7 receptor (P2X7R) at a high level.19,20
Extracellular ATP is a very potent stimulus for IL-1β processing and release by acting at the P2X7R,21–23 although the mechanism involved is as yet obscure. MacKenzie et al24 showed that P2X7R activation in THP-1 macrophages triggers shedding of plasma membrane–derived, IL-1β–loaded microvesicles. A similar pathway for IL-1β release has also been described in microglia.25 However, in a recent elegant study, Andrei et al26 provided clear evidence that in human monocytes pro–IL-1β may also be targeted to secretory lysosomes and is then converted into mature IL-1β and secreted in response to extracellular ATP.26
DCs release IL-1β upon stimulation by T cells or bacterial products. Here we show that stimulation of DCs with ATP, or the more potent analog BzATP, causes microvesicle shedding from immature and mature DCs. However, whereas microvesicles from mature DCs contain a large amount of IL-1β, those from immature DCs contain trace amounts of this cytokine. Microvesicles from either DC type also contain caspase-1 and caspase-3 and cathepsin D and, besides MHC II molecules, express the P2X7R. Finally, increasing the ionic strength of the incubation medium substantially reduces both microvesicle shedding and IL-1β release. However under these conditions, microvesicle loading with IL-1β is increased, showing that fewer vesicles are shed but with a higher cytokine content. These findings show that microvesicle shedding is a relevant mechanism for IL-1β release also in DCs, the key cell type in the initiation of the immune response, and provide clear evidence that other factors relevant for inflammation (eg, caspases) are released via this route. Microvesicles might be a novel pathway for the delivery of boluses of immunomodulatory factors to target cells.
Materials and methods
Reagents
BzATP (2′,3′-(4-benzoyl-benzoyl)-ATP), oxidized ATP (oATP), bacterial endotoxin (LPS) from Escherichia coli (serotype 055:B5), benzamidine, and phenylmethylsulfonyl fluoride (PMSF) were from Sigma Aldrich (Milan, Italy). Ficoll and Percoll were purchased from Pharmacia Biotech AB (Uppsala, Sweden). KN-62 was from Calbiochem-Novabiochem (La Jolla, CA). Ac-Tyr-Val-Ala-Asp-chloromethylketone (Y-VAD–CMK) was from Bachem (Bubendorf, Switzerland). Bromoenol lactone (BEL) was from Alexis Biochemicals (San Diego, CA).
Cell culture
Human monocytes were from buffy coats (kindly provided by the blood bank, Arcispedale S. Anna, Ferrara, Italy) and purified by Ficoll and Percoll gradients.27 To induce monocyte-to-DC differentiation, cells were cultured in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) complemented with 10% heat-inactivated fetal calf serum (FCS), 1% nonessential amino acids, 1 mM sodium pyruvate, 0.050 mM 2-mercaptoethanol (all from Gibco, Milan, Italy), 200 U/mL penicillin, and 20 μg/mL streptomycin, in the presence of 100 ng/mL GM-CSF and 200 U/mL IL-4 (Peprotech, Rocky Hill, NJ). Cells were maintained in Petri dishes (Bibby Sterilin, Stone, United Kingdom) at 37°C in 5% CO2. After 6 days, DCs were separated from lymphocytes with monoclonal antibody–coated beads specific for CD19 and CD2 antigens (Dynal, Oslo, Norway). Mature DCs were obtained by an overnight incubation of immature DCs with 10 μg/mL LPS. The day after, medium was changed to LPS-free culture medium, and 24 hours later cells were used for experiments.
Microscopic analysis
DCs were detached from the Petri dishes by using 2-mM EDTA–containing cold PBS and then plated (2 × 105) onto 24-mm glass coverslips (Merck Eurolabs, Lutterworth, United Kingdom). Experiments were performed in a saline solution, heretofore referred to as standard saline solution, containing 125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM NaH2PO4, 20 mM Hepes, 5.5 mM glucose, 5 mM NaHCO3, and 1 mM CaCl2 (pH 7.4) with NaOH. Stimulation of cells in Ca2+-free conditions was performed in standard saline solution without CaCl2 and in the presence of 0.5 mM EGTA. DCs were also stimulated in Na+-free saline solutions, where Na+ was replaced by sucrose (300 mM sucrose, 1 mM K2HPO4, 1 mM MgSO4, 5.5 mM glucose, 20 mM Hepes, and 1 mM CaCl2 [pH 7.4] with KOH) or potassium (130 mM KCl, 1 mM MgSO4, 1 mM glucose, 5 mM KHCO3, 20 mM Hepes, and 1 mM CaCl2 [pH 7.4] with KOH). Morphologic changes and microvesicle shedding were analyzed by mounting coverslips in a thermostatted Leyden chamber (set at 38°C; model TC-202A; Medical Systems, Greenvale, NY) placed on the stage of an inverted Nikon Eclipse TE300 microscope equipped with a 100×/0.5-1.3 NA oil Iris objective (Nikon, Tokyo, Japan). The images were captured by a back-illuminated charge-coupled device (CCD) camera (Princeton Instruments, Trenton, NJ) using the Metamorph software (Universal Imaging, West Chester, PA). Cell-surface changes were measured with the LSM5 Image Examiner Program from Zeiss (Carl Zeiss SpA, Arese, Milano, Italy). DCs for electron microscope analysis were detached from flasks with 2-mM EDTA–containing cold PBS, and cell pellets were fixed with 2.5% glutaraldehyde. Samples for electron microscopy were processed by the Centro di Microscopia Elettronica of the University of Ferrara (Ferrara, Italy).
Vesicle purification
For characterization of cell-derived vesicles, immature and mature DCs were plated in the different incubation buffers at a concentration of 107/flask and challenged with 200 μM BzATP for 10 minutes at 37°C. After incubation the solution was collected and the protease inhibitors PMSF and benzamidine and 1 mM EDTA were added. The solution was then centrifuged at 160g for 5 minutes in a Heraeus Centrifuge equipped with a CWS T110-S342 rotor (Thermo Electron, Cologno Monzese, Italy) to eliminate detached cells and debris. Vesicles were then purified by centrifuging supernatants at 100 000g in a Beckman L8-M Ultracentrifuge equipped with a 70Ti rotor (Beckman Coulter SpA, Milano, Italy) for 90 minutes at 4°C. Microvesicle pellets were resuspended in 40 to 50 μL of a sucrose saline solution containing PMSF and benzamidine (protease inhibitors were not included in the preparations for measurement of caspase activity).
Quantitation of microvesicle release
For quantitation of microvesicle release in the absence of extracellular Ca2+, DCs were stimulated with nucleotides in Ca2+-free saline solution supplemented with 500 μM EGTA. Intracellular Ca2+ was chelated by treatment of the cells for 30 minutes with 5 μM BAPTA/AM (Molecular Probes, Eugene, OR). To inhibit P2X7R, DCs were preincubated for 2 hours with 600 μM oATP or treated with 100 nM KN-62, a P2X7R competitive inhibitor. Alternatively, DCs were also incubated overnight in the presence of an inhibitory monoclonal antibody raised against the extracellular domain of human P2X7R,28,29 kindly provided by Dr Gary Buell (Ares-Serono Pharmaceutical Research Institute, Geneva, Switzerland). Controls were run in the presence of irrelevant mouse IgG (Sigma Aldrich).
Cytokine and caspase measurement
Vesicles from immature and mature DCs were analyzed for IL-1β content using an R&D Systems enzyme-linked immunosorbent assay (ELISA) kit (Minneapolis, MI), according to the manufacturer's instructions. Caspase-1 and caspase-3 activities were measured as ability to hydrolyze the fluorogenic substrates Y-VAD-7-amino-4-trifluoromethyl coumarin and Z-DEVD-Rhodamine 110, respectively. The fluorometric assay kits were from MBL (Woburn, MA) for caspase-1 and from Molecular Probes (Leiden, The Netherlands) for caspase-3.
Western-blot analysis
Sample protein concentration was measured with the Bradford method. Samples were solubilized in O solution (10% wt/vol glycerol, 5% vol/vol 2-mercaptoethanol, 2.3% wt/vol SDS, 62.5 mM Tris, 0.003% bromophenol blue). Vesicle proteins and trichloroacetic acid–concentrated supernatants were separated on 4% to 12% Bis-Tris NuPAGE acrylamide gels (Life Technologies) and blotted for 2 hours onto a nitrocellulose paper (Schleicher and Schull Italia, Legnano, Italy). Aspecific binding sites were blocked for 1 hour with 10% skim milk in TBS buffer (10 mM Tris-Cl; 150 mM NaCl, pH 8.0) except for membranes developed for IL-1β detection, which were blocked for 1 hour with 2% nonfat milk. The rabbit polyclonal anti-P2X7 serum and the mouse monoclonal anti–cathepsin D were purchased from Calbiochem; the rabbit anti-P2Y2 antibody was from Sigma Aldrich; the monoclonal mouse anti-CD39 was from Alexis Biochemicals; the monoclonal anti–IL-1β antibody 3ZD was provided by the Biological Resources Branch of the National Cancer Institute–Frederick Cancer Research and Development Center (Bethesda, MD); the mouse anti–MHC II antibody was kindly provided by Prof Fabio Malavasi (University of Torino, Italy). The primary antibodies were diluted in TBS-t buffer (TBS plus 0.1% Tween 20), sometimes in the presence of 1% BSA as protein carrier, when indicated by manufacturers, and were incubated overnight. Finally, membranes were incubated for 1 hour in the presence of the secondary reagent: protein A (from Staphylococcus aureus) peroxidase linked for P2Y2, and goat anti–mouse HRP–conjugated antibody (BioRad, Hercules, CA) for IL-1β, CD39, MHC II, and cathepsin D.
Results
BzATP triggers microvesicle shedding from human monocyte-derived DCs
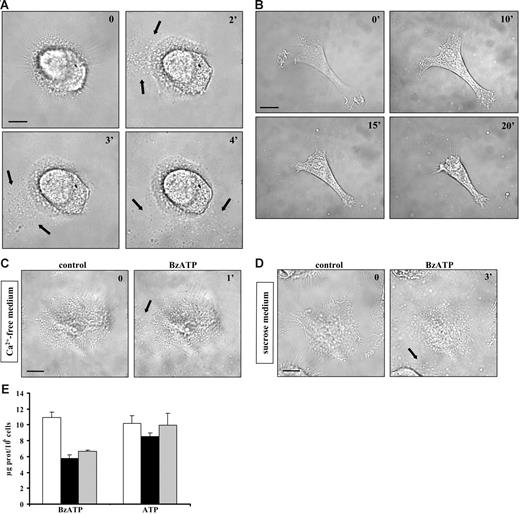
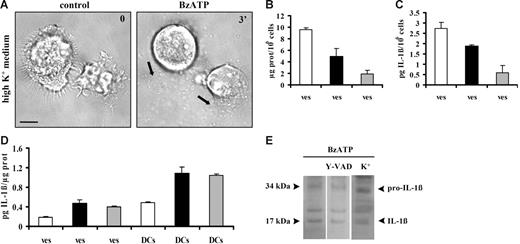
It has been reported that P2X7R stimulation triggers shedding of plasma membrane microvesicles containing the proinflammatory cytokine IL-1β from THP-1 monocytes and microglia.24,25 This process is thought to be relevant for the release of inflammatory mediators but it is not known whether it is also present in DCs, key immunomodulatory elements characterized by high P2X7R expression levels. Figure 1A (black arrows) shows that stimulation of immature DCs with the potent ATP analog BzATP induces massive microvesicle shedding. ATP is also a stimulus for microvesicle release but at a much higher concentration (3 mM; not shown). Microvesicle shedding starts between 15 and 120 seconds after addition of the stimulus, often originating at 1 pole of the cell (Video S1, available on the Blood website; see the Supplemental Video link at the top of the online article). After shedding, microvesicles quickly diffuse throughout the culture dish. Microvesicle shedding is paralleled by dendrite retraction and dramatic cell shrinkage. Figure 1B shows an immature DC that undergoes up to 53% cell-surface reduction 20 minutes after stimulation with BzATP. Cell shrinkage is not followed by cell death for at least an hour (as evaluated by Tunel) and is reversible upon nucleotide removal (not shown).
Extracellular BzATP triggers microvesicle shedding, dendrite retraction, and cell shrinkage in human DCs. Immature monocyte-derived DCs seeded on a glass coverslip at a concentration of 105/mL were treated with BzATP at 37°C in standard saline solution (A-B); Ca2+-free, 500-μM EGTA–supplemented saline solution (C); or sucrose solution (D-E) and stimulated with either 30 (A) or 200 μM BzATP (B-D). Images were acquired at 5-second intervals with the Nikon Eclipse T-300 microscopy setup described in “Materials and methods,” under “Microscopic analysis.” Bar = 10 μm. In panel E, DCs were placed in culture flasks (see “Materials and methods”) and stimulated with BzATP (200 μM) or ATP (3 mM) in sucrose saline solution (□); in Ca2+-free, 500-μM EGTA–containing sucrose saline solution (▪); or in the absence of extracellular Ca2+, after incubation of the cells for 30 minutes in the presence of 5 μM BAPTA/am (⊡). Black arrows in A, C, and D indicate shed microvesicles. Data are averages ± SE of 3 experiments performed with cells from 3 different donors.
Extracellular BzATP triggers microvesicle shedding, dendrite retraction, and cell shrinkage in human DCs. Immature monocyte-derived DCs seeded on a glass coverslip at a concentration of 105/mL were treated with BzATP at 37°C in standard saline solution (A-B); Ca2+-free, 500-μM EGTA–supplemented saline solution (C); or sucrose solution (D-E) and stimulated with either 30 (A) or 200 μM BzATP (B-D). Images were acquired at 5-second intervals with the Nikon Eclipse T-300 microscopy setup described in “Materials and methods,” under “Microscopic analysis.” Bar = 10 μm. In panel E, DCs were placed in culture flasks (see “Materials and methods”) and stimulated with BzATP (200 μM) or ATP (3 mM) in sucrose saline solution (□); in Ca2+-free, 500-μM EGTA–containing sucrose saline solution (▪); or in the absence of extracellular Ca2+, after incubation of the cells for 30 minutes in the presence of 5 μM BAPTA/am (⊡). Black arrows in A, C, and D indicate shed microvesicles. Data are averages ± SE of 3 experiments performed with cells from 3 different donors.
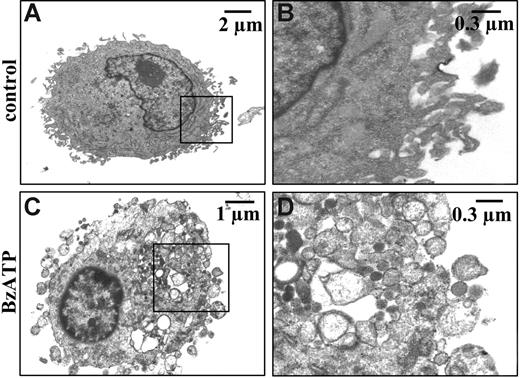
As shown in Figure 1C, chelation of extracellular Ca2+ does not fully prevent P2X7-dependent microvesicle release. Likewise, microvesicle shedding is not inhibited by incubation of the cells in a medium where Na+ is replaced by sucrose (Figure 1D). We observed that microvesicle recovery and further biochemical analysis are much facilitated when microvesicles are shed in low ionic strength (sucrose medium) as opposed to standard medium, thus most of the following experiments are performed in sucrose medium, if not otherwise indicated. In Figure 1E, we quantitatively compare microvesicle release under different conditions of Ca2+ chelation. In the virtual absence of extracellular Ca2+ (no Ca2+ added, 0.5 mM EGTA; Figure 1E ▪), microvesicle release is decreased by 50% with BzATP, but not ATP, as a stimulus. Chelation of intracellular Ca2+ with BAPTA/AM does not further reduce microvesicle shedding. The total amount of microvesicles released in sucrose buffer is rather variable, depending on the donor, and averages 16 ± 6 μg protein/106 cells (average of 17 different donors). The threshold of BzATP, which induces microvesicle release, is between 10 and 200 μM, depending on the donor sensitivity. DCs matured with LPS and stimulated with BzATP release about the same amount of microvesicles as immature DCs (immature DCs 18 ± 7 μg protein/106 cells vs mature DCs 15 ± 6 μg protein/106 cells; averages of 7 different donors). Electron microscopy confirmed that P2X7 stimulation causes a dramatic process of microvesicle budding from the plasma membrane as well as cytoplasmic vacuolization. Microvesicles are very heterogenous in size, ranging between 100 and 1000 nm (Figure 2).
Electron microscopy analysis of BzATP-stimulated DCs. Immature DCs in sucrose solution at a concentration of 106/mL at 37°C were left untreated (A-B) or stimulated with 200 μM BzATP (C-D) for 10 minutes. Cells were then detached from flasks, fixed with glutaraldehyde, and processed for electron microscopy. Morphometric analysis indicated a microvesicle size in the range between 100 nm and 1 μm.
Electron microscopy analysis of BzATP-stimulated DCs. Immature DCs in sucrose solution at a concentration of 106/mL at 37°C were left untreated (A-B) or stimulated with 200 μM BzATP (C-D) for 10 minutes. Cells were then detached from flasks, fixed with glutaraldehyde, and processed for electron microscopy. Morphometric analysis indicated a microvesicle size in the range between 100 nm and 1 μm.
Microvesicle shedding is partially P2X7R dependent
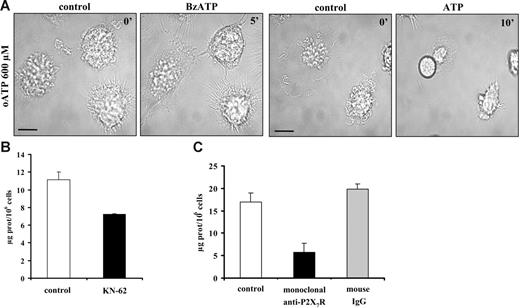
Microvesicle shedding has been shown to be dependent on activation of the P2X7R subtype.24,25 In support of this observation, we found that DC microvesicle shedding is fully blocked by the covalent P2XR blocker oATP, whether the stimulus is BzATP or ATP (Figure 3A). The competitive inhibitor KN-62 also inhibits vesicle release but to a lesser extent (about 35% decrease of microvesiculation; Figure 3B ▪). To better clarify the role of the P2X7R in microvesicle shedding, we used an inhibitory monoclonal antibody raised against the extracellular domain of this receptor.28,29 As shown in Figure 3C (▪), this antibody reduces microvesicle release substantially but not entirely (by about 70%). Finally, UTP and ADP, which are inactive at P2X7R, are unable to cause microvesicle shedding (not shown).
Oxidized ATP, but not KN-62, prevents microvesicle shedding. Immature DCs in standard saline solution were seeded on a glass coverslip at 37°C at a concentration of 105/mL and were preincubated for 2 hours with the covalent P2XR blocker oATP prior to stimulation with 100 μM BzATP or 3 mM ATP (A). Image acquisition and analysis were as in Figure 1. Blocking of P2X7R was also run by supplementing sucrose saline solution with 100 nM KN-62 (B, ▪) or by overnight incubation of DCs with 10 μg/mL of a monoclonal anti-P2X7R (C, ▪). The negative control was run in the presence of irrelevant mouse IgG (⊡). Bar = 10 μm. Data are averages ± SE of 3 experiments performed with cells from 3 different donors.
Oxidized ATP, but not KN-62, prevents microvesicle shedding. Immature DCs in standard saline solution were seeded on a glass coverslip at 37°C at a concentration of 105/mL and were preincubated for 2 hours with the covalent P2XR blocker oATP prior to stimulation with 100 μM BzATP or 3 mM ATP (A). Image acquisition and analysis were as in Figure 1. Blocking of P2X7R was also run by supplementing sucrose saline solution with 100 nM KN-62 (B, ▪) or by overnight incubation of DCs with 10 μg/mL of a monoclonal anti-P2X7R (C, ▪). The negative control was run in the presence of irrelevant mouse IgG (⊡). Bar = 10 μm. Data are averages ± SE of 3 experiments performed with cells from 3 different donors.
Microvesicles from mature DCs contain IL-1β
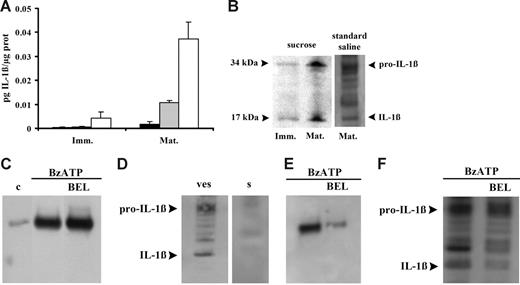
Microvesicle release could be a general pathway for release of cytoplasmic proteins, such as IL-1β, that lack leader sequences and therefore might also have a role in IL-1β release from DCs. It was previously demonstrated that ATP induces a fast IL-1β release from DCs that is compatible with the fast microvesicle shedding,11 therefore we investigated whether DC microvesicles contain IL-1β. In the experiment shown in Figure 4A, microvesicles were collected from the supernatants of immature (Imm) or mature (Mat) DCs and then assayed for IL-1β content before and after freeze-thawing. The microvesicle-free supernatants (Figure 4A ▪) from immature or mature DCs contain no or very little IL-1β. Intact microvesicles (Figure 4A ⊡) from immature DCs also show little IL-1β immunoreactivity, whereas a slightly higher IL-1β content is found in the intact microvesicles from mature DCs. Freeze-thawing (Figure 4A □) causes a large release of immunoreactive IL-1β that is much higher in the microvesicles derived from mature compared with immature DCs. This suggests that IL-1β is contained within the microvesicles and that immunoreactive IL-1β found in intact microvesicles is likely due to leakage from damaged microvesicles. Microvesicular content of IL-1β is confirmed by Western blot (Figure 4B). Western blot also confirms the higher cytokine content of microvesicles derived from mature DCs. IL-1β is synthesized as a precursor molecule (pro–IL-1β, 31-34 kDa), which is processed upon secretion into the mature form (17-18 kDa) by the protease caspase-1. Figure 4B shows that microvesicles contain IL-1β in both the procytokine and the mature, biologically active form, as previously reported by MacKenzie et al.24 Both IL-1β forms are present in microvesicles whether DC stimulation was performed in low or high salt solution. Andrei et al26 recently showed that in human monocytes IL-1β colocalizes with lysosomal markers and is secreted alongside with cathepsin D. Thus, we checked whether cathepsin D is also released in DCs in response to ATP together with IL-1β. As shown in Figure 4C, BzATP stimulation of DCs incubated in standard saline causes accumulation of cathepsin D into the supernatants. Similar results are obtained if stimulation is performed in sucrose medium (not shown). However, as reported for incubation in sucrose medium (Figure 4A), we were also unable to detect IL-1β in the supernatants of DCs incubated in standard saline (Figure 4D), indicating that if release of soluble IL-1β does occur, it must be quantitatively much less than that of cathepsin D. We then investigated whether cathepsin D is contained in the shed microvesicle fraction. Figure 4E shows that this hydrolytic enzyme can indeed be detected in the microvesicle fraction together with IL-1β (see also Figure 4F). Treatment with BEL, an inhibitor of calcium-independent phospholipase A2 previously reported to block pro–IL-1β processing without affecting IL-1β and cathepsin D secretion, has little effect on release of cathepsin D into the supernatants (Figure 4C) but strongly decreases the cathepsin D microvesicle content (Figure 4E). Due to the frustratingly low amount of soluble IL-1β (Figure 4D), we were unable to check the effect of BEL on the IL-1β content of the supernatants; however, we did detect a small effect on the microvesicle content of mature IL-1β (Figure 4F).
Microvesicles contain both immature and mature IL-1β. In panel A, immature (Imm) and mature (Mat) DCs were seeded in culture flasks at a concentration of 106/mL and treated with 200 μM BzATP at 37°C for 10 minutes in sucrose solution. Interleukin-1β was measured in vesicle-free DC supernatant (▪), intact microvesicles (⊡), and microvesicles lysed by 3 freeze-thawing cycles (□). Data are averages ± SE of 3 experiments from 3 different donors each performed in triplicate. Interleukin-1β content was also assessed by Western blot in microvesicles from immature and mature DCs treated with BzATP in sucrose or in standard saline (B); in panel D, secretion of the cytokine was analyzed in the supernatant (s) of mature DCs treated with BzATP in standard saline; microvesicle content of IL-1β is shown for comparison (ves). Release of cathepsin D from mature DCs has been assessed in supernatants and vesicles (C and E, respectively) in the absence or presence of 20 μM BEL (c indicates control). Panel F shows the effect of BEL on microvesicle IL-1β content. Cells were incubated in the presence of BEL in RPMI 1640 medium for 1 hour at 37°C.
Microvesicles contain both immature and mature IL-1β. In panel A, immature (Imm) and mature (Mat) DCs were seeded in culture flasks at a concentration of 106/mL and treated with 200 μM BzATP at 37°C for 10 minutes in sucrose solution. Interleukin-1β was measured in vesicle-free DC supernatant (▪), intact microvesicles (⊡), and microvesicles lysed by 3 freeze-thawing cycles (□). Data are averages ± SE of 3 experiments from 3 different donors each performed in triplicate. Interleukin-1β content was also assessed by Western blot in microvesicles from immature and mature DCs treated with BzATP in sucrose or in standard saline (B); in panel D, secretion of the cytokine was analyzed in the supernatant (s) of mature DCs treated with BzATP in standard saline; microvesicle content of IL-1β is shown for comparison (ves). Release of cathepsin D from mature DCs has been assessed in supernatants and vesicles (C and E, respectively) in the absence or presence of 20 μM BEL (c indicates control). Panel F shows the effect of BEL on microvesicle IL-1β content. Cells were incubated in the presence of BEL in RPMI 1640 medium for 1 hour at 37°C.
Pro–IL-1β processing is thought to occur at the same moment as release, thus we investigated whether microvesicle release is caspase dependent and whether microvesicles contain caspase activity. Neither the caspase-1–selective inhibitor Y-VAD nor the wide-range inhibitor Z-VAD is able to prevent microvesicle release (not shown). However, microvesicles from immature and mature DCs contain caspase-1 and -3 activity. Microvesicle content of caspase-1 is slightly but significantly (P < .05, 2-tailed Student t test for paired samples) higher in immature compared with mature DCs (Figure 5A-C), whereas caspase-3 content does not significantly differ between DCs at the 2 stages of maturation (Figure 5D-F). In order to check whether IL-1β delivery to microvesicles is caspase-1 dependent, incubation of LPS-treated DCs was performed in the presence of 100 μM Y-VAD, but little inhibition of cytokine release is detected by ELISA (not shown) or immunoblot (Figure 6E).
Active caspase-1 and -3 are present inside microvesicles from immature and mature DCs. Immature and mature DCs from 3 different donors were seeded in culture flasks at a concentration of 106/mL and stimulated with 200 μM BzATP in sucrose solution at 37°C. Microvesicles from both immature (○) and mature (□) DCs were recovered and assayed for caspase-1 (A-C) or caspase-3 (D-F) content.
Active caspase-1 and -3 are present inside microvesicles from immature and mature DCs. Immature and mature DCs from 3 different donors were seeded in culture flasks at a concentration of 106/mL and stimulated with 200 μM BzATP in sucrose solution at 37°C. Microvesicles from both immature (○) and mature (□) DCs were recovered and assayed for caspase-1 (A-C) or caspase-3 (D-F) content.
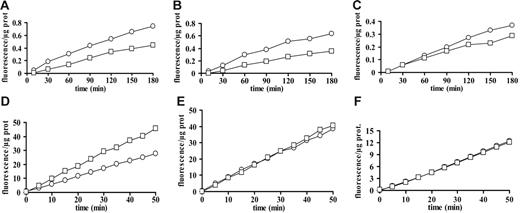
Microvesicle shedding is enhanced in sucrose solution, whereas microvesicle IL-1β loading is facilitated in the presence of high extracellular K+. In panel A, mature DCs were stimulated with 60 μM BzATP in high-K+ medium. In all other experiments, DCs, whether in high-K+ medium, sucrose solution, or standard saline, were stimulated with 200 μM BzATP. Bar = 10 μm. Panel B shows microvesicle protein content (ves) of supernatants from DCs incubated in sucrose solution (□), high-K+ medium (▪), or standard saline (⊡). Panel C shows vesicular IL-1β release/106 cells from DCs incubated in the 3 different incubation media (bars as in panel B). In panel D, DCs were incubated in sucrose, high-K+ solution, or standard saline solution and stimulated with BzATP. Microvesicles were recovered and IL-1β content of microvesicles (ves) and DCs (DCs) was expressed as pg of cytokine/μg of microvesicle protein or pg of cytokine/μg of cellular protein, respectively (bars as in panel B). Data are averages ± SE of 3 experiments performed with cells from 3 different donors. In panel E, DCs were treated with BzATP in standard saline solution after overnight incubation in the presence or the absence of the irreversible caspase-1 inhibitor Y-VAD–CMK (100 μM). Mature DCs from the same donor were also treated with BzATP in high-K+ medium. The purified microvesicles were analyzed by Western blot for the presence of IL-1β. Black arrows in A indicate shed microvesicles.
Microvesicle shedding is enhanced in sucrose solution, whereas microvesicle IL-1β loading is facilitated in the presence of high extracellular K+. In panel A, mature DCs were stimulated with 60 μM BzATP in high-K+ medium. In all other experiments, DCs, whether in high-K+ medium, sucrose solution, or standard saline, were stimulated with 200 μM BzATP. Bar = 10 μm. Panel B shows microvesicle protein content (ves) of supernatants from DCs incubated in sucrose solution (□), high-K+ medium (▪), or standard saline (⊡). Panel C shows vesicular IL-1β release/106 cells from DCs incubated in the 3 different incubation media (bars as in panel B). In panel D, DCs were incubated in sucrose, high-K+ solution, or standard saline solution and stimulated with BzATP. Microvesicles were recovered and IL-1β content of microvesicles (ves) and DCs (DCs) was expressed as pg of cytokine/μg of microvesicle protein or pg of cytokine/μg of cellular protein, respectively (bars as in panel B). Data are averages ± SE of 3 experiments performed with cells from 3 different donors. In panel E, DCs were treated with BzATP in standard saline solution after overnight incubation in the presence or the absence of the irreversible caspase-1 inhibitor Y-VAD–CMK (100 μM). Mature DCs from the same donor were also treated with BzATP in high-K+ medium. The purified microvesicles were analyzed by Western blot for the presence of IL-1β. Black arrows in A indicate shed microvesicles.
Clear evidence from experiments performed in monocyte/macrophages supports a role for K+ efflux in the stimulation of IL-1β release triggered via the P2X7R.30–32 As shown in Figure 6A, incubation in K+ medium does not prevent BzATP-stimulated microvesicle release. However, the amount of microvesicles released from LPS-treated DCs in high K+ (Figure 6B ▪) and standard saline (Figure 6B ⊡) is strongly reduced compared with that obtained in sucrose containing medium (Figure 6B □). Accordingly, total IL-1β released via microvesicles is higher in sucrose than in high-K+ or standard medium (Figure 6C). Nevertheless, both cellular and microvesicle IL-1β content (normalized to cellular and microvesicle protein concentration, respectively) are higher in DCs stimulated in high K+ medium or in standard saline solution than in sucrose solution (Figure 6D).
Microvesicle shedding and cytokine release (measured by ELISA) are prevented neither by incubation of DCs in the presence of the irreversible caspase-1 inhibitor Y-VAD nor by stimulation in high-K+ medium, another experimental condition in which caspase-1 activation is inhibited. Therefore, we checked if the release of the mature form of IL-1β is prevented in these experimental conditions. As shown in Figure 6E, the 17-kDa form of the cytokine is contained to about the same level within microvesicles released either from Y-VAD–treated DCs or from cells stimulated in high-K+ saline solution. The small amount of total IL-1β released by DCs makes it difficult to unequivocally assess whether in the presence of Y-VAD or in high-K+ medium there is a statistically significant reduction of the 17-kDa versus the 34-kDa form. This result is rather surprising, as the inhibitory effect of these manipulations is well known.23,26 We may hypothesize that in DCs caspase-1 is not easily accessible to Y-VAD or to inhibition by high K+ (which may depend on the intracellular location of the enzyme). Alternatively, it may be that since the microvesicles contain an active caspase-1 (Figure 5), IL-1β is processed during the time needed for microvesicle harvesting and centrifugation, as during these manipulations microvesicles are transferred to low-K+–Y-VAD–free buffer, although the Y-VAD form used (Y-VAD–CMK) should in principle cause irreversible caspase-1 inhibition. Finally, we wish to stress that, as shown in Figure 6D, overall total DC content of IL-1β is increased in high-K+ medium, as shown by ELISA.
These results suggest that in DCs incubation in media at high ionic strength indirectly down-modulates IL-1β release by decreasing microvesicle shedding. However, high ionic strength increases both intracellular IL-1β accumulation and microvesicle loading. The net result of manipulation of the ionic composition of the incubation medium is that in sucrose medium DCs release a higher amount of microvesicles with a lower IL-1β content, whereas in high-K+ or standard Na+-containing saline fewer microvesicles are released but with a higher IL-1β content.
ATP triggers IL-1β release from microvesicles
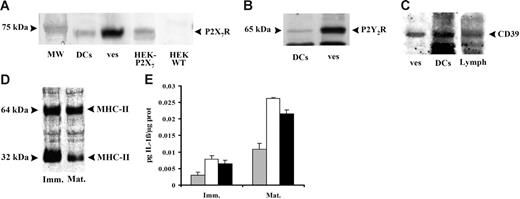
The microvesicle pathway allows rapid export of IL-1β, but how do the vesicles release their cargo? It is obvious that, as shown in Figure 4, so far the microvesicles are intact and very little bioactive IL-1β is available to the target cell. We found that DCs and microvesicles contain the P2X7R (Figure 7A) and other receptors involved in purinergic signaling such as the P2Y2R (Figure 7B) and the ecto-ATPase CD39 (Figure 7C). However, no relevant difference is present in the level of expression of these receptors between immature and mature DCs. We also found expression on both DCs and the microvesicles of the P2Y1R and the P2Y11R (not shown). Interestingly, microvesicles also express known immune cell markers such as MHC II (Figure 7D). These findings suggest an obvious mechanism for delivery of intravesicular IL-1β: lysis of vesicles by extracellular ATP. In Figure 7E, we show that incubation of microvesicles in the presence of cytolytic concentrations of ATP triggers an IL-1β release comparable to that caused by freeze-thawing. A similar mechanism for IL-1β export from microvesicles has been recently described by Bianco et al25 in microglia.
Expression of P2X7R confers to DC-derived microvesicles susceptibility to ATP-dependent lysis and triggers IL-1β release. Immature DCs were stimulated with 200 μM BzATP in sucrose saline solution and the expression of P2X7R (A), P2Y2R (B), and CD39 (C) were analyzed by Western blot both in untreated cells (DCs) and in microvesicles released (ves) after stimulation with 200 μM BzATP. The expression of MHC II (D) was also detected in microvesicles from immature (Imm) and mature (Mat) DCs. HEK-293 transfected with P2X7 cDNA and HEK293 wild type were used as positive and negative controls for P2X7R expression, respectively; human lymphocytes (Lymph) were used as positive control for CD39 expression. Panel E shows IL-1β content of intact microvesicles (⊡), microvesicles lysed by freeze thawing (□), or microvesicles treated with 3 mM ATP at 25°C for 20 minutes (▪). Data are averages ± SE of 3 experiments performed with cells from 3 different donors.
Expression of P2X7R confers to DC-derived microvesicles susceptibility to ATP-dependent lysis and triggers IL-1β release. Immature DCs were stimulated with 200 μM BzATP in sucrose saline solution and the expression of P2X7R (A), P2Y2R (B), and CD39 (C) were analyzed by Western blot both in untreated cells (DCs) and in microvesicles released (ves) after stimulation with 200 μM BzATP. The expression of MHC II (D) was also detected in microvesicles from immature (Imm) and mature (Mat) DCs. HEK-293 transfected with P2X7 cDNA and HEK293 wild type were used as positive and negative controls for P2X7R expression, respectively; human lymphocytes (Lymph) were used as positive control for CD39 expression. Panel E shows IL-1β content of intact microvesicles (⊡), microvesicles lysed by freeze thawing (□), or microvesicles treated with 3 mM ATP at 25°C for 20 minutes (▪). Data are averages ± SE of 3 experiments performed with cells from 3 different donors.
Discussion
Release of intact vesicles is increasingly recognized as a major avenue for the delivery of extracellular signals or bioactive factors.24,33 Virtually all cells are able to release small-size vesicles (microvesicles) derived from the plasma membrane (microparticles) or from multivesicular endocytic compartments (exosomes).34,35 Microparticles have been described in detail especially in platelets,36 where they are thought to be involved in hemostasis, whereas exosomes, initially identified in neoplastic cells,37 have been most thoroughly characterized in immune cells.35 Release of microparticles and exosomes is constitutive but can also be triggered by ligands of plasma membrane receptors.38
A recent study by MacKenzie et al24 identified the P2X7R as a major stimulus for microvesicle shedding from the human macrophage cell line THP-1 and from HEK-293 cells transfected with the P2X7R. At about the same time, we and others showed that DCs express several P2Rs and the P2X7R at an unusually high level.11,14,39,40 Here we show that stimulation of DC P2X7R causes rapid and massive shedding of microvesicles of a rather heterogenous size (from small microvesicles with a diameter of about 100 nm to larger ones with a diameter up to 1 μm). The smaller microvesicles have a size compatible with the larger exosomes, whereas the size of the larger microvesicles is compatible with that of the microparticles. These microvesicles express MHC II molecules as well as the P2X7R, P2Y1R, P2Y2R, P2Y11R, and CD39, but we have not carried out a detailed biochemical analysis to fully characterize microvesicle surface markers.
The mechanism by which microvesicles are released is poorly known. Removal of extracellular Ca2+ was shown to abolish or drastically decrease microvesicle shedding triggered by BzATP or ATP from THP-1 macrophages or microglia.24,25 In DCs, we found that chelation of extracellular or intracellular Ca2+ only partially (by 50%) reduces vesicle shedding with BzATP as an agonist, whereas no inhibition at all is present with ATP as a stimulus. These rather surprising findings suggest that the intracellular machinery responsible for this event is rather more complex than previously thought. Additional Ca2+-independent signals such as activation of intracellular Rho-dependent kinases41,42 are likely to be involved in microvesicle as well as membrane bleb formation. The interplay between these pathways and Ca2+ are still to be fully clarified. An additional puzzling finding is that while previous reports24,25 established a key role for the P2X7R, we are unable to fully block microvesicle release with the potent P2X7R blocker KN-62. However, other P2X7R-dependent processes in DCs, such as plasma membrane depolarization or ethidium bromide uptake, were inhibited by KN-62 by only 35% to 40% (not shown). The other commonly used P2X7R blocker oATP completely abrogates microvesicle release, but this ATP analog is known to bind other plasma membrane receptors besides the P2X7R,43 thus it is of little help to rigorously identify a unique role for the P2X7R. To better clarify this issue we have used a blocking monoclonal antibody raised against the outer domain of this receptor.28,29 Even by using this highly specific reagent we are able to decrease microvesicle release by only 65% to 70%, which suggests that other P2Rs may have a role in this process. However, while other P2Rs may participate in microvesicle shedding, there are few doubts that the P2X7R remains the only P2R coupled to IL-1β processing and release, as shown by experiments performed in P2X7R-less cells and in P2X7R-knockout (KO) mice.22,44 A key question is whether microvesicle shedding is the main or the only route by which DCs release IL-1β. In THP-1 macrophages and in mouse microglia it appears that virtually all bioactive IL-1β is contained within microvesicles.24,25 This also seems to occur in DCs, however we wish to stress that DCs release modest amounts of this cytokine compared with macrophages and microglia (pg/106 versus tens of ng/106, respectively), thus making its accurate detection in the cell supernatants rather difficult. Therefore, we cannot exclude that IL-1β is also released in part via nonmicrovesicular pathways, for example via the late lysosomal pathway recently described by Andrei et al.26 As reported by these authors in human macrophages, we found that P2X7R stimulation causes cathepsin-D secretion, a marker of late lysosomes. However, we also detected cathepsin D in the microvesicle fraction, a finding not entirely surprising, as cathepsins were reported to associate with exosomes.45
Ability of mononuclear cells to release membranous vesicles either constitutively or in response to LPS stimulation has been known for a few years.46,47 Interest in microvesicles has mainly focused on their possible role in coagulation as a site of prothrombinase activity or as a mechanism for the dissemination of tissue factor. More recently it has also been suggested that microvesicles might be involved in the release of IL-1β, a cytokine lacking a leader sequence.24 Microvesicles released from monocytes expose phosphatidylserine on the outer leaflet, another factor that amplifies thrombin generation and thus reinforces the postulated function of shed microvesicles in blood coagulation.33 However, the observation that a key proinflammatory cytokine such as IL-1β is also contained in the vesicles expands the relevance of this process in immunity and suggests that it might be relevant in focal diseases (eg, atherosclerosis) in which the coagulation and inflammatory pathways are closely interrelated.
Previous works showed that microparticles expose CD11a/CD18 and CD14, which endows them with a cell adhesion potential.47 Thus, their cargo of coagulation factors and cytokines could be efficiently delivered to selected tissue or cellular targets. However, it is unclear how factors trapped to the lumen are released once they reach the target. Of course, if one postulates an intracellular site of action, then the lumenal factors may be released after the vesicles are endocytosed and digested by the target cells, but this would be an ineffective procedure if the site of action is extracellular.
Exposure of functional P2X7R affords an efficient mean for vesicle content release. Our data show that stimulation of the P2X7R with extracellular ATP causes microvesicle lysis and IL-1β release, suggesting a mechanism for lumenal content release based on P2X7R activation. Thus, extracellular ATP might participate in IL-1β release at 2 crucial steps: firstly at the level of microvesicles release, secondly at the level of delivery of microvesicle content.
One of the best-studied stimuli for microvesicle release from monocytes is LPS.46,47 This bacterial factor also causes membrane blebbing, another striking consequence of monocyte activation by ATP. The mechanism by which LPS causes these membrane phenomena is unknown, but based on the ability of LPS to cause ATP secretion from macrophages and microglia,48,49 an obvious mechanism would implicate the LPS-stimulated release of ATP followed by activation of the P2X7R. Interestingly, LPS does not cause microvesiculation in peripheral human lymphocytes,46 a cell type with low P2X7R expression.19
ATP release appears to be a widespread phenomenon, as it is triggered not only by LPS48 but also by membrane stretching,50 swelling,51 mechanical stimulation,52 cytokines,53 or endogenous bactericidal factors.54 ATP in the extracellular space might also serve the role of maturation/differentiation factor for DCs, as several laboratories have demonstrated that this nucleotide can induce an alternative pathway of DC maturation characterized by an up-regulation of costimulatory molecules but lack of IL-12 and inflammatory cytokines and chemokines.16,17,55,56 The 2 effects of ATP, the early activatory effect leading to fast secretion of IL-1β and the delayed modulatory effect leading to distorted DC maturation, have a different ATP dose requirement. The former is triggered by large increases in the extracellular ATP concentration (> 500 μM), whereas the latter occurs in the presence of ATP concentrations in the 100- to 250-μM range.16 Both the dose requirement and the pharmacology suggest that the P2 receptors involved are likely to be different: P2X7 for the fast activatory and P2Y11 for delayed modulatory response.
In conclusion, we have described a process whereby human DCs stimulated by extracellular ATP via the P2X7R disseminate into the pericellular space microvesicles containing IL-1β that can be released upon further incubation of the microvesicles in the presence of ATP. This mechanism might be relevant for release and targeting of cytokines during inflammation.
Authorship
Contribution: C.P. designed research, performed experiments, and analyzed data; D.F. designed research and analyzed data; P.C. performed experiments; E.A. performed experiments; D.S. contributed vital reagents; E.S. performed experiments; F.D.V. designed research, analyzed data, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Di Virgilio, Department of Experimental and Diagnostic Medicine, Section of General Pathology, University of Ferrara, I-44100 Ferrara, Italy; e-mail: fdv@unife.it.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants by the Italian Ministry of Education, University and Scientific Research (MIUR), the Italian Association for Cancer Research (AIRC), the Italian Space Agency (ASI), Telethon of Italy, and Institutional funds from the University of Ferrara.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal