Abstract
Defects in apoptosis mechanisms play important roles in malignancy and autoimmunity. Orphan nuclear receptor Nur77/TR3 has been demonstrated to bind antiapoptotic protein Bcl-2 and convert it from a cytoprotective to a cytodestructive protein, representing a phenotypic conversion mechanism. Of the 6 antiapoptotic human Bcl-2 family members, we found that Nur77/TR3 binds strongest to Bcl-B, showing selective reactivity with Bcl-B, Bcl-2, and Bfl-1 but not Bcl-XL, Mcl-1, or Bcl-W. Nur77 converts the phenotype of Bcl-B from antiapoptotic to proapoptotic. Bcl-B is prominently expressed in plasma cells and multiple myeloma. Endogenous Bcl-B associates with endogenous Nur77 in RPMI 8226 myeloma cells, where RNA interference experiments demonstrated dependence on Bcl-B for Nur77-induced apoptosis. Furthermore, a Nur77-mimicking peptide killed RPMI 8226 myeloma cells through a Bcl-B–dependent mechanism. Because Bcl-B is abundantly expressed in plasma cells and some myelomas, these findings raise the possibility of exploiting the Nur77/Bcl-B mechanism for apoptosis for eradication of autoimmune plasma cells or myeloma.
Introduction
Insufficient apoptosis is a causative or contributing factor to malignant and autoimmune diseases.1,2 Bcl-2 family proteins play important roles in controlling cell life and death decisions. The human genome contains at least 6 identified genes encoding antiapoptotic members of this protein family: Bcl-2 (BCL2), Bcl-XL (BCL2L1), Mcl-1 (MCL1), Bcl-W (BCL2L2), Bfl-1 (BCL2A1), and Bcl-B (BCL2L10).3 Recently, a novel mechanism of modulating Bcl-2 activity has been elucidated in which the phenotype of Bcl-2 is reversed from antiapoptotic to proapoptotic by binding to Bcl-2 of an orphan member of the nuclear receptor family, Nur77/TR3.4 Stimuli that affect phosphorylation of Nur77 can cause accumulation of this transcription factor in the cytosol,5,6 where it binds Bcl-2 at the surface of mitochondrial membranes.4 Evidence has been presented that Nur77 induces a profound conformational change in Bcl-2, causing it to expose a normally buried BH3 domain and thus allowing it to mimic the actions of proapoptotic Bcl-2 family proteins that use BH3-mediated dimerization to thwart the actions of antiapoptotic members of the family.4
In this report, we have investigated the interactions and effects of Nur77 on the entire family of antiapoptotic human Bcl-2 proteins. Our results show that Nur77 interacts selectively with Bcl-2, Bfl-1, and Bcl-B but not Bcl-XL, Mcl-1, and Bcl-W. Nur77 converts the phenotypes of Bfl-1 and Bcl-B from protector to killer, implying a similar mechanism as reported previously for Bcl-2. Because Nur77 appeared to bind strongest to Bcl-B among the Bcl-2 family members, we performed an in-depth analysis of Bcl-B, including assessing for the first time what types of cells express Bcl-B protein. We determined that plasma cells represent one of the few cell types that prominently express this protein in vivo in human tissues and observed that endogenous Nur77 interacts with endogenous Bcl-B in myeloma cell lines. The findings may have implications for understanding the biology of apoptosis regulation of plasma cells as well as for devising new therapeutic strategies for eliminating autoantibody-producing or malignant plasma cells.
Materials and methods
Informed consent was obtained in accordance with the Declaration of Helsinki. This study was approved by the institutional review board from each institution that participated.
Reagents and antibodies
Phorbol 12-myristate 13-acetate (TPA), ionomycin, sodium fluoride (NaF), sodium orthovanadate (Na3VO4), phenylmethylsulfonyl fluoride (PMSF), aprotinin, and leupeptin were purchased from Sigma-Aldrich (St Louis, MO). Benzyloxycarbonyl-Val-Ala-Asp (O-methyl)-fluoromethyl ketone (zVAD-fmk) was purchased from Bachem (King of Prussia, PA). Antibodies employed for antigen detection included polyclonal anti–Bcl-W antibody (Nventa, Vancouver, BC, Canada), anti-Myc–HRP (Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal rabbit anti-GST antibody produced in our laboratory, monoclonal anti–Bcl-2 antibody (DAKO, Carpinteria, CA), monoclonal anti-Hsp60 antibody (Nventa), monoclonal antitubulin antibody (Sigma), and monoclonal anti-GFP antibodies (Santa Cruz Biotechnology). A polyclonal antibody specific for Bcl-B (BR-49) was raised in rabbit using affinity-purified recombinant GST–Bcl-B protein as the immunogen. Commercial mouse monoclonal anti-CD138 antibody (Serotec, Raleigh, NC) was applied for a colocalization study.
Plasmid constructions and directed mutagenesis
pEGFP expression plasmids incorporating the N-terminal green fluorescent protein (GFP) epitope tag and encoding Nur77, Nur77-ΔDBD, Nur77-1-168, Nur77-DC1, and Nur77-ΔDBD-ΔDC1 proteins have been previously described.4 pcDNA3 expression plasmids incorporating the N-terminal Myc epitope tag and encoding Bcl-2, Bcl-B, Bcl-BΔTM, Bcl-XL, Bfl-1, Bcl-W, and Mcl-1 have been also previously described.7
Cell culture and transfections
HEK293T, HeLa, and COS-7 cells were cultured in DMEM high-glucose medium (Irvine Scientific, Santa Ana, CA) containing 10% fetal bovine serum (FBS), 100 units mL−1 penicillin, and 100 μg mL−1 streptomycin at 37°C. Transfection of HEK293T, HeLa, and COS-7 cells was performed using Lipofectamine PLUS reagent or Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RPMI 8226, U266B1, MM1-S, ARH-77, OPM-2, NCI-H929, Ramos, and Jurkat cells were cultured in RPMI high-glucose medium (Irvine Scientific) containing 10% FBS, 100 units mL−1 penicillin, and 100 μg mL−1 streptomycin at 37°C. Electroporation of RPMI 8226 cells was performed using Nucleofector system (Amaxa, Gaithersburg, MD) according to the manufacturer's instructions. Briefly, 2.5 × 106 cells were electroporated with plasmids encoding either GFP or GFP-Nur77ΔDBD proteins using nucleofector kit V and program G-015. Cells were then suspended in 2 mL RPMI 10% FCS media and cultured for 18 hours at 37°C.
Immunoblotting and immunoprecipitations
Immunoblotting was performed as described previously8 using cell lysates normalized for total protein content. For coimmunoprecipitations, cells were cultured in 50 μM zVAD-fmk to prevent apoptosis. Cells were suspended in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 20 mM EDTA, 50 mM NaF, 0.5% NP-40, 0.1 mM Na3VO4, 20 μg mL−1 leupeptin, 20 μg mL−1 aprotinin, 1 mM dithiothreitol, and 1 mM PMSF). Lysates (500 μL) were then incubated with 1 μg monoclonal anti-Myc antibody (Roche, Milan, Italy), polyclonal rabbit anti–Bcl-W antibody (Nventa), polyclonal rabbit anti–Bcl-2 antibody,9 or monoclonal anti-Nur77 antibody (R&D Systems, Minneapolis, MN) and 20 μL protein G–Sepharose (Zymed, South San Francisco, CA) at 4 °C overnight. Beads were washed 5 times with 1 mL lysis buffer before boiling in Laemmli sample buffer and performing SDS–polyacrylamide gel electrophoresis (PAGE), transfer to nitrocellulose membranes, and immunoblotting. Antibodies employed for antigen detection are described under “Reagents and antibodies.”
Caspase activity measurements
Caspase-3–like protease activity in cell lysates was measured using a fluorometric substrate Ac-DEVD-AFC (Alexis, San Diego, CA), as described before.8 Data are reported as relative fluorescence units (RFUs) of product produced per minute per 10 μg total protein. Data were analyzed using the PRISM Statistics software package employing an unpaired t test method.
Apoptosis assay
Both floating and adherent cells (after trypsinization) were collected, washed with PBS, fixed with PBS containing 3.7% formaldehyde, and stained with 1 μg/mL DAPI in PBS to visualize the nuclei by UV microscopy. Percentages of apoptotic cells were determined by counting 200 GFP-positive cells, scoring cells having nuclear fragmentation and/or chromatin condensation as apoptotic (mean SD; n = 3).
siRNA experiments
RPMI 8226 cells (2.5 × 106) were electroporated as described in “Cell culture and transfections” with 5 μg control small interfering RNA (siRNA) or siRNA targeting either Bcl-B or Bcl-2 proteins. Bcl-B sense siRNA strand was (5′→3′): GGCUUUUCUGUCAUGCUUGTT; Bcl-B antisense siRNA strand (5′→3′): CAAGCAUGACAGAAAAGCCTG; Bcl-2 sense siRNA strand (5′→3′): GGAUUGUGGCCUUCUUUGATT; Bcl-2 antisense siRNA strand (5′→3′): UCAAAGAAGGCCACAAUCCTC. The siRNA was purchased from Ambion (Austin, TX). Cells are suspended in 2 mL RPMI 10% FCS media and cultured for 72 hours at 37°C.
Expression and purification of recombinant Bcl-2-family proteins
Glutatione S-transferase (GST) fusion proteins were produced in bacteria and purified by affinity chromatography as described10 using glutathione-Sepharose. GST fusion proteins containing Bcl-XL, Bcl-2, Bcl-W, Bcl-B, Bfl-1, and Mcl-1 are lacking their C-terminal transmembrane domains (about the last 20 amino acids) (“ΔTM”).
Protein binding assays
Cell extracts (200 μg protein per condition) from HEK293T cells overexpressing GFP-Nur77 protein were incubated with 2 μg immobilized GST or GST fusion proteins corresponding to various Bcl-2 family proteins. After 5 washes, proteins associated with glutathione-Sepharose were analyzed by immunoblotting using anti-GFP or anti-GST antibodies.
Peptide synthesis
Peptides were synthesized on Rink amide p-methylbenzhydrylamine resin using Fmoc synthesis and diisopropylcarbodiimide/1-hydroxybenzotriazole (DIC/HOBt) coupling using an Advanced ChemTech (Louisville, KY) 396 multiple peptide synthesizer. Peptides were acetylated on their N termini and amidated on their C termini. Standard deprotection solutions were used with an extended treatment of 6 hours to remove Pbf from multiple arginines. Peptides were purified by high-performance liquid chromatography (HPLC) on C18 columns and confirmed by matrix-assisted laser desorption/ionization (MALDI) mass analysis. Peptides with C-terminal cysteines were covalently linked to chloroacetylated N-aminocaproic acid in a displacement reaction.
Fluorescence polarization assays
Fluorescence polarization assays (FPAs) were performed according to procedures published previously.10 Briefly, various concentrations of GST protein or GST fusion Bcl-2 family proteins were incubated with 20 nM FITC–Nur77 peptide. Fluorescence polarization was measured in phosphate-buffered saline (PBS) [pH 7.4] after 10 minutes using an Analyst TM AD Assay detection system (LJL Biosystems, Sunnyvale, CA).
Human tissues
For immunohistochemistry, tissue microarrays were produced for 26 cases of Crohn disease that had been obtained from the Yonsei University by Dr H. Kim under institutional review board approval. Normal bone marrow biopsies were provided by University of Texas, M. D. Anderson Cancer Center, Houston (Dr L. J. Medeiros), and myeloma specimens were obtained from VA Hospital of Los Angeles. A polyclonal antibody specific for Bcl-B (BR-49) was raised in rabbit using affinity-purified recombinant GST–Bcl-B protein as the immunogen. Dewaxed tissue sections were immunostained using methods previously described.11 For the antibody specificity control, the immunostaining procedure was performed in parallel using preimmune Bcl-B serum and immune serum (1:1000 dilution) preabsorbed with 10 μg GST–Bcl-B protein or Bcl-W peptide immunogens.
For the double-labeling procedure, tissue sections were stained using rabbit Bcl-B antiserum (DAB chromagen; DAKOCytomation; brown) followed by mouse monoclonal CD138 antibody (Serotec) (SG chromagen; Vector Laboratories, Burlingame, CA; black). Nuclear Red (DAKOCytomation) was used for nuclear counterstaining of the double-labeled slides. Automated image analysis system (Aperio Technology, Vista, CA) was employed to visualize Bcl-B and CD138 staining separately, applying a color deconvolution algorithm.12 Quantification of immunohistochemical staining was performed using color translation and an automated thresholding algorithm (Aperio Technology).
Results
Nur77 binds selectively to Bcl-2, Bfl-1, and Bcl-B
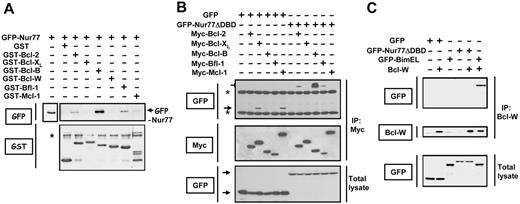
In vitro binding of full-length Nur77 protein to the 6 human antiapoptotic Bcl-2-family proteins was explored using GST fusion proteins (Figure 1A). GST–Bcl-2, GST–Bcl-B, and GST–Bfl-1 bound in vitro to Nur77 protein (provided in cell lysates and expressed as a GFP fusion protein). GST–Bcl-B showed the strongest binding by far. GST–Bcl-XL and GST–Bcl-W displayed no detectable binding in vitro to Nur77, while GST–Mcl-1 showed very weak binding. Analysis of the GST fusion proteins showed that 5 of the 6 were largely intact and applied in comparable amounts, with the exception of GST–Mcl-1, and that all were capable of binding BH3 peptides with high affinity.10
Nur77 binds selectively to Bcl-2, Bfl-1, and Bcl-B. (A) GST fusion proteins representing Bcl-2 members (2 μg) were incubated overnight at 4°C with lysates from HEK 293T cells transfected with plasmid encoding GFP-Nur77. GST proteins were recovered on glutathione-Sepharose, and associated proteins were analyzed by SDS-PAGE immunoblot using rabbit anti-GFP (top blot) and anti-GST (bottom blot) antibodies. (B) 293T cells were cotransfected with plasmids encoding either GFP or GFP-Nur77ΔDBD (a mutant of Nur77 lacking the DNA binding domain that allows Nur77 to enter the cytoplasm without requiring additional stimulation) in combination with plasmids encoding Myc-tagged versions of various Bcl-2 family members. After 24 hours, immunoprecipitation (IP) was performed using anti-Myc antibody, and immune-complexes were analyzed by immunobloting using anti-GFP (top blot) or anti-Myc (middle blot) antibodies. Cell lysates (50 μg) were analyzed directly (bottom blot). (C) For Bcl-W, we performed immunoprecipitation using anti–Bcl-W antibody, and immune-complexes were analyzed by immunobloting using anti-GFP (top blot) and anti–Bcl-W (middle blot) antibodies. BimEL serves as a positive control. Lysates (50 μg) were also analyzed directly in gels to confirm protein production (“total lysate”) (bottom). Asterisks indicate nonspecific bands.
Nur77 binds selectively to Bcl-2, Bfl-1, and Bcl-B. (A) GST fusion proteins representing Bcl-2 members (2 μg) were incubated overnight at 4°C with lysates from HEK 293T cells transfected with plasmid encoding GFP-Nur77. GST proteins were recovered on glutathione-Sepharose, and associated proteins were analyzed by SDS-PAGE immunoblot using rabbit anti-GFP (top blot) and anti-GST (bottom blot) antibodies. (B) 293T cells were cotransfected with plasmids encoding either GFP or GFP-Nur77ΔDBD (a mutant of Nur77 lacking the DNA binding domain that allows Nur77 to enter the cytoplasm without requiring additional stimulation) in combination with plasmids encoding Myc-tagged versions of various Bcl-2 family members. After 24 hours, immunoprecipitation (IP) was performed using anti-Myc antibody, and immune-complexes were analyzed by immunobloting using anti-GFP (top blot) or anti-Myc (middle blot) antibodies. Cell lysates (50 μg) were analyzed directly (bottom blot). (C) For Bcl-W, we performed immunoprecipitation using anti–Bcl-W antibody, and immune-complexes were analyzed by immunobloting using anti-GFP (top blot) and anti–Bcl-W (middle blot) antibodies. BimEL serves as a positive control. Lysates (50 μg) were also analyzed directly in gels to confirm protein production (“total lysate”) (bottom). Asterisks indicate nonspecific bands.
These in vitro binding experiments were extended to examine Nur77 interactions in cells by coimmunoprecipitation assays using HEK293T cells transfected with expression plasmids encoding the relevant proteins (Figure 1B-C). For these experiments, we employed a Nur77 mutant lacking the DNA-binding domain (Nur77ΔDBD) because it constitutively accumulates in cytosol,4,5 thus improving potential for interactions with Bcl-2 family proteins. GFP-Nur77ΔDBD (but not GFP control protein) was specifically coimmunoprecipitated with Bcl-2, Bfl-1, and Bcl-B, with Bcl-B again showing the strongest binding. In contrast, no association of Nur77ΔDBD was detected for Bcl-XL, Bcl-W, or Mcl-1. However, Bcl-XL and Mcl-1 displayed nonspecific interactions with GFP under these coimmunoprecipitation conditions. Immunoblot analysis showed that the 6 Bcl-2 family proteins were produced at comparable levels, with the exception of Bfl-1 (Figure 1B), which is known to be a short-lived protein.13 Control coimmunoprecipitation experiments using BH3-containing proteins such as BimEL confirmed that the experimental conditions were capable of detecting interactions of all Bcl-2 family proteins with known interacting proteins (Figure 1C and data not shown). Nur77ΔDBD also interacts with the mouse ortholog of Bcl-B, Boo/Diva, as determined by coimmunoprecipitation experiments (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The selective association of Nur77ΔDBD with Bcl-2, Bfl-1, and Bcl-B was also demonstrated in intact cells by confocal microscopy analysis of protein colocalization in COS-7 cells. Previous studies showed that in the absence of overexpressed Bcl-2, GFP-tagged Nur77ΔDBD is present in a diffuse cytosolic pattern, whereas coexpression of Bcl-2 causes GFP-Nur77ΔDBD to localize to membranous organelles where Bcl-2 resides.4 Similarly, GFP-Nur77ΔDBD was predominantly found in an organellar pattern in COS-7 cells expressing Bfl-1 or Bcl-B but not Bcl-XL, Mcl-1, or Bcl-W (Figure S2). GFP control protein, in contrast, was present in a diffuse pattern regardless of coexpression of various Bcl-2 family members (not shown). Two-color microscopy analysis in which Bcl-2 family proteins were visualized by immunofluorescence showed colocalization of Bcl-2, Bfl-1, and Bcl-B with GFP-Nur77ΔDBD but not with Bcl-XL, Mcl-1, or Bcl-W. Three-color microscopy studies, adding anti-Hsp60 as a mitochondrial marker, showed that much of the GFP-Nur77ΔDBD is targeted to mitochondria when either Bcl-2 or Bcl-B is coexpressed (Figure S3). We conclude, therefore, that Nur77 binds selectively to Bcl-2, Bfl-1, and Bcl-B but not Bcl-XL, Mcl-1, or Bcl-W.
Nur77 collaborates with Bcl-2, Bfl-1, and Bcl-B to induce apoptosis
Because coexpression of Nur77ΔDBD with Bcl-2 induces apoptosis,4 we compared the effects of Nur77ΔDBD on the other antiapoptotic Bcl-2 family proteins. When expressed without Nur77ΔDBD in HeLa cells (Figure 2) or HEK293T cells (not shown), none of the 6 antiapoptotic Bcl-2 family proteins induced significant apoptosis, and all 6 of these proteins suppressed apoptosis induced by staurosporine (not shown). Expression of GFP-Nur77ΔDBD by itself also did not induce significant apoptosis of HeLa cells. In contrast, coexpressing GFP-Nur77ΔDBD with various Bcl-2 family members resulted in striking increases in percentage apoptosis for Bcl-2, Bfl-1, and Bcl-B but not Bcl-XL, Mcl-1, or Bcl-W (Figure 2). GFP control protein did not produce this effect, confirming specificity. Immunoblot analysis showed that GFP-Nur77ΔDBD did not alter levels of the Bcl-2 family proteins. We conclude, therefore, that Nur77 displays the ability to convert Bcl-2, Bfl-1, and Bcl-B (but not Bcl-XL, Mcl-1, or Bcl-W) from antiapoptotic to proapoptotic, correlating with the protein binding data.
Nur77 collaborates with Bcl-2, Bfl-1, and Bcl-B to induce apoptosis. HeLa cells in 12-well plates were cotransfected with 0.3 μg plasmids encoding either GFP or GFP-Nur77ΔDBD in combination with 1 μg plasmids encoding the 6 antiapoptotic Bcl-2 family members. After 1 day, cells were washed with PBS, fixed with 3.7% formaldehyde, and stained with DAPI to visualize nuclei by UV microscopy. The percentages of apoptotic cells were determined by counting 200 GFP-positive cells, scoring cells having nuclear fragmentation and/or chromatin condensation. Data are reported as mean ± SE (n = 3). For assessing protein expression (3 lower panels), lysates were prepared from transfected cells, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using Bcl-2, Myc, and GFP antibodies.
Nur77 collaborates with Bcl-2, Bfl-1, and Bcl-B to induce apoptosis. HeLa cells in 12-well plates were cotransfected with 0.3 μg plasmids encoding either GFP or GFP-Nur77ΔDBD in combination with 1 μg plasmids encoding the 6 antiapoptotic Bcl-2 family members. After 1 day, cells were washed with PBS, fixed with 3.7% formaldehyde, and stained with DAPI to visualize nuclei by UV microscopy. The percentages of apoptotic cells were determined by counting 200 GFP-positive cells, scoring cells having nuclear fragmentation and/or chromatin condensation. Data are reported as mean ± SE (n = 3). For assessing protein expression (3 lower panels), lysates were prepared from transfected cells, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using Bcl-2, Myc, and GFP antibodies.
Membrane targeting is required for Nur77-mediated conversion of Bcl-B
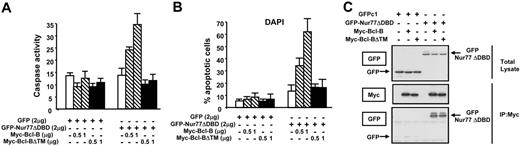
Previous studies showed that deleting the C-terminal transmembrane (TM) domain of Bcl-2 prevents its localization to mitochondria and abrogates its ability to collaborate with Nur77 to induce apoptosis.4 We therefore explored the consequences of removing the TM domain from Bcl-B (Figure 3). Previously, we showed that full-length Bcl-B targets to mitochondria, while Bcl-BΔTM is found in the cytosol.14 When GFP-Nur77ΔDBD was expressed in HEK293T cells with full-length Bcl-B, caspase activation (Figure 3A) and apoptosis (Figure 3B) were induced. In contrast, coexpressing Nur77ΔDBD with Bcl-BΔTM did not activate caspases or induce apoptosis. Immunoblot analysis showed that Bcl-B and Bcl-BΔTM were expressed at comparable levels. Moreover, both proteins associated with GFP-Nur77ΔDBD as determined by coimmunoprecipitation experiments (Figure 3C). However, confocal microscopy studies showed that while GFP-Nur77ΔDBD was targeted to membranous organelles in Bcl-B–expressing cells, GFP-Nur77ΔDBD was predominantly found in a diffuse cytosolic distribution in cells expressing Bcl-BΔTM (Figure S4). Similar conclusions were reached by subcellular fractionation analysis showing that expression of Bcl-B increased while Bcl-BΔTM decreased the proportion of GFP-Nur77ΔDBD present in mitochondria-containing heavy membrane fractions relative to cytosolic fractions (Figure S5).
Membrane targeting is required for Bcl-B–induced apoptosis with Nur77. (A) HEK293T cells were transfected with 2 μg of either pEGFP-C1 or pEGFP-Nur77ΔDBD together with 0.5 to 1 μg plasmid DNA encoding a tagged Bcl-B or Bcl-BΔTM. After 24 hours, cells were lysed and caspase activity was assessed using DEVD-AFC substrate (mean ± SD; n = 3). (B) HEK293T cells were transfected as described for panel A. After 48 hours, fixed cells were stained with DAPI to visualize nuclei by UV microscopy. The percentages of apoptotic cells were determined by counting 200 GFP-positive cells (mean ± SE; n = 3). (C) HEK293T cells were transfected with pEGFP-C1 or pEGFP-Nur77ΔDBD in combination with Myc–Bcl-B or Myc–Bcl-BΔTM in the presence of 50 μM z-VAD-fmk. Cell lysates were prepared, and immunoprecipitation was performed with monoclonal anti-Myc monoclonal antibody. The immunoprecipitates or the lysates were immunoblotted with anti-GFP or anti-Myc antibodies.
Membrane targeting is required for Bcl-B–induced apoptosis with Nur77. (A) HEK293T cells were transfected with 2 μg of either pEGFP-C1 or pEGFP-Nur77ΔDBD together with 0.5 to 1 μg plasmid DNA encoding a tagged Bcl-B or Bcl-BΔTM. After 24 hours, cells were lysed and caspase activity was assessed using DEVD-AFC substrate (mean ± SD; n = 3). (B) HEK293T cells were transfected as described for panel A. After 48 hours, fixed cells were stained with DAPI to visualize nuclei by UV microscopy. The percentages of apoptotic cells were determined by counting 200 GFP-positive cells (mean ± SE; n = 3). (C) HEK293T cells were transfected with pEGFP-C1 or pEGFP-Nur77ΔDBD in combination with Myc–Bcl-B or Myc–Bcl-BΔTM in the presence of 50 μM z-VAD-fmk. Cell lysates were prepared, and immunoprecipitation was performed with monoclonal anti-Myc monoclonal antibody. The immunoprecipitates or the lysates were immunoblotted with anti-GFP or anti-Myc antibodies.
Endogenous Bcl-B interacts with endogenous Nur77 in cells of plasma cell lineage
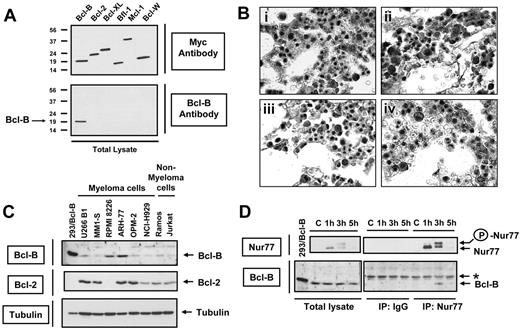
To extend these studies from transfection to endogenous proteins, we produced monospecific antibodies recognizing Bcl-B protein (Figure 4A) and surveyed human tissues for Bcl-B expression by immunohistochemistry, thus determining the cell types that normally express Bcl-B in vivo. Specific immunostaining was detected in liver, renal tubules, ovarian epithelium, bronchial epithelium, and some neurons, with the strongest intensity of Bcl-B immunoreactivity found in plasma cells (M.K., manuscript in preparation). Essentially all plasma cells appeared to contain strong Bcl-B immunoreactivity. Comparisons of preimmune and immune serum, as well as antigen preadsorption experiments, confirmed the specificity of the plasma cell immunostaining (Figure 4B). Two-color immunohistochemical analysis using anti-CD138 as a plasma cell marker confirmed expression of Bcl-B in these cells (Figure S6). Bcl-B immunostaining was also detected in 73 of 165 (44%) multiple myeloma (MM) patient specimens examined (as defined by more than 20% immunopositivity), a plasma cell malignancy. High levels of Bcl-B protein were detected by immunoblotting in lysates prepared from 2 of 6 MM cell lines (Figure 4C).
Endogenous Nur77 binds endogenous Bcl-B in cells of plasma cell lineage. (A) HEK293T cells were transfected with 1 μg plasmids encoding Myc-tagged Bcl-B, Bcl-2, Bcl-XL, Bfl-1, Mcl-1, and Bcl-W proteins. After 24 hours, cells were lysed and analyzed by SDS-PAGE/immunoblotting using anti-Myc (top blot) and anti–Bcl-B (bottom blot) antibodies. Molecular weight markers are shown in kilodaltons (kDa). (B) Immunohistochemical detection of Bcl-B in normal human bone marrow. Serial sections of bone marrow biopsy were stained with (i) preimmune serum, (ii) anti–Bcl-B antiserum, (iii) anti–Bcl-B antiserum preadsorbed with GST–Bcl-B, and (iv) anti–Bcl-B serum preadsorbed with Bcl-W peptide. Specimens were counterstained with nuclear red dye. Original magnifications are × 1000. (C) Lysates from various myeloma and nonmyeloma cell lines were analyzed by SDS-PAGE/immunoblotting using Bcl-B, Bcl-2, and tubulin antibodies. (D) RPMI 8226 cells were stimulated for 1, 3, or 5 hours with TPA (100 ng/mL) and ionomycin (1 μM). Lysates were prepared, and immunoprecipitation was performed with monoclonal anti-Nur77 monoclonal antibody or mouse IgG. Immunoprecipitates and lysates were analyzed by SDS-PAGE/immunobloting using anti-Nur77 (top blot) or anti–Bcl-B (bottom blot) antibodies. In panels C and D, “293/Bcl-B” represents lysate (20 μg) from HEK 293T cells transfected with plasmid encoding Bcl-B protein, used as a positive control. Asterisk indicates a nonspecific band. Phospho form of Nur77 is indicated by the circled P.
Endogenous Nur77 binds endogenous Bcl-B in cells of plasma cell lineage. (A) HEK293T cells were transfected with 1 μg plasmids encoding Myc-tagged Bcl-B, Bcl-2, Bcl-XL, Bfl-1, Mcl-1, and Bcl-W proteins. After 24 hours, cells were lysed and analyzed by SDS-PAGE/immunoblotting using anti-Myc (top blot) and anti–Bcl-B (bottom blot) antibodies. Molecular weight markers are shown in kilodaltons (kDa). (B) Immunohistochemical detection of Bcl-B in normal human bone marrow. Serial sections of bone marrow biopsy were stained with (i) preimmune serum, (ii) anti–Bcl-B antiserum, (iii) anti–Bcl-B antiserum preadsorbed with GST–Bcl-B, and (iv) anti–Bcl-B serum preadsorbed with Bcl-W peptide. Specimens were counterstained with nuclear red dye. Original magnifications are × 1000. (C) Lysates from various myeloma and nonmyeloma cell lines were analyzed by SDS-PAGE/immunoblotting using Bcl-B, Bcl-2, and tubulin antibodies. (D) RPMI 8226 cells were stimulated for 1, 3, or 5 hours with TPA (100 ng/mL) and ionomycin (1 μM). Lysates were prepared, and immunoprecipitation was performed with monoclonal anti-Nur77 monoclonal antibody or mouse IgG. Immunoprecipitates and lysates were analyzed by SDS-PAGE/immunobloting using anti-Nur77 (top blot) or anti–Bcl-B (bottom blot) antibodies. In panels C and D, “293/Bcl-B” represents lysate (20 μg) from HEK 293T cells transfected with plasmid encoding Bcl-B protein, used as a positive control. Asterisk indicates a nonspecific band. Phospho form of Nur77 is indicated by the circled P.
Using RPMI 8226 myeloma cells, which express endogenous Bcl-B, we tested for interactions with endogenous Nur77. Like most cells, RPMI 8226 cells do not contain Nur77 protein under normal culture conditions, but stimulation with phorbol ester (TPA) and Ca2+ ionophore (ionomycin) induced Nur77 protein production, as determined by immunoblotting (Figure 4D). Moreover, a slower migrating form of Nur77 was also detected by SDS-PAGE/immunoblotting, consistent with the phosphorylation events that are known to cause Nur77 accumulation in cytosol.6 Coimmunoprecipitation experiments showed that endogenous Bcl-B associates with endogenous Nur77 in RPMI 8226 cells following stimulation with TPA/ionomycin (Figure 4D).
Endogenous Bcl-B mediates Nur77-induced apoptosis in myeloma cells
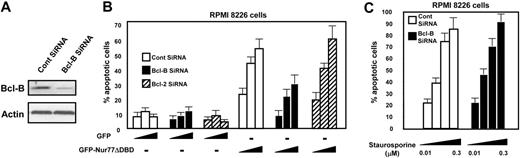
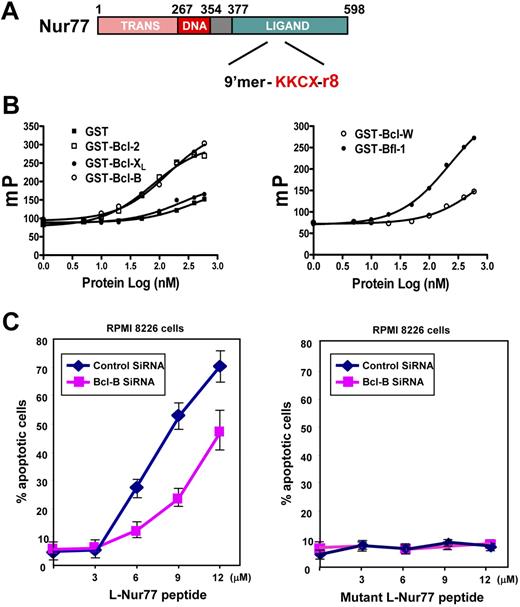
We used siRNA technology to reduce Bcl-B protein expression in RPMI 8226 myeloma cells (Figure 5A). RPMI 8226 cells were selected because they express Bcl-B but not Bcl-2, thus allowing us to focus on Bcl-B. Although TPA/ionomycin induces Nur77–Bcl-B interaction, this was not sufficient to induce apoptosis in these MM cells, possibly because endogenous Nur77 failed to reach critical levels for induction of apoptosis. We therefore introduced GFP-Nur77ΔDBD expression plasmid into RPMI 8226 cells by electroporation to achieve robust cytosolic Nur77 levels. GFP-Nur77ΔDBD induced dose-dependent apoptosis of RPMI 8226 cells, whereas GFP did not (Figure 5B). Treatment with siRNA targeting Bcl-B reduced the apoptotic response to GFP-Nur77ΔDBD by more than half. In contrast, Bcl-2–targeting siRNA had no effect, consistent with the absence of Bcl-2 protein expression in RPMI 8226 cells (Figure 4C). While Bcl-B siRNA reduced apoptosis induced by Nur77 transfection, apoptosis induced by staurosporine was not altered (Figure 5C), demonstrating selectivity.
Endogenous Bcl-B mediates Nur77-induced apoptosis in myeloma cells. (A) RPMI 8226 myeloma cells were electroporated with either control or Bcl-B siRNA. After 72 hours, cells were harvested and lysates (100 μg) were analyzed by SDS-PAGE/immunoblotting using Bcl-B (top blot) and actin (bottom blot) antibodies. (B) RPMI 8226 cells were electroporated with either control, Bcl-B, or Bcl-2 siRNA. Then, 72 hours later, cells were transfected with plasmids (0.1 to 1 μg) encoding GFP or GFP-Nur77ΔDBD proteins. After 18 hours, fixed cells were stained with DAPI to visualize nuclei by UV microscopy and determine percentages of apoptotic cells by counting 200 GFP-positive cells (mean ± SE; n = 3). (C) RPMI 8226 cells were electroporated with either control or Bcl-B siRNA. Cells were treated 72 hours later with staurosporine (0.01 to 0.3 μM) for 18 hours. Apoptosis was determined as in panel B.
Endogenous Bcl-B mediates Nur77-induced apoptosis in myeloma cells. (A) RPMI 8226 myeloma cells were electroporated with either control or Bcl-B siRNA. After 72 hours, cells were harvested and lysates (100 μg) were analyzed by SDS-PAGE/immunoblotting using Bcl-B (top blot) and actin (bottom blot) antibodies. (B) RPMI 8226 cells were electroporated with either control, Bcl-B, or Bcl-2 siRNA. Then, 72 hours later, cells were transfected with plasmids (0.1 to 1 μg) encoding GFP or GFP-Nur77ΔDBD proteins. After 18 hours, fixed cells were stained with DAPI to visualize nuclei by UV microscopy and determine percentages of apoptotic cells by counting 200 GFP-positive cells (mean ± SE; n = 3). (C) RPMI 8226 cells were electroporated with either control or Bcl-B siRNA. Cells were treated 72 hours later with staurosporine (0.01 to 0.3 μM) for 18 hours. Apoptosis was determined as in panel B.
Nur77-mimicking peptide induces Bcl-B–dependent apoptosis of myeloma cells
As an alternative to expressing Nur77ΔDBD in myeloma cells, we also employed a synthetic peptide that mimics the effects of Nur77 protein on Bcl-2. In this regard, previous studies showed that the segment of Nur77 necessary and sufficient for inducing Bcl-2 conversion maps within the C-terminal ligand-binding domain in the DC1 region.4 We confirmed that the DC region of Nur77 is also involved in Bcl-B binding (Figure S7). In separate studies, a 9 amino acid peptide from this region of human Nur77 (Figure 6A) was shown to bind Bcl-2 and convert it from a protector to killer.
Nur77-mimicking peptide induces Bcl-B–dependent apoptosis of myeloma cells. (A) Schematic representation of Nur77 protein and the corresponding Nur77 9′mer peptide. “GX” corresponds to the linker, and “r8” corresponds to 8 arginines that endow the peptide with cell penetration activity. (B) A serial concentration of GST or GST–Bcl-2, GST–Bcl-XL, or GST–Bcl-B (left panel) or GST–Bcl-W, GST–Bfl-1, (right panel) or GST–Mcl-1 was incubated with 20 nM FITC–Nur77 peptides in PBS buffer. Fluorescence polarization was measured after 10 minutes. (C) RPMI 8226 cells were electroporated with either control or Bcl-B siRNA. After 72 hours, cells were treated for 18 hours with either Nur77 peptide or corresponding inactive mutant (3 to 12 μM). Then, cells were washed with PBS, fixed with 3.7% formaldehyde, and stained with DAPI to visualize nuclei by UV microscopy. The percentages of apoptotic cells were determined by counting 200 GFP-positive cells (mean ± SE; n = 3).
Nur77-mimicking peptide induces Bcl-B–dependent apoptosis of myeloma cells. (A) Schematic representation of Nur77 protein and the corresponding Nur77 9′mer peptide. “GX” corresponds to the linker, and “r8” corresponds to 8 arginines that endow the peptide with cell penetration activity. (B) A serial concentration of GST or GST–Bcl-2, GST–Bcl-XL, or GST–Bcl-B (left panel) or GST–Bcl-W, GST–Bfl-1, (right panel) or GST–Mcl-1 was incubated with 20 nM FITC–Nur77 peptides in PBS buffer. Fluorescence polarization was measured after 10 minutes. (C) RPMI 8226 cells were electroporated with either control or Bcl-B siRNA. After 72 hours, cells were treated for 18 hours with either Nur77 peptide or corresponding inactive mutant (3 to 12 μM). Then, cells were washed with PBS, fixed with 3.7% formaldehyde, and stained with DAPI to visualize nuclei by UV microscopy. The percentages of apoptotic cells were determined by counting 200 GFP-positive cells (mean ± SE; n = 3).
We tested the Nur77 9′mer for binding to the antiapoptotic Bcl-2 family proteins using GST fusions proteins and fluorochrome-conjugated Nur77 peptide for FPAs (Figure 6B). Like full-length Nur77 protein, the Nur77 peptide was determined to bind Bcl-2, Bfl-1, and Bcl-B in vitro. In contrast, FITC-Nur77 did not bind Bcl-XL or Bcl-W. Equivocal results were obtained for Mcl-1 (not shown). In competition experiments, unlabeled Nur77 peptide reduced binding of FITC-Nur77 in a concentration-dependent manner, while a Nur77 peptide with alanine substitutions in the first and last positions of the peptide had far less effect on FITC-Nur77 binding (Figure 6B). The binding of Nur77 9′mer peptide to Bcl-B was also confirmed independently by a time-resolved fluorescence energy transfer (TR-FRET) method (not shown).
For cell-based experiments, we used a version of Nur77 peptide with 8 arginines to endow the peptide with membrane penetrating functionality. When added to cultures of RPMI 8226 cells, Nur77 peptide induced apoptosis in a concentration-dependent fashion. Furthermore, pretreatment of RPMI 8226 myeloma cells with Bcl-B–targeting siRNA reduced Nur77 peptide–induced apoptosis compared with cells treated with control synthetic RNA (Figure 6C). In contrast, culturing RPMI 8226 with mutant Nur77 peptide that does not bind Bcl-2 family proteins failed to induce apoptosis. Thus, Bcl-B is required for optimal induction of apoptosis by Nur77 peptide in these myeloma cells.
Discussion
Here we show that orphan nuclear receptor Nur77 binds selectively to members of the Bcl-2 family of antiapoptotic proteins, Bcl-2, Bfl-1, and Bcl-B, with binding to Bcl-B apparently the strongest of these protein interactions. Coexpression of cytosolic Nur77 with Bcl-2, Bfl-1, or Bcl-B induced apoptosis, while coexpression with other nonbinding members of the Bcl-2 family, Bcl-XL, Mcl-1, Bcl-W, did not promote cell death. Nur77 has been shown to translocate from nucleus to cytosol,4,5 binding to Bcl-2 and converting its phenotype from antiapopptotic to proapoptotic in association with conformational changes that expose the BH3 domain of Bcl-2.4 The BH3 domain of Bcl-2 family proteins mediates their homodimerization and heterodimerization.15,16 We presume a similar conversion mechanism applies to Bfl-1 and Bcl-B, but conformation-specific antibodies that can assess the status of BH3 domain exposure are lacking for these proteins. Our findings imply that Bcl-2, Bfl-1, and Bcl-B have 2 phenotypic states depending on whether they are bound by Nur77. Indeed, circumstances in which Bcl-2 expression increases rather than decreases cell death are well documented (reviewed by Chen et al,17 Cheng et al,18 and Uhlmann et al19 ). Interestingly, the closest homolog of Bcl-B in mice, Boo/Diva, has been reported to have either proapoptotic or antiapoptotic activity,20–22 further supporting the notion that certain members of the Bcl-2 family have biphenotypic properties.18,19,23–29
The membrane-anchoring domain of Bcl-B is required for its collaboration with Nur77/TR3 to induce apoptosis, similar to observations previously made for Bcl-2.4 Without the C-terminal TM domain, Bcl-B failed to recruit Nur77/TR3 to mitochondria and failed to promote apoptosis. Although other explanations are plausible, one reason membrane targeting may be required for phenotypic conversion of antiapoptotic Bcl-2 family proteins by Nur77/TR3 is that the conformational changes required for BH3 domain exposure are expected to expose hydrophobic regions of these α-helical proteins. Thus, apposition to membranes may be required for the Nur77/TR3-mediated conformational transformation, shielding the hydrophobic face of amphipathic α-helices in Bcl-2 family proteins from the aqueous environment. Indeed, nuclear magnetic resonance (NMR) studies of interactions of Bcl-2 family proteins with membranes support this concept.30
Important species-specific differences exist among several of the Bcl-2-family proteins, even in mammals (reviewed by Reed et al31 ). For instance, in mice, the apparent ortholog of Bfl-1 is A1, which has been amplified in the mouse genome to result in 4 genes, A1a, A1b, A1c, and A1d.32 The closest ortholog of Bcl-B in mice is Boo/Diva, with 49% amino acid identity. However, Boo/Diva expression in mice appears to be limited to ovary and testis, suggesting that the murine gene is regulated differently than its closest human counterpart.
Using monospecific antibodies, we observed that human Bcl-B protein is strongly expressed in plasma cells and MM cell lines. Reducing Bcl-B expression in a myeloma cell line decreased killing induced by expression of cytosolic Nur77 and killing induced by a peptide that mimics Nur77, implying that endogenous Bcl-B plays a role in Nur77-mediated apoptosis induction in these cells. These findings may have ramifications for developing strategies for counteracting long-lived plasma cells that secrete autoantibody in autoimmune disease and for treating plasma cell malignancies. With regard to myeloma, while only a subset of primary MM specimens examined abundantly expressed Bcl-B, many MMs express Bcl-2 or Bfl-1,33,34 which are also targets of Nur77 and Nur77-mimicking peptides. Thus, stabilized versions of cell-permeable Nur77 peptides or small-molecule nonpeptidyl compounds that mimic them might be useful for eradicating multiple myeloma cells.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John C. Reed, Burnham Institute for Medical Research, 10901 N Torrey Pines Rd, La Jolla, CA 92037; reedoffice@burnham.org.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the National Institutes of Health (NIH) (GM60554), the SASS Foundation of America, and the Philippe Foundation. We thank A. H. Olson (Aperio Technology) for color deconvolution algorithms, L. J. Mederios (M. D. Anderson) for bone marrows, and M. Hanaii and J. Valois for manuscript preparation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal