Abstract
The Dutch-Belgian Hemato-Oncology Cooperative Group and the Swiss Group for Clinical Cancer Research (HOVON-SAKK) collaborative study group evaluated outcome of patients (pts) with acute myeloid leukemia (AML) in first remission (CR1) entered in 3 consecutive studies according to a donor versus no-donor comparison. Between 1987 and 2004, 2287 pts were entered in these studies of whom 1032 pts (45%) without FAB M3 or t(15;17) were in CR1 after 2 cycles of chemotherapy, received consolidation treatment, and were younger than 55 years of age and therefore eligible for allogeneic hematopoietic stem cell transplantation (allo-SCT). An HLA-identical sibling donor was available for 326 pts (32%), whereas 599 pts (58%) lacked such a donor, and information was not available in 107 pts. Compliance with allo-SCT was 82% (268 of 326). Cumulative incidences of relapse were, respectively, 32% versus 59% for pts with versus those without a donor (P < .001). Despite more treatment-related mortality (TRM) in the donor group (21% versus 4%, P < .001), disease-free survival (DFS) appeared significantly better in the donor group (48% ± 3% versus 37% ± 2% in the no-donor group, P < .001). Following risk-group analysis, DFS was significantly better for pts with a donor and an intermediate- (P = .01) or poor-risk profile (P = .003) and also better in pts younger than 40 years of age (P < .001). We evaluated our results and those of the previous MRC, BGMT, and EORTC studies in a meta-analysis, which revealed a significant benefit of 12% in overall survival (OS) by donor availability for all patients with AML in CR1 without a favorable cytogenetic profile.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-SCT) following myeloablative cytoreductive therapy has been established as a notably powerful treatment modality to reduce the risk of relapse in patients with acute myeloid leukemia (AML) in first complete remission (CR1).1 At the same time, it is also well established that a significant part of the beneficial effect of allo-SCT is offset by an increase in treatment-related mortality (TRM) that is typically associated with allo-SCT.2 As a result, it has been difficult to firmly demonstrate that allo-SCT results in superior disease-free survival (DFS) and overall survival (OS) as compared with autologous SCT or consolidation chemotherapy in patients with AML in CR1.3–10 Cytogenetic analysis has allowed for distinguishing categories of AML with widely different prognosis, and, currently, 3 cytogenetic prognostic profiles (favorable, intermediate, adverse) are commonly used. For an assessment of the value of allo-SCT in each of these categories with sufficient statistical power, large databases are evidently required.11–14
Various attempts have been made to assess the value of allo-SCT in AML in CR1 in the context of prospective studies. Randomized controlled trials (RCTs) are the gold standard for the evaluation of treatment efficacy. One disease area in which RCTs have not proved feasible is the assessment of the role of SCT in hematologic malignancy. As an alternative to RCTs, a genetic randomization has been proposed and has been applied by different cooperative groups.8–10 As the availability of a matched sibling donor is essentially a random process, the presence or absence of a donor can be used as a surrogate for randomization.15,16 Although such an analysis has the advantage of avoiding selection bias, it may have the disadvantage of underestimating the effects of allo-SCT when low numbers of patients with a sibling donor actually receive the transplant planned.17 Furthermore, these studies obviously require considerable numbers of patients as well as mature follow-up to evaluate the net effect of (long-term) adverse and favorable effects with sufficient power. Recently, 3 larger studies of donor versus no donor have suggested that allo-SCT results in superior DFS in patients with AML in CR1.8–10 Although the MRC study showed improved survival in patients with an intermediate-risk AML, the EORTC/GIMEMA study rather suggested a beneficial effect restricted to AML with a poor-risk profile.8,9 The recent BGMT study, using an adapted risk index, showed an advantage for intermediate-risk patients.10 None of the latter studies demonstrated an evident improvement of OS in patients with a donor.
Here, we set out to address the question whether the use of allo-SCT in patients with AML in CR1 has a favorable impact on DFS and OS in general as well as in each of the 3 commonly accepted risk categories. Hence, we compared outcome of patients with AML in CR1 with an HLA-identical sibling donor with outcome of patients without a donor. The analysis is based on patients enrolled in 3 successive AML studies conducted by the HOVON/SAKK cooperative groups.18–21 In these studies the majority of patients were evaluated for the presence of a sibling donor, and a comparatively high percentage of patients with a donor actually received their intended allograft. In addition, we present a meta-analysis based on the data of the present HOVON/SAKK study and those from the previously published EORTC, MRC, and BGMT studies.
Patients, materials, and methods
Approval was obtained from the Rotterdam Medical Ethical Committee as well as the institutional review boards of all the participating centers in the HOVON 4, 29, and 42 studies. Informed consent was obtained from all participating patients in accordance with the Declaration of Helsinki.
The study population for this study consists of patients with newly diagnosed AML included in the consecutive AML HOVON-SAKK studies AML4,18,20 AML29,19 and AML42 between October 1987 and December 31, 2003, with the following additional selection criteria: no FAB M3 or acute promyelocytic leukemia; age younger than or equal to 50 years for patients in AML4 and AML4A, because patients older than the age of 50 were not eligible for allo-SCT in these early studies and age younger than or equal to 55 for patients in the later studies AML29 and AML42; with CR reached after cycle I or cycle II; completion of 2 cycles of induction chemotherapy; having received consolidation treatment with chemotherapy and/or transplantation.
In these studies a questionnaire was used concerning the search for a donor and the result of typing for patients who had reached a complete remission on protocol and who were eligible for postremission treatment. On the basis of the information from the questionnaire and the actual consolidation treatment given the patients from the study population were classified in 3 groups: the donor group, the no-donor group, and a group of patients with insufficient information. A patient was classified in the donor group if the patient had received an allogeneic transplant from an HLA-matched related donor in first complete remission, or if the search for a donor resulted in a syngeneic twin, a genotypically or phenotypically identical sibling, an HLA-identical sibling (without further specification), or a matched related donor (eg, a father or mother, which was the case in 2 patients). Another requirement for the donor group was that the donor was willing to serve as a donor and that there were no medical contraindications for stem cell collection. Those not fulfilling the criteria for the donor group were classified in the no-donor group. Thus, the no-donor group included patients with no HLA-identical siblings or no siblings available for typing, those with siblings with one or more mismatches, as well as patients with a (mis)matched unrelated donor or those for whom a search for an unrelated donor was initiated. If an HLA-identical sibling was found, but this sibling was not available as a donor because of refusal, living far away, family problems, or contraindications (concomitant disease), the patient was also classified in the no-donor group. Patients who could not be classified in the donor group or the no-donor group were classified in the no-information group.
Treatment protocols
Treatment in the AML4/4A,18,20 AML29,19 and AML42 studies involved one cycle of induction with an anthracycline (daunorubicin or idarubicin) in combination with cytarabine (200 mg/m2 for 7 days) and a second cycle of amsacrine with intermediate-dose cytarabine (1000 mg/m2 every 12 hours for 6 days). If CR occurred, patients in the AML4/4A protocol were randomly assigned after a third cycle of chemotherapy (mitoxantrone and etoposide) to treatment with high-dose busulfan and cyclophosphamide followed by autologous SCT or no additional treatment. In the AML29 and AML42 studies, patients in CR after 2 cycles of chemotherapy were randomly assigned to treatment with a third cycle of chemotherapy (mitoxantrone and etoposide) without SCT or high-dose chemotherapy with busulfan and cyclophosphamide followed by autologous SCT. When an HLA-identical sibling donor was available, eligible patients proceeded to allogeneic SCT after induction cycles I and II. The AML4A study addressed a question of therapy with granulocyte macrophage colony-stimulating factor (molgrastim; Sandoz, Basel, Switzerland) during and/or after chemotherapy.18 The AML29 and AML42 trials addressed the question of the value of growth factor priming by adding granulocyte colony-stimulating factor (lenograstim; Aventis, Hoevelaken, The Netherlands) to the induction program on the days of chemotherapy.19 In addition, the AML42 trial addressed the question of the value of intensifying cytarabine during induction cycles I and II.
Risk groups
Patients were classified as good, intermediate, and poor risk on the basis of cytogenetic abnormalities, the white blood cell (WBC) count at diagnosis, and whether CR was reached after cycle I or after cycle II (Table 1). Cytogenetic abnormalities t(8;21)(q22;q22) and inv(16) or t(16;16)(p13;q22) were considered favorable. Complex cytogenetic abnormalities (ie, defined as at least 3 unrelated cytogenetic clones) and −5q, −7q, abn(3q), t(6;9)(q23;q34), abn(11q23), and t(9;22)(q34;q11) were considered unfavorable. Patients without favorable or adverse abnormalities or without karyotype information were classified as intermediate cytogenetic risk. Patients were classified as good risk in case of cytogenetic abnormality t(8;21)(q22;q22) with WBC count at diagnosis less than 20 × 109/L, or in case of inv(16) or t(16;16)(p13;q22) without additional adverse cytogenetic abnormalities. Patients were classified as poor risk in case of adverse cytogenetic abnormalities or in case of intermediate cytogenetic risk and a late CR, reached after cycle II. All other patients were classified in the intermediate-risk group.
Risk group classification
| Group . | Definition . |
|---|---|
| Good | t(8;21) and WBC count ≤ 20 × 109/L and no additional unfavorable cytogenetic abnormalities; inv/del(16) and no additional unfavorable cytogenetic abnormalities |
| Intermediate | Patients not assigned to good or poor-risk groups |
| Poor | Unfavorable cytogenetics: complex karyotypes (≥3); del(5q)/−5; del(7q)/−7; abn(3q); t(6;9)/t(9;22); abn(11q23); and late CR* |
| Group . | Definition . |
|---|---|
| Good | t(8;21) and WBC count ≤ 20 × 109/L and no additional unfavorable cytogenetic abnormalities; inv/del(16) and no additional unfavorable cytogenetic abnormalities |
| Intermediate | Patients not assigned to good or poor-risk groups |
| Poor | Unfavorable cytogenetics: complex karyotypes (≥3); del(5q)/−5; del(7q)/−7; abn(3q); t(6;9)/t(9;22); abn(11q23); and late CR* |
CR indicates complete remission; WBC, white blood cell.
*Except patients with favorable cytogenetics
End points and statistical methods
OS and DFS were measured from the date of start of consolidation treatment. The event for OS was death whatever the cause, and patients were censored at the date of last contact if alive. The events for DFS were death in CR1, considered as TRM, or relapse. The cumulative risks of relapse and TRM over time were calculated as competing risks with actuarial methods where patients alive in continuing first complete remission were censored at the date of last contact. Any actuarial probability mentioned in this study is the probability at 4 years. Multivariable Cox regression analysis for OS, DFS, relapse, and TRM was applied on an intention-to-treat basis to calculate hazard ratios for the donor group compared with the no-donor group, for intermediate and unfavorable risk compared with favorable risk and for age older than 40 compared with age younger than 40, where the cut point 40 was chosen as the rounded number closest to the median age of 39 years. All P values for tests that compare the outcomes in the donor and no-donor group were based on log likelihood ratio tests, except when explicitly stated otherwise. Log likelihood ratio tests were also used to test for interactions (ie, to test for differences in the donor effect between risk groups and between younger and older patient for each of the end points OS, DFS, relapse, and TRM). P values of these test for interaction will only be mentioned in the “Results” when smaller than 0.10. Hazard ratio estimates with 95% confidence intervals (CIs) comparing the donor group with the no-donor group were also obtained by log-rank analysis in subgroups stratified by risk and by age younger than and older than 40 years. These estimates were combined with similar estimates from the MRC, EORTC, and BGMT studies8–10 in a meta-analysis. In that analysis the effects were also considered in subgroups split by age (younger than and older than 35 years) and split by cytogenetic risk. The cut point of 35 years was chosen because the same cut point had been used in the EORTC and MRC studies. The cytogenetic risk classifications used in all 4 studies were fairly similar, although not entirely identical. Because there was more variation between the studies in the way in which early or late achievement of CR or a high WBC count were incorporated in the overall risk classification, we have not considered these factors in the overview. The criteria for favorable cytogenetics were highly similar in all studies: t(8;21) and inv(16) abnormalities were considered favorable, but there were some dissimilarities depending on the presence of additional unfavorable cytogenetic abnormalities. All 4 studies considered complex abnormalities, abn 3q, −5, 5q−, −7, abn 11q23, t(6;9), and t(9;22) as unfavorable, but abnormality 7q− as such was not uniformly considered unfavorable in all studies. In the EORTC study all patients without cytogenetic abnormalities or with −Y only were considered intermediate, whereas all others were considered bad or very bad. These patient groups (bad/very bad from EORTC, and patients with unfavorable cytogenetics from HOVON-SAKK/MRC/BGMT) were grouped together in the meta-analysis as unfavorable. The variance estimates for the log-rank statistics reported in the EORTC study could not be derived from the publication but were kindly provided by Stefan Suciu. The variance estimates for the log-rank statistics for the age and cytogenetic risk subgroups of the BGMT study were kindly provided by Didier Blaise.
Results
Donor availability and consolidation treatment applied
Between October 1987 and January 1, 2004, 2287 patients were included in consecutive AML HOVON-SAKK studies AML4, AML29, and AML42. One thousand two hundred fifty-five patients were excluded from the study population because of FAB M3 or t(15;17) (n = 112), age older than 55 (n = 612), no CR after 2 cycles of induction chemotherapy (n = 336), or no consolidation treatment (n = 195). Detailed information about the presence and availability of an HLA-identical sibling donor was obtained in 90% (n = 925) of the remaining 1032 patients. An HLA-identical sibling donor was available in 326 patients (32%), whereas 599 patients (58%) lacked such a donor as a result of absence of siblings, HLA-incompatibility, or ineligibility of a potential donor. Information about the presence of siblings and/or results of HLA typing was lacking in 107 patients, which constituted the no information group. Consolidation therapy in the latter group consisted of chemotherapy in 83 and autologous SCT in 25 patients. These patients are not included in the analysis, because our study concentrates entirely on the comparison between the donor and no-donor groups. At the time of the data analysis, the median follow-up of patients alive from start consolidation was 63 months. Patient characteristics of the donor and no-donor groups are presented in Table 2. Both groups are comparable with respect to age, FAB type, WBC count, number of cycles to achieve remission, and cytogenetic risk distributions and prognostic risk score.
Patient characteristics
| Parameter . | Subgroup of patients . | |
|---|---|---|
| Donor . | No donor . | |
| Number | 326 | 599 |
| Median age, y (range) | 39 (15–55) | 39 (16–55) |
| Sex | ||
| Male | 163 | 296 |
| Female | 163 | 303 |
| FAB type, n (%) | ||
| M0 | 6 (2) | 18 (3) |
| M1 | 56 (17) | 118 (20) |
| M2 | 101 (31) | 160 (27) |
| M4 | 66 (20) | 139 (23) |
| M5 | 75 (23) | 124 (21) |
| M6 | 8 (3) | 21 (4) |
| M7 | 1 (0.5) | 2 (0.5) |
| Unknown | 13 (4) | 17 (3) |
| WBC count, × 109/L, n (%) | ||
| No more than 20 | 175 (54) | 316 (53) |
| 20–100 | 117 (36) | 212 (35) |
| More than 100 | 33 (10) | 71 (12) |
| Cytogenetic classification, n (%) | ||
| Favorable | 38 (12) | 84 (14) |
| Intermediate | 225 (69) | 382 (64) |
| Unfavorable | 36 (11) | 71 (12) |
| Unknown | 27 (8) | 62 (10) |
| Cycles to CR, n (%) | ||
| 1 | 221 (68) | 427 (71) |
| 2 | 105 (32) | 172 (29) |
| Prognostic risk category, n (%)* | ||
| Good | 32 (10) | 73 (12.1) |
| Intermediate | 178 (55) | 333 (56) |
| Poor | 116 (36) | 193 (32) |
| Parameter . | Subgroup of patients . | |
|---|---|---|
| Donor . | No donor . | |
| Number | 326 | 599 |
| Median age, y (range) | 39 (15–55) | 39 (16–55) |
| Sex | ||
| Male | 163 | 296 |
| Female | 163 | 303 |
| FAB type, n (%) | ||
| M0 | 6 (2) | 18 (3) |
| M1 | 56 (17) | 118 (20) |
| M2 | 101 (31) | 160 (27) |
| M4 | 66 (20) | 139 (23) |
| M5 | 75 (23) | 124 (21) |
| M6 | 8 (3) | 21 (4) |
| M7 | 1 (0.5) | 2 (0.5) |
| Unknown | 13 (4) | 17 (3) |
| WBC count, × 109/L, n (%) | ||
| No more than 20 | 175 (54) | 316 (53) |
| 20–100 | 117 (36) | 212 (35) |
| More than 100 | 33 (10) | 71 (12) |
| Cytogenetic classification, n (%) | ||
| Favorable | 38 (12) | 84 (14) |
| Intermediate | 225 (69) | 382 (64) |
| Unfavorable | 36 (11) | 71 (12) |
| Unknown | 27 (8) | 62 (10) |
| Cycles to CR, n (%) | ||
| 1 | 221 (68) | 427 (71) |
| 2 | 105 (32) | 172 (29) |
| Prognostic risk category, n (%)* | ||
| Good | 32 (10) | 73 (12.1) |
| Intermediate | 178 (55) | 333 (56) |
| Poor | 116 (36) | 193 (32) |
FAB indicates French-American-British classification; CR, complete remission; WBC, white blood cell.
*Based on cytogenetics, WBC count, and early or late attainment of CR (see “Patients, materials, and methods”).
Patients in complete remission following 2 induction cycles in the HOVON-SAKK AML studies received the following consolidation: a third cycle of chemotherapy, or high-dose cytotoxic therapy followed by an autograft or an allograft following ablative conditioning (Table 3). Among the donor group 82% of the patients received an allo-SCT from an HLA-identical sibling, whereas 17% received a third cycle of chemotherapy and 1% an autograft. In contrast, 65% of the patients in the no-donor group were treated with chemotherapy consolidation, 28% received an autologous stem cell graft, whereas 8% received an allo-SCT using stem cells of mismatched-related or unrelated donors (Table 3).
Distribution of consolidation therapies (third cycle of chemotherapy, autologous SCT, or allo-SCT), applied to patients in donor and no donor groups
| Type of therapy . | Group of patients . | |
|---|---|---|
| Donor . | No donor . | |
| All patients | 326 | 599 |
| Chemotherapy, n (%)* | 55 (17) | 398 (65) |
| Autologous SCT, n (%)† | 3 (1) | 165 (28) |
| Allogeneic SCT, n (%)† | ||
| HLA-identical sibling | 268 (82) | 0 |
| Unrelated donor | 0 | 40 (7) |
| Mismatched family donor | 0 | 5 (1) |
| Type of therapy . | Group of patients . | |
|---|---|---|
| Donor . | No donor . | |
| All patients | 326 | 599 |
| Chemotherapy, n (%)* | 55 (17) | 398 (65) |
| Autologous SCT, n (%)† | 3 (1) | 165 (28) |
| Allogeneic SCT, n (%)† | ||
| HLA-identical sibling | 268 (82) | 0 |
| Unrelated donor | 0 | 40 (7) |
| Mismatched family donor | 0 | 5 (1) |
SCT indicates stem cell transplantation; HLA human leukocyte antigen.
*Third cycle of chemotherapy.
†Preceded by high-dose cytotoxic therapy.
Relapse, treatment-related mortality, and survival
At the time of the analysis, 155 (48%) of 326 patients in CR1 with a donor and 218 (36%) of 599 patients in the no-donor group were alive in continuous complete remission. The details of the comparison of the donor and no-donor group are shown in Table 4. The risk of relapse was significantly less in the donor group (32% versus 59% at 4 years, with hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.37-0.57; P < .001; Table 4). The reduction in risk of relapse was observed in all prognostic risk categories, that is, in good risk (HR, 0.57; 95% CI, 0.24-1.31; P = .17), and was significant in intermediate-risk (HR, 0.47; 95% CI, 0.34-0.64; P < .001) and poor-risk (HR, 0.42; 95% CI, 0.30-0.59; P < .001) AML. The reduction in risk of relapse was significant and similar for patients younger and older than the age of 40 years (Table 4). The hazard ratio as regards risk of relapse for donor versus no-donor groups in younger patients was 0.41 (P < .001) and in older patients it was 0.51 (P < .001). The TRM was clearly worse in the donor group (25% versus 4%, P < .001), without evidence of a difference between the 3 AML risk groups. In patients older than the age of 40 years, the risk of TRM in the donor group was more increased (HR, 6.1; 95% CI, 3.0-12.2; P < .001) as compared with younger patients (HR, 2.7; 95% CI, 1.5-4.9; P = .002).
Effect of donor availability on outcome in AML in CR1
| Outcome . | Donor . | No donor . | P* . | HR (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | No. of events . | Probability of outcome at 4 y ± SE, % . | n . | No. of events . | Probability of outcome at 4 y ± SE, % . | |||
| All patients | 326 | 599 | ||||||
| Survival | 157 | 54 ± 3 | 326 | 46 ± 2 | .09 | 0.85 (0.70–1.03) | ||
| DFS | 171 | 48 ± 3 | 381 | 37 ± 2 | < .001 | 0.71 (0.59–0.85) | ||
| Relapse | 103 | 32 ± 3 | 354 | 59 ± 2 | < .001 | 0.46 (0.37–0.57) | ||
| TRM | 68 | 21 ± 2 | 27 | 4 ± 1 | < .001 | 3.99 (2.55–6.25) | ||
| Good risk | 32 | 73 | ||||||
| Survival | 9 | 84 ± 6 | 17 | 78 ± 5 | .99 | 1.01 (0.45–2.27) | ||
| DFS | 11 | 72 ± 8 | 28 | 64 ± 6 | .40 | 0.74 (0.37–1.50) | ||
| Relapse | 7 | 22 ± 7 | 24 | 32 ± 6 | .17 | 0.57 (0.24–1.31) | ||
| TRM | 4 | 6 ± 4 | 4 | 4 ± 2 | .42 | 1.80 (0.44–7.31) | ||
| Intermediate risk | 178 | 333 | ||||||
| Survival | 76 | 57 ± 4 | 172 | 48 ± 3 | .23 | 0.85 (0.65–1.11) | ||
| DFS | 82 | 53 ± 4 | 199 | 41 ± 3 | .014 | 0.73 (0.56–0.94) | ||
| Relapse | 50 | 28 ± 3 | 188 | 55 ± 3 | < .001 | 0.47 (0.34–0.64) | ||
| TRM | 32 | 19 ± 3 | 11 | 3 ± 1 | < .001 | 5.13 (2.58–10.2) | ||
| Poor risk | 116 | 193 | ||||||
| Survival | 72 | 40 ± 5 | 137 | 30 ± 4 | .17 | 0.82 (0.62–1.09) | ||
| DFS | 78 | 33 ± 4 | 154 | 17 ± 3 | .003 | 0.67 (0.51–0.88) | ||
| Relapse | 46 | 39 ± 5 | 142 | 77 ± 3 | < .001 | 0.43 (0.31–0.60) | ||
| TRM | 32 | 28 ± 4 | 12 | 6 ± 2 | < .001 | 3.47 (1.78–6.77) | ||
| Age younger than 40 y | 174 | 316 | ||||||
| Survival | 73 | 61 ± 4 | 164 | 49 ± 3 | .015 | 0.71 (0.54–0.94) | ||
| DFS | 81 | 55 ± 4 | 204 | 37 ± 3 | < .001 | 0.59 (0.46–0.77) | ||
| Relapse | 51 | 28 ± 3 | 187 | 58 ± 3 | < .001 | 0.41 (0.30–0.56) | ||
| TRM | 30 | 17 ± 3 | 17 | 5 ± 1 | .002 | 2.6 (1.4–4.75) | ||
| Age older than 40 y | 152 | 283 | ||||||
| Survival | 84 | 44 ± 4 | 162 | 42 ± 3 | .84 | 0.97 (0.75–1.27) | ||
| DFS | 90 | 39 ± 4 | 177 | 36 ± 3 | .15 | 0.83 (0.64–1.07) | ||
| Relapse | 52 | 35 ± 4 | 167 | 60 ± 3 | < .001 | 0.51 (0.37–0.70) | ||
| TRM | 38 | 25 ± 4 | 10 | 4 ± 1 | < .001 | 6.07 (3.0–12.24) | ||
| Outcome . | Donor . | No donor . | P* . | HR (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | No. of events . | Probability of outcome at 4 y ± SE, % . | n . | No. of events . | Probability of outcome at 4 y ± SE, % . | |||
| All patients | 326 | 599 | ||||||
| Survival | 157 | 54 ± 3 | 326 | 46 ± 2 | .09 | 0.85 (0.70–1.03) | ||
| DFS | 171 | 48 ± 3 | 381 | 37 ± 2 | < .001 | 0.71 (0.59–0.85) | ||
| Relapse | 103 | 32 ± 3 | 354 | 59 ± 2 | < .001 | 0.46 (0.37–0.57) | ||
| TRM | 68 | 21 ± 2 | 27 | 4 ± 1 | < .001 | 3.99 (2.55–6.25) | ||
| Good risk | 32 | 73 | ||||||
| Survival | 9 | 84 ± 6 | 17 | 78 ± 5 | .99 | 1.01 (0.45–2.27) | ||
| DFS | 11 | 72 ± 8 | 28 | 64 ± 6 | .40 | 0.74 (0.37–1.50) | ||
| Relapse | 7 | 22 ± 7 | 24 | 32 ± 6 | .17 | 0.57 (0.24–1.31) | ||
| TRM | 4 | 6 ± 4 | 4 | 4 ± 2 | .42 | 1.80 (0.44–7.31) | ||
| Intermediate risk | 178 | 333 | ||||||
| Survival | 76 | 57 ± 4 | 172 | 48 ± 3 | .23 | 0.85 (0.65–1.11) | ||
| DFS | 82 | 53 ± 4 | 199 | 41 ± 3 | .014 | 0.73 (0.56–0.94) | ||
| Relapse | 50 | 28 ± 3 | 188 | 55 ± 3 | < .001 | 0.47 (0.34–0.64) | ||
| TRM | 32 | 19 ± 3 | 11 | 3 ± 1 | < .001 | 5.13 (2.58–10.2) | ||
| Poor risk | 116 | 193 | ||||||
| Survival | 72 | 40 ± 5 | 137 | 30 ± 4 | .17 | 0.82 (0.62–1.09) | ||
| DFS | 78 | 33 ± 4 | 154 | 17 ± 3 | .003 | 0.67 (0.51–0.88) | ||
| Relapse | 46 | 39 ± 5 | 142 | 77 ± 3 | < .001 | 0.43 (0.31–0.60) | ||
| TRM | 32 | 28 ± 4 | 12 | 6 ± 2 | < .001 | 3.47 (1.78–6.77) | ||
| Age younger than 40 y | 174 | 316 | ||||||
| Survival | 73 | 61 ± 4 | 164 | 49 ± 3 | .015 | 0.71 (0.54–0.94) | ||
| DFS | 81 | 55 ± 4 | 204 | 37 ± 3 | < .001 | 0.59 (0.46–0.77) | ||
| Relapse | 51 | 28 ± 3 | 187 | 58 ± 3 | < .001 | 0.41 (0.30–0.56) | ||
| TRM | 30 | 17 ± 3 | 17 | 5 ± 1 | .002 | 2.6 (1.4–4.75) | ||
| Age older than 40 y | 152 | 283 | ||||||
| Survival | 84 | 44 ± 4 | 162 | 42 ± 3 | .84 | 0.97 (0.75–1.27) | ||
| DFS | 90 | 39 ± 4 | 177 | 36 ± 3 | .15 | 0.83 (0.64–1.07) | ||
| Relapse | 52 | 35 ± 4 | 167 | 60 ± 3 | < .001 | 0.51 (0.37–0.70) | ||
| TRM | 38 | 25 ± 4 | 10 | 4 ± 1 | < .001 | 6.07 (3.0–12.24) | ||
HR indicates hazard ratio for donor compared with no donor from multivariate Cox model adjusted for risk and age.
P from likelihood ratio test.
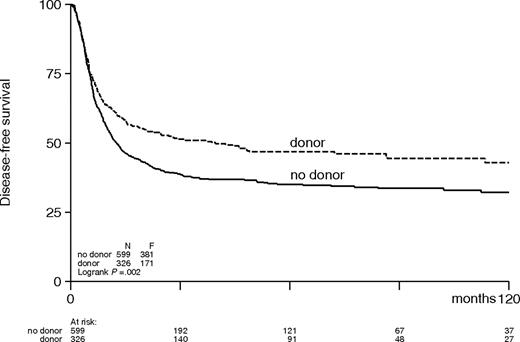
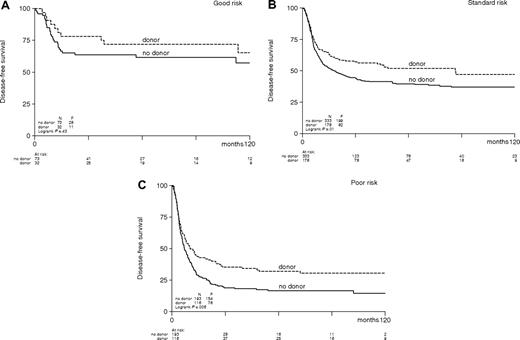
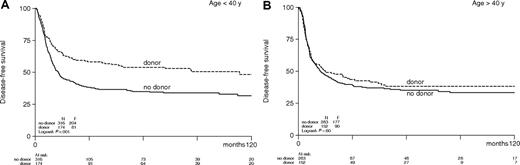
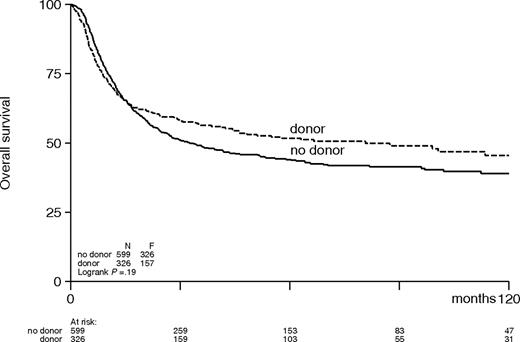
The combined effects of a lower relapse rate and an increased TRM in the donor group resulted in a significantly better DFS in the donor group compared with the no-donor group (48% versus 27%; HR, 0.70; 95% CI, 0.59-0.84; P < .001; Table 4; Figure 1). The improvement in DFS was observed in all AML prognostic risk categories with estimated hazard ratios, ranging between 0.74 and 0.67 (Table 4; Figure 2) but were only significant in intermediate-risk and poor-risk patients. The improvement of DFS was most pronounced in patients younger than the age of 40 with a donor (HR, 0.59; 95% CI, 0.46-0.77; P < .001) and less in adults 40 to 55 years (HR, 0.83; 95% CI, 0.64-1.07; P = .15; Figure 3). The improved DFS in the donor group did translate in a better OS, but the difference was less pronounced: 54% versus 46% OS at 4 years in the donor group compared with the no-donor group (HR, 0.84; 95% CI, 0.69-1.02; P = .07) (Table 4; Figure 4). The improvement in OS was observed in intermediate- and poor-risk categories, but it was not apparent in good-risk patients (HR, 1.01; 95% CI, 0.44-2.28; P = .99). The full details of the multivariable Cox regression analyses are shown in Table 5. Intermediate- and poor-risk features appeared as strong adverse risk factors for both DFS and OS. Furthermore, age significantly affected survival. OS was 53% (± 3%) at 4 years for patients younger than 40 years, and it was 45% (± 3%) for older patients (> 40 years) (P < .001). Other multivariable Cox regression analyses with end points OS and DFS were performed by adding more variables to the model: sex, WBC count at diagnosis, cycles of chemotherapy needed to obtain CR1, and time from start of treatment to start consolidation. Of these, only a higher WBC count was associated with worse OS and DFS. The addition of these covariates did not change the HR for donor availability on the end points OS and DFS.
Actuarial rates of disease-free survival of patients with acute myeloid leukemia in first complete remission according to donor availability.
Actuarial rates of disease-free survival of patients with acute myeloid leukemia in first complete remission according to donor availability.
Actuarial disease-free survival of patients with acute myeloid leukemia in first complete remission according to risk category and donor availability. (A) Good risk (P = .43), (B) intermediate risk (P = .01), (C) poor risk (P = .006).
Actuarial disease-free survival of patients with acute myeloid leukemia in first complete remission according to risk category and donor availability. (A) Good risk (P = .43), (B) intermediate risk (P = .01), (C) poor risk (P = .006).
Actuarial disease-free survival of patients with acute myeloid leukemia in first complete remission according to age category and by donor availability.
Actuarial disease-free survival of patients with acute myeloid leukemia in first complete remission according to age category and by donor availability.
Actuarial overall survival of patients with acute myeloid leukemia in first complete remission according to donor availability.
Actuarial overall survival of patients with acute myeloid leukemia in first complete remission according to donor availability.
Results of multivariate analysis in which donor availability, prognostic category, and age were considered
| Parameter . | Overall survival . | Disease-free survival . | Relapse . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Donor availability* | 0.85 | (0.70–1.03) | .09 | 0.71 | (0.59–0.85) | < .001 | 0.46 | (0.37–0.57) | < .001 |
| Prognostic category† | < .001 | < .001 | < .001 | ||||||
| Intermediate | 2.50 | (1.67–3.76) | 1.83 | (1.30–2.56) | 1.97 | (1.35–2.87) | |||
| Poor risk | 4.53 | (3.01–6.83) | 3.49 | (2.48–4.90) | 3.67 | (2.51–5.38) | |||
| Age older than 40‡ | 1.39 | (1.16–1.67) | < .001 | 1.23 | (1.03–1.45) | .02 | 1.19 | (0.98–1.43) | .07 |
| Parameter . | Overall survival . | Disease-free survival . | Relapse . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Donor availability* | 0.85 | (0.70–1.03) | .09 | 0.71 | (0.59–0.85) | < .001 | 0.46 | (0.37–0.57) | < .001 |
| Prognostic category† | < .001 | < .001 | < .001 | ||||||
| Intermediate | 2.50 | (1.67–3.76) | 1.83 | (1.30–2.56) | 1.97 | (1.35–2.87) | |||
| Poor risk | 4.53 | (3.01–6.83) | 3.49 | (2.48–4.90) | 3.67 | (2.51–5.38) | |||
| Age older than 40‡ | 1.39 | (1.16–1.67) | < .001 | 1.23 | (1.03–1.45) | .02 | 1.19 | (0.98–1.43) | .07 |
Prognostic category according to cytogenetics, WBC count, and early or late attainment of CR (see “Patients, materials, and Methods”).
HR indicates hazard ratio; CI, confidence interval.
*In comparison with no donor group.
†In comparison with good-risk group.
‡In comparison with age younger than 40 group.
One hundred five patients received an allogeneic peripheral blood stem cell graft, 165 patients received an allogeneic bone marrow graft, and the source of stem cells was not detailed in 10 patients. No differences with respect to relapse, TRM, and DFS were noted between recipients of blood stem cell and bone marrow grafts. TRM was also evaluated in early and late trial eras. No improvement of TRM over time could be demonstrated in recipients who had received their allograft after the median date of inclusion (date not shown) as compared with the early transplants.
Salvage treatment after relapse
The smaller difference in OS between the donor and no-donor group compared with the difference in DFS can be explained by the effect of salvage treatment. Most of the 457 relapsed patients received salvage treatment (88%), either chemotherapy (51%) or an autologous or allogeneic transplant (37%). Forty percent of the relapsing patients reached a second CR (CR2, n = 184), but most of these either relapsed again (93 of 184; 51%) or died in CR2 (46 of 184; 25%). At the time of analysis 69 of the relapsed patients were still alive of whom 45 were in continued CR2. The actuarial probability of survival after relapse at 3 years was 16% in the donor group and 15% in the no-donor group. Because in the donor group relatively more patients died in CR1 and fewer relapsed as compared with the no-donor group, the net contribution of salvage treatment to OS was larger in the no-donor group.
Actual application of intended allogeneic SCT
The donor/no-donor analysis was performed on an intention-to-treat basis. On one hand an intention-to-treat analysis may underestimate the beneficial effect of allo-SCT on relapse, but on the other hand it may also underestimate the adverse effects of allo-SCT on TRM.17 In this study, 268 patients (82%) in the donor group (n = 326) actually received an HLA-identical sibling SCT in CR1. Reasons for not performing the intended transplantation included predominantly early relapse, poor condition, and toxicity. The 268 actual transplant recipients experienced a DFS of 53% (± 3%), as a result of a relapse rate of 22% (± 3%) and a TRM of 24% at 4 years. The DFS of the patients in the donor group, who did not proceed to transplantation, was only 22%. These results obviously indicate that the actual transplant recipients represent a positively selected group of patients. As the relapse rate was only 22% (± 3%) in the 268 allo-SCT recipients, but estimated 31% (± 3%) in the whole group of patients with a donor, especially the beneficial effect of allo-SCT on relapse may be underestimated by an intention-to-treat analysis, as was suggested before.17
Discussion
The role of allo-SCT in younger adults with AML in CR1 has remained the subject of scientific debate for more than 2 decades. A limited number of large studies, using an intention-to-treat analysis compared outcome of patients with a donor versus those without a donor. As the availability of a sibling donor is essentially a random process, a donor versus no-donor study serves as a valuable alternative for true randomized studies.15,16 Some earlier reports had provided evidence for improved DFS. However, in none of the studies evaluating allo-SCT was a benefit in OS demonstrated.3–9,13 In addition, discrepant results were obtained in cytogenetically defined subsets of AML with marked differences in relapse risk.8,9,13 Although the necessity to study these categories is undisputed, breaking down a large cohort of patients into smaller subgroups unavoidably results in loss of statistical power to detect a difference. Therefore, prospective studies of appreciable size, which allow for comparison with other studies addressing a comparable question, and which permit a meta-analysis remain of importance to more definitely establish the role of allo-SCT as a consolidation therapy in AML in first remission.
The HOVON-SAKK cooperative consortium has conducted 3 successive upfront trials in adults with AML aged younger than 60 years, in which allo-SCT has consistently been standard treatment for patients achieving CR and having an HLA-identical sibling donor.18–21 To deliver allo-SCT shortly after the second course of chemotherapy as scheduled in these studies, HLA-typing of the patient and his or her relatives was performed early after study entry, that is, during the phase of induction chemotherapy. The present study shows that more than 90% of patients were indeed evaluated for the presence of a donor, which subsequently resulted in a high access (82%) to allo-SCT on achieving remission. According to the intention-to-treat analysis, a strong reduction of relapse was observed in the patients with a donor, which translated into enhanced DFS for the donor group and resulted in an appreciable net gain of 10% in DFS (Table 4; Figure 1). That favorable effect appeared most pronounced in patients with intermediate or poor risk, while no statistically significant benefit was obtained in patients with good risk. Currently, myeloablative allo-SCT is not universally recommended for patients with AML in CR1 with favorable subtypes of AML, where the risk of relapse is 35% or less.22,23 This applies to the so-called core binding factor leukemias [AML t(8;21), AML inv(16)], and acute promyelocytic leukemia where allo-SCT is usually reserved for relapse. This is done to avoid the unnecessary risks of TRM and chronic graft-versus-host disease, which may lead to excess mortality and adversely affect quality of life, in a considerable number of patients, who may be cured with chemotherapy alone. Would it perhaps also be better to postpone transplantation in patients with AML of intermediate or poor risk until the signs of first relapse? There are no data allowing a direct and unequivocal answer to this question. An approach of a purposeful delay of allogeneic SCT has not prospectively been studied in patients with intermediate- or poor-risk AML. However, a purposeful delay of upfront autologous SCT in first CR has been studied in a randomized way in the HOVON 29 and 42 studies. Patients without a sibling donor were randomly assigned between an autograft or third cycle of chemotherapy, with the intention to reserve the graft for the treatment of relapse. In that setting, approximately 20% (W.P., unpublished observations, HOVON-SAKK, May 2006) of relapsing patients ultimately received their preserved autograft in second CR. A high-resistance rate and enhanced toxicity had prohibited a higher accessibility to a late autograft. By extrapolation, this observation suggests that a strategy of postponing allo-SCT may result in a considerable risk of in fact loosing the opportunity of allo-SCT. If the assessment of minimal residual disease (MRD), however, would be validated in the future as a reliable and generally applicable method for identifying patients with AML with either a high or very low risk of relapse, then the issue of studying a purposeful delay of allo-SCT might regain urgency in patients with AML of intermediate or poor risk, but with a very low risk of relapse based on MRD assessment.
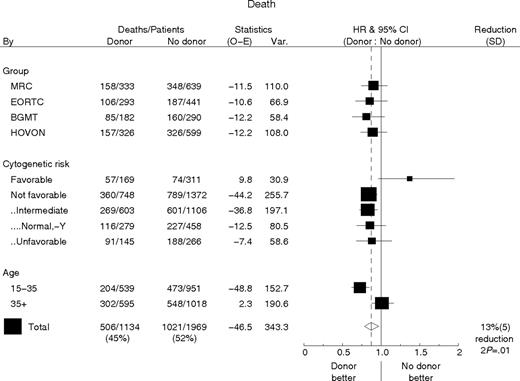
Although the beneficial effect of allo-SCT on DFS was most prominent in intermediate-risk AML in the MRC study,8 the EORTC-GIMEMA study had shown that DFS was better in patients with a donor only in case of poor-risk AML.9 Given these not entirely concordant results and the absence of a significant benefit in OS, we performed a meta-analysis with the HOVON-SAKK data and the data of the MRC, EORTC, and BGMT studies using numbers and figures as presented in their initial reports,8–10 or kindly supplemented on request. It offered the opportunity for a study in an accumulated number of more than 4000 patients with AML in CR1 with markedly enhanced statistical power. DFS and OS results were analyzed by donor availability and broken down for cytogenetic risk category as well as age category. Figures 5 and 6 show the forest plots of the hazard ratios for DFS and OS of donor versus no donor, split by study, cytogenetic risk, and age group and the overall estimate together with 95% confidence intervals. The findings from all 4 studies are highly consistent, and the confidence intervals of the hazard ratio estimates for the separate studies all contain the pooled estimate. With end point DFS the pooled estimate of the hazard ratio is 0.79 (95% CI, 0.72-0.88; P < .001) (Figure 5). However this benefit is not observed in the small subgroup of patients with favorable cytogenetics, whereas the benefit appeared similar in various subgroups of patients without favorable cytogenetics. The benefit in DFS for patients with a donor is most pronounced in younger patients younger than age 35 (HR, 0.67; 95% CI, 0.58-0.78) and smaller, but not absent for older patients (HR, 0.92; 95% CI, 0.80-1.05).
Disease-free survival by donor availability with the hazard ratio (HR) plots for study group, risk, and age subgroups. The percentage reduction is equal to 100 × (1 minus] HR). Patients without cytogenetic information are not included in a cytogenetic risk subgroup. The not-favorable cytogenetic group consists of all patients lacking favorable cytogenetic abnormalities. Within that group several subgroups are considered. All patients not classified as favorable or unfavorable were considered intermediate risk. Normal, −Y is a subgroup of the intermediate group and contains all patients without cytogenetic abnormalities or with only −Y, as was derived from the EORTC, BGMT, and HOVON series. The unfavorable group includes all patients classified as bad or very bad in the EORTC series or as unfavorable in the other studies (MRC, HOVON-SAKK, BGMT). The pooled estimate of the HR of donor availability for DFS for all patients irrespective of age or cytogenetic subgroup is 0.79 (95% CI, 0.72-0.88; P < .001).
Disease-free survival by donor availability with the hazard ratio (HR) plots for study group, risk, and age subgroups. The percentage reduction is equal to 100 × (1 minus] HR). Patients without cytogenetic information are not included in a cytogenetic risk subgroup. The not-favorable cytogenetic group consists of all patients lacking favorable cytogenetic abnormalities. Within that group several subgroups are considered. All patients not classified as favorable or unfavorable were considered intermediate risk. Normal, −Y is a subgroup of the intermediate group and contains all patients without cytogenetic abnormalities or with only −Y, as was derived from the EORTC, BGMT, and HOVON series. The unfavorable group includes all patients classified as bad or very bad in the EORTC series or as unfavorable in the other studies (MRC, HOVON-SAKK, BGMT). The pooled estimate of the HR of donor availability for DFS for all patients irrespective of age or cytogenetic subgroup is 0.79 (95% CI, 0.72-0.88; P < .001).
Overall survival by donor availability with the hazard ratio (HR) plots for study group, risk, and age subgroups. The percentage reduction is equal to 100 × (1 − HR). Patients without cytogenetics are not included in a cytogenetic risk subgroup. The not-favorable cytogenetic group consists of all patients without favorable cytogenetics (see legend Figure 5 for subgroup classification). The pooled estimates of the HR of donor availability for all patients irrespective of age and or cytogenetic subgroup for OS is 0.87 (95% CI, 0.79-0.97; P = .01) and 0.84 for the group of patients without favorable cytogenetics (95% CI, 0.74-0.95; P < .01).
Overall survival by donor availability with the hazard ratio (HR) plots for study group, risk, and age subgroups. The percentage reduction is equal to 100 × (1 − HR). Patients without cytogenetics are not included in a cytogenetic risk subgroup. The not-favorable cytogenetic group consists of all patients without favorable cytogenetics (see legend Figure 5 for subgroup classification). The pooled estimates of the HR of donor availability for all patients irrespective of age and or cytogenetic subgroup for OS is 0.87 (95% CI, 0.79-0.97; P = .01) and 0.84 for the group of patients without favorable cytogenetics (95% CI, 0.74-0.95; P < .01).
With end point OS the pooled estimate of the hazard ratio for donor availability is 0.87 (95% CI, 0.79-0.97; P = .01; Figure 6). Also with respect to OS the benefit is restricted to patients without favorable cytogenetics and is most pronounced in younger patients (HR, 0.73; 95% CI, 0.621-0.85) and absent in older (> 35 years) patients (HR, 1.01; 95% CI, 0.88-1.17). Although this analysis strengthens the HOVON-SAKK observation of enhanced DFS in patients with either intermediate- or poor-risk AML with a sibling donor, it also demonstrates a statistically significant OS benefit of 12% (HR, 0.84; 95% CI, 0.74-0.95) for all patients without favorable cytogenetics, including those with a normal or −Y karyotype. Collectively, these results indicate that the beneficial effect of allo-SCT becomes apparent as soon as the risk of relapse exceeds approximately 35%, irrespective of the specific type of underlying cytogenetic abnormality that causes the higher relapse rate. Only favorable-risk patients with a risk of relapse below 35% do not profit from allo-SCT in terms of overall survival because TRM attenuates the beneficial effect of allo-SCT in those patients. Whether that conclusion also applies to patients, who may be further subclassified on the basis of molecular abnormalities such as mutations of CEBPα, Flt3, and NMP1, remains to be demonstrated. Those molecular data were not available in the present study with long-term follow-up, but will be the subject of future study.
Apart from the well-known adverse effect of an intermediate- or poor-risk profile on outcome, also age emerged as an important risk factor following multivariable analysis (Table 6). Both the results of the HOVON-SAKK study and the meta-analysis indicate that the advantage of an available donor is at best modest in patients older than the age of 40 years (Figures 5 and 6). Suciu et al9 had earlier reported that the beneficial effect of allo-SCT in poor-risk patients was most prominent in younger patients. The current scientific challenge to make allo-SCT a safer treatment modality in older patients seems a priority issue that already concerns those aged 40 and older. The adverse affect of increasing age on outcome of allo-SCT was especially expressed in an age-related rise of treatment-related complications. Although other studies have suggested a progressive reduction of TRM during the past decades,24,25 no such effect could be demonstrated in the present study. It may suggest that improved supportive care has only moderately affected overall outcome following myeloablative allo-SCT. In contrast, several recent studies have suggested that TRM may significantly be reduced in older (> 50 years) patients with AML following allo-SCT and reduced intensity conditioning (RIC).26,27 Mohty et al28 recently reported the first donor versus no-donor analysis in older patients with AML, for whom an RIC allo-SCT was intended following the identification of a matched sibling donor. Although the study included only a limited number of patients, improved DFS was suggested for patients with a donor. Meanwhile, other studies have shown that RIC allo-SCT using matched unrelated donors may result in comparable overall outcome as RIC allo-SCT using sibling donors.29–32
In conclusion, HLA-matched sibling allo-SCT following myeloablative conditioning should currently be considered as the treatment of choice in younger adults with intermediate or poor-risk AML in CR1. Therefore, an early evaluation of donor availability and subsequent commitment to proceed to allo-SCT in CR1 would be strongly recommended in these categories of AML patients.
Authorship
Contribution: J.J.C., W.L.J.vP., B.L. created the initial design of the present analysis, performed the actual evaluation, and wrote the manuscript; L.F.V., M.T., E.J., S.M.G.D., M.vM.K., P.W., H.S., P.C.H., H.vdL., M.F., A.F., J.M., A.G., J.J.C., W.L.J.vP., and B.L. designed the HOVON studies (4, 29, 42) treated patients, critically reviewed the manuscript, made suggestions for additional analysis, and finalized the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the HOVON-SAKK study group appears in Document S1 (available on the Blood website; see the Supplemental Document link at the top of the online article).
Correspondence: Jan J. Cornelissen, Erasmus University Medical Center, Department of Hematology, Groene Hilledijk 301, 3075 EA Rotterdam, The Netherlands; e-mail: j.cornelissen@erasmusmc.nl.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Stefan Suciu (EORTC) and Didier Blaise (BGMT) for providing details that enabled us to do the meta-analysis and Janine Vrij for secretarial assistance.
This work was supported by the Queen Wilhelmina Fund (KWF)–Kankerbestrijding for support of data management.

![Figure 5. Disease-free survival by donor availability with the hazard ratio (HR) plots for study group, risk, and age subgroups. The percentage reduction is equal to 100 × (1 minus] HR). Patients without cytogenetic information are not included in a cytogenetic risk subgroup. The not-favorable cytogenetic group consists of all patients lacking favorable cytogenetic abnormalities. Within that group several subgroups are considered. All patients not classified as favorable or unfavorable were considered intermediate risk. Normal, −Y is a subgroup of the intermediate group and contains all patients without cytogenetic abnormalities or with only −Y, as was derived from the EORTC, BGMT, and HOVON series. The unfavorable group includes all patients classified as bad or very bad in the EORTC series or as unfavorable in the other studies (MRC, HOVON-SAKK, BGMT). The pooled estimate of the HR of donor availability for DFS for all patients irrespective of age or cytogenetic subgroup is 0.79 (95% CI, 0.72-0.88; P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-06-025627/4/m_zh80090700230005.jpeg?Expires=1767771617&Signature=V48mPn~ZkKBhfpvanl8pxBDvMFsQaCoDqF84m4-r9QDbvaVaZo2S23PZDKMxw2EqXhp7Wk4YV1aaux8-YIgVquvI7cUrPvMifMUsmHAWJnqrXUjGfCyxA8KZ0oX7dYj6ziELNzxqYBGjlVptUT1IMpImWFSiJSZtFbQlrtK2uKpFZv-ZlnL4z2nX8KKtSKjtBdFyT7Y1J64RCmLsw~~QiR3V11obC7Y42WeJsCWzn8u6gswsROX7AImv4lcoBd0dWLoFsQm3jJBO4xi3VT9JEgztKqFMuP3~cnq8dUzb2XM1yV-rYuQOPmIMf8SQcMenhSkkjMnvZTKfmWEpjTmgfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal