Abstract
Prior to 1990, many patients with inherited bleeding disorders were infected with hepatitis C virus (HCV). This study assessed the risk of end-stage liver disease (ESLD) in patients with hemophilia with chronic hepatitis C. Patients were infected between 1961 and 1990 and were followed up to August 2005. Of 847 anti-HCV+ patients, 160 (19%) spontaneously cleared HCV and 687 (81%) developed chronic hepatitis C. Coinfection with HIV was present in 210 patients. After 35 years of infection the cumulative incidence of ESLD was 11.5% (95% CI, 8.2%-14.8%) in HIV− patients and 35.1% (95% CI, 29.2%-41.0%; P < .001) in patients coinfected with HIV. Independent risk factors of ESLD were HIV coinfection (hazard ratio 13.8; 95% CI, 7.5-25.3), older age at infection (hazard ratio 2.3 per 10 years; 95% CI, 2.0-2.8), alcohol abuse (hazard ratio 4.9; 95% CI, 2.5-9.6), and presence of HCV genotype 1 (hazard ratio 2.2; 95% CI, 1.1-4.2). With longer duration of HCV infection, the risk of developing ESLD is emerging in patients with inherited bleeding disorders. Risk factors for rapid progression to ESLD are alcohol abuse, coinfection with HIV, older age at infection, and presence of HCV genotype 1.
Introduction
Prior to 1990, many patients with hereditary bleeding disorders were infected with hepatitis C virus (HCV) through replacement therapy with inadequately or non–virus-inactivated clotting factor products during the 1970s and 1980s.1–4 Once infected, about 80% of patients develop chronic hepatitis C.5 After 20 years of infection, 10% of patients progress to cirrhosis and an additional 10% to end-stage liver disease (ESLD; ie, liver failure, hepatocellular carcinoma, liver-related death).6 HIV coinfection, alcohol abuse, older age at infection, and male sex are the main determinants associated with faster progression of liver disease.7–11 Whether highly active antiretroviral therapy (HAART) improves the natural course in patients coinfected with HCV/HIV remains controversial.12–15
Growing evidence indicates that progression of fibrosis in patients with chronic hepatitis C may not be linear.16,17 Patients infected for several decades may be at higher risk for developing ESLD. These findings indicate the need for new studies on patients infected for longer than 25 years.
To determine the natural history of HCV infection, the onset of infection must be identified, and information on its full course and its potential modifiers must be obtained.18 These criteria may be met in a cohort of patients with inherited bleeding disorders because data on exposures to clotting products are recorded and patients are regularly tested for viral infections.19 Furthermore, these patients are seen on a yearly basis for their bleeding problems, resulting in a reliable follow-up independent of HCV status or liver disease. In addition, they form a homogeneous group with the same route of infection and are almost all male. The aim of the present study was to assess ESLD in a cohort of HCV-infected patients with inherited bleeding disorders.
Patients, materials, and methods
Study design and participants
This international, multicenter cohort study constitutes all HCV antibody-positive patients with congenital bleeding disorders from hemophilia treatment centers of Sheffield, United Kingdom (Royal Hallamshire Hospital), London, United Kingdom (Royal Free Hospital), and Utrecht, The Netherlands (Van Creveldkliniek). Patients were seen at least annually, and blood samples, ultrasounds of liver and spleen, and physical examination were routinely performed. Patients or their guardians gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the institutional review boards at all participating centers.
Laboratory methods
HCV and HIV antibody, HCV RNA, and HCV genotype tests were performed in the local hospital laboratories as described previously.8,9,20 Hepatitis C status was defined as “spontaneous clearance” (positive anti-HCV antibodies but negative HCV RNA tests on at least 2 occasions at least 6 months apart), or “chronic hepatitis C” (positive anti-HCV antibodies and persistently positive HCV RNA).
Definition of HCV infection, natural history, and ESLD
The date of first exposure to large pool concentrates or cryoprecipitate was assumed to be the date of infection. For 152 patients (18%) the precise date was unknown and the date of July 1977 (London, United Kingdom), January 1972 (Sheffield, United Kingdom), or January 1970 (Utrecht, The Netherlands) was taken. These different dates correspond to the median date of introduction of clotting factor in the different centers. For patients born after these dates it was assumed to be the date of their first birthday. Patients were infected between 1961 and 1990.
The natural history started at the date of HCV infection and ended at the date of ESLD, death, start of antiviral therapy for HCV, or last clinical evaluation, whichever came first. Thus, ESLD over HCV infection time was studied in untreated patients. ESLD was defined as the occurrence of liver failure (ascites, bleeding esophageal varices, hepatic encephalopathy), hepatocellular carcinoma, or liver-related death. Alcohol abuse was defined as intake of more than 20 units alcohol per week or 3 or more units per day.
Data analysis
To estimate the cumulative incidence of ESLD over time since infection, the Kaplan-Meier survival table method was used. The cumulative incidence is the probability of ESLD over follow-up time, taking censoring into account (eg, proportion of patients with ESLD after 30 years of infection). The analysis was stopped when fewer than 10% of the patients remained under observation. The hazard rates (= incidence rates) for ESLD were calculated as the number of cases divided by the patient-years at risk and expressed in cases per 100 person-years (ie, the probability of ESLD in a certain period, given that the patient is ESLD free at the beginning of that period). Cumulative incidences and hazard rates for ESLD were both calculated for the total cohort of patients with chronic hepatitis C and after stratification for HIV. In addition, hazard rates were also calculated for the first, second, and third decade of infection.
Cox proportional hazards models were used to assess the effect of several determinants on the risk of ESLD. The model yields a hazard ratio, which is a ratio of the incidence of ESLD with a specific determinant over the incidence without that determinant. The natural history (ie, untreated infection time) was the survival time variable. Determinants used for the analyses were age at infection, history of alcohol abuse, HCV genotype, and HIV/HAART. Since only 2.8% of patients were coinfected with hepatitis B, this determinant was not used in the analyses. Patients were not tested for hepatitis D virus. Nevertheless, although HDV is relatively frequent in Mediterranean countries, its prevalence is very low in Northern European countries. CD4+ lymphocyte counts were not used in the analyses due to incomplete data or the availability of CD4 counts only at time points when patients had already developed ESLD or AIDS.
To prevent survival bias, HAART was considered as a time-dependent variable, taking the value of 0 prior to the time HAART was given and 1 thereafter. Genotype was not available for 20% of patients. Therefore, we used imputation techniques (missing value analysis, SPSS 12.0, Chicago, IL) to predict the missing genotypes using regression models. We performed the analyses on ESLD both without those patients and with imputation of missing genotype. Because results were similar, further analyses were performed with imputed genotype.
Results
Patient characteristics
The present cohort comprises all 847 patients with HCV antibodies evaluated up to August 2005. Patient characteristics are shown in Table 1. The median age at first exposure was 14 years (range, < 1-77 years) and the median age at end of follow-up was 43 years (range, 11-87 years). The median follow-up time since infection was 27 years (range, 3-42 years) and total follow-up time since infection was 22 259 person-years. Patients suffered predominantly from hemophilia A and B (91%). A history of alcohol abuse was present in 70 patients.
Patient characteristics
| No. of patients | 847 |
| Age at end of follow-up, y* | 43 (11-87) |
| Male sex, no. (%) | 799 (94) |
| Diagnosis, no. (%) | |
| Hemophilia A | 640 (76) |
| Hemophilia B | 127 (15) |
| Other | 80 (9) |
| Total no. of deaths | 199 (24) |
| Cause of death, no. (%)† | |
| AIDS | 73 (37) |
| Liver-related | 55 (28) |
| Other | 71 (36) |
| Hepatitis C genotype, no. (%)‡ | |
| Type 1 | 361 (53) |
| Type 2-5 | 179 (27) |
| Missing | 147 (20) |
| Time from HCV infection to end of follow-up, y | 27 (3-42) |
| ESLD, no. | 71 |
| Liver failure | 59 |
| Hepatocellular carcinoma | 13 |
| Liver transplantation | 9 |
| Liver-related death | 55 |
| Coinfection with HIV, no. (%) | 210 (25) |
| HIV treatment | 161 of 210 (77) |
| HAART§ | 78 of 116 (67) |
| History of alcohol abuse, no. (%) | 70 (8) |
| No. of patients | 847 |
| Age at end of follow-up, y* | 43 (11-87) |
| Male sex, no. (%) | 799 (94) |
| Diagnosis, no. (%) | |
| Hemophilia A | 640 (76) |
| Hemophilia B | 127 (15) |
| Other | 80 (9) |
| Total no. of deaths | 199 (24) |
| Cause of death, no. (%)† | |
| AIDS | 73 (37) |
| Liver-related | 55 (28) |
| Other | 71 (36) |
| Hepatitis C genotype, no. (%)‡ | |
| Type 1 | 361 (53) |
| Type 2-5 | 179 (27) |
| Missing | 147 (20) |
| Time from HCV infection to end of follow-up, y | 27 (3-42) |
| ESLD, no. | 71 |
| Liver failure | 59 |
| Hepatocellular carcinoma | 13 |
| Liver transplantation | 9 |
| Liver-related death | 55 |
| Coinfection with HIV, no. (%) | 210 (25) |
| HIV treatment | 161 of 210 (77) |
| HAART§ | 78 of 116 (67) |
| History of alcohol abuse, no. (%) | 70 (8) |
Values are numbers (%) or medians (ranges).
*End of follow-up is death or last evaluation.
Proportion of patients died.
Genotype of HCV of 687 patients with chronic hepatitis C.
A total of 116 patients with HIV were alive when HAART became available.n
Viral status
Of all 847 patients, 687 (81%) developed chronic hepatitis C; 210 patients (25%) were coinfected with HIV. In total, 160 (of 847, 19%) spontaneously cleared HCV. At introduction of HAART in 1996-1997, 116 patients with HIV were still alive and 78 of them (67%) have since received HAART.
Cause of death
Overall, 199 patients (24%) died; 73 patients (37%) died of HIV/AIDS, 55 (28%) of liver disease, and 71 (36%) due to other causes.
ESLD
In total, 71 patients developed ESLD. One patient who cleared HCV spontaneously developed ESLD after long-term alcohol abuse. The remaining 70 patients with chronic hepatitis C developed ESLD after a median of 21 years (range, 8-36 years) of infection. Of these, 50 had 2 or more features of ESLD. Liver failure was present in 59, hepatocellular carcinoma in 13, and liver-related death in 55 patients. Nine patients with ESLD underwent liver transplantation.
Of the 70 patients with chronic hepatitis C who developed ESLD, 58 patients had not been treated with anti-HCV therapy (ie, developed ESLD during natural history). The remaining 12 patients developed ESLD after unsuccessful anti-HCV therapy. So only the 58 untreated patients were the ‘cases' in the Kaplan-Meier survival table, the Hazard rate, and the Cox proportional hazards model.
Cumulative incidence of ESLD
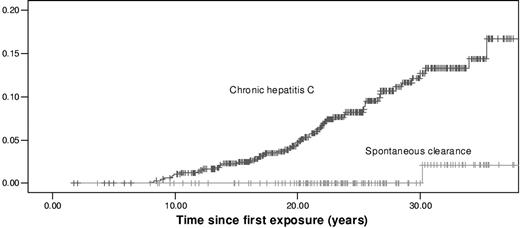
After 35 years of infection, the cumulative incidence of ESLD in all patients with chronic hepatitis C was 17.1% (95% CI, 14.0%-20.2%). In contrast, it was 2.1% (95% CI, 1.0%-3.2%) in patients who spontaneously cleared HCV (P < .001; Figure 1).
Cumulative incidences of ESLD in 687 patients with chronic hepatitis C and in 160 patients who spontaneously cleared HCV.
Cumulative incidences of ESLD in 687 patients with chronic hepatitis C and in 160 patients who spontaneously cleared HCV.
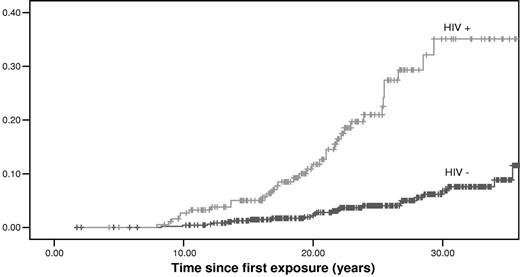
HIV was an important determinant of outcome; the cumulative incidence of ESLD increased from 11.5% (95% CI, 8.2%-14.8%) in HIV− patients to 35.1% (95% CI, 29.2%-41.0%) in patients coinfected with HIV (P < .001; Figure 2).
Cumulative incidences of ESLD in patients with chronic hepatitis C according to their HIV status. A total of 190 patients were HIV+ and 497 HIV−.
Cumulative incidences of ESLD in patients with chronic hepatitis C according to their HIV status. A total of 190 patients were HIV+ and 497 HIV−.
Hazard rate of ESLD
The risk of developing ESLD increased with duration of infection. In patients with chronic hepatitis C (n = 687), the overall hazard rate of ESLD was 0.36/100 person-years (Table 2). However, when stratified per 10 years of infection it increased from 0.10 in the first 10 years of infection to 0.90 after more than 20 years of infection. For HIV− patients with chronic hepatitis C (n = 497), the hazard rate increased from 0.04 in the first 10 years to 0.53 after more than 20 years. In patients coinfected with HIV (n = 190), it increased from 0.27 to 2.63.
Hazard rates of ESLD (per 100 person-years) for 687 patients with chronic hepatitis C
| . | Duration of infection . | |||
|---|---|---|---|---|
| Fewer than 10 y . | 10-20 y . | Longer than 20 y . | Total y . | |
| Chronic hepatitis C (n = 687) | 7/6820 = 0.10 | 20/5973 = 0.33 | 31/3460 = 0.90 | 58/16253 = 0.36 |
| HIV− (n = 497) | 2/4939 = 0.04 | 7/4504 = 0.15 | 15/2851 = 0.53 | 24/12294 = 0.20 |
| HIV+ (n = 190) | 5/1881 = 0.27 | 13/1469 = 0.88 | 16/608 = 2.63 | 34/3958 = 0.86 |
| . | Duration of infection . | |||
|---|---|---|---|---|
| Fewer than 10 y . | 10-20 y . | Longer than 20 y . | Total y . | |
| Chronic hepatitis C (n = 687) | 7/6820 = 0.10 | 20/5973 = 0.33 | 31/3460 = 0.90 | 58/16253 = 0.36 |
| HIV− (n = 497) | 2/4939 = 0.04 | 7/4504 = 0.15 | 15/2851 = 0.53 | 24/12294 = 0.20 |
| HIV+ (n = 190) | 5/1881 = 0.27 | 13/1469 = 0.88 | 16/608 = 2.63 | 34/3958 = 0.86 |
Risk of ESLD related to duration of infection. The risk of ESLD increased with infection time.
Risk of ESLD
The Cox proportional hazards model was used to analyze the effect of age at infection, HCV genotype, HIV and HAART status, and alcohol abuse on the development of ESLD (Table 3). In the univariate analysis, HAART appeared to be associated with reduced risk of ESLD, but this was not statistically significant. In the multivariate analysis, ESLD risk was increased with HIV infection (hazard ratio 13.8; 95% CI, 7.5-25.3), that is, independent of other risk factors, patients with HIV had a 13.8 times higher incidence of ESLD compared to HIV− patients. Older age at infection (hazard ratio 2.3/10 years; 95% CI, 2.0-2.8), presence of genotype 1 (hazard ratio 2.2; 95% CI, 1.1-4.2), and alcohol abuse (hazard ratio 4.9; 95% CI, 2.5-9.6) were also associated with a higher risk of ESLD.
Determinants associated with ESLD in patients with chronic hepatitis C
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age at infection, per 10 y | 1.8 (1.6-2.1) | < .001 |
| HCV genotype | .06 | |
| Type 2-5 | 1.0 | |
| Type 1 | 1.9 (1.0-3.6) | |
| History of alcohol abuse | .01 | |
| No | 1.0 | |
| Yes | 2.5 (1.3-4.7) | |
| HIV status | < .001 | |
| Negative | 1.0 | |
| Positive | 5.5 (3.3-9.3) | |
| HAART* | .37 | |
| No | 1.0 | |
| Yes | 0.8 (0.5-1.3) | |
| Multivariate analysis | ||
| Age at infection, per 10 y | 2.3 (2.0-2.8) | < .001 |
| HCV genotype | .02 | |
| Type 2-5 | 1.0 | |
| Type 1 | 2.2 (1.1-4.2) | |
| History of alcohol abuse | < .001 | |
| No | 1.0 | |
| Yes | 4.9 (2.5-9.6) | |
| HIV status | < .001 | |
| Negative | 1.0 | |
| Positive | 13.8 (7.5-25.3) | |
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age at infection, per 10 y | 1.8 (1.6-2.1) | < .001 |
| HCV genotype | .06 | |
| Type 2-5 | 1.0 | |
| Type 1 | 1.9 (1.0-3.6) | |
| History of alcohol abuse | .01 | |
| No | 1.0 | |
| Yes | 2.5 (1.3-4.7) | |
| HIV status | < .001 | |
| Negative | 1.0 | |
| Positive | 5.5 (3.3-9.3) | |
| HAART* | .37 | |
| No | 1.0 | |
| Yes | 0.8 (0.5-1.3) | |
| Multivariate analysis | ||
| Age at infection, per 10 y | 2.3 (2.0-2.8) | < .001 |
| HCV genotype | .02 | |
| Type 2-5 | 1.0 | |
| Type 1 | 2.2 (1.1-4.2) | |
| History of alcohol abuse | < .001 | |
| No | 1.0 | |
| Yes | 4.9 (2.5-9.6) | |
| HIV status | < .001 | |
| Negative | 1.0 | |
| Positive | 13.8 (7.5-25.3) | |
Use of HAART was evaluated in HIV+ patients and considered as a time-dependant variable.
When we stratified for HIV, the effect of genotype 1 (adjusted for age at infection and alcohol abuse) appeared to be stronger in HIV+ patients (hazard ratio 3.3; 95% CI, 1.3-8.1) than in HIV− patients (hazard ratio 1.3; 95% CI, 0.5-3.7).
Discussion
This study showed that 17% of patients with hemophilia with chronic hepatitis C developed ESLD after 35 years of infection. However, HIV was an important determinant of outcome; cumulative incidence of ESLD ranged from 12% in HIV− patients to 35% in patients coinfected with HIV. Duration of HCV infection was also strongly associated with an increased risk of ESLD; hazard rates for all patients with chronic hepatitis C increased from 0.10 in the first decade of infection to 0.90 after more than 20 years of infection. ESLD was independently associated with older age at infection, HIV coinfection, alcohol abuse, and HCV genotype 1.
The present study has several limitations. First, these findings do not necessarily apply to patients without hemophilia because almost all patients were male, were repeatedly infected through repeated infusions with clotting factor, and were, in general, infected at a relatively young age. Secondly, we were not able to determine the incidence of severe fibrosis or compensated cirrhosis because liver biopsies have been performed in only a minority of our cohort patients. Finally, because we were not able to accurately assess the effect of CD4+ lymphocyte counts, we could not corroborate the findings of Goedert et al that lower CD4+ lymphocyte counts in patients coinfected with HIV/HCV were associated with an increased risk of ESLD.11 This reported association has to be further investigated because conflicting data exist. Brau et al reported that progression of fibrosis in patients coinfected with HCV/HIV was independently associated with HIV viral load, necroinflammation on liver biopsy, and age at HCV infection, but not with CD4+ lymphocyte counts.12 They suggest that rather the quality (ie, HCV specificity and magnitude of this immune response) than the quantity of CD4+ lymphocytes is predictive for progression of hepatitis C.
The present study is strengthened by the large number of patients with long follow-up. Furthermore, date of infection, HCV and HIV status, data on start of HAART, HCV genotype, and history of alcohol abuse were precisely recorded in the large majority of patients. In addition, the time variable used in the analyses was time since infection up to ESLD, death, last evaluation, or start of antiviral therapy (ie, the natural history without interference of interferon), minimizing the risk of confounding by indication. For the same reason, HAART was introduced as a time-dependent variable. Finally, only solid, clinical endpoints for ESLD were used, leaving little space for misclassification.
In general, 2 types of classifications have been used in studies on the natural history of HCV infection in patients with inherited bleeding disorders. Some authors define severe liver disease (especially cirrhosis) as the presence of certain laboratory, clinical, and ultrasonographic findings.19,21 These authors found a proportion of patients with severe liver disease ranging from 5% to 16% after 2 decades of infection. Other reports, however, described the natural history of hepatitis C using histologic or hard clinical outcomes (liver failure, hepatocellular carcinoma, liver-related death).7–11 In studies from London and Sheffield, histologic diagnosis of cirrhosis was made in 30% to 41% of patients, after 20 to 25 years of infection.7,8 The discrepancy in proportions of patients with cirrhosis between noninvasive and histologic methods of diagnosis adds to the hypothesis that the risk of cirrhosis may be underestimated in nonhistologic studies.
In a large, multicenter cohort in hemophilia patients (Multicenter Hemophilia Cohort Study [MHCS]), Goedert reported cumulative incidences of ESLD of 15% for patients coinfected with HCV/HIV and of 3% for patients only HCV seropositive after 16 years of follow-up.11 We found much higher cumulative incidences in our cohort; 35% for those coinfected with HIV and 12% for those infected only with HCV. Several factors may account for these differences. First, the follow-up of the MHCS started from May 1982 (the median HIV seroconversion date) or the patients' birth date. This results in a different follow-up, that is, time since HIV infection instead of time since HCV infection. The difference in start of follow-up indicates that a head-to-head comparison of our study with the MHCS has it limitations. Secondly, the age at end of follow-up in our cohort was much higher (43 versus 20 years) indicating that duration of infection was probably longer in our study. In addition, age at infection was expected to be lower in the MHCS, resulting in lower risk for ESLD.8,9 Indeed, in our study risk of ESLD increased strongly with older age at infection. Finally, we assessed the risk of ESLD in patients with chronic hepatitis C, that is, HCV RNA+ patients. In the MHCS paper, incidences of ESLD were determined in HCV-seropositive patients. However, the cumulative incidence in the MHCS would probably have been higher when only HCV RNA+ patients were taken into account because about 20% of patients with an HCV infection spontaneously clear HCV (even a higher percentage when infected at a younger age).9,22
In the present study, risk of ESLD was associated with older age at infection. Several factors may play a role. There is a reduced availability of antioxidizing systems with increasing age, which may lead to a more rapid progression to advanced liver disease.23–25 Furthermore, older patients may have used alcohol for a longer time, which may lead to a less favorable course of hepatitis C.26 Finally, hepatic steatosis may play a role in age-related deterioration of the liver. The amount of fibrosis correlates with body mass index and steatosis.27 In general, older patients have a higher body mass index and may therefore be more vulnerable for HCV-induced liver damage.
Infection with HIV has a major impact on the development of ESLD.10 Whether HAART could improve this detrimental course remains unclear. Early reports showed no or even a negative effect of HAART on HCV viremia.14,28 Others suggested an increased risk of hepatotoxicity in patients using HAART.29 However, several authors reported a beneficial effect of HAART on fibrosis progression and development of severe fibrosis and cirrhosis.12,15 Our findings could not corroborate these reports; HAART appeared to slightly reduce the risk of ESLD but this was not statistically significant. The low number of patients receiving HAART and the relative short follow-up after start of HAART might have contributed to this.
The role of genotype on the clinical course of hepatitis C remains controversial. In the present study, the incidence of ESLD was increased 2-fold with HCV genotype 1. Genotype 1 may be associated with a less robust immune response to HCV and may have a great replication competence, resulting in more severe liver disease (especially in patients with HIV).9 Several studies have also shown that presence of genotype 1 results in a higher risk of ESLD.9,30,31 Others suggest that there is no association between genotype and risk of advanced liver disease.11,25
Future studies must be performed to study the effect of interferon-based regimens on the development of ESLD. In addition, it would be interesting to study the effect of low and moderate alcohol intake on progression of liver disease. Finally, it is important to assess the proportion of hemophilia patients with significant fibrosis or (compensated) cirrhosis by new, noninvasive techniques (eg, Fibroscian).32,33 Especially in patients withgenotype 1 and moderate or severe fibrosis, state-of-the-art antiviral therapy is highly cost effective, preventing progression to ESLD.
In conclusion, after 35 years of infection 12% of HIV− patients with chronic hepatitis C developed ESLD compared to 35% in patients coinfected with HIV. Risk of ESLD increased with duration of HCV infection and was associated with older age at infection, history of alcohol abuse, HCV genotype 1, and presence of HIV.
Authorship
Contribution: D.P. performed research, collected data, analyzed data, and wrote the paper; M.M., T.T.Y., K.F., and E.P.M.-B. designed research, analyzed data, and wrote the paper; J.J.vV. and A.G. collected data and wrote the paper; K.J.vE. designed research and the wrote paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eveline P. Mauser-Bunschoten, Van Creveldkliniek, University Medical Center Utrecht, The Netherlands; e-mail: e.mauserbunschoten@umcutrecht.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank S. C. Gouw for her statistical advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal